Abstract
Several patterns of brain allometry previously observed in mammals have been found to hold for sharks and related taxa (chondrichthyans) as well. In each clade, the relative size of brain parts, with the notable exception of the olfactory bulbs, is highly predictable from the total brain size. Compared with total brain mass, each part scales with a characteristic slope, which is highest for the telencephalon and cerebellum. In addition, cerebellar foliation reflects both absolute and relative cerebellar size, in a manner analogous to mammalian cortical gyrification. This conserved pattern of brain scaling suggests that the fundamental brain plan that evolved in early vertebrates permits appropriate scaling in response to a range of factors, including phylogeny and ecology, where neural mass may be added and subtracted without compromising basic function.
Keywords: chondrichthyan, cerebellar foliation, allometry, mammal, neuroevolution
The allometric relationship of brain parts to overall brain size has been studied and debated extensively (1–7). At the core of the debate lies the question of whether the brain is best characterized as a collection of independently varying structures/devices evolved for particular behavioral requirements or niches or as a single coordinated processing structure/device in which adaptation for species-specific behavioral capacities occurs without the production of delineable modules (8, 9). Many methodological issues have arisen as well, including what about a brain should be quantified [cells or volumes (10)], what should be compared and how, and how to take into account the statistical dependence of both structural and species relationships (11).
Until recently, a single data corpus comprising primates, bats, and insectivorous mammals was the sole source for comparison (2), leaving the question of whether these mammals represented all vertebrates, or even all other mammals, unresolved. The addition of carnivorous mammals (including marine mammals), ungulates, xenarthrans, and the manatee demonstrated that the original conclusions drawn from primates, bats, and insectivores could be extended to this larger data set (8, 12). These studies revealed that mammalian brain structure exhibits a pattern of variation containing two principal components. The first component, accounting for ≈96% of the total variance of related brain parts to total brain size, loads most highly on neocortex and cerebellum. The second component loads most highly on the olfactory bulb and associated limbic structures and accounts for ≈3% of the original variance. Each brain part also has a characteristic slope with respect to absolute brain size, such that every large mammalian brain is composed disproportionately of neocortex and cerebellum. The remaining 1% of the variance must subsume all remaining sources, including niche, sex and individual differences, and measurement error. This 1% contribution is large in one sense: In two species with the same brain size, a single structure might differ by a factor of 2.5. The total range of structure sizes may differ by a factor of 100,000 or more between the smallest and largest mammalian brains, however.
Cartilaginous fishes occupy a basal position in the evolutionary tree of gnathostomes, and extending these analyses to species in such a key phylogenetic position would be highly instructive regarding the cross-vertebrate generality of the mammalian brain plan (13). For many reasons, brains of sharks, skates and rays (batoids), and holocephalans—a group collectively referred to as chondrichthyans—could demonstrate more diversity in basic brain plans than is seen in the highly covarying mammals. As a group, these species sit closer to the first divergence of vertebrates, where multiple solutions to fundamental adaptive problems might have emerged and been stabilized, with mammals representing only one branch of this early tree. Chondrichthyans occupy a wide range of aquatic niches (14), have an extremely wide range of body size (15), and exhibit substantial variations in brain size and brain organization (16–21). Because neurons are generated widely within the fish brain throughout life (22, 23), engagement of neurogenesis in some aspect of later life history, which is not a major factor in mammals, also might alter numerical relationships established in early development (24). Consequently, we have examined the overall scaling relationships in chondrichthyan brains, duplicating the analytical techniques of the mammalian work but also using other quantitative methods to address some persistent problems in allometric analyses.
Results
Basic Scaling by Order.
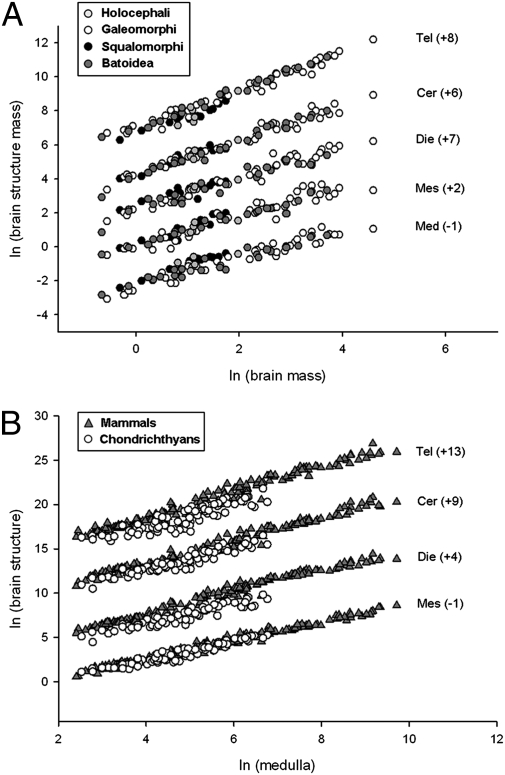
Of the six major brain components (telencephalon, diencephalon, mesencephalon, cerebellum, medulla oblongata, and olfactory bulbs), only the first five scaled strongly with one another. We studied this scaling in 10 orders of chondrichthyans, totaling 81 species (Fig. 1A). These data are visualized as a regression against either total brain mass or the medulla, which allows the easiest visual comprehension of the data structure (not identical to the inferential statistical analysis, as detailed in SI Materials and Methods). SI Materials and Methods also provides details of the brain divisions, explanations of how data were collected from original reports (16–20) and how new data on the mass of the olfactory bulbs were collected in a subset of these species, and the phylogenetic relationships of the chondrichthyans used for independent contrast analyses.
