Abstract
It is well established that CD4+ CD8+ thymocytes are more sensitive to myriad death stimuli than CD4+ or CD8+ single positive (SP) thymocytes. The mechanism behind this hypersensitivity to apoptosis of CD4+ CD8+ thymocytes is not understood. To test whether the difference lay in the apoptotic preset of mitochondria, established by the BCL-2 family of proteins, we developed a method, FACS-based BH3 profiling. Using this tool, we could discriminate thymocyte subpopulations and demonstrate that mitochondria in double positive (DP) thymocytes are more primed for death than those in single positive counterparts. Loss of proapoptotic BIM, known to cause autoimmunity, also causes loss of “priming.” Priming is a phenotype with physiologic consequences, which can be measured at the single-cell level in complex samples using FACS-based BH3 profiling.
Keywords: BCL-2 |, BIM, negative selection
During thymic development, roughly 98% of CD4+CD8+ double positive (DP) thymocytes undergo apoptosis before maturing to the CD4 or CD8 single positive (SP) stage. CD4+ CD8+ cells are also more sensitive to glucocorticoid and radiation-induced apoptosis than are either CD4 or CD8 SP cells (1, 2). The mechanism by which DP cells are selectively hypersensitive to diverse death signals is unknown.
Apoptosis, a genetically controlled programmed cell death, is a response to many different physiologic and toxic stimuli. Whether a cell will undergo apoptosis is based on competing pro- and antiapoptotic signaling converging at the mitochondrion. The decisive event that commits the cell to death via the intrinsic, or mitochondrial, pathway of apoptosis is mitochondrial outer membrane permeabilization (MOMP) (3). Members of the BCL-2 family of proteins are the primary regulators of MOMP, and can be broken into three groups (4) (Fig. 1A). The multidomain proapoptotic group includes BAX and BAK, which are required for MOMP (5, 6). When they are activated, BAX and BAK undergo conformational changes, homo-oligomerize, and drive formation of pores in the mitochondrial outer membrane that allow the egress of proapoptotic factors, including cytochrome c, SMAC/DIABLO, and omi from the mitochondrial intermembrane space (7). These factors cooperate in the elaboration of many apoptotic phenotypes including the activation of caspases, cysteine proteases that cleave and disable many proteins vital to cellular function. In most circumstances, MOMP may be considered the point of irreversible commitment to apoptosis.
Fig. 1.
BCL-2 family interactions govern MOMP. (A) Death decision circuitry controlled by the BCL-2 family. (B) Interaction map for BH3 peptides and the antiapoptotic BCL-2 members. Red spaces indicate tight binding with a Kd of less than 100 nM as determined by fluorescence polarization. Antiapoptotic BCL-2 proteins have unique binding signatures used to determine their identity in BH3 profiling (13).
The prodeath BH3-only proteins form another group in the family. These are named for their possession of a single region of homology with the rest of the family, the BH3 (BCL-2 homology 3) region. This region is an amphipathic α-helix, the hydrophobic face of which is necessary for interaction with the multidomain pro- and antiapoptotic proteins (8). The BH3 domains are required for the prodeath function of this group of proteins. Furthermore, the BH3 domains by themselves, in the form of oligopeptides, can perform proapoptotic functions. On the basis of function, the BH3 domains and the proteins possessing them can be segregated into two subgroups: activators and sensitizers (9). Activators, which include BID and BIM, can directly activate BAX and BAK, inducing conformational change and oligomerization (10–12). Sensitizers cannot activate BAX or BAK, but exert proapoptotic function by binding to antiapoptotic proteins and displacing either activators or activated, monomeric BAX or BAK (9, 13, 14).
The third group, the multidomain antiapoptotic proteins, includes BCL-2, BCL-XL, MCL-1, BCL-w, and BFL-1. These proteins, localized primarily to the mitochondrion, oppose apoptosis by binding and sequestering activator BH3-only proteins or monomeric, activated BAX and BAK (13, 15, 16). Antiapoptotic function may be competitively antagonized by sensitizer BH3 domains, but interactions between the two groups are not completely promiscuous. Rather, each antiapoptotic protein interacts with a select subgroup of sensitizer BH3 domains (13, 14, 17, 18) (Fig. 1B).Mitochondria on which antiapoptotic proteins are loaded with abundant activators, or perhaps activated BAX or BAK, are particularly sensitive to exposure to sensitizer BH3 domains (13, 19). We refer to such mitochondria, or the cells containing them, as “primed for death” or “primed.” The pattern of response to BH3 peptides can identify the participation of individual antiapoptotic proteins in maintaining cell survival using the interaction pattern in Fig. 1B (13).
