Abstract
The transcription factor Osterix (Osx) is required for osteoblast differentiation and bone formation during embryonic development, but it is not known whether Osx has an essential function in postnatal bone growth and in bone homeostasis. Conditional deletion of Osx at several time points postnatally revealed that Osx was essential for osteoblast differentiation and new bone formation in growing and adult bones. Additionally, inactivation of Osx in bones severely disrupted the maturation, morphology, and function of osteocytes. These findings identify Osx as having an essential role in the cell-specific genetic program of osteocytes. Interestingly, Osx inactivation also led to the massive accumulation of unresorbed calcified cartilage in a large area below the growth plate of endochondral bones. This specific area was also marked by an unanticipated almost complete lack of bone marrow cells and a marked decrease in the density and size of osteoclasts. This diminished density of osteoclasts could contribute to the lack of resorption of mineralized cartilage. In addition, we speculate that the abnormally accumulated, mainly naked cartilage represents an unfavorable substrate for osteoclasts. Our study identifies Osx as an essential multifunctional player in postnatal bone growth and homeostasis.
Keywords: osteoblast differentiation, skeletal homeostasis, transcription factor, osteocyte, cartilage resorption
The identification of master transcription factors essential for osteoblast differentiation and bone formation during embryonic development has greatly advanced our knowledge of bone biology. However, the transcriptional control of postnatal bone formation and homeostasis remain poorly understood. Normal skeletal growth and homeostasis depends on the coordinated activities of three types of bone cells: osteoblasts, osteoclasts, and osteocytes. Factors secreted by the mesenchyme-derived osteoblasts also control the differentiation and activity of the bone-resorbing osteoclasts derived from hematopoietic stem cells. Conversely, bone resorption by osteoclasts releases factors important for bone formation. Osteocytes, which make up over 90–95% of all bone cells in adult animals, are derived from mature osteoblasts and are embedded inside the bone matrix. It has been suggested that osteocytes are mediators of mechanical and hormonal stimulations to control the activity of both osteoblasts and osteoclasts (1). Osteocytes are regulators of mineralization and mineral homeostasis (2, 3), and by controlling Sost expression also act as modulators of Wnt signaling (4).
Three transcription factors [β-catenin, Runx2, and Osterix (Osx)] are required for osteoblast differentiation and bone formation during embryonic development (5–9). Osx, which acts downstream of Runx2, is a zinc-finger-containing transcription factor essential for embryonic osteoblast differentiation and bone formation (8). During development, Osx is specifically expressed in osteoblast lineage cells and, at lower levels, in prehypertrophic chondrocytes, but not in osteoclasts. Osx-null mutant mice, which die at birth, develop a complete cartilaginous skeleton, but no bone formation takes place in either the endochondral or membranous skeleton. All skeletal elements of these Osx-null mice are characterized by the presence of Runx2-expressing precursor cells, which are arrested in their differentiation and unable to express osteoblast markers. Recent studies have shown that genetic variants in the region of Osx are associated with bone mineral density (BMD) in both children and adults, suggesting that Osx may continue to play an important role in the postnatal skeleton (10, 11). However, despite the crucial role of Osx in osteoblast differentiation and bone formation during development, an essential role for Osx in postnatal bone growth and homeostasis has not yet been demonstrated.
To investigate the functions of Osx in skeletal growth and homeostasis, we conditionally ablated Osx postnatally through tamoxifen activation of the CreER recombinase (12). Our findings suggest that Osx acts as an essential and central factor of bone homeostasis after birth, because it is required not only for new bone formation, osteocyte maturation, and function, but also for cartilage resorption.
Results
Postnatal Osx Inactivation Leads to Severely Altered Bone Structures.
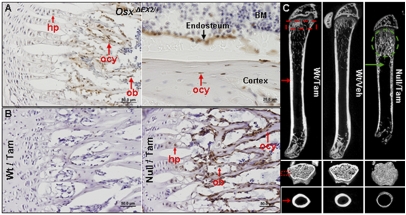
In the floxed Osx allele, a cassette containing IRES-EGFP preceded by a LOXP site was inserted 3′ to the poly-A site, whereas the other LOXP site was in the first intron of the Osx gene. In mice harboring this allele, EGFP expression occurs only in Osx-expressing cells when the LoxP sites recombine (13). Immunohistochemical (IHC) analyses showed that there were abundant EGFP-positive cells on the surfaces of trabeculae and cortex in the humerus of 1-mo-old OsxΔEX2/+ heterozygous mice (Fig. 1A). Prehypertrophic and hypertrophic chondrocytes were also positive for EGFP in these mice. This extends previous findings (8) indicating that Osx continues to be expressed in osteoblasts and hypertrophic chondrocytes postnatally. In addition, EGFP-positive cells were seen embedded inside the cortex and trabeculae, indicating that Osx is expressed in osteocytes as well (Fig. 1A). Overall expression of Osx is highly specific for osteoblasts, osteocytes, and (pre)hypertrophic chondrocytes.