Fig. 1.
Scaling of the relative sizes of five major brain structures plotted as a function of total brain mass across 81 chondrichthyan species (51 sharks, 24 batoids, and 6 holocephalans) (A) and plotted as a function of medulla size, comparing brain structure scaling in chondrichthyans and mammals (B). For the purpose of visualization, an arbitrary constant (in parentheses in each scatterplot) was added to each brain structure. Tel, telencephalon; cer, cerebellum; die, diencephalon; mes, mesencephalon; med, medulla oblongata. The slopes and intercepts (without arbitrary constants) for each regression are given in Table 1 for A and in Table 2 for B.
Here we present five major analyses. First, we explore covariation in the volume of brain parts by factor analysis. Second, we investigate the incidence of hyperallometry (i.e., relatively greater increase in the size of any one brain part with respect to the others), using a statistical test that has rarely, if ever, been used by neuroscientists but is used widely in other areas of biology. Third, we analyze “grade shifts,” or alterations of the patterns of brain allometry appearing at taxonomic boundaries, both within chondrichthyans and between chondrichthyans and mammals. Fourth, we investigate niche-specific brain alterations and factorial structure. Finally, we analyze the relationship between brain structure size and cerebellar foliation within species occupying specialized habitats.
Covariation in structure volumes.
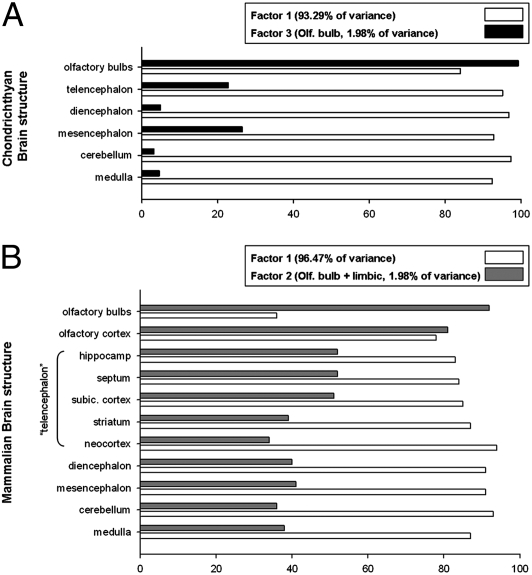
Fig. 1A shows that the sizes of brain components are closely predicted by total brain volume in chondrichthyans and align very well with those of mammals (Fig. 1B). Factor analyses to quantify this claim are restricted to 51 of the 81 chondrichthyans for which data on the size of the olfactory bulbs were available. This analysis yielded three factors. Factor 1, comprising the telencephalon, diencephalon, and cerebellum, accounts for 93.29% of the total variance; factor 2, comprising the medulla and mesencephalon, accounts for 3.27% of the variance; factor 3, consisting of the olfactory bulbs, accounts for 1.98% of the variance. These results are close, but not identical, to those found in mammals, in which the first factor accounts for 96.47% of the variance and the second factor loads highest on the olfactory bulb and limbic system (2.6%) (8). In mammals, the first two chondrichthyan factors appear to be a single factor on which nearly all brain components load highly (Fig. 2B). The first two chondrichthyan factors are closely correlated; across 50 contrasts in the phylogenetic tree of chondrichthyans, the first two factors correlate at a level of 0.820 with each other, but each correlates at only about 0.6 with the “olfactory factor.” The relative loading of factor 1 versus the olfactory factor (factor 2 in mammals, factor 3 in chondrichthyans) is shown in Fig. 2 A and B.
Fig. 2.
Relative loadings of the two factors of the principal components analysis on 6 major brain areas across chondrichthyans (A) and 11 major brain areas across mammals (B). For both groups, the first principal component is plotted (factor 1), which accounts for the vast majority of the variance. For mammals, the second principal component (the olfactory bulb and limbic system, accounting for 2.6% of the variance) is plotted and compared with the third principal component in chondrichthyans (accounting for 1.98% of the variance), both of which load high on the olfactory bulb(s).
Hyperallometry.
For all six structures examined in chondrichthyans, we tested for hyperallometry between each pair of structures. How we accounted for multiple comparisons in these analyses is discussed in SI Materials and Methods. Ignoring the olfactory bulbs initially, our results suggested the same division of structures that had been suggested by the factor analysis. Each of the three structures in factor 1 (see above) was significantly hyperallometric relative to each of the two structures in factor 2 at a higher level of significance than was found in any test between two structures in the same factor. The telencephalon was hyperallometric relative to all of the other structures in factors 1 and 2, but was not significantly different from the diencephalon. The olfactory bulbs were hyperallometric relative to all other structures, although it was not