Abstract
Drug-induced dyskinesias in dopamine-denervated animals are known to depend on both pre- and postsynaptic changes of the nigrostriatal circuitry. In lesion models used thus far, changes occur in both of these compartments and, therefore, it has not been possible to dissect the individual contribution of each compartment in the pathophysiology of dyskinesias. Here we silenced the nigrostriatal dopamine neurotransmission without affecting the anatomical integrity of the presynaptic terminals using a short-hairpin RNA-mediated knockdown of tyrosine hydroxylase enzyme (shTH). This treatment resulted in significant reduction (by about 70%) in extracellular dopamine concentration in the striatum as measured by on-line microdialysis. Under these conditions, the animals remained nondyskinetic after chronic L-DOPA treatment, whereas partial intrastriatal 6-hydoxydopamine lesioned rats with comparable reduction in extracellular dopamine levels developed dyskinesias. On the other hand, apomorphine caused moderate to severe dyskinesias in both groups. Importantly, single-dose L-DOPA challenge in apomorphine-primed shTH animals failed to activate the already established abnormal postsynaptic responses. Taken together, these data provide direct evidence that the status of the presynaptic, DA releasing compartment is a critical determinant of both the induction and maintenance of L-DOPA–induced dyskinesias.
Keywords: adeno-associated virus, dopamine, Parkinson's disease, RNA interference, shRNA
Treatment-induced motor complications are a major problem in management of patients suffering from Parkinson's disease (PD) (1). Dyskinesias induced by L-DOPA, in particular, constitute a significant challenge that impacts a higher proportion of the treated patients with treatment duration. Essentially all patients are expected to develop dyskinesias within a decade from onset of treatment (2). The underlying mechanisms of L-DOPA–induced dyskinesias (LIDs) are still not fully understood. Current views suggest that both presynaptic (i.e., production, storage, controlled release, and reuptake of dopamine by nigrostriatal dopaminergic neurons) and postsynaptic (i.e., status of receptors and second messenger signaling pathways in striatal neurons) components are critical in induction and maintenance of dyskinesias (3–5). However, the destruction of the presynaptic dopamine (DA) terminals, typically obtained by administration of a specific neurotoxin in animals, and the plastic changes induced in the postsynaptic striatal neurons occur at the same time. Moreover, synaptic changes secondary to chronic drug treatment further complicate the interpretation of the observations made in studies using animal models of PD (4–6).
The aim of this study was to tease apart the contribution of the pre- and postsynaptic compartments in the pathophysiology of LIDs in the parkinsonian brain. The abnormal response of the striatal neurons to DA receptor stimulation following chronic DA depletion could be determined solely by mechanisms intrinsic to the striatal cells or alternatively, the functional activity of the presynaptic compartment could determine whether or not L-DOPA treatment results in development of dyskinsias. We hypothesized that the response of the postsynaptic striatal neuron remains under the control of the presynaptic DA terminals even in dyskinetic animals. However, this hypothesis could not be tested using the classical neurotoxin lesion paradigms.
Recent advances in in vivo gene transfer techniques using viral vectors and targeted silencing of specific gene expression using RNA interference mechanisms have created a unique opportunity to tackle this question (7–10). Combining these two technologies provides a tool to knockdown the tyrosine hydroxylase (TH) expression, known to be the rate-limiting enzyme in synthesis of DA, selectively in the nigrostriatal projection system (11, 12). Here, we have expressed a short-hairpin RNA-mediated TH knockdown construct (shTH) using a recombinant adeno-associated virus carrying AAV5 capsid protein (rAAV5). In this model, the DA synthesis machinery is functionally silenced in a targeted manner in nigral DA neurons, whereas the structural integrity of the cells and their terminals are maintained (12). This unique experimental system allowed us to explore the specific contribution of the presynaptic compartment in the induction and maintenance of LIDs.
Results
To provide conclusive data to prove or dispute the hypothesis, we first investigated whether two critical conditions were met following rAAV5-mediated shTH expression: (i) the structural integrity of the nigral DA neurons and their terminals needed to be maintained such that DA stores in these cells could be replenished by peripheral L-DOPA supplement; and (ii) under baseline conditions, extracellular DA levels in vivo had to be at subphysiological levels such that the postsynaptic cells would develop supersensitivity to direct DA receptor stimulation. Thus, we first tested the validity of these conditions.
Long-Term rAAV5-Mediated shTH Expression in Nigral DA Neurons Leads to a Functional Knockdown of DA Input in the Striatum.
We verified the transduction efficacy and the specific TH knockdown by performing triple immunohistochemical staining against the DAergic markers TH and vesicular monoamine transporter-2 (VMAT2) as well as the GFP marker protein coexpressed by rAAV5-shTH and rAAV5-shTHscr vector constructs (Fig. S1). Injection of rAAV5 vectors encoding for both shTH and shTHscr resulted in expression of the transgene in vast majority of the cells. shTH expression led to specific down-regulation of the TH protein, whereas in the shTHscr group all three proteins were coexpressed in the DAergic neurons.
