Abstract
The glycolipid α-galactosylceramide (α-GalCer) has been shown to bind CD1d molecules to activate invariant natural killer T (iNKT) cells, and subsequently induce activation of various immune-competent cells, including dendritic cells, thereby providing a significant adjuvant effect for various vaccines. However, in phase I clinical trials, α-GalCer was shown to display only marginal biological activity. In our search for a glycolipid that can exert more potent stimulatory activity against iNKT cells and dendritic cells and produce an adjuvant effect superior to α-GalCer, we performed step-wise screening assays on a focused library of 25 α-GalCer analogues. Assays included quantification of the magnitude of stimulatory activity against human iNKT cells in vitro, binding affinity to human and murine CD1d molecules, and binding affinity to the invariant t cell receptor of human iNKT cells. Through this rigorous and iterative screening process, we have identified a lead candidate glycolipid, 7DW8-5, that exhibits a superior adjuvant effect than α-GalCer on HIV and malaria vaccines in mice.
Alpha-Galactosylceramide (α-GalCer), a glycolpid composed of α-linked sugar and lipid moieties, is a well known specific lipid antigen for invariant natural killer T (iNKT) cells (1). This α-GalCer is presented by CD1d, a MHC class I–like molecule, which is then recognized by the invariant t cell receptor (invTCR) of iNKT cells. After recognition, α-GalCer activates iNKT cells to rapidly produce large quantities of Th1 and Th2 cytokines (2), and subsequently induces a cascade of activation of various immune competent cells, including dendritic cells (DC) (3, 4), natural killer (NK) cells (5, 6), B cells (7, 8), and CD4+ and CD8+ T cells (9, 10). α-GalCer can therefore be used not only as a potential therapy for autoimmune diseases, infectious diseases, and cancer (11, 12), but also as an adjuvant to enhance the efficacy of various vaccines (13). We have previously shown that coadministration of α-GalCer with malaria vaccines enhanced their immunogenicity and efficacy and that the adjuvant effect of α-GalCer was mediated by IFN-γ (14). Other research has shown that mice immunized with DNA vaccines or proteins coinjected with α-GalCer developed antibody titers 1 to 2 logs higher than those induced by DNA vaccines or proteins alone (15, 16).
Several studies regarding the structure–activity relationship (SAR) of glycolipid binding to CD1d have shown that alterations in glycolipid structure will result in different biological activities. For example, truncation of the fatty acyl chain or the phytosphingosine group of the glycolipid has been shown to decrease Th1 response (17, 18), whereas introduction of an aromatic group to the fatty acyl chain or sphingosine tail has been shown to enhance the production of Th1 cytokines (19, 20).
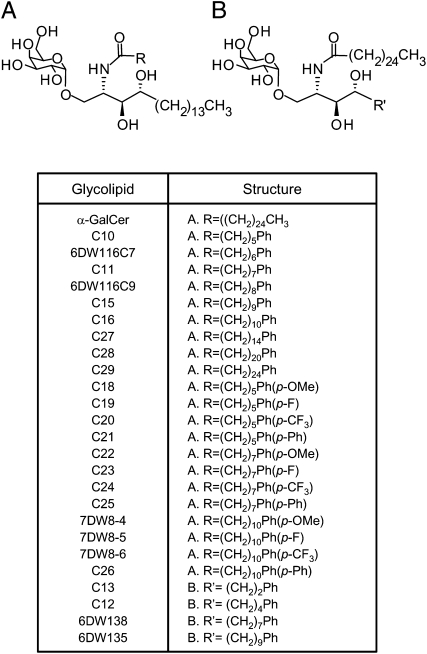
In this study, we generated a library of 25 synthetic analogues of α-GalCer according to previously established SAR of the glycolipids (Fig. 1 and Fig. S1). We then screened this focused library to identify a glycolipid that can potently activate iNKT cells and dendritic cells (DCs) and thus display robust adjuvant activity. It is our goal that the discovery of such a glycolipid-based adjuvant will ultimately be applicable to a wide variety of DNA and viral vaccines in humans.
Fig. 1.
Structure of α-GalCer analogues. Analogues in group A have a modification at a fatty acyl chain, whereas analogues in group B have a terminal benzene ring at phytosphingosine chain. Ph indicates a phenyl ring. The synthesis of these analogues is summarized in Fig. S1.
Results
Profile of IFN-γ and IL-4 Production by Human iNKT Cells in Response to Glycolipids Presented by HeLa-hCD1d.