Different mitochondria can vary greatly in their receptiveness to prodeath signaling. Therefore, mitochondrial preconditions can be a major determinant of whether a cell lives or dies in response to individual perturbations. A summary parameter of this mitochondrial preset might thus serve as a powerful predictor of cellular response to many perturbations, including cancer response to chemotherapy (19).We have standardized the comparison of such mitochondrial sensitivity to prodeath signaling using a tool we call BH3 profiling (13, 19). The central principle of BH3 profiling is to expose mitochondria to standardized death signals in the form of a panel of BH3 domain peptides, and measure MOMP. Primed mitochondria respond more robustly to both the activator and sensitizer peptides and are more predisposed toward apoptosis. Additionally, primed cells are more sensitive than relatively unprimed cells to myriad insults including DNA damaging agents, microtubule disrupting agents, and the small molecule BCL-2-antagonist ABT-737 (13, 19–21).
Because differences in mitochondrial priming can mediate differences in cellular sensitivity to myriad toxic insults, we hypothesized that DP thymocytes might possess mitochondria that are more primed than in SP counterparts. To test this question required comparing thymocyte subsets by BH3 profiling. However, our previous methods of BH3 profiling could not distinguish subsets in heterogeneous samples as it delivered a bulk measurement of mitochondrial priming. To test this hypothesis we therefore developed a method of BH3 profiling on the basis of fluorescence-activated cell sorting (FACS) that permitted simultaneous single-cell measurement of priming and cell surface marking.
Results
BH3 Profiling in Permeabilized Whole Cells.
To compare the priming by BH3 profiling of thymocyte subsets required developing a method for distinguishing subpopulations in a thymocyte preparation while simultaneously measuring MOMP in single cells. Because FACS is an established method for distinguishing fractions in a heterogeneous mixture, we adapted our prior BH3 profiling methods to FACS. First, we required a fluorescent signal that correlated with MOMP. A typical BH3 profile that measures MOMP by cytochrome c release quantified by ELISA (heavy membrane cytochrome c assays or HM-CC assay, Fig. 2A and Fig. S1A) requires between 107 and 108 viable cells. Originally, heavy membranes were isolated to make the mitochondria accessible to the noncell permeant peptides. MOMP was measured by cytochrome c release from mitochondria, quantified by ELISA (13, 19). We have subsequently tested the dual emission fluorescent probe JC-1, which fluoresces red when concentrated in the matrix of healthy mitochondria (i.e., with normal ΔΨm), and green otherwise, as a measure of MOMP in isolated mitochondria. We found excellent correlation with our HM-CC results (Fig. S1 B and C).
Fig. 2.
ΔΨm measurements in digitonin-permeabilized cells correlate with cytochrome c release from mitochondria. (A) Heavy membrane cytochrome c (HM-CC) BH3 profiling. Tissue samples are broken into single-cell suspensions, then disrupted to create a heavy membrane preparation. Samples are added to peptides in separate reactions and the assay terminated by separation of supernatant and pellet fractions. The fractions are then applied to an ELISA to determine relative cytochrome c release. (B) Whole cell ΔΨm (WC-JC-1) BH3 profiling. Single-cell suspensions are permeabilized via digitonin and stained with JC-1. Permeabilized cells are then exposed to peptides in a 384-well format and the decay of ΔΨm measured as fluorescence at 590 nm. (C) HM-CC BH3 profiling. BCL-2 1863, MCL-1 1780, KM12, or MDA-MB-231 cell lines were incubated with 100 μM peptides in experimental buffer for 45 min at RT. Two Upper panels are done in singlicate; two Lower in triplicate; means shown with bars for SD. (D) WC-JC-1 BH3 profiling at 90 min. Shown are means of three experiments. Bars show SD. (E) Kinetic measurement of cells in B. Fluorescence at 590 nm was used to measure ΔΨm at 5-min intervals. Loss of ΔΨm in whole cells occurs only with peptides that also cause cytochrome c release. Shown are means of three experiments.