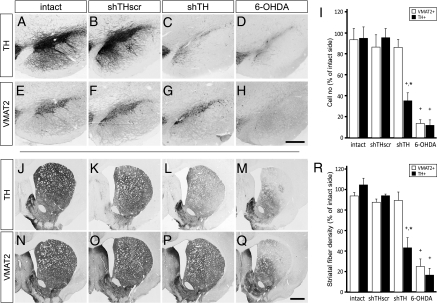
We confirmed the maintenance of long-term down-regulation of TH expression in the nigral DAergic neurons in the absence of any structural damage using two sets of tools. First, at the histological level, we quantified the numbers of TH and VMAT2-positive cells in the substantia nigra pars compacta (SN) and the density of fiber innervation in the striatum. Estimation of the total number of nigral DAergic cells using stereological techniques showed that the TH- and VMAT2-positive cell population in the SN remained unchanged in the intact and shTHscr groups (Fig. 1 A, B, E, F, and I). As expected, the 6-OHDA lesion led to 88.0 ± 5.2% and 86.3 ± 3.6% decrease in TH- and VMAT2-positive cells in the SN, respectively (Fig. 1 D and H). In the shTH group, on the other hand, there was a 64.6 ± 7.7% reduction in TH-positive cells, whereas the VMAT2-positive neuron counts were not different from the normal controls confirming that the DAergic cells that no longer expressed the TH enzyme remained viable throughout the experiment (Fig. 1 C and G).
Fig. 1.
Long-term down-regulation of TH by rAAV-mediated shRNA expression in the SN and striatum. Both TH and VMAT2 phenotypic markers were maintained at normal levels (intact; A, E, J, and N) after expression of scrambled control sequence (shTHscr; B, F, K, and O), whereas in the active knockdown group (shTH), TH protein was selectively reduced in the cell bodies (C) and at the fiber terminals (L), whereas the VMAT2 expression remained unchanged (G and P) In the 6-OHDA lesioned rats, on the other hand, neurodegeneration in the SN was accompanied with loss of both TH (D and M) and VMAT2 (H and Q) immunostaining. The magnitude and specificity of the down-regulation in TH enzyme is shown by stereological quantification of TH and VMAT2-positive cell numbers in the SN (I) and semiquantitative optical densitometry measurements in the striatum (R). Data are analyzed by two way factorial ANOVA [I, group vs. phenotypic marker effect F(7,27) = 16.497, P < 0.0001; R, group vs. phenotypic marker effect F(7,27) = 26.841, P < 0.0001], followed by Tukey's HSD post hoc. Error bars represent ± SEM. *Different from intact and shTHscr control group within the same phenotypic marker; +TH-positive cells or fiber density different from the corresponding VMAT2-positive cells or fiber density in the same group. [Scale bars, 50 μm (in H for A–H) and 100 μm (in Q for J–Q).]
At the striatal terminal level, TH and VMAT2 fiber densities in the two control groups were not different between the two sides of the brain (Fig. 1 J, K, N, O, and R). There was a clear loss of both the TH- and VMAT2-positive fibers on the lesioned side in the 6-OHDA lesioned rats. Reduction in the fiber density in this group was 83.4 ± 6.5% and 74.9 ± 7.3% compared with the uninjected control side in TH and VMAT2 stained sections, respectively (Fig. 1 M and Q). The dissociation between the two markers was obvious in the shTH-expressing rats (Fig. 1 L and P), as TH-positive fiber density was reduced by 56.7 ± 9.8%, whereas VMAT2-positive fiber density remained unchanged (Fig. 1R).
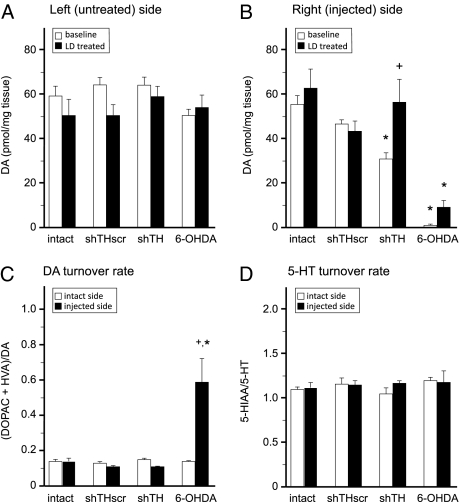
The second set of analysis was carried out at the biochemical level. Striatal samples from each group were processed for HPLC analysis to measure the tissue content of DA, serotonin (5-HT), and their metabolites under baseline conditions, and in separate animals, after L-DOPA treatment (Fig. 2). The levels of DA on the uninjected side of the brain were not significantly different in any group, nor were they modified by L-DOPA treatment (Fig. 2A). On the injected side, however, DA levels in the shTH and 6-OHDA groups were lower than the control groups. Only in the shTH group, L-DOPA administration resulted in significant reconstitution of DA levels. In fact, after L-DOPA treatment, the values were no longer different from controls providing evidence that complete reconstitution of DA stores were possible in the shTH treated animals. On the other hand, in the 6-OHDA group, where the terminals were structurally damaged and removed, this capacity was lost (Fig. 2B). Moreover, in the 6-OHDA, but not in the shTH group, DA turnover rates were abnormally high (Fig. 2C). 5-HT turnover was not modified in any of the groups illustrating that the changes in the DA system were specific (Fig. 2D). Complete list of all biochemical analysis is given in Tables S1–S4.
Fig. 2.