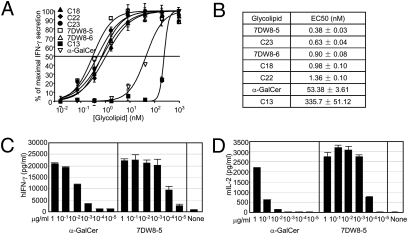
From the first set of screening assays, in which a human iNKT cell line was cocultured with HeLa cells transfected with human CD1d molecules in the presence of each glycolipid listed in Fig. 2, we found that several analogues induced significantly higher IFN-γ secretion than α-GalCer (Fig. S2A). Interestingly, C27, C28, and C29, which have a longer fatty acyl chain than α-GalCer, exhibited noticeably decreased IFN-γ production (Fig. S2A). In addition, compared with analogues that have a single benzene ring, those having two benzene rings (C21, C25, and C26) failed to stimulate human iNKT cells. Finally, 6DW135, 6DW138, C12, and C13, all of which have a terminal phenyl ring at the end of a truncated phytosphingosine chain, were unable to stimulate human iNKT cells to produce a substantial amount of IFN-γ (Fig. S2A). As for IL-4 production, although similar trends regarding SAR were observed, the difference was much less compared with that seen in IFN-γ production (Fig. S2B). Based on the level of IFN-γ produced by the glycolipids, we selected nine analogues—C11, C18, C22, C23, C24, 6DW116C9, 7DW8-4, 7DW8-5, and 7DW8-6—as initial candidates for further assessment of their biological activities. We also included α-GalCer and 13C, which has a modified and truncated phytosphingosine chain and shows minimal activity, as controls.
Fig. 2.
Cytokine production by human iNKT cells in response to glycolipids presented by HeLa-hCD1d. (A and C) Human iNKT cells (2 × 104) were cocultured with 2 × 104 HeLa-hCD1d cells in the presence of escalating doses of each glycolipid as described previously (21, 23). After 24 h incubation, the cultured supernatants were collected and the concentration of human IFN-γ was determined by ELISA. The level of IFN-γ secretion was then plotted against glycolipid concentration. Sigmoid dose–response curves were fitted by GraphPad Prism software. EC50 was calculated with sigmoid dose–response formula by GraphPad Prism software (B). (D) Mouse iNKT hybridoma cells (2 × 104) were cocultured with 2 × 104 A20-mCD1d in the presence of indicated concentrations of each glycolipid as described previously (21, 23). After 24 h incubation, the culture supernatants were collected and the concentrations of murine IL-2 in the supernatants were determined by ELISA. In A, C, and D, the results represent one of two similar experiments.
Level of Cytokines Produced by Human iNKT Cells in Response to Glycolipids Presented by Autologous DCs.
As immature DCs are known to efficiently present α-GalCer in vivo (13), we used autologous immature DCs as antigen-presenting cells (APCs). It was found that coculture of human iNKT cells and autologous DCs secreted IFN-γ, IL-4, GM-CSF, and IL-12 in the presence of all of the selected analogues. In particular, C18, C22, C23, 7DW8-5, and 7DW8-6 were shown to induce a significantly higher level of all of the Th1-related cytokines, IFN-γ, IL-12, and GM-CSF, than the parental compound, α-GalCer (Fig. S3). The control, C13, having a modification in the phytosphingosine chain, failed to elicit any cytokines in vitro. Collectively, these results indicate that DCs cannot only present the selected compounds to human iNKT cells and activate them, but may, in turn, get activated to secrete IL-12 and GM-CSF. Based on the results from this set of assays, we selected five analogues, which include C18, C22, C23, 7DW8-5, and 7DW8-6, for further assessment of their biological activities.
Level of Stimulatory Activity of Selected Glycolipids Against Human iNKT Cells.
To determine the EC50 value of each of the five selected glycolipids, we cocultured a human iNKT cell line and HeLa-hCD1d in the presence of a wide range of concentration of each glycolipid, ranging from 10−2 to 103 nM. Twenty-four hours later, the concentration of IFN-γ in the cultured supernatants was determined by ELISA, and the level of IFN-γ secretion was plotted against glycolipid concentration (Fig. 3A). As shown in Fig. 2B, all the selected compounds have lower EC50 values than α-GalCer. In particular, 7DW8-5 has the lowest EC50 with regard to its stimulatory activity against human iNKT cells, and the EC50 value is almost 140-fold lower than that of α-GalCer. The rest of the selected glycolipids also showed a much lower EC50 than that of α-GalCer. We then determined a comprehensive dose–response comparison of 7DW8-5 versus α-GalCer by adding from 10 pg/mL to 1 μg/mL of each to the coculture of a human iNKT cell line and HeLa-hCD1d. We found that 7DW8-5 could stimulate human iNKT cells to produce a similar level of IFN-γ to those induced by a 100-fold higher amount of α-GalCer (Fig. 3C), thus indicating that 7DW8-5 has 100-fold higher dose-sparing effect than α-GalCer. Similarly, when we added a wide range of the two glycolipids to the coculture of a mouse iNKT hybridoma and A20 cells expressing mouse CD1d, we found that 7DW8-5 could exhibit more than 1,000-fold higher dose-sparing effect than α-GalCer (Fig. 3D).
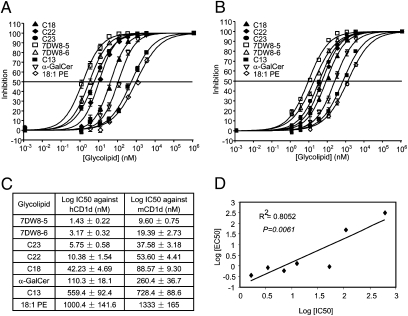
Fig. 3.