Fig. 1.
Osx expression pattern and skeletal phenotypes of Osxpostnatal mutant mice. (A) anti-eGFP IHC analysis on frozen humerus sections of 1-mo-old OsxΔEX2/+ mouse. (B) anti-eGFP IHC analysis showed that Osx was effectively deleted in Osx-expressing cells in the humeri of 2-mo-old Osxpostnatal mutant. hp, hypertrophic chondrocyte; ob, osteoblast; ocy, osteocyte. (C) μCT images of the femurs of 2-mo-old mice. Massive accumulation of mineralized tissue (green dotted circle) and complete absence of trabeculae (green arrow). For schedules of tamoxifen injections, see Materials and Methods. Wt/Tam, tamoxifen-treated wild-type mice; Wt/Veh, vehicle-treated wild-type mice; Null/Tam, tamoxifen-treated Osxpostnatal mutant mice.
To inactivate Osx postnatally, CAG-CreER; Osxfloxed/− mice were injected with tamoxifen starting at several different time points after birth. IHC analyses with an anti-EGFP antibody showed that there were abundant EGFP-positive cells on the surface of the trabeculae and cortex, as well as embedded inside the bone matrix (Fig. 1B). Hypertrophic chondrocytes were also weakly positive for EGFP. This findings confirms that the floxed Osx allele was efficiently removed in Osx-expressing bone cells upon tamoxifen injections.
The Osxpostnatal mutants grew at slower rates than the tamoxifen- or vehicle-treated wild-type controls (Fig. S1A). In these mice, radiography showed the presence of dense mineralized tissue under the growth plates of all long bones (Fig. S1B). Microcomputed tomography (μCT) images of the femurs of Osxpostnatal mutants revealed a number of marked phenotypic changes. First, there was a zone of intensely mineralized tissue extending from right beneath the growth plate into the metaphysis. In contrast, there was a complete absence of trabeculae below this zone of hypermineralization, and a much thinner and porous cortical bone. There was also a complete absence of cortex around and beyond the primary spongiosa (Fig. 1C). These phenotypes were observed in the P24 Osxpostnatal mutants 10 d after the first tamoxifen injection. The abnormal mineralized tissue progressively increased in size toward the diaphysis of femurs and also in lumbar vertebrae of Osxpostnatal mutants from P24 to 3 mo. Also, when Osx was inactivated at 4 mo, and the mice were killed 3 mo later, the very thin cortical bone showed multiple microfractures (Fig. S2A). Evidently, postnatal Osx inactivation led to severely altered bone structures, suggesting that Osx continues to play a crucial role in the postnatal skeleton.
Osx Is Required for Osteoblast Differentiation and Bone Formation During and After the Postnatal Growth Period.
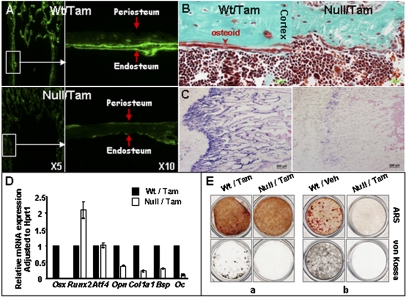
To examine the role of Osx in osteoblast differentiation and bone formation during and after the postnatal growth period, the CAG-CreER;Osxfloxed/− mice and wild-type controls were injected with tamoxifen or vehicle starting at weaning or at 4 mo, and then with calcein shortly before sacrifice at 1.5 mo or 7 mo, respectively. In the femurs and vertebrae of both tamoxifen- and vehicle-treated wild-type controls, bone formation was clearly indicated by the double calcein fluorescent lines lining the cortexes and trabeculae. In contrast, in the Osxpostnatal mutants, there were almost no intact double or single lines on the surfaces of the trabeculae and cortex, except a few faint, short, single lines and dots (Fig. 2A and Fig. S2B). Histomorphometry of lumbar vertebrae of 1.5-mo-old mice (Table S1) provided further evidence that new bone formation in Osxpostnatal mutants was dramatically reduced. The absence of new bone formation was also supported by the greatly decreased levels of Col1a1 mRNA throughout long bones, as seen by in situ hybridization (Fig. 2C). Histological analyses further showed that there were very few morphologically mature osteoblasts and almost no osteoid on the endosteum in the Osxpostnatal mutants (Fig. S2C and Fig. 2B). Along with drastically decreased Osx expression (∼1% of the controls), qPCR measurements of osteoblast-specific marker RNAs, such as Col1a1, Bsp, and Oc, were all markedly reduced in the Osxpostnatal mutants, despite elevated Runx2 expression and sustained Atf4 expression (Fig. 2D). Furthermore, primary osteoblast lineage cells isolated either from calvariae or from the bone marrow mesenchymal progenitor cells of the Osxpostnatal mutants completely failed to form any mineralized nodules in vitro (Fig. 2E). No difference, however, was seen in the number of bone marrow-derived CFU fibroblasts (CFU-Fs) between tamoxifen-treated wild-type and Osxpostnatal mutant mice (Fig. S2D), suggesting that progenitor cells were not affected. Together, these data provided solid evidence that Osx is required for osteoblast differentiation and bone formation far beyond birth and the initial growth period.