Use of a convenient fluorescent MOMP measure allowed us to perform BH3 profiling on whole cells. For peptides to gain access to mitochondria in this case, the cell membrane was permeabilized, creating a novel whole cell JC-1-based BH3 profiling assay (WC-JC-1, Fig. 2B) (22). Final digitonin concentrations of 0.001–0.005% were effective in gently permeabilizing the plasma membrane without compromising mitochondrial potential for up to 3 h in cell lines and primary samples. To stabilize mitochondria in permeabilized cells we also changed our buffer to a trehalose-based buffer described by the Newmeyer laboratory, referred to here as T-EB (23).
We tested WC-JC-1 on four cell lines chosen for their distinct BH3 profiles originally identified using the HM-CC technique. BCL-2 1863 and MCL-1 1780 are murine leukemia lines driven by the overexpression of Myc and either BCL-2 or MCL-1. These two cell lines showed, in agreement with our prior results primed, BCL-2- and MCL-1-dependent profiles (22). KM12, a poorly primed human colon cancer cell line showed almost no cytochrome c release from any peptides. MDA-MB-231, a human breast cancer line, showed a primed, BCL-XL-dependent profile.
HM-CC assays were compared with WC-JC-1 at a single timepoint, with excellent agreement between the two techniques (Fig. 2 C and D). Loss of ΔΨm consistently lagged cytochrome c release by ≈45 min, so a 45-min timepoint is used for HM-CC and a 90-min timepoint for WC-JC-1 readings. An advantage to the WC-JC-1 technique is that kinetic information could also be obtained (Fig. 2E). Furthermore, far fewer cells are required in the whole-cell assay. Although a cytochrome c release assay required ≈108 cells, only 2.5–5 × 104 cells per peptide treatment are needed for the JC-1-based assay. A total of 106 cells are typically sufficient for replicate measurements of all peptides. However these numbers were still too large to permit us to conveniently use a strategy of sorting thymocyte subsets and profiling them separately, a strategy that would moreover subject the cells to longer and more strenuous ex vivo handling.
BH3 Profiling Heterogeneous Samples at the Single-Cell Level by FACS.
Although both our HM-CC and WC-JC-1 BH3 profiling methods can determine apoptotic potential of mitochondria in a sample, the bulk measurement provided cannot distinguish behavior of subpopulations in a heterogeneous sample. Fluorescent detection of MOMP via ΔΨm loss allowed us to adapt WC-JC-1 BH3 profiling to FACS analysis, a method well established to perform single-cell fluorescent measurement (WC-JC-1/FACS, Fig. 3A).
Fig. 3.
FACS BH3 reveals variable priming in perturbed cell lines and simultaneously profiles subpopulations of heterogeneous samples. (A) WC-JC-1/FACS BH3 profiling. Single cell suspensions are cell-surface labeled, washed, and then permeabilized and exposed to peptides. JC-1 is added to stain the mitochondria and their potential is determined by FACS. Individual populations can be gated and profiled simultaneously. (B) FSC vs. SSC was used to exclude cellular debris from analysis (Left). Intact, permeabilized cells were then gated based on SSC vs. pacific blue (anti-B220) staining (Right). Lower B220+ murine leukemia cells could be discerned clearly from B220− adherent human lines. (C) The green:red fluorescence ratio of JC-1 as measured in FITC and PE channels, respectively, was used to determine ΔΨm. DMSO and FCCP/valinomycin controls are shown. A low green to red ratio indicates higher ΔΨm as shown for DMSO. Full depolarization was defined by the FCCP/valinomycin control. (D) Simultaneous profiling of mixed primed and unprimed populations. The percent of the population in the depolarized gate determined the response to peptide treatments. Each cell line's unique BH3 profile was extracted from the mixed populations and corresponds with earlier HM-CC and WC-JC-1 assays. Shown are means of five separate experiments; bars indicate SD. Peptides are used at a final concentration of 100 μM. (E) Simultaneous profiling of mixed primed populations with differing antiapoptotic dependence. Profiling was conducted as in D and bars show the mean of five separate experiments with bars indicating SD.