Quantification of monoamines in striatal tissue samples using HPLC. Two groups of animals were analyzed to determine the concentrations of DA and its metabolites under baseline conditions or following L-DOPA treatment on the uninjected control side (A) and injected side (B). In addition the turnover rate for DA (C) and 5-HT (D) were calculated. Data are analyzed by two-way factorial ANOVA [B, treatment vs. group effect F(7,37) = 13.479, P < 0.0001; C, group vs. side effect F(7,41) = 5.194, P < 0.0001], followed by Tukey's HSD post hoc test. Error bars represent ± SEM. *Different from intact and shTHscr control groups within the same condition (A and B) or side (C and D). +Different from baseline condition (B) or intact side (C).
rAAV5-Mediated Knockdown Leads to a Significant Decrease in the Extracellular DA Levels in the Striatum.
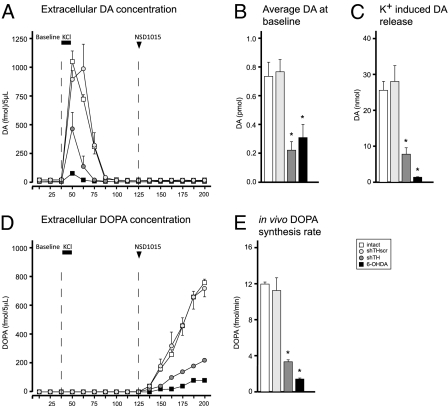
To test the validity of the second assumption, i.e., that the extracellular DA concentration in the shTH group in vivo would be reduced, we performed a microdialysis experiment using an online HPLC system with high sensitivity (design illustrated in Fig. S2). Using this system, we analyzed the extracellular DA and DOPA content in the striatum by using a three-phase microdialysis protocol. In phase I, we monitored the extracellular DA concentrations under baseline conditions (Fig. 3A), and found that they were reduced by 69.6 ± 8.2% and 57.7 ± 12.5% in the shTH and 6-OHDA groups, respectively (Fig. 3B). This suggested that, although the DA loss in the two experimental treatment groups is mechanistically different from each other, at the functional level, the extracellular DA depletion as measured by microdialysis were comparable. In phase II, we applied high concentration of KCl in dialysate and found that the readily releasable pool of DA was reduced by 69.4 ± 7.0% and 94.5 ± 0.3% in the shTH and 6-OHDA groups, respectively (Fig. 3C). In phase III, we inhibited the aromatic acid decarboxylase (AADC) enzyme in the brain by administering 3-hydroxybenzylhydrazine dihydrochloride (NSD-1015; 100 mg/kg i.p. injection) and monitored the in vivo build-up of DOPA in the striatum. Inhibition of the AADC enzyme led to accumulation of DOPA in all groups. However, the rate was significantly decreased in shTH and 6-OHDA groups (71.4 ± 1.0% and 87.2 ± 0.5%, respectively; Fig. 3 D and E).
Fig. 3.
Online in vivo microdialysis. Baseline (B) and KCl-induced (C) DA release were estimated by calculating the area under the curve in A at the respective time intervals. In the third phase, the animals received a systemic injection of NSD-1015, and in vivo accumulation of DOPA was monitored via the microdialysis probe (D) to estimate the DOPA synthesis rate (E). Statistical comparisons were performed by one-way ANOVA [B, F(3,10) = 11.975, P < 0.01; C, F(3,10) = 19.354, P < 0.0001; E, F(3,10) = 126.806, P < 0.0001] followed by Tukey's HSD post hoc test. Error bars represent ± SEM. *different from intact and shTHscr controls.
Primed Striatal Neurons in Dyskinetic Rats Remain Responsive to Normal Modulation of Activity by DA Released from DAergic Terminals.
The results of the biochemical analysis provided evidence that both assumptions held true in this experimental setting and thus allowed us to test the hypothesis that striatal neurons would remain responsive to modulation by DA terminals even after maladaptive plasticity had developed.
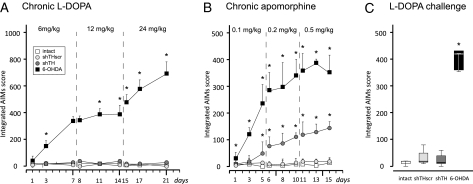
We addressed this issue in two consecutive steps. First, as the biochemical data suggested that shTH expressing rats had a very good buffering capacity for handling the newly synthesized DA upon exogenous L-DOPA administration, we expected that these animals would be resistant to develop dyskinesias despite chronic treatment with L-DOPA. The experimental results supported this view. L-DOPA treatment was carried out so that the animals received daily injections in an escalating dose regimen of 6, 12, and 24 mg/kg s.c. over a 3-wk treatment period. As expected, the 6-OHDA lesioned rats gradually developed dyskinesias in a time and dose dependent manner, as measured using a well established abnormal involuntary movements (AIMs) scale (Fig. 4A) (13, 14). On the other hand, the shTH group remained similar to the control groups and did not show any abnormal movements throughout the 3-wk testing period. Conversely, as the extracellular DA levels were reduced in both shTH and 6-OHDA treated rats, we expected that both groups would be sensitive to treatment with apomorphine, a direct D1/D2 receptor agonist, targeting the postsynaptic site. This was indeed the case. Apomorphine treatment was given at escalating doses (0.1, 0.2, and 0.5 mg/kg) over three 5-d blocks. During this treatment period not only the 6-OHDA–lesioned rats, but also the shTH group, developed dyskinesias upon apomorphine treatment, albeit at different severities, whereas control groups did not exhibit any AIMs (Fig. 4B). In the shTH treated rats orolingual dyskinesias and axial dystonia were the predominant manifestations, whereas 6-OHDA lesioned animals displayed high frequency and severity of dyskinesias of all three components, involving also the abnormal limb movements. Both groups of animals developed similar level of locomotive dyskinesia, seen as full body rotation (Fig. S3).