Assessment of binding affinity of each glycolipid against CD1d molecules. (A and B) Binding affinity of glycolipids to human or mouse CD1d molecules was determined by a competitive ELISA. After coating with antimouse IgG1 antibody overnight, Maxisorp ELISA plates were incubated with hCD1d:mIgG dimer (A) or mCD1d:mIgG dimer (B) for 2 h. Serially diluted glycolipids were then added to the plates in the presence of 1 μg/mL biotinylated 18:1 PE lipid. After 24 h incubation, bound biotinylated 18:1 PE lipid was determined with Streptavidin-HRP and tetramethylbenzidine substrate. Inhibition curves were fitted with sigmoid dose–response formula using Prism 4.0 software (GraphPad). (C) IC50 values represent the average of three independent assays expressed as mean ± SD and (D) IC50 values against EC50 values obtained in Fig. 2B were plotted. The relationships were obtained by fitting the lines between them. Statistical analysis on the correlation of the two values was evaluated by a Student t test.
IFN-γ Production from Human PBMCs in Response to Glycolipids Presented by Autologous DCs.
To determine the relative number of peripheral blood mononuclear cells (PBMCs) that react with glycolipids, an ELISPOT assay was performed. We cocultured human PBMCs and autologous immature DCs as APCs in the presence of the glycolipids for 24 h in ELISPOT plates. As human PBMCs were used rather than iNKT cell lines, this assay more closely approximates the biological activity of glycolipids in humans. Based on the results with the use of PBMCs from two different donors, we found that 7DW8-5 consistently stimulated the highest number of PBMCs to secrete IFN-γ, which was significantly higher than that induced by α-GalCer (Fig. S4). The other four selected analogues also induced a significantly higher number of human PBMCs secreting IFN-γ than α-GalCer (Fig. S4).
Maturation/Activation of Human Immature DCs by Glycolipids.
The five selected analogues—C18, C22, C23, 7DW8-5, and 7DW8-6—were also subjected to an assay that determines their abilities to induce maturation/activation of human DCs in vitro. For this purposes, we isolated immature human DCs and cocultured them with human iNKT cells in the presence or absence of the glycolipids, as described (21). By FACS analysis, we found that the expression of DC maturation/activation markers, specifically CD86 (Fig. S5A) and HLA-DR (Fig. S5B), was strongly up-regulated upon incubation with all five glycolipids, and the mean fluorescence intensity of the expression of both molecules was higher than that with α-GalCer. These results are in agreement with those obtained by measuring the levels of IL-12 and GM-CSF secreted by autologous DCs as shown in Fig. S3, and together indicate that the five selected glycolipids are potent inducers of human DC maturation and activation to a degree stronger than α-GalCer.
Binding Affinity of Glycolipids to CD1d Molecules.
We have recently developed a competitive binding ELISA to investigate the binding affinity between CD1d and glycolipids independent of invTCR recognition (22). In this competition assay, we used biotinylated 18:1 PE lipid as an indicator because 18:1 PE lipid was shown to bind CD1d molecules without being recognized by iNKT cells (23). Through this assay, we were able to determine the binding of selected analogues to both human and mouse CD1d molecules (Fig. 3 A and B), per the IC50 values presented in Fig. 4C. In short, 7DW8-5 displayed the lowest IC50 among the analogues to human and mouse CD1d molecules in the competitive binding ELISA (Fig. 3C), indicating that, of all analogues tested, 7DW8-5 has the strongest affinity to CD1d molecules of human or mouse origin. As expected, C13, which contains a modification at a truncated phytosphingosine chain, has a lower affinity to both CD1d molecules compared with α-GalCer (Fig. 4C). We have previously shown that IFN-γ mediates the adjuvant activity of α-GalCer on malaria vaccines in mice (14). Therefore, we calculated the correlation between the binding affinity of glycolipids to CD1d molecules and their biological activity, as indicated by the EC50 values shown in Fig. 2B. We found that a slight correlation (R2 = 0.8052) exists between the two values (Fig. 3D), with a P value of 0.061.
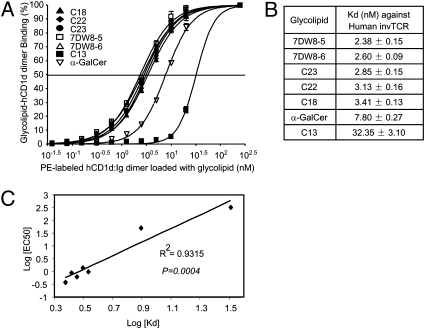
Fig. 4.