To test whether WC-JC-1/FACS can distinguish different BH3 profiles in heterogeneous populations of cells, we mixed cells with distinct BH3 profiles and tested the ability of WC-JC-1/FACS BH3 profiling to deconvolute their profiles. Both BCL-2 1863 and MCL-1 1780 cell lines are B220+ murine lymphocytic leukemia cells, allowing us to identify them using anti-B220 pacific blue staining. BCL-2 1863 cells were mixed with KM12 cells to produce a heterogeneous mixture of B220+ primed BCL-2 cells and B220−unprimed cells. (Fig. 3 B–D and Fig. S2). The more primed B220+ population can be seen by the mitochondrial depolarization induced by PUMA and other peptides in the B220+ but not the B220− population (compare Fig. 3D with Fig. 2). Without this discrimination, BH3 profiling of the bulk sample yields an intermediate profile that is inconsistent with that of either of the constituent samples.
WC-JC-1/FACS also distinguishes differences between primed populations that are dependent on different antiapoptotic proteins. MCL-1 1780 was mixed with MDA-MB-231 to produce a primed MCL-1-dependent B220+ population and a primed BCL-XL-dependent B220− population. Again, WC-JC-1/FACS could distinguish the different BH3 profiles within the heterogeneous sample, even when both cell types are primed (Fig. 3E and Fig. S3; note especially the NOXA and BAD signals).
The WC-JC-1/FACS requires ≈106 total cells per replicate profile. It is possible to reduce this by a factor of 2–4 times depending on the volume of cells used, making it useful for samples with limited availability. The assay can be run within 4 h, and two samples can be assayed in parallel, allowing for rapid analysis of samples without need for ex vivo subculture.
WC-JC-1/FACS Detects Differences in Thymocyte Subpopulations.
Having validated the ability of WC-JC-1/FACS to distinguish the BH3 profile of subpopulations of a heterogeneous cell mixture, we turned our attention to measuring the BH3 profiles of thymocyte subpopulations. In the course of T cell development, cells proceed from CD4− CD8− double negative precursors to DP cells before undergoing negative selection. This selection produces CD4+ and CD8+ SP cells. It has long been known that DP thymocytes are more sensitive to myriad toxic stimuli compared with single positive thymocytes, but the mechanism has remained obscure (1, 2). To test the hypothesis that differences in mitochondrial priming underlies the differences in sensitivity to stimuli, we performed WC-JC-1/FACS on murine thymocytes using CD4 and CD8 staining to segregate the relevant single and double positive populations.
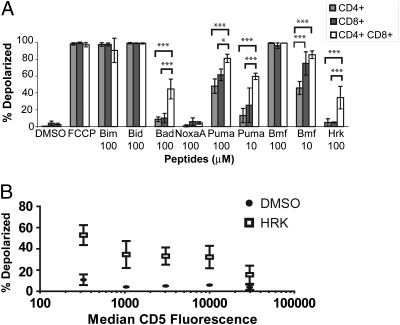
BH3 profiling of thymocyte subpopulations revealed that DP cells were significantly more primed than the mature single positive cells. Of particular note are the increased responses to the sensitizer peptides BAD, PUMA, and HRK (Fig. 4 and Fig. S4 for individual gated FACS readings). Our results demonstrate that the DP population is more sensitive to apoptotic signaling at the mitochondrion. This means that a different mitochondrial apoptotic preset is at least partly responsible for the hypersensitivity to noxious stimuli in DP thymocytes. When the DP population is further subdivided on the basis of CD5 surface marking, which has been found to correspond to maturation in the DP compartment (24), we found that dependence on BCL-XL declined, as indicated by the loss of sensitivity to the HRK peptide (Fig. 4B and Fig. S5). This is consistent with the loss of BCL-XL as cells mature into SP thymocytes.
Fig. 4.
FACS BH3 profiling reveals developmentally controlled priming of thymocytes. (A) Data are the average of three independent experiments and error bars indicate the SD. *P < 0.05 and ***P < 0.001 as determined by two-way ANOVA. (B) DP thymocytes were stained with CD5 as a marker of maturation. Loss of HRK sensitivity corresponds with CD5 expression and the loss of BCL-XL dependence. n = 3. Bars indicate SD.
Prior studies have found that CD8 SP thymocytes are lost when MCL-1 is deleted and that numbers of all thymocytes are dramatically reduced when MCL-1 and BCL-XL are both deleted. We found, however, that NOXA, a selective antagonist of MCL-1, had surprisingly little effect on CD8 SP cells. A likely explanation lies in the fact that BH3 profiling takes a snapshot at a single point in time. Deletion strategies, on the other hand, integrate over the lifetime of the mouse, so that if a CD8 SP cell was ever MCL-1 dependent, it would be deleted. One can imagine that many developmental or immunologic stresses might be brought to bear on a lymphocyte at different times so that a transient MCL-1 dependence might result. Evidence for this might be found in the modest population of CD8 SP cells that are found to depolarize in response to the NOXA peptide (Fig. 5 and Fig. S4B). The ability of the combination of BAD and NOXA peptides to very efficiently depolarize mitochondria is consistent with the He laboratory's observation (25) that combined MCL-1 and BCL-XL deletion is particularly lethal for thymocytes (Fig. S6).