Fig. 4.
Induction of dyskinesias by daily L-DOPA and apomorphine treatment using a dose-escalation regimen. The animals were scored three times at each dose level for development of abnormal involuntary movements (AIMs). Chronic L-DOPA administration was carried out over 21 d of three 7-d treatment blocks or 6 mg/kg, 12 mg/kg, and 24 mg/kg (A). DA receptor stimulation was done by administration of apomorphine at 0.1, 0.2, and 0.5 mg/kg doses over 15 d (B). Some animals that have completed the apomorphine sensitization regimen were challenged with a single dose of 24 mg/kg L-DOPA on day 16 (C). Data are shown as median values in all panels. In A and B the error bars show 75% percentiles, whereas in C box plots mark the 50% percentiles and the whiskers indicate 95% percentiles. Statistical comparisons in A and B were performed using Friedman test, time effect P < 0.0001, group effect P < 0.0001. Individual comparisons in A, B, and C were performed by Kolmogorov-Smirnov test and P values were compensated for false discovery rates. *Different from intact and shTHscr controls.
These results led us to the second step where, after 15 d of apomorphine injections, we administered a single high dose of L-DOPA (24 mg/kg) in a subset of animals. In this scenario, both the shTH and 6-OHDA treated rats had been primed with apomorphine and displayed clear dyskinetic behaviors. As expected, in the 6-OHDA group, all of the animals responded to L-DOPA with equally severe dyskinesias, whereas in the shTH group no abnormal behaviors were seen (Fig. 4C). The shTH expressing remained indistinguishable from both the intact and shTHscr treated control animals during the entire observation period of 150 min after the drug administration.
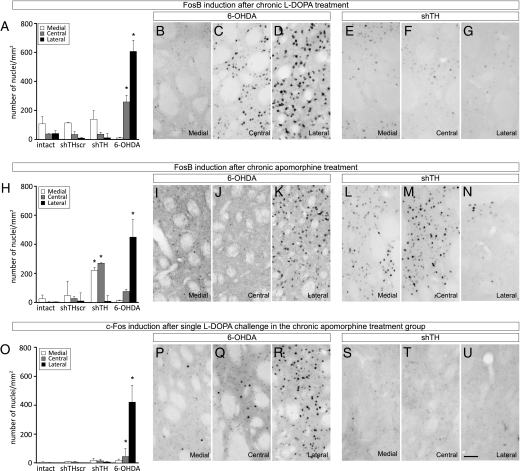
To generate the final data to support the hypothesis, we analyzed the immediate early gene expression in the striatum of all animals that received both drug treatments. For this purpose the striatal sections were immunostained using antibodies against FosB (to illustrate the effect of the chronic drug treatment) and against c-Fos (to illustrate the effect of the acute drug challenge on the last day), and quantified the numbers of immunopositive cells for each marker in the lateral, central, and medial striatum separately (the placement of regions of interest are shown in Fig. S4). Chronic L-DOPA treatment resulted in an increased number of FosB-positive nuclei in the 6-OHDA lesioned animals, which was most prominent in the central and lateral striatum (Fig. 5 C and D; quantified in A). We did not observe any significant FosB induction in the shTH group (Fig. 5 E–G), supporting the observations at the behavioral level, nor did we see FosB induction in intact and shTHscr control groups following chronic L-DOPA treatment (Fig. 5A).
Fig. 5.
Analysis of FosB and c-Fos induction following L-DOPA and apomorphine treatment. The numbers of c-Fos and FosB-positive cells were assessed on three coronal sections in the striatum as shown in Fig. S4. (A–G FosB induction after chronic L-DOPA treatment; (H–N) FosB induction after chronic apomorphine treatment; (O–U) c-Fos induction in the striatum after a single dose 24 mg/kg L-DOPA challenge in the animals that were primed with chronic apomorphine treatment. Note that the FosB and c-Fos photomicrographs in Middle and Bottom rows are taken from adjacent series of sections processed from the same animal. The contrast between 6-OHDA lesions and rAAV5-mediated TH knockdown is illustrated with two panels under each condition representing the medial, central, and lateral striatal expression of the two gene products. Quantification of the FosB (A and H) and c-Fos (O) positive nuclei after chronic L-DOPA (A), chronic apomorphine (H), and acute L-DOPA (O) treatments are illustrated as bar graphs. Statistical comparisons for each striatal area were performed by one-way ANOVA [A, lateral striatum F(3,21) = 47.425, P < 0.0001; central striatum F(3,21) = 6.065, P < 0.05; H, lateral striatum F(3,14) = 8,124, P < 0.005; central striatum F(3,14) = 3.478, P < 0.05; medial striatum F(3,14) = 3,719, P < 0.05; O, lateral striatum F(3,13) = 4.977, P < 0.05] followed by Tukey's HSD post hoc test. Error bars represent ± SEM. *Different from the number of positive-nuclei from the corresponding striatal region in other groups. (Scale bar, 50 μm in U for B–G, I–N and P–U.)