Assessment of binding affinity of each glycolipid against invTCR of human iNKT cells. (A) Binding affinity to invTCR of human iNKT cells was determined by staining human iNKT cells with a serially diluted phycoerythrin-labeled CD1d–glycolipid complex. Various doses of phycoerythrin-labeled hCD1d dimer loaded with each glycolipid ranging from 10−1.5 to 102.5 nM, and 10 μg/mL of FITC-labeled anti-CD3ε mAb were incubated with 2 × 105 human iNKT cells on ice for 30 min. After washing the cells, the stained cells were gated with CD3 and analyzed by a flow cytometric analysis. Binding curves were fitted by GraphPad Prism software. (B) Binding isotherms were then subjected to a Scatchard transformation to access the apparent equilibrium dissociation constant characteristic of the avidity, and generated Kd value. (C) Kd values against EC50 obtained in Fig. 2B were plotted. The relationships were obtained by fitting the lines between them. Statistical analysis on the correlation of the two values was evaluated by a Student t test.
Binding Affinity of Glycolipid–CD1d Complex to invTCR of Human iNKT cells.
To determine the binding affinity of glycolipid–CD1d complex to invTCR of iNKT cells, we have used a FACS-based method to evaluate the avidity between glycolipid-CD1d complex and invTCR, as previously described (23, 24). Because glycolipid-loaded hCD1d dimer stains iNKT cells through interacting with their invTCR, it is reasonable to assume that this method can assess the binding of glycolipid–hCD1d complex to the TCR. We found that hCD1d:Ig dimers loaded with 7DW8-5 exhibited the strongest avidity to human iNKT cells (Fig. 4A), with a Kd value that is more than threefold lower than that of α-GalCer (Fig. 4B). C13 displayed the lowest avidity to the invTCR of human iNKT cells (Fig. 4B). When we calculated the correlation between the affinity of glycolipids to the invTCR of human iNKT cells and their stimulatory activity against human iNKT cells as determined by the EC50 value in Fig. 2B, we found a strong inverse correlation between the two parameters (R2 = 0.9315), with a P value of 0.0004 (Fig. 4C). From the aforementioned assays, we selected one leading candidate glycolipid, 7DW8-5, for further assessment of its adjuvant activity.
Adjuvant Effects of 7DW8-5 on HIV Vaccines.
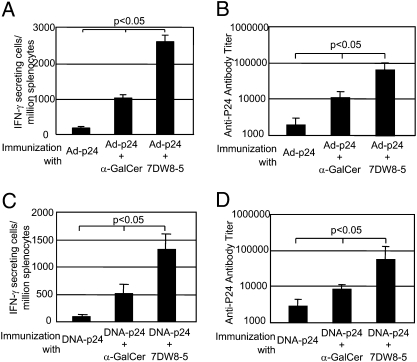
To test the adjuvant activity of 7DW8-5 and compare it with that of its parental compound, α-GalCer, we evaluated the level of their adjuvant activity by using a recombinant adenovirus expressing HIV p24 antigen, Ad-p24, as well as naked DNA plasmid encoding p24—DNA-p24—as a template vaccine. With regard to Ad-p24 immunization regimen, groups of BALB/c mice received coadministration of a suboptimal dose of the Ad-p24 and 1 μg of either glycolipid, and 2 wk later, the relative number of epitope-specific CD8+ T cells secreting IFN-γ was determined by an ELISPOT assay. We also measured the anti-p24 antibody titer in the sera by an ELISA. We found that the 7DW8-5 was able to display an adjuvant effect more potent than that of α-GalCer, increasing by more than twofold the level of p24-specific CD8+ T cell response (Fig. 5A). 7DW8-5 was also able to exhibit a stronger adjuvant effect on the humoral response to Ad-p24, although the difference was less pronounced (Fig. 5B). Similarly, when coadministered with DNA-p24, 7DW8-5 displayed a more potent adjuvant effect on both the p24-specific CD8+ T cell response and anti-p24 antibody response (Fig. 5 C and D), again, the difference being more evident for CD8+ T cell response.
Fig. 5.
Adjuvant effect of 7DW8-5 on HIV vaccines in mice. (A and B) Groups of BALB/c mice (n = 5) were immunized intramuscularly with 5× 107 pfu of Ad-p24 together with 1 μg of glycolipid, and 2 wk later, splenocytes and sera were collected from immunized mice. (A) Splenocytes were used to determine the relative number of IFN-γ–secreting CD8+ T cells by an ELISPOT assay, and in B, sera were used to determine the titers of anti-p24 antibody by ELISA. (C and D) Groups of BALB/c mice (n = 5) were immunized intramuscularly with 50 μg of DNA-p24 together with 1 μg of each glycolipid, and 3 wk later, the mice were boosted with 50 μg of DNA-p24 alone. Two weeks after the boost, the relative number of IFN-γ–secreting CD8+ T cells (C), as well as the titers of anti-p24 antibody (D) were determined as in A and B, respectively. In all experiments, the data were expressed as the mean ± SD of five mice, and results represent one of three similar experiments.
Adjuvant Effects of 7DW8-5 on HIV Vaccines in CD1d-Deficient Mice.