Fig. 5.
BH3 profile correlates with protein levels in thymocytes. (A) Primary wild type or BIM−/− thymocytes were profiled, and loss of BIM correlates with decrease in priming in each population. Average and SD of n = 6 for WT, n = 4 for BIM−/−. *P < 0.05 and ***P < 0.001 as determined by two-way ANOVA. PUMA and BMF peptides are used at 10 μM to stay in dynamic range and all other peptides are used at 100 μM final concentration. (B) Intracellular staining for BIM, BCL-XL, and BCL-2 show differential expression of antiapoptotic proteins in wild-type thymocytes and comparable BIM levels. Average and SD of three samples.
BIM is an essential mediator of apoptosis in thymocytes. Loss of BIM leads to loss of negative selection (Fig. S7), autoimmunity, and resistance to diverse apoptotic stimuli (26, 27). Our BH3 profiling results suggest that the highly primed state of DP cells is what makes them sensitive to apoptotic signaling during negative selection. Our model therefore predicts that loss of BIM would cause a significant reduction in priming. To test this hypothesis, we compared BH3 profiles of thymocytes of BIM−/− and WT mice. (Fig. 5A) Whereas all populations displayed reduced priming and response to all sensitizer peptides, BIM loss caused the most significant decrease in priming in CD4+ CD8+. That some priming still persists is indicated only by a residual signal from the BMF peptide. This result suggest that other proapoptotic proteins may be responsible for a minor portion of the priming, but are inadequate to fully cover the loss of BIM.
In addition to their difference in priming, DP cells also display a difference in their antiapoptotic dependence. Only the DP cells display a strong response to the BAD and HRK peptides, indicating dependence on BCL-XL (Fig. 1B). The prior observation that elimination of BCL-XL by gene targeting selectively shortens survival of DP thymocytes therefore provides validation of the accuracy of FACS-based BH3 profiling (28). The SP cells show little signal from the more selective BAD, HRK, and NOXA peptides, suggesting that no single antiapoptotic protein plays a dominant role in sequestering prodeath proteins.
Previous work has found that BCL-XL is up-regulated while BCL-2 is down-regulated in the CD4+ CD8+ compared with the single positive cells (1, 28–30). To verify this, we conducted intracellular staining for BCL2, BCLXL, and BIM (Fig. 5B and Fig. S8) in wild-type thymocytes. Comparable levels of BIM were found in single and double positive populations. Therefore, BIM levels alone seem an inadequate explanation for differences in priming between single and double positive thymocytes. Instead, a much more complex interplay among pro- and antiapoptotic BCL-2 family proteins is likely responsible. Both CD4 and CD8 SP population showed strong staining for BCL-2, which was nearly undetectable in DP cells. Similarly, staining for BCL-XL was strongest in DP cells, corresponding with their HRK signal in the BH3 profile.
Discussion
Lymphocytic systems have played a very important role in the study of apoptosis, just as apoptosis plays a critical role in the development of lymphocytes. However, study of control of lymphocytic apoptosis in vivo has been hampered by the rapid clearance of apoptotic cells from the circulation and immune organs. The result is that in vivo studies of the apoptosis of lymphocyte populations is largely based on observation of their loss. Here, using FACS-based BH3 profiling, we directly study the apoptotic preset of thymocytes and show that mitochondria of DP cells are inherently more sensitive to BH3-only death signals than are mitochondria from single positive cells . This is important in vivo support for the concept that differences in how primed mitochondria are for death can have critical physiologic significance. When priming is lost via loss of BIM, for instance, negative selection suffers and autoimmunity can result (27).