Fig. 2.
Osx is required for osteoblast differentiation and bone formation during and after the postnatal growth period. (A) Calcein incorporation. The second of two calcein injections was performed 5 d after the first injection and 2 d before sacrifice. (B) Goldner staining. (C) Col1a1 in situ hybridization on femur sections of 6-wk-old tamoxifen-treated control and Osxpostnatal mutant. In A and B, plastic sections were prepared from 6-wk-old tamoxifen-treated control and Osxpostnatal mutant. (D) Quantitative PCR analysis of osteoblast markers. RNA was isolated from the humeri of P24 tamoxifen-treated mice. (E) In vitro osteogenic assays. (a) Primary osteoblasts were isolated from the calvariae of P24 tamoxifen-treated Osxpostnatal mutant and control mice. (b) BMSC was isolated from 2-mo-old tamoxifen-treated Osxpostnatal mutant and vehicle-injected controls. The cells were cultured in osteogenic media for 14 d. ARS, alizarin red staining.
Major Role of Osx in Both Osteocyte Maturation and Function.
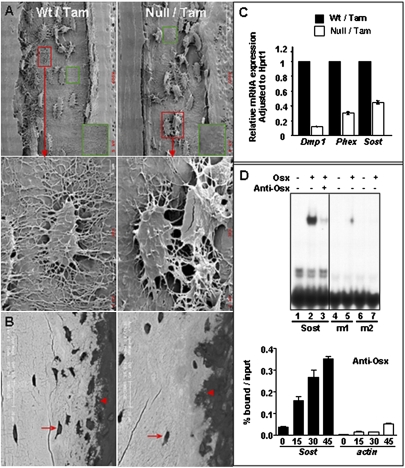
We examined the morphology of the Osx-null osteocytes using acid-etched scanning electronic microscopy (SEM). As shown in Fig. 3A, the osteocytes in the Osxpostnatal mutant were markedly deformed. There was a decreased number of osteocytes close to both periosteum and endosteum in the mutant. Moreover, these osteocytes were covered with very few dendrites. The number of dendrites in osteocytes found in the middle of the mutant cortex was also noticeably decreased, and the overall density of the dendrite network in the mutant cortex was much reduced. In addition, the expression levels of Dmp1, Phex, and Sost, which are highly expressed in normal osteocytes, were significantly reduced in the Osxpostnatal mutants (Fig. 3C). Fgf23 expression was elevated in bones of Osxpostnatal mutants (Fig. S3A) but to a lesser extent than in Dmp1-null and Hyp mice (2, 3), Serum levels of both phosphorus and calcium were, however, unchanged (Fig. S3A). Figure 3B showed that in the Osxpostnatal mutant there were very few mineral spherical particles in the process of being incorporated into the bone matrix, and the bone mineral density was lower than in the wild-type controls, indicating that the mineralization process in the Osxpostnatal mutants was seriously compromised. Furthermore, transmission electron microscopy (TEM) images revealed that the collagen fibers surrounding osteocytes in the Osxpostnatal mutant were disorganized, unlike in the wild-type controls (Fig. S3B).
Fig. 3.
Osx is required for osteocyte maturation and functions. (A) SEM images of the cortexes of humeri of 6-wk-old mice. (B) Back-scattered SEM images. Arrow, osteocytes; arrowhead, mineral spherical vaterites. (C) Quantitative PCR analysis of osteocyte markers. RNA was extracted from the humeri of tamoxifen-treated P24 mice. (D) Sost is a direct target of Osx. (Upper) EMSA using wild-type, m1, and m2 Sost oligos. Recombinant Osx was made in baculovirus (23). For oligo sequences, see Fig. S3C. (Lower) ChIP assay. Chromatin samples were prepared from BMP-2 treated MC3T3-E1 cells. Cells were harvested at 0, 15, 30, and 48 h after BMP-2 addition. The data are presented as percent of input after subtracting control IgG values.