To confirm that the adjuvant effect of 7DW8-5 on HIV vaccines is mediated by CD1d molecule, CD1d-deficient mice with a BALB/c background that also lack iNKT cells, as well as WT BALB/c mice, were immunized with HIV vaccines coadministered with 7DW8-5 or α-GalCer, and the level of p24-specific CD8+ T cell response was measured by an ELISPOT assay. As expected, 7DW8-5 greatly enhanced the p24-specific CD8+ T cell response induced by either DNA-p24 or Ad-p24 in WT BALB/c mice, but failed to display any adjuvant effect in CD1d-deficient mice immunized with DNA-p24 or Ad-p24 (Fig. S6).
Adjuvant Effects of 7DW8-5 on Malaria Vaccines.
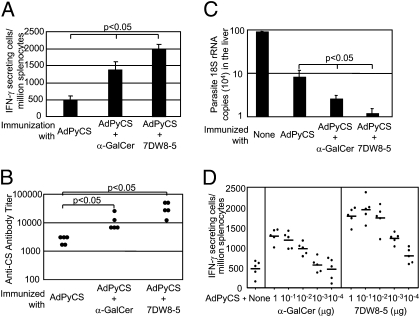
Because of the lack of an appropriate challenge model for HIV vaccines, we opted to use a malaria challenge model to determine the adjuvant effect of 7DW8-5 on the efficacy of a malaria vaccine. For this purpose, mice were immunized with a suboptimal dose of a recombinant adenovirus expressing a Plasmodium yoelii CS protein, AdPyCS, together with 1 μg of either glycolipid. One group of immunized mice were killed to determine the base level of PyCS-specific CD8+ T cell response, as well as the anti-PyCS antibody response. Another group of immunized mice, as well as naive mice, were challenged with P. yoelii sporozoites 2 wk later, and the amounts of parasite burden in the liver were measured by real-time PCR as previously described (14). We found that 7DW8-5 enhanced the malaria-specific CD8+ T cell response significantly more than α-GalCer (Fig. 6A) and it also enhanced the malaria-specific humoral response equally, if not slightly stronger than α-GalCer (Fig. 6B). Most importantly, 7DW8-5 was able to display a significantly stronger adjuvant effect than α-GalCer in enhancing protective efficacy of AdPyCS after a single immunizing dose (Fig. 6C). We have shown in Fig. 2B that the EC50 of 7DW8-5 is almost 140-fold lower than that of α-GalCer. Therefore, we decided to determine a comprehensive dose–response relationship of the adjuvant effect of 7DW8-5 compared with that of α-GalCer by coadministering a wide range of serially diluted amounts of glycolipid (100 pg to 1 μg/mL) to mice vaccinated with a suboptimal dose of AdPyCS (5 × 106 pfu). We found that coadministration of 1 ng of 7DW8-5 elicited a similar level of PyCS-specific CD8+ T cell response compared with that induced by 100 ng of α-GalCer (Fig. 6D), thus indicating the approximately 100-fold higher dose sparing adjuvant effect of 7DW8-5 compared with α-GalCer. In addition to this dramatic dose sparing effect, 7DW8-5 displayed a more potent adjuvant capacity; inducing a peak level of PyCS-specific CD8+ T cell response that is approximately 50% greater than that of α-GalCer (Fig. 6D).
Fig. 6.
Adjuvant effect of 7DW8-5 on a malaria vaccine in mice. (A and B) Groups of BALB/c mice (n = 5) were immunized intramuscularly with 5 × 106 pfu of AdPyCS together with 1 μg of glycolipid, and 2 wk later, splenocytes and sera were collected from immunized mice. (A) Splenocytes were used to determine the relative number of IFN-γ–secreting CD8+ T cells by an ELISPOT assay, and (B) sera were used to determine the titers of anti-p24 antibody by ELISA. (C) Some groups of AdPyCS-immunized, glycolipid-treated mice were challenged with 104 infective sporozoites of P. yoelii 2 wk later. Forty-two hours after challenge, the amount of parasite-specific rRNA in the liver was measured by a real-time PCR, and RNA copies were calculated by relative quantification based on endogenous GAPDH RNA. The quantity of parasite rRNA in nonimmunized mice was used as a control. (D) Groups of BALB/c mice (n = 5) were immunized with AdPyCS together with indicated amounts of each glycolipid, and 2 wk later, splenocytes were collected to determine the relative number of IFN-γ–secreting CD8+ T cells by an ELISPOT assay. (A and C) Data expressed as the mean ± SD of five mice. In all experiments, the results represent one of two similar experiments.
Discussion
We have previously shown that α-GalCer can be used as an adjuvant for malaria vaccines in the mouse model, and that the adjuvant effect is mediated by IFN-γ. In this regard, the identification of a glycolipid that can trigger a robust IFN-γ response may lead us to discover a more potent glycolipid-based adjuvant platform. In this study, we screened a focused library consisting of 25 analogues of α-GalCer listed in Fig. 1 through a series of step-wise bioassays and identified a lead clinical candidate, named 7DW8-5, that displays superior adjuvant activity on HIV and malaria vaccines in mice. Structurally, 7DW8-5 possesses a fluorinated benzene ring at the end of C8 length fatty acyl chain. 7DW8-5 was able to exert a 140-fold higher dose sparing effect than its parental compound, α-GalCer, in stimulating human iNKT cells (Fig. 2B), and a 100-fold higher dose sparing effect than α-GalCer in displaying the adjuvant effect (Fig. 6D). In addition, coadministration of 7DW8-5 with a malaria vaccine induced an approximately 50% higher peak level of PyCS-specific CD8+ T cell ELISPOT response than that displayed by α-GalCer. Finally, 7DW8-5 displayed the strongest binding affinity to human and mouse CD1d molecules, as well as invTCR of human iNKT cells, which are well correlated with their EC50 values for stimulating human iNKT cells to secrete IFN-γ.