Chronic apomorphine treatment, on the other hand, led to increased FosB-positive cells both in 6-OHDA and shTH, but not in the control groups (Fig. 5H). In the 6-OHDA group, the induction was almost exclusively in the lateral striatum (Fig. 5, compare K with I and J), whereas in the shTH treated rats, the FosB-positive cells were mostly in the medial and central areas (Fig. 5, compare L, M, and N). A single 24-mg/kg L-DOPA injection after chronic apomorphine treatment led to a comparable level of induction of c-Fos in the 6-OHDA group (Fig. 5 Q and R; quantified in O), whereas in the shTH group, no acute c-Fos induction was seen after L-DOPA treatment (Fig. 5 S–U). This observation constituted the final piece of evidence showing that presynaptic DA terminals could retain the functional control of the postsynaptic striatal neurons even after the establishment of dysplastic changes in these neurons.
Discussion
This study was designed to address a critical yet unanswered question regarding the relative contribution of the pre- and postsynaptic compartments in induction and maintenance of drug-induced dyskinesias in PD. The difficulty in assessing the impact of a single compartment on the occurrence of motor complications originates from the inability to dissociate changes induced by each compartment independently (15). Thus the novelty of our current approach was to use viral vector-mediated long-term shRNA expression to functionally knockdown DA synthesis, without destruction of the presynaptic terminals. As the manipulation is unilateral and involves only the nigral DA neurons, these rats eat and drink normally and maintain a normal general health status. Using this experimental model, we showed that chronic stimulation of the postsynaptic DA receptors by a direct D1/D2 agonist (apomorphine) induced dyskinesias, whereas administration of L-DOPA failed to induce any abnormal movements. Moreover, rats that have been already primed with apomorphine treatment did not display any dyskinesias upon an acute high dose challenge with L-DOPA. These behavioral observations were correlated with immediate early gene activation seen in the respective groups. Taken together, in the present model where the structural integrity of the DA terminals is maintained, the response of the postsynaptic striatal neurons to stimulation of DA receptors was strictly controlled by the presynaptic activity. Thus, it appears from our data that the state of the presynaptic machinery is a critical determinant of the induction and maintenance of the LIDs.
Experimental studies have thus far focused on changes that occur at the postsynaptic site as the leading mechanism for induction and maintenance of dyskinesias. The data supporting this view argue that upon lesion of DAergic input to the striatum, and following treatment with L-DOPA or direct DA-agonists, striatal neurons undergo dysplastic changes (16). Alterations in postsynaptic DA receptors on striatal neurons have been formulated as denervation supersensitivity (17–19). Additionally there are also changes in the activity of other neurotransmitter systems such as glutamate, 5-HT, acetylcholine, and adenosine (16, 20–23). It is thought that the changes at the receptor level cause further modification of downstream cascades including second messenger systems and signaling pathways (3, 24). Based on the structural and functional changes described above, it has been argued that postsynaptic mechanisms are critical for the development of dyskinesias in PD.
The alternative view emphasizes the importance of the presynaptic compartment in the pathophysiology of motor complications following chronic L-DOPA treatment and is primarily based on observations in PD patients. In vivo PET studies comparing PD patients with and without dyskinesias did not reveal any specific changes in either D1 or D2 receptor binding potentials (25, 26). Furthermore, in a recent PET study it has been shown that PD patients with dyskinesias have reduced DA transporter expression, supporting the role of presynaptic alterations in the appearance of dyskinesias (27). Finally, clinical observations from DOPA-responsive dystonia show that, despite the fact that these patients have similar levels of striatal DA depletion, dyskinesias do not occur even after long-term (essentially life-long) L-DOPA treatment (28, 29). As these patients do not develop DA neurodegeneration, it appears that under conditions when the presynaptic DA compartment is structurally intact, dysplastic changes in the postsynaptic striatal neurons do not lead to development of motor complications. Thus, proponents of the so-called “presynaptic hypothesis of dyskinesias” argue that the postsynaptic plastic changes are secondary, rather than causally linked to LIDs (4).
The unregulated DA production and release via alternative routes following degeneration of the nigrostriatal neurons provide further evidence for the critical role of a functionally intact presynaptic DA terminals in the development of the LIDs. In the parkinsonian brain, as the presynaptic DAergic neurodegeneration progresses, other cell types start to take part in handling exogenously administered L-DOPA and its conversion to DA. In particular, 5-HT cells are known to play a critical role. These cells contain the enzymes AADC and VMAT2 and therefore possess not only the capability to convert L-DOPA to DA but also store it in synaptic vesicles as a false neurotransmitter (30). However, because they do not express D2 autoreceptors and DA transporter, which are essential for the normal autoregulatory feedback control of DA release from the presynaptic terminal, their activity leads to uncontrolled swings in extracellular DA concentrations (31). Importantly, it has been recently shown that DA released from 5-HT terminals was responsible for the appearance of LIDs in parkinsonian rats (32). Furthermore, either a lesion of 5-HT system by specific toxins, or pharmacological silencing of these neurons by selective 5-HT1A and 5-HT1B agonists dramatically reduced or even completely abolished LIDs in 6-OHDA lesioned rats and MPTP-treated monkeys (33). These studies point to the deterministic role of the presynaptic DA releasing compartment on the occurrence of dyskinesias.