Moreover, we found that Osx can activate the 2-kb Sost promoter (Fig. S3C) and specifically bind to a DNA fragment located within the promoter (Fig. 3D). Mutations in this binding site that prevented Osx binding inhibited activation of this promoter by Osx. A chromatin immunoprecipitation (ChIP) assay showed that Osx was able to interact with the same DNA fragment in the chromatin of intact cells (Fig. 3D), indicating that Sost is a direct target of Osx. Collectively, our findings suggest that Osx is needed for the maturation and function of osteocytes postnatally.
We noted that the number of BrdU-positive cells in the primary spongiosa of the Osxpostnatal mutants was significantly higher than in the wild-type controls (Fig. S3Da). In contrast, the number of BrdU-positive cells in proliferating chondrocytes was unchanged in Osxpostnatal mutants, implying that the function of these cells was unaffected. The increase in BrdU-positive cells was paralleled by an increase in Runx2-positive cells, which are likely to be preosteoblasts, in the primary spongiosa of the mutant (Fig. S3Db). It has been shown that SOST, which is an antagonist of Wnt signaling, was able to inhibit osteoblast proliferation in cell cultures (14). Given that Osx was highly expressed in osteocytes, and that loss of Osx led to decreased Sost expression, we speculated that the increased number of Runx2-positve preosteoblasts might be linked to the lower levels of Sost expression in osteocytes.
Osx Inactivation Leads to Massive Accumulation of Calcified Cartilage.
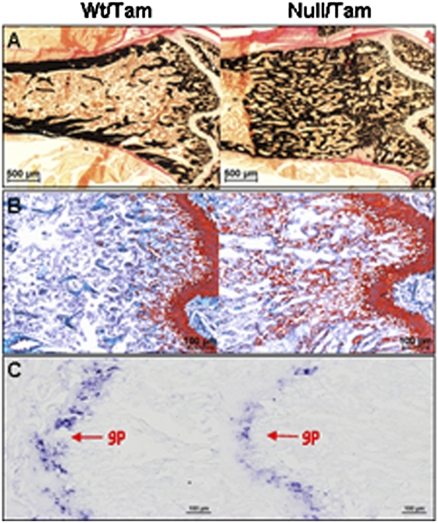
Although postnatal Osx inactivation in osteoblast lineage cells caused the arrest of osteoblast differentiation and bone formation, we observed a large excess of mineralized tissue in the long bones of the Osxpostnatal mutants. Both Safranin O and IHC analyses with antibodies against cartilage-specific matrix proteins (type X collagen and Aggrecan) revealed that the large accumulation of mineralized tissue in both the lumbar vertebrae (Fig. S4A) and the femurs (Fig. 4 A and B and Fig. S4B) of the Osxpostnatal mutants was mainly calcified cartilage matrix.
Fig. 4.
Inactivation of Osx led to massive accumulation of calcified cartilage below the growth plate. (A) von Kossa. (B) Safranin O (SafO) staining. Plastic sections of femurs of tamoxifen-treated 2-mo-old mice were used for staining. (C) In situ hybridization of Col2a1 RNA. Decalcified frozen sections were prepared from the humeri of tamoxifen-treated 6-wk-old mice. gp, growth plate.
By in situ hybridization we found no ectopic Col2a1 and no Col10a1 expression in the zone of excess cartilage of the Osxpostnatal mutant (Fig. 4C and Fig. S4C). This finding ruled out the possibility that the abnormal accumulation of cartilage tissue was produced by Osx-null preosteoblasts.
Mmp13 is expressed by hypertrophic chondrocytes and osteoblasts, in which Osx is also expressed. We reasoned that deletion of Osx in hypertrophic chondrocytes and osteoblasts may cause reduced Mmp13 expression and consequently hinder cartilage ECM remodeling. Indeed, we found that Mmp13 expression in the long bones and calvariae and of the Osxpostnatal mutants was significantly decreased (Fig. S4D). However, IHC analysis with an antibody that specifically recognizes the MMP-cleaved Aggrecan neopeptide revealed that Aggrecan was cleaved in the Osxpostnatal mutants despite decreased Mmp13 expression, presumably by other MMPs, such as MMP9, whose expression was unaffected in the Osxpostnatal mutants (Fig. S4D).
Taken together, our findings suggest that inactivation of Osx results in the abnormal accumulation of calcified cartilage matrix, which is not caused by ectopic production of cartilage or by defective cleavage of Aggrecan. Thus, accumulation of mineralized cartilage tissue is very likely due to defective resorption.