BH3 profiling is a functional tool. Although it does not directly identify all of the many BCL-2 family proteins involved in this increased priming of DP cells, it is interesting to note, and is a validation of the method, that BH3 profiling detects in particular an increase in sensitivity of DP mitochondria to the HRK peptide. The HRK peptide interacts with high affinity only with BCL-XL among the antiapoptotic proteins. Taken with the rest of the BH3 profile, this is diagnostic of a singular dependence on BCL-XL in the DP cells. This is notable because it has been shown previously as well as here (Fig. 5B) that among all of the stages of T cell development, it is at the CD4+ CD8+ stage that BCL-XL is most highly expressed (1, 28).
Moreover, the significance of the finding that all of the thymic lymphocytes studied here are primed for death, although to varying degrees, should not be overlooked. Others have found that in peripheral T cells BIM constitutively binds BCL-2 (31), consistent with a primed, BCL-2-dependent phenotype we have observed in circulating mononuclear cells (20). Therein may lie the basis for two basic, broadly accepted observations. First, there is the observation of the chemosensitivity of normal lymphoid cells. Transient lymphopenia is a common side effect of many types of chemotherapy. Second, there is the general chemosensitivity of lymphoid malignancies as a group. Many lymphoid cancers are curable with chemotherapy alone, and almost all demonstrate an initial response to chemotherapy. This property may arise from the fact that lymphoid malignancies are derived from normal cells that are already primed to be receptive to apoptotic signaling. For instance, we have found that primary CLL cells and lymphoma cell lines tend to be highly primed (19, 20).
Our studies of CD4+ CD8+ lymphocytes required a different technology, FACS-based BH3 profiling (WC-JC-1/FACS). During our development of this technology, we had to switch from a MOMP readout of cytochrome c release to one of ΔΨm loss using the fluorescent JC-1 dye. There is ongoing study in the field of apoptosis of how closely linked mitochondrial permeabilization is to ΔΨm loss (32–34). It is therefore worth noting that our studies on dozens of cell lines, including this study, show a very tight correlation between the two events, although ΔΨm loss invariably lags behind cytochrome c release by 30–60 min in isolated mitochondria and permeabilized cells. For comparison with prior experiments, it is worth noting that we used oligomycin to prohibit regeneration of ΔΨm via the reversible ATP synthase.
This tool allows us to compare sensitivity to apoptotic signaling of heterogeneous cells in virtually any kind of tissue or blood sample that can be converted to a single cell suspension. We believe that this technology allows us to explore an entirely new facet of in vivo biology, so the potential applications are quite numerous. As there is no need for ex vivo subculture, one can obtain a direct view of apoptotic signaling as it occurs in vivo. An immediate application of FACS-based BH3 profiling is the study of blood cells. Immunology seems a particularly obvious application, because the cell surface marking of many functionally disparate subsets of cells has been a major focus of this discipline over decades. Now we have the capacity to ask how different subsets differ in their propensity to undergo mitochondrial apoptosis. We can also ask how different perturbations, such as antigen exposure, activation, or cytokine exposure affects different subsets in vitro or in vivo. Prior study of apoptotic signaling of blood cells in vivo has been difficult because early apoptotic leukocytes are cleared extremely rapidly. FACS-based BH3 profiling permits the study of apoptotic signaling at a point before elaboration of signals that induce rapid phagocytosis.
Whether or not to undergo apoptosis is one of the most important decisions a cell can make. We thus anticipate exploiting FACS-based BH3 profiling in the study of cancer cells and chemosensitivity, as well as in the study of nonmalignant physiologic systems subjected to a variety of stresses. FACS-based BH3 profiling provides a convenient measure of sensitivity to apoptotic signaling at the single-cell level in any tissue. This allows for the analysis of heterogeneity of apoptotic response in many types of cell preparations, including cancer cells, where heterogeneity of response is such a critical issue. We think this technology has the potential to find application in a wide range of clinical, biological, and translational investigation.
Materials and Methods
WC-JC1-BH3 assays were conducted on a Tecan Safire 2 with Ex 545 ± 20 nM and Em 590 ± 20 nm. Statistics were calculated in GraphPad Prism 5. Anti-B220 pacific blue, anti-CD19-pacific blue, anti-CD5 biotin, anti-CD4-Alexa647, anti-CD8-APC-Cy7, anti-BCL2-PE (BD Pharmingen), anti-BIM-PE (Santa Cruz), and anti-BCL-XL-FITC (Cell Signaling), and streptavidin-pacific blue were used. A total of 20 mg/mL oligomycin (Sigma), 100 μM JC-1 (Invitrogen), and 5% digitonin (Sigma) in DMSO were stored at −20 °C. A combination of 1 mM FCCP and 0.5 mM valinomycin (Sigma) in DMSO was stored at −20 °C. Peptides were synthesized and HPLC purified at the Tufts University Physiology Core Facilty. A total of 10 mM peptide solutions in DMSO were stored at −80 °C. Trehalose, KCl, malate, glutamate, sucrose, EDTA, EGTA, Tris base, Mops acid, BSA, and succinic acid (Sigma) were used as received.