To compare the pathophysiological mechanisms of dyskinesias, we used two different experimental models. On one hand, the TH knockdown approach allowed us to generate a functional presynaptic DA depletion creating an isolated postsynaptic dysfunction. On the other hand, the 6-OHDA lesion model, which disrupted DAergic input structurally, led to a combined pre- and postsynaptic dysfunction. The validity of the comparison, however, depends on the assumption that the presence of DAergic neuronal death is the main difference between these two conditions. The preserved release of factors from the DA neurons and terminals (e.g., BDNF and glutamate) in the shTH group might, however, contribute to the behavior seen in TH knockdown animals. In addition, there is evidence to suggest that structural destruction of the DA terminals can generate changes in postsynaptic neurons, which involves modifications of spine morphology and distribution, as well as alterations in synapse structure and changes in electrophysiological and electrochemical properties of the cells (34–37). It is not clear, at this point, if the shRNA expression that silence DA production chronically results in development of similar changes over time. Nevertheless, some of the changes that are seen in 6-OHDA lesioned rats such as the reduction in spine numbers and alterations in miniature excitatory postsynaptic currents in medium spiny neurons have been shown to occur in reserpinized rats, which leads to DA depletion that last several days or weeks (38–40). In our experiments all behavioral tests were carried out 6–8 mo after transduction, which argues that such secondary modifications should have taken place at the time of analysis.
Finally, strong evidence supporting the presynaptic hypothesis comes from transplantation studies. In animal models of PD, embryonic ventral mesencephalon rich in DA neurons results in reconstitution of the DA terminal network capable of restoring feedback-controlled release of DA in the striatum (41). Importantly DA cell rich grafts have been shown to improve LIDs in these animals (42). On the contrary, transplantation of 5-HT neurons, which cannot release DA in a regulated fashion, worsens the dyskinetic side effects of L-DOPA medication (43, 44). It is probable that the worsening of dyskinesias in grafted patients might be a consequence of abnormal DA handling in the presynaptic compartment(s) possibly due to presence of serotonin neurons in the graft and/or ongoing inflammatory processes in the host brain (45).
In conclusion, by using a model system allowing us to functionally deplete striatal DA while maintaining the structural integrity of the presynaptic compartment, we have shown that postsynaptic plastic changes caused by DA receptor stimulation occur as a consequence of the lack of DA and do not require structural damage to the presynaptic DAergic terminals (46). Importantly, although dyskinesias may be elicited as a function of the intrinsic cellular changes of the postsynaptic compartment, the striatal neurons respond normally to regulated DA release from an adequate presynaptic compartment. These observations have implications for the interpretation of the concept of priming and support the view that this phenomenon may be the direct consequence of loss of DA while essentially nonphysiological DAergic pharmacotherapy merely unravels this behavior. Reconstitution of the DA machinery by cell- or gene-based therapies can reverse or even prevent development of L-DOPA-induced dyskinesias in PD patients.
Methods
A total of 117 young female Sprague–Dawley rats were used for rAAV5 vector injections and 3 × 7 μg striatal 6-OHDA lesions. rAAV5 vector constructs carried either a functional shRNA coding sequence to knockdown TH enzyme (shTH) or the scrambled sequence as shRNA control. Moreover a GFP marker protein was encoded under the control of a chicken-β-actin promoter. The efficacy of the viral transduction in the nigral DA neurons was confirmed by triple immunohistochemistry on coronal sections. The down-regulation of TH and cell survival in the nigra were assessed by unbiased stereological quantification method based on optical fractionator principle on histological sections stained for TH and VMAT2. The extracellular monoamine and metabolite levels were measured using online microdialysis and the tissue levels of DA, 5-HT, and metabolites were assessed using HPLC. A subset of animals received chronic injections of apomorphine (s.c. 0.1–0.5 mg/kg) or L-DOPA (s.c. 6–24 mg/kg, plus 10mg/kg benserozide) and the development of abnormal involuntary movements were scored. Induction of immediate-early genes in the striatum after chronic and acute L-DOPA or apomorphine treatment were analyzed by quantifying the FosB or c-Fos positive nuclei on histology specimens using image analysis. These animals were perfused 2 h following the final drug injections. Detailed information on the experimental group design and procedures can be found in the SI Methods.
Supplementary Material
Acknowledgments
We thank Anneli Josefsson and Ulla Samuelsson for technical assistance, Åsa Petersén and Manolo Carta for valuable discussions, and Shane Grealish for proofreading. We acknowledge the financial support from the Swedish Research Council (K2009-61P-20945-03-1), Crafoord Foundation, and Parkinsonfonden.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003432107/-/DCSupplemental.