In theory, tamoxifen administration will activate CAG-CreER in all cell types, including hematopoietic stem cell-derived osteoclasts. RNA analysis of in vitro differentiated osteoclasts indicated that these cells did not express Osx, suggesting that deletion of Osx in the cells of this lineage will not affect their osteoclastogenic potential in a cell-autonomous manner. Indeed, the monocytes isolated from the bone marrow cells of Osxpostnatal mutants were able to form tartrate-resistant acid phosphatase (TRAP)-positive multinucleated osteoclasts as efficiently as the monocytes from the wild-type controls (Fig. S5A).
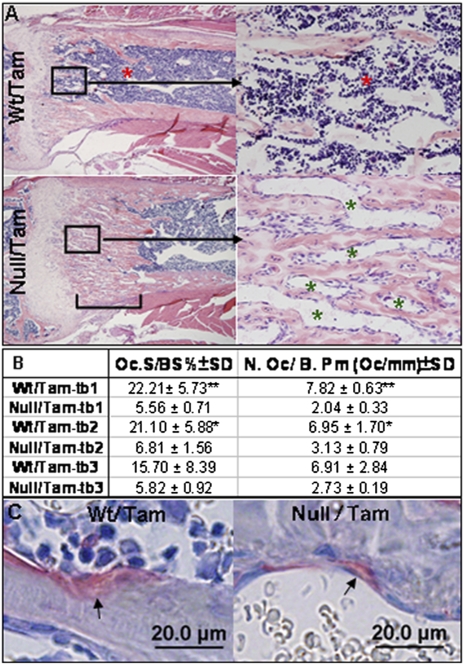
The findings in Fig. 4A and Fig. S4A show that while calcified cartilage was accumulating in the Osxpostnatal mutants, the preexisting bone trabeculae were diminishing, as indicated by the absence of trabeculae right below the zone of calcified cartilages in the femurs and the markedly reduced trabeculae volume in the center of the lumbar vertebrae. In addition, the cortical bones of Osxpostnatal mutants were much thinner and porous. These results suggested that the resorption defect was more severe in the mineralized cartilage than in the bone matrix. Histomorphometry analysis revealed that the density of osteoclasts in the ectopically accumulated mineralized cartilage was reduced at least three times in the femurs of Osxpostnatal mutants compared with the same area below the growth plate in control mice (Fig. 5B). Moreover, the average size of osteoclasts on the surface of this ectopic mineralized cartilage was reduced by about half compared with that of osteoclasts in control bones (Fig. 5C and Fig. S5B).
Fig. 5.
Absence of bone marrow cells and reduced density of osteoclasts in the region of ectopic mineralized cartilage. (A) H&E staining showed that in the zone of mineralized cartilage (marked by the bracket) in the femur of 6-wk-old Osxpostnatal mutant, there are almost no bone marrow cells (red asterisks). The spaces between the mineralized cartilages are filled by capillaries (green asterisks). Bone marrow cells are found below the zone of mineralized cartilage in the mutant. (B) Histomorphometry analysis of osteoclasts (Oc) in metaphysis (excluding cortex) of femurs of tamoxifen-treated 2-mo-old control and Osxpostnatal mutants. BS represents mineralized tissue surface. Tb-1: the area measured from the growth plate to 500 μm distally; tb-2: the area measured from the end of tb-1 to 500 μm distally; tb-3: the area measured from the end of tb-2 to 700 μm distally. n = 3/genotype group. *P < 0.05, **P < 0.001. (C) TRAP staining showed that the size of osteoclasts found in the Osxpostnatal mutant was much smaller than the ones in the tamoxifen-treated control mice. Arrows indicate osteoclasts.
In the cortex, however, the distribution rather than the total number of osteoclasts was changed. There were many more osteoclasts inside the thinner and porous cortical bone, but much fewer in the periosteum of the Osxpostnatal mutants than in control bones The total number of TRAP-positive osteoclasts in the cortical bone region was, however, similar in mutants and control mice (Fig. S5C and Table S2), although the size of the osteoclasts and the intensity of TRAP staining were reduced in the mutants.
We also noted that the ratio of Opg/Rankl expression in long bones was higher in the Osxpostnatal mutants than in the wild-type controls (Fig. S5D). This could account for the overall decrease in the size of osteoclasts, and the reduced TRAP (Acp5) expression in the femur of Osxpostnatal mutants (Fig. S5E).
Strikingly, in the area of excess mineralized cartilage in the femurs of Osxpostnatal mutants, there was an almost complete absence of bone marrow cells in contrast to the presence of bone marrow cells in the same area under the growth plate of control femurs (Fig. 5A and Fig. S5F). There was also an increase in blood vessels stained by anti-collagen type IV in the area of mineralized cartilage in Osxpostnatal mutants (Fig. S5F). However, in the marrow cavity of the remainder diaphysis below the zone of excess cartilage, bone marrow cells were present in apparently normal amounts. Thus in the endochondral bones of Osxpostnatal mutant mice, bone marrow cells were largely absent in the area where accumulation of unresorbed cartilage was found.