From a vaccine development perspective, it is imperative to develop an adjuvant agent that enables vaccines to induce high levels of the cell-mediated and humoral immune responses, which results in more potent overall protective immunity. Recently, there have been increasing numbers of strategies to discover new adjuvant agents. These approaches are based on proteins, DNA, RNA, lipids, or sugars, which can be a ligand for Toll-like receptors, Notch-like receptors, Fc receptors, or other receptors expressed by professional APCs (25–27). The advantage of discovering a new adjuvant agent based on a CD1d-binding, iNKT-stimulatory glycolipid, like α-GalCer, is that the phenotypic and functional properties of the CD1d molecules and invTCR of iNKT cells have been conserved between humans and mice, thereby allowing us to predict the activity of glycolipids in humans through mouse studies to a certain extent. In addition, the parental glycolipid, α-GalCer, has been approved and well characterized in terms of safety and activity in humans through previously completed phase I clinical trials. As such, we anticipate that 7DW8-5 would have a similar safety profile in humans because of the same clear CD1d-dependent adjuvant activity of this structurally similar α-GalCer analogue. Additionally, glycolipids used as vaccine adjuvant agents would be administered in much smaller quantities intramuscularly than the large doses currently dispensed i.v. for cancer therapy, further minimizing the potential systemic side effects.
Recent crystal structure studies of glycolipid–CD1d complexes have revealed that the lipid portion of glycolipids fits into the CD1d binding groove with some diversity (28–31). Biological studies on α-GalCer analogues have shown that the modification of the lipid chain determines Th1/Th2 balance (32, 33). Therefore, attempts have been made to synthesize α-GalCer analogues with modifications in the lipid portion aiming to selectively induce Th1 cytokine secretions. In this study, our search for α-GalCer analogues that induce a Th1-biased response and as well as a potent adjuvant effect, allowed us to identify several rules that govern their SAR.
First, by testing the activities of analogues that have a terminal phenyl group after various carbon spacer lengths on a fatty acyl chain, we found that, for glycolipids to display optimal biological activity, particularly the ability to stimulate iNKT cells to produce IFN-γ, the carbon length of the acyl chain should be between 6 and 11. If the carbon length of the fatty acyl chain is longer than 15, e.g., C27, C28, and C29, the glycolipid drastically loses its ability to induce IFN-γ. This may be because the A′ pocket in the CD1d hydrophobic groove is unable to accommodate a glycolipid with a fatty acyl chain having a terminal benzene ring beyond a 15-carbon spacer length. Second, modification of a terminal benzene ring at the fatty acyl chain of analogues appears to alter the ability to induce IFN-γ. By adding –OMe, –F, or –CF2 to the terminal benzene ring of compounds, C10 (6-carbon length), C11 (8-carbon length), and C16 (11-carbon length), glycolipids were able to induce a higher level of IFN-γ, whereas the introduction of an additional benzene ring to the terminal benzene ring of these compounds, dramatically reduced the ability to induce IFN-γ secretion. Our previous docking model study had indicated that introduction of a terminal phenyl group in the α-GalCer analogues appears to promote additional hydrogen bonding between the benzene ring of fatty acyl chain and the aromatic residues that are present on the wall of A′ pocket in the CD1d hydrophobic groove (19). It is likely that an addition of a small radical group to the benzene ring enhances this interaction and forms a more stable glycolipid–CD1d complex. The recent crystal structures of the complexes consisting of mouse CD1d and analogues C10, C11, C15, or C23, show that the terminal phenyl ring of the acyl chain formed a higher number of van der Waals contacts with A′-pocket than the alkyl fatty acyl chain, and, furthermore, the appended phenyl atoms were restrained in conformation, which decreased the loss of entropy upon binding (34). These findings suggest that α-GalCer analogues with a terminal phenyl ring in the acyl chain may have more steady binding with CD1d molecules. In addition to the van der Waals contacts, a fluorine, inserted at a paraposition of the phenyl ring of C11, also strengthened the acyl chain binding of C23 with CD1d molecules by forming a neutral hydrogen bond (34). In contrast, because of the limited space in the A′ pocket of the binding groove, a fatty acyl chain carrying double benzene rings became too bulky to be contained. Third and finally, the introduction of a terminal benzene ring to the truncated phytosphingosine chain decreased the ability of glycolipids to bind CD1d molecules and produce cytokines. In particular, the analogue, named C13, which carries a very short phytosphingosine chain with a terminal benzene ring, failed to stimulate human iNKT cells and a mouse iNKT hybridoma to secrete IFN-γ/IL-4 or IL-2, respectively, and has also been shown to have a very weak binding affinity to CD1d molecules. The weakened binding affinity may be explained by the fact that the F′ pocket of the CD1d binding groove, in which phytosphingosine chain is inserted, has less space than the A′ pocket and is unable to accommodate even a small aromatic group.