Flow Cytometry.
FACS measurements were conducted on a BD FACS Canto II with lasers at 407, 488, and 633 nm and FACS Diva version 6.1.1. Pacific blue was measured on the 407-nm laser with a 450/50-nm filter, JC-1 from the 488-nm laser using a 530/30-nm filter (FITC) and a 585/42-nm filter (PE), APC and APC-Alexa750 from the 633-nm laser using a 660/20-nm (APC) and a 780/60-nm (APC-Cy7) filter, respectively. JC-1 ratio measurements were calculated in Diva as the FITC/PE ratio at 25% scaling.
ΔΨm BH3 Profile Using Whole Cells (WC-JC-1).
Cell densities range from 1 × 104 to 5 × 104 cells/well for adherent or suspension lines respectively.15 μL of 200 μM peptides in T-EB (300 mM Trehalose, 10 mM hepes-KOH pH 7.7, 80 mM KCl, 1 mM EGTA, 1 mM EDTA, 0.1% BSA, 5 mM succinate) (23) were deposited per well in a black 384-well plate (BD Falcon no. 353285). Single cell suspensions were washed in T-EB before being resuspended at 4× their final density. One volume of the 4× cell suspension was added to one volume of a 4× dye solution containing 4 μM JC-1, 40 μg/mL oligomycin, 0.02% digitonin, 20 mM 2-mercaptoethanol in T-EB. This 2× cell/dye solution stood at RT for 5–10 min to allow permeabilization and dye equilibration. A total of 15 μL of the 2× cell/dye mix was then added to each treatment well of the plate, shaken for 15 s inside the reader, and the fluorescence at 590 nm monitored every 5 min at RT.
WC-JC-1/FACS for Cell Lines.
Single-cell suspensions of human colon or breast cancer lines were mixed with a roughly equal number of B220+ murine leukemia cells. Cells were washed in 1% BSA/PBS and resuspended at a density of 1 × 107/mL on ice. Anti-B220 pacific blue (1:400) was added, and the cells stained in the dark at 4 °C for 30 min. The cells were washed in T-EB and resuspended at a density of 5 × 105 cells/mL 100 μL of cell suspension was added to 100 μL of a solution containing 0.002% digitonin, 20 μg/mL oligomycin, and 200 μM peptides in a FACS tube to give a final density of 2.5 × 104 cells/mL in 100 μM peptide/0.001% digitonin/10 μg/mL oligomycin in T-EB. Cells were incubated at RT for 90 min in the dark before 25 μL of 900 nM JC-1 was added and the solution gently mixed. Cells were JC-1 stained for 30 min before FACS. A medium flow rate was used to avoid low count rates or lost cells. FSC vs. SSC gating excluded debris, and pacific blue vs. SSC identified the two cell lines. The green:red fluorescence ratio was measured using the FITC and PE channels. Complete depolarization was defined by a gate setting the FCCP/valinomycin control as 98–100% depolarized. Response to each treatment was listed as the percentage of cells within the depolarized gate.
WC-JC-1/FACS of Primary Thymocytes.
Single suspension of murine thymocytes was prepared from wild-type C57BL6/J or BIM−/− B6/129 mice age 6–8 wk. Cells were washed in 1% BSA/PBS and resuspended in 1% BSA/PBS containing a 1:100 dilution of anti-CD19-pacific blue, anti-CD4-Alexa647, and anti-CD8-APC-Cy7 for 30 min on ice in the dark. For CD5, 1:100 CD5-biotin replaced CD19 followed by 1:400 streptavidin-pacific blue for 15 min. After staining, cells were washed in PBS, suspended in T-EB at 1 × 106 cells/mL, and 100 μL of this solution was added to each FACS tube containing 100 μL 0.002% digitonin, 20 μg/mL oligomycin, and 20 μM peptides in T-EB. The cells were incubated at RT for 90 min before adding 25 μL of 900 nM JC-1 in T-EB. Thirty minutes after JC-1 addition, the cells were analyzed by FACS for loss of ΔΨm as indicated by the ratio of FITC to PE channels.