References
- 1.Obeso JA, Olanow CW, Nutt JG. Levodopa motor complications in Parkinson's disease. Trends Neurosci. 2000;23(10, Suppl):S2–S7. doi: 10.1016/s1471-1931(00)00031-8. [DOI] [PubMed] [Google Scholar]
- 2.Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- 3.Cenci MA, Lundblad M. Post- versus presynaptic plasticity in L-DOPA-induced dyskinesia. J Neurochem. 2006;99:381–392. doi: 10.1111/j.1471-4159.2006.04124.x. [DOI] [PubMed] [Google Scholar]
- 4.de la Fuente-Fernández R. Presynaptic mechanisms of motor complications in Parkinson disease. Arch Neurol. 2007;64:141–143. doi: 10.1001/archneur.64.1.141. [DOI] [PubMed] [Google Scholar]
- 5.Linazasoro G. Pathophysiology of motor complications in Parkinson disease: Postsynaptic mechanisms are crucial. Arch Neurol. 2007;64:137–140. doi: 10.1001/archneur.64.1.137. [DOI] [PubMed] [Google Scholar]
- 6.Lee CS, Kumar A. Reply to: PET studies and physiopathology of motor fluctuations in Parkinson's disease. Brain. 2004;127:E16. doi: 10.1093/brain/awh228. [DOI] [PubMed] [Google Scholar]
- 7.Lundberg C, et al. Applications of lentiviral vectors for biology and gene therapy of neurological disorders. Curr Gene Ther. 2008;8:461–473. doi: 10.2174/156652308786847996. [DOI] [PubMed] [Google Scholar]
- 8.Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulusoy A, Bjorklund T, Hermening S, Kirik D. In vivo gene delivery for development of mammalian models for Parkinson's disease. Exp Neurol. 2008;209:89–100. doi: 10.1016/j.expneurol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Grimm D. Small silencing RNAs: State-of-the-art. Adv Drug Deliv Rev. 2009;61:672–703. doi: 10.1016/j.addr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med. 2003;9:1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- 12.Ulusoy A, Sahin G, Björklund T, Aebischer P, Kirik D. Dose optimization for long-term rAAV-mediated RNA interference in the nigrostriatal projection neurons. Mol Ther. 2009;17:1574–1584. doi: 10.1038/mt.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkler C, Kirik D, Björklund A, Cenci MA. L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of parkinson's disease: Relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2002;10:165–186. doi: 10.1006/nbdi.2002.0499. [DOI] [PubMed] [Google Scholar]
- 14.Lundblad M, et al. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson's disease. Eur J Neurosci. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- 15.Nadjar A, Gerfen CR, Bezard E. Priming for l-dopa-induced dyskinesia in Parkinson's disease: A feature inherent to the treatment or the disease? Prog Neurobiol. 2009;87:1–9. doi: 10.1016/j.pneurobio.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch EC. Nigrostriatal system plasticity in Parkinson's disease: Effect of dopaminergic denervation and treatment. Ann Neurol. 2000;47(4 Suppl 1):S115–S120. [PubMed] [Google Scholar]
- 17.Pycock CJ, Marsden CD. Central deopaminergic receptor supersensitivity and its relevance to Parkinson's disease. J Neurol Sci. 1977;31:113–121. doi: 10.1016/0022-510x(77)90009-0. [DOI] [PubMed] [Google Scholar]
- 18.Rinne UK, et al. Positron emission tomography demonstrates dopamine D2 receptor supersensitivity in the striatum of patients with early Parkinson's disease. Mov Disord. 1990;5:55–59. doi: 10.1002/mds.870050114. [DOI] [PubMed] [Google Scholar]
- 19.Antonini A, et al. [11C]raclopride and positron emission tomography in previously untreated patients with Parkinson's disease: Influence of L-dopa and lisuride therapy on striatal dopamine D2-receptors. Neurology. 1994;44:1325–1329. doi: 10.1212/wnl.44.7.1325. [DOI] [PubMed] [Google Scholar]
- 20.Guerra MJ, Liste I, Labandeira-Garcia JL. Effects of lesions of the nigrostriatal pathway and of nigral grafts on striatal serotonergic innervation in adult rats. Neuroreport. 1997;8:3485–3488. doi: 10.1097/00001756-199711100-00014. [DOI] [PubMed] [Google Scholar]
- 21.Chase TN, Oh JD. Striatal dopamine- and glutamate-mediated dysregulation in experimental parkinsonism. Trends Neurosci. 2000;23(10, Suppl):S86–S91. doi: 10.1016/s1471-1931(00)00018-5. [DOI] [PubMed] [Google Scholar]
- 22.Pisani A, et al. Role of tonically-active neurons in the control of striatal function: Cellular mechanisms and behavioral correlates. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:211–230. doi: 10.1016/s0278-5846(00)00153-6. [DOI] [PubMed] [Google Scholar]
- 23.Fredduzzi S, et al. Persistent behavioral sensitization to chronic L-DOPA requires A2A adenosine receptors. J Neurosci. 2002;22:1054–1062. doi: 10.1523/JNEUROSCI.22-03-01054.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calon F, et al. Dopamine-receptor stimulation: Biobehavioral and biochemical consequences. Trends Neurosci. 2000;23(10, Suppl):S92–S100. doi: 10.1016/s1471-1931(00)00026-4. [DOI] [PubMed] [Google Scholar]