Although the measurement of in vitro cytokine production induced by glycolipid analogues allowed for the selection of the best lead adjuvant candidate for testing, as shown in Fig. 2 and Fig. S2 and Fig. S3, the binding affinity of glycolipids to the invTCR of human iNKT cells (Fig. 4C), to the contrary, provided the better correlate to the biological activity of selected glycolipids—even more so than the binding affinity to murine and human CD1d molecules (Fig. 3D). This indicates that a glycolipid's binding affinity to human iNKT cells may have a better predictive value for its biological function than its binding affinity to CD1d molecules. Thus future screening assays may incorporate invTCR binding affinity as a selection criterion for more potent glycolipid-based adjuvants.
In summary, through a multitude of step-wise in vitro and in vivo biological assays, the present study has identified a lead candidate glycolipid as a vaccine adjuvant from a focused library of 25 synthetic analogues of α-GalCer. We have indentified that a glycolipid, named 7DW8-5, shows the strongest biological activity against human and mouse iNKT cells in vitro, the highest affinity against human and mouse CD1d molecules, as well as invTCR of human iNKT cells, and, ultimately, the most potent adjuvant activity on HIV/malaria vaccines in mice. This lead glycolipid will be tested for its adjuvant effect on a candidate vaccine under a phase I clinical trial in the near future.
Materials and Methods
Isolation and Generation of Immature DC and iNKT Cell Lines from Human PBMCs.
For generating immature DCs, CD14+ monocytes were isolated from PBMCs by using CD14-magnetic microbeads (Miltenyi Biotech), and then were cultured for 5 d in the presence of 20 ng/mL human GM-CSF (R&D Systems) and 25 ng/mL human IL-4 (R&D Systems). Human iNKT cell lines, expressing the Vα24 TCR, were generated as previously published (21, 23, 35).
Assessment of Antigen-Specific Cellular and Humoral Immune Responses.
The numbers of antigen-specific, IFN-γ–secreting CD8+ T cells in the spleens of immunized mice were determined by an ELISPOT assay, using a synthetic peptide corresponding to the CD8+ T cell epitope within the respective antigen, as previously described (14). Antigen-specific humoral responses were determined by ELISA as described (15). All of the peptides were synthesized by Biosyntheis.
Sporozoite Challenge and Assessment of Protection.
Sporozoite were obtained and challenge experiments were performed as described previously (14). Mice were injected with 2 × 104 live P. yoelii sporozoites via tail vein, and 42 h later, the parasite burden in the liver was determined by measuring parasite-specific rRNA by using a model 7300 real-time PCR system (Applied Biosystems). Parasite burden was described as a ratio of the absolute copy number of parasite rRNA to that of mouse GAPDH mRNA (14).
Data Analysis.
All data were expressed as the mean ± SD of triplicate wells from each sample. Statistical analysis of experimental and control data were evaluated by one-way ANOVA and Student t test. A P value lower than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Takayuki Shiratsuchi (Aaron Diamond AIDS Research Center, NY) for providing hCD1d: mIgG dimer, Dr. Ana Rodriguez (New York University, NY) for providing P. yoelii sporozoites, and Dr. Vincent Sahi for assisting with FACS analysis. We also thank Mr. Chui Ng for reviewing the manuscript. This work was partially supported by National Institutes of Health Grants AI070258 (to M.T.) and AI072155 (to C.-H.W.) and Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery Grant 38648 (to D.D.H), and by the Irene Diamond Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006662107/-/DCSupplemental.
References
- 1.Tsuji M. Glycolipids and phospholipids as natural CD1d-binding NKT cell ligands. Cell Mol Life Sci. 2006;63:1889–1898. doi: 10.1007/s00018-006-6073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kronenberg M. Toward an understanding of NKT cell biology: Progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 3.Chung Y, Chang WS, Kim S, Kang CY. NKT cell ligand alpha-galactosylceramide blocks the induction of oral tolerance by triggering dendritic cell maturation. Eur J Immunol. 2004;34:2471–2479. doi: 10.1002/eji.200425027. [DOI] [PubMed] [Google Scholar]
- 4.Vincent MS, et al. CD1-dependent dendritic cell instruction. Nat Immunol. 2002;3:1163–1168. doi: 10.1038/ni851. [DOI] [PubMed] [Google Scholar]
- 5.Metelitsa LS, et al. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167:3114–3122. doi: 10.4049/jimmunol.167.6.3114. [DOI] [PubMed] [Google Scholar]
- 6.Carnaud C, et al. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 7.Galli G, et al. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J Exp Med. 2003;197:1051–1057. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitamura H, et al. alpha-galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell Immunol. 2000;199:37–42. doi: 10.1006/cimm.1999.1602. [DOI] [PubMed] [Google Scholar]
- 9.Stober D, Jomantaite I, Schirmbeck R, Reimann J. NKT cells provide help for dendritic cell-dependent priming of MHC class I-restricted CD8+ T cells in vivo. J Immunol. 2003;170:2540–2548. doi: 10.4049/jimmunol.170.5.2540. [DOI] [PubMed] [Google Scholar]
- 10.Singh N, et al. Cutting edge: Activation of NK T cells by CD1d and alpha-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J Immunol. 1999;163:2373–2377. [PubMed] [Google Scholar]
- 11.Yu KO, Porcelli SA. The diverse functions of CD1d-restricted NKT cells and their potential for immunotherapy. Immunol Lett. 2005;100:42–55. doi: 10.1016/j.imlet.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Berkers CR, Ovaa H. Immunotherapeutic potential for ceramide-based activators of iNKT cells. Trends Pharmacol Sci. 2005;26:252–257. doi: 10.1016/j.tips.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Aseguinolaza G, et al. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195:617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, et al. Enhancement of HIV DNA vaccine immunogenicity by the NKT cell ligand, alpha-galactosylceramide. Vaccine. 2008;26:1807–1816. doi: 10.1016/j.vaccine.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Galli G, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci USA. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oki S, Chiba A, Yamamura T, Miyake S. The clinical implication and molecular mechanism of preferential IL-4 production by modified glycolipid-stimulated NKT cells. J Clin Invest. 2004;113:1631–1640. doi: 10.1172/JCI20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goff RD, et al. Effects of lipid chain lengths in alpha-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 19.Fujio M, et al. Structure-based discovery of glycolipids for CD1d-mediated NKT cell activation: Tuning the adjuvant versus immunosuppression activity. J Am Chem Soc. 2006;128:9022–9023. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- 20.Chang YJ, et al. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc Natl Acad Sci USA. 2007;104:10299–10304. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XGC, Garcia-Navarro R, Franck RW, Tsuji M. Identification of C-glycoside analogues that display a potent biological activity against murine and human invariant natural killer T cells. Immunology. 2009;127:216–225. doi: 10.1111/j.1365-2567.2008.02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiratsuchi T, Schneck J, Kawamura A, Tsuji M. Human CD1 dimeric proteins as indispensable tools for research on CD1-binding lipids and CD1-restricted T cells. J Immunol Methods. 2009;345:49–59. doi: 10.1016/j.jim.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, et al. Invariant TCR rather than CD1d shapes the preferential activities of C-glycoside analogues against human versus murine iNKT cells. J Immunol. 2009;183:4415–4421. doi: 10.4049/jimmunol.0901021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sidobre S, et al. The T cell antigen receptor expressed by Valpha14i NKT cells has a unique mode of glycosphingolipid antigen recognition. Proc Natl Acad Sci USA. 2004;101:12254–12259. doi: 10.1073/pnas.0404632101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkinson T. The future of toll-like receptor therapeutics. Curr Opin Mol Ther. 2008;10:21–31. [PubMed] [Google Scholar]
- 26.Jensen MA, Arnason BG, White DM. A novel Fc gamma receptor ligand augments humoral responses by targeting antigen to Fc gamma receptors. Eur J Immunol. 2007;37:1139–1148. doi: 10.1002/eji.200636321. [DOI] [PubMed] [Google Scholar]
- 27.Wheeler AW, Woroniecki SR. Allergy vaccines–new approaches to an old concept. Expert Opin Biol Ther. 2004;4:1473–1481. doi: 10.1517/14712598.4.9.1473. [DOI] [PubMed] [Google Scholar]
- 28.Zajonc DM, Savage PB, Bendelac A, Wilson IA, Teyton L. Crystal structures of mouse CD1d-iGb3 complex and its cognate Valpha14 T cell receptor suggest a model for dual recognition of foreign and self glycolipids. J Mol Biol. 2008;377:1104–1116. doi: 10.1016/j.jmb.2008.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zajonc DM, Ainge GD, Painter GF, Severn WB, Wilson IA. Structural characterization of mycobacterial phosphatidylinositol mannoside binding to mouse CD1d. J Immunol. 2006;177:4577–4583. doi: 10.4049/jimmunol.177.7.4577. [DOI] [PubMed] [Google Scholar]
- 30.Koch M, et al. The crystal structure of human CD1d with and without alpha-galactosylceramide. Nat Immunol. 2005;6:819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 31.Zajonc DM, et al. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat Immunol. 2005;6:810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang PH, et al. Quantitative microarray analysis of intact glycolipid-CD1d interaction and correlation with cell-based cytokine production. J Am Chem Soc. 2008;130:12348–12354. doi: 10.1021/ja8012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarthy C, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204:1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiefner A, Fujio M, Wu D, Wong CH, Wilson IA. Structural Evaluation of Potent NKT Cell Agonists: Implications for Design of Novel Stimulatory Ligands. J Mol Biol. 2009;394:71–82. doi: 10.1016/j.jmb.2009.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinjo Y, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.