Discussion
Our findings indicate that Osx has multiple essential functions in postnatal bone growth and homeostasis. First, several lines of evidence show that inactivation of Osx during and after the major postnatal growth period causes an arrest of osteoblast differentiation and of new bone formation. Our study provides clear evidence that a specific transcription factor essential for embryonic skeletal development is equally essential for osteoblast differentiation and new bone formation postnatally.
To study the Osx postnatal functions, we inactivated Osx after birth using CAG-CreER, a ubiquitously expressed rather than osteoblast-specific Cre recombinase. Inactivation of Osx by a ubiquitously expressed recombinase allowed us to explore the potential function of Osx in cells of the osteoblast lineage before expression of osteoblast-specific marker genes A recent study showed that mice, in which the conditional allele of Osx was inactivated by using a Cre transgene driven by a osteoblast-specific Col1a1 promoter (15), developed a much milder phenotype compared with the Osxpostnatal mutant mice described here. Unlike our Osxpostnatal mutant mice, in which osteoblast differentiation and bone formation was completely arrested, these mice exhibited only a moderately decreased osteoblast activity. One major difference between these two conditional mice models is that in the Col1a1-Cre model, Osx was ablated only in differentiated Col1a1-expressing osteoblasts, whereas in the CAG-CreER model, Osx was deleted in all cell types, including cells involved in all stages of osteoblast differentiation. Another difference is that in the Osx flox/−;Col1a1-Cre mice, inactivation of Osx began around E14.5 when the osteoblast-specific Col1a1 promoter became active, in contrast to the Osxpostnatal mutants described here. A subsequent study that used a Col1a1-CreERT2 transgene to delete Osx postnatally produced only a modest decrease in bone formation and bone mineral density (16). No osteocyte anomalies and no abnormal cartilage accumulation were described in either of these two studies; furthermore, the number and function of osteoclasts was also unchanged. The phenotype of the Osxflox/−;Col1a1-Cre mice indicated that Osx was needed for the optimal function of Col1a1-expressing osteoblasts, whereas the present mouse model demonstrated that Osx was not only essential for osteoblast differentiation and bone formation in postnatal mice, but also for cartilage resorption and osteocyte maturation and function.
During bone formation, osteoblasts first deposit an unmineralized bone matrix called osteoid, which mainly consists of type I collagen. The osteoid subsequently becomes mineralized by incorporation of small mineralized particles. This mineralization process is believed to be principally regulated by osteocytes (1). In the present mouse model, the abnormal Osx-null osteocytes could be derived from either existing osteocytes or Osx-null osteoblasts, which already had begun to express Col1a1 (15) at the time of tamoxifen injections. The fact that some of the abnormal osteocytes in Osxpostnatal mutants were surrounded by unorganized collagen fibers (Fig. S3B) suggested that some abnormal osteocytes might be derived from Osx-null mature osteoblasts. This finding suggested that Osx plays a key role in the maturation of osteocytes. Other lines of evidence support the notion that Osx is also required for osteocyte maintenance and functions. The defective mineralization process in the Osxpostnatal mutants was likely due to the combined decreased expression of Dmp1 and Phex, not to hypophosphatemia, because the blood phosphate levels were normal. Furthermore, the finding that Osx interacted with a specific site in the sclerostin promoter both in EMSA experiments and in intact cells, and activated this promoter in transfection assays, suggested that Osx is also a player in mature osteocytes. Preliminary results showed that inactivation of Osx by Dmp1-Cre, which is highly expressed in osteocytes postnatally, led to morphological osteocyte abnormalities similar to those in Osxpostnatal mutants. Overall, our findings identify Osx as a critical transcription factor required for the maturation and function of osteocytes.
The endochondral bones of Osxpostnatal mutants were also characterized by a massive accumulation of calcified cartilage, which during the growth period extended progressively more deeply toward the diaphysis. This process was driven by the continuous activity of growth plate-proliferating chondrocytes. The unique characteristic of these mice was that the growth plate chondrocytes continued their activity, but no new bone was being formed. The zone of excess cartilage did not contain Col2a1- or Col10a1-expressing cells, or active osteoblasts, as indicated by the virtual absence of Col1a1-expressing cells. This zone did contain cells in which the Osx promoter was active, as illustrated by EGFP positivity and also an abundance of Runx2-positive cells. Our findings clearly indicate that cartilage resorption is severely defective in Osxpostnatal mutants. It is unlikely that this lack of resorption would be due to a selective increase in OPG production by hypertrophic chondrocytes, because OPG immunohistochemistry did not show a significant increase in staining in the hypertrophic zone of Osxpostnatal mutant mice (Fig. S5G). Therefore, we suggest that the process of cartilage resorption is tightly coupled to Osx-dependent bone formation.