- 25.de la Fuente-Fernández R, et al. Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson's disease: Implications for dyskinesias. Brain. 2004;127:2747–2754. doi: 10.1093/brain/awh290. [DOI] [PubMed] [Google Scholar]
- 26.Linazasoro G, et al. Levodopa-induced dyskinesias in parkinson disease are independent of the extent of striatal dopaminergic denervation: A pharmacological and SPECT study. Clin Neuropharmacol. 2009;32:326–329. doi: 10.1097/WNF.0b013e3181b52792. [DOI] [PubMed] [Google Scholar]
- 27.Troiano AR, et al. PET demonstrates reduced dopamine transporter expression in PD with dyskinesias. Neurology. 2009;72:1211–1216. doi: 10.1212/01.wnl.0000338631.73211.56. [DOI] [PubMed] [Google Scholar]
- 28.Nygaard TG, Marsden CD, Fahn S. Dopa-responsive dystonia: Long-term treatment response and prognosis. Neurology. 1991;41:174–181. doi: 10.1212/wnl.41.2_part_1.174. [DOI] [PubMed] [Google Scholar]
- 29.Nutt JG, Nygaard TG. Response to levodopa treatment in dopa-responsive dystonia. Arch Neurol. 2001;58:905–910. doi: 10.1001/archneur.58.6.905. [DOI] [PubMed] [Google Scholar]
- 30.Arai R, Karasawa N, Geffard M, Nagatsu T, Nagatsu I. Immunohistochemical evidence that central serotonin neurons produce dopamine from exogenous L-DOPA in the rat, with reference to the involvement of aromatic L-amino acid decarboxylase. Brain Res. 1994;667:295–299. doi: 10.1016/0006-8993(94)91511-3. [DOI] [PubMed] [Google Scholar]
- 31.Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- 32.Bishop C, et al. Contribution of the striatum to the effects of 5-HT1A receptor stimulation in L-DOPA-treated hemiparkinsonian rats. J Neurosci Res. 2009;87:1645–1658. doi: 10.1002/jnr.21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muñoz A, et al. Combined 5-HT1A and 5-HT1B receptor agonists for the treatment of L-DOPA-induced dyskinesia. Brain. 2008;131:3380–3394. doi: 10.1093/brain/awn235. [DOI] [PubMed] [Google Scholar]
- 34.Galarraga E, Bargas J, Martínez-Fong D, Aceves J. Spontaneous synaptic potentials in dopamine-denervated neostriatal neurons. Neurosci Lett. 1987;81:351–355. doi: 10.1016/0304-3940(87)90409-5. [DOI] [PubMed] [Google Scholar]
- 35.Nitsch C, Riesenberg R. Synaptic reorganisation in the rat striatum after dopaminergic deafferentation: An ultrastructural study using glutamate decarboxylase immunocytochemistry. Synapse. 1995;19:247–263. doi: 10.1002/syn.890190404. [DOI] [PubMed] [Google Scholar]
- 36.Ingham CA, Hood SH, Taggart P, Arbuthnott GW. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J Neurosci. 1998;18:4732–4743. doi: 10.1523/JNEUROSCI.18-12-04732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picconi B, et al. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003;6:501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- 38.Day M, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 39.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taverna S, Ilijic E, Surmeier DJ. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson's disease. J Neurosci. 2008;28:5504–5512. doi: 10.1523/JNEUROSCI.5493-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moukhles H, Forni C, Nieoullon A, Daszuta A. Regulation of dopamine levels in intrastriatal grafts of fetal mesencephalic cell suspension: An in vivo voltammetric approach. Exp Brain Res. 1994;102:10–20. doi: 10.1007/BF00232434. [DOI] [PubMed] [Google Scholar]
- 42.Lee CS, Cenci MA, Schulzer M, Björklund A. Embryonic ventral mesencephalic grafts improve levodopa-induced dyskinesia in a rat model of Parkinson's disease. Brain. 2000;123:1365–1379. doi: 10.1093/brain/123.7.1365. [DOI] [PubMed] [Google Scholar]
- 43.Carlsson T, Carta M, Winkler C, Björklund A, Kirik D. Serotonin neuron transplants exacerbate L-DOPA-induced dyskinesias in a rat model of Parkinson's disease. J Neurosci. 2007;27:8011–8022. doi: 10.1523/JNEUROSCI.2079-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlsson T, et al. Impact of grafted serotonin and dopamine neurons on development of L-DOPA-induced dyskinesias in parkinsonian rats is determined by the extent of dopamine neuron degeneration. Brain. 2009;132:319–335. doi: 10.1093/brain/awn305. [DOI] [PubMed] [Google Scholar]
- 45.Hedlund E, Perlmann T. Neuronal cell replacement in Parkinson's disease. J Intern Med. 2009;266:358–371. doi: 10.1111/j.1365-2796.2009.02155.x. [DOI] [PubMed] [Google Scholar]
- 46.Kim DS, Palmiter RD, Cummins A, Gerfen CR. Reversal of supersensitive striatal dopamine D1 receptor signaling and extracellular signal-regulated kinase activity in dopamine-deficient mice. Neuroscience. 2006;137:1381–1388. doi: 10.1016/j.neuroscience.2005.10.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.