Intracellular Protein Staining and FACS.
Fresh thymocytes were counted and suspended in 1% BSA/PBS at 107 cells/mL and stained with anti-CD19, anti-CD8, and anti-CD4 at 1:100 dilution for 30 min on ice. Cells were pelleted and washed in 1% BSA-PBS before fixation in 250 μL perm/fix buffer (BD Bioscience) per 106 cells. Cells were washed twice in perm/wash buffer, blocked with 2% normal rabbit sera and normal hamster IgG, and stained with anti-BCL2, anti-BCL-XL, anti-BIM or their corresponding isotype controls for 1 h at RT followed by two washed in perm/wash buffer and a final was in PBS before FACS.
Supplementary Material
Acknowledgments
We thank John Daley for sharing FACS machine expertise, Joe Opferman and Nick Souders for advice, and Glenn Dranoff for very helpful critical comments. A.L. and J.A.R. gratefully acknowledge support from National Institutes of Health Grant R01CA129974. A.L. is a Leukemia and Lymphoma Society scholar.
Footnotes
Conflict of interest statement: A.L. is both a cofounder of and equity holder in Eutropics Pharmaceuticals, which has a license for the BH3 profiling technology. J.A.R. has consulted for Eutropics Pharmaceuticals.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914878107/-/DCSupplemental.
References
- 1.Grillot DA, Merino R, Núñez G. Bcl-XL displays restricted distribution during T cell development and inhibits multiple forms of apoptosis but not clonal deletion in transgenic mice. J Exp Med. 1995;182:1973–1983. doi: 10.1084/jem.182.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 3.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 4.Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 5.Lindsten T, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei MC, et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 8.Boyd JM, et al. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995;11:1921–1928. [PubMed] [Google Scholar]
- 9.Letai A, et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 10.Gavathiotis E, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovell JF, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Wei MC, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 13.Certo M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Kuwana T, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Cheng EH, Levine B, Boise LH, Thompson CB, Hardwick JM. Bax-independent inhibition of apoptosis by Bcl-XL. Nature. 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 16.Cheng EH, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Opferman JT, et al. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 19.Deng J, et al. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Del Gaizo Moore V, et al. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood. 2008;111:2300–2309. doi: 10.1182/blood-2007-06-098012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunelle JK, Ryan J, Yecies D, Opferman JT, Letai A. MCL-1-dependent leukemia cells are more sensitive to chemotherapy than BCL-2-dependent counterparts. J Cell Biol. 2009;187:429–442. doi: 10.1083/jcb.200904049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi R, et al. Mitochondria frozen with trehalose retain a number of biological functions and preserve outer membrane integrity. Cell Death Differ. 2007;14:616–624. doi: 10.1038/sj.cdd.4402035. [DOI] [PubMed] [Google Scholar]
- 24.Punt JA, Suzuki H, Granger LG, Sharrow SO, Singer A. Lineage commitment in the thymus: Only the most differentiated (TCRhibcl-2hi) subset of CD4+CD8+ thymocytes has selectively terminated CD4 or CD8 synthesis. J Exp Med. 1996;184:2091–2099. doi: 10.1084/jem.184.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dzhagalov I, Dunkle A, He YW. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol. 2008;181:521–528. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouillet P, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 27.Bouillet P, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 28.Ma A, et al. Bclx regulates the survival of double-positive thymocytes. Proc Natl Acad Sci USA. 1995;92:4763–4767. doi: 10.1073/pnas.92.11.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gratiot-Deans J, Ding L, Turka LA, Nuñez G. bcl-2 proto-oncogene expression during human T cell development: Evidence for biphasic regulation. J Immunol. 1993;151:83–91. [PubMed] [Google Scholar]
- 30.Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993;151:2546–2554. [PubMed] [Google Scholar]
- 31.Zhu Y, et al. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc Natl Acad Sci USA. 2004;101:7681–7686. doi: 10.1073/pnas.0402293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colell A, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 33.Ricci JE, et al. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Zamzami N, Kroemer G. Methods to measure membrane potential and permeability transition in the mitochondria during apoptosis. Methods Mol Biol. 2004;282:103–115. doi: 10.1385/1-59259-812-9:103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.