In osteopetrotic bones, cartilage and bone tissues accumulate and completely fill the marrow cavity (17). This suggests that osteoclasts are the cells that resorb both cartilage and bone matrices. In Osxpostnatal mutant endochondral bones the overall number of osteoclasts and their size, as well as TRAP expression, was reduced. The reduction in osteoclast numbers was especially marked in the zone of excessive accumulated cartilage. Despite the overall reduction in TRAP expression and activity, our findings suggested that osteoclasts were functional in bone resorption. Indeed, the intrinsic ability of precusor cells from Osxpostnatal mutants to differentiate into osteoclasts in culture was very similar to that of control cells. In addition, the presence of numerous osteoclasts inside the thin and porous cortical bones, and the gradual disappearance of preexisting bone trabeculae once Osx was ablated, strongly supported this view.
During the normal process of endochondral bone formation in the primary spongiosa, osteoblasts first use the cartilage as a scaffold to deposit a bone-specific matrix. During bone remodeling, αvβ3 integrin, which is expressed at high levels in osteoclasts, has a major role in the attachment of osteoclasts to this bone matrix and their subsequent function. αvβ3 integrin interacts with several bone matrix proteins, including bone sialoprotein (BSP), which is expressed at especially high levels in the primary spongiosa. Both loss- and gain-of-function mouse genetic experiments strongly support a role for Bsp in bone resorption (18, 19). Expression of Bsp and several other ECM components was markedly reduced in Osxpostnatal-null bones. The existence of an essentially naked cartilage scaffold not covered by bone was a unique abnormality in Osxpostnatal-null endochondral bones. We propose that the absence of bone formation on the surface of the cartilage scaffold and the reduced number and size of osteoclasts in the area of excess mineralized cartilage could account for the defective resorption of this cartilage.
The reduction in the overall number and size of osteoclasts and in TRAP expression in Osxpostnatal-null long bones could be attributed to an increase in the ratio of Opg to Rankl expression, which itself was a likely consequence of the observed decrease in Sost expression.
One other potential mechanism that might also have accounted for the failure of cartilage resorption is much less likely. We found that the expression of Mmp13 in the femurs and calvariae of the Osxpostnatal mutant mice was significantly reduced (20, 21). Nevertheless, despite decreased Mmp13 expression, the specific cleavage of Aggrecan by MMPs in the Osxpostnatal mutant mice took place.
In Osxpostnatal-null mutants—specifically in the region of ectopic mineralized cartilage—we observed a clear correlation between the lack of new bone and the absence of bone marrow cells. These data suggested that in the primary spongiosa, Osx-dependent endochondral bone formation was required for the formation of bone marrow cells. Our findings are in agreement with a recent study that grafted endochondral bone progenitor cells under the kidney capsule (22). In this assay, these cells had an essential role in the generation of host-derived bone marrow and knockdown of Osx in these progenitor cells, which inhibited donor-derived osteogenesis and abolished formation of the hematopoietic stem cell niche and bone marrow cells in the host (22). Our findings represent the in vivo skeletal equivalent of the graft study. We speculate that the absence of mature osteoblasts and the lack of bone in the zone of ectopic mineralized cartilage causes the localized absence of bone marrow cells.
The Osxpostnatal-null mutant mice grew slower than wild-type controls. The moderate expansion of the hypertrophic zone of these mice could be due to reduced expression of Mmp13. Because the blood levels of phosphate were normal in Osxpostnatal-null mice, the slower growth and the expanded hypertrophic zone cannot be due to hypophosphatemia, as is the case in Dmp1-null mice. We propose that the decreased growth of Osxpostnatal-null limbs could be accounted for by the increase in size of the hypertrophic zone, lack of new bone formation, and the accumulation of unresorbed mineralized cartilage.
In summary, our findings suggest that postnatally, Osx has an essential role in osteoblast differentiation and bone formation. Osx is also needed for the maturation and full expression of the genetic program and hence the function of osteocytes after birth. Furthermore, our data strongly suggest that cartilage resorption is coupled to Osx-dependent endochondral bone formation. The lack of resorption of the accumulated unresorbed mineralized cartilage under the growth plate in the Osxpostnatal mutants could be accounted for in part by the decrease in osteoclast function and density in this area and by the inefficiency of osteoclasts to resorb cartilage in absence of bone deposition on the surface of the cartilage scaffold. Our study thus identifies Osx as an indispensable multifunctional actor in postnatal skeletal growth and homeostasis.
Materials and Methods
Generation of Osxpostnatal Mutant Mice.
The OsxΔEX2/+ mice were generated by crossing Osxfloxed/floxed and Prm-Cre mice. CAG-CreER transgenic mice (12) were purchased from Jackson Laboratory. The tamoxifen-treated CAG-CreER;Osxfloxed/− mice are referred to throughout the text as Osxpostnatal mutant mice; the vehicle or tamoxifen-treated CAG-CreER;Osxfloxed/+ mice are referred to as wild-type controls. Mice were injected intraperitoneally with tamoxifen 1.5–3.0 mg/10 g body weight (Sigma-Aldrich) or vehicle (corn oil containing 10% ethanol) at the desired postnatal days. Osxpostnatal mutant mice and wild-type controls were injected three times with either tamoxifen or vehicle from P15 to P20 and killed at P24; four times (twice with 3 mg/10 g body weight and twice with 1.5 mg/10 g body weight) from P16 to P26 and killed at 6 wk; or four times (3 mg/10 g body weight) from P21 to P30 and killed at 2 mo. These mice are designated P24, 6 wk, and 2 mo.
X-ray, μCT, and Histomorphometry Analyses.
See SI Materials and Methods for the following procedures: RNA isolation and quantitative PCR analyses; EMSA and transfection; in vitro osteoclastogenesis and in vitro osteoblastogenesis; immunohistochemical analysis and in situ hybridization; EM analysis of osteocytes; chromatin immunoprecipitation (ChIP) assay; and statistical analyses.
Supplementary Material
Acknowledgments
We thank Dr. Klaus von der Mark (Nikolaus Fiebiger Centre of Molecular Medicine, University of Erlangen-Nurenberg) for providing anti-ColX antibody, M. Starbuck (Bone Histomorphometry Core, M. D. Anderson Cancer Center) for histomorphometry analyses , and E. M. Johnson (Small Animal Imaging Facility, M. D. Anderson Cancer Center) for μCT imaging services. This work was supported by National Institutes of Health Grant AR049072.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0912855107/-/DCSupplemental.
References
- 1.Bonewald LF. Mechanosensation and transduction in osteocytes. Bonekey Osteovision. 2006;3:7–15. doi: 10.1138/20060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strom TM, et al. Pex gene deletions in Gy and Hyp mice provide mouse models for X-linked hypophosphatemia. Hum Mol Genet. 1997;6:165–171. doi: 10.1093/hmg/6.2.165. [DOI] [PubMed] [Google Scholar]
- 3.Feng JQ, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Komori T, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 8.Nakashima K, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 9.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 10.Timpson NJ, et al. Common variants in the region around Osterix are associated with bone mineral density and growth in childhood. Hum Mol Genet. 2009;18:1510–1517. doi: 10.1093/hmg/ddp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Styrkarsdottir U, et al. New sequence variants associated with bone mineral density. Nat Genet. 2009;41:15–17. doi: 10.1038/ng.284. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 13.Akiyama H, et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci USA. 2005;102:14665–14670. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutherland MK, et al. Sclerostin promotes the apoptosis of human osteoblastic cells: A novel regulation of bone formation. Bone. 2004;35:828–835. doi: 10.1016/j.bone.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Baek WY, et al. Positive regulation of adult bone formation by osteoblast-specific transcription factor osterix. J Bone Miner Res. 2009;24:1055–1065. doi: 10.1359/jbmr.081248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baek WY, de Crombrugghe B, Kim JE. Postnatally induced inactivation of Osterix in osteoblasts results in the reduction of bone formation and maintenance. Bone. 2010;46:920–928. doi: 10.1016/j.bone.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novack DV, Teitelbaum SL. The osteoclast: Friend or foe? Annu Rev Pathol. 2008;3:457–484. doi: 10.1146/annurev.pathmechdis.3.121806.151431. [DOI] [PubMed] [Google Scholar]
- 18.Valverde P, et al. Overexpression of bone sialoprotein leads to an uncoupling of bone formation and bone resorption in mice. J Bone Miner Res. 2008;23:1775–1788. doi: 10.1359/JBMR.080605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malaval L, et al. Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J Exp Med. 2008;205:1145–1153. doi: 10.1084/jem.20071294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stickens D, et al. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131:5883–5895. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inada M, et al. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci USA. 2004;101:17192–17197. doi: 10.1073/pnas.0407788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan CK, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, et al. Inhibition of Wnt signaling by the osteoblast-specific transcription factor Osterix. Proc Natl Acad Sci USA. 2008;105:6936–6941. doi: 10.1073/pnas.0710831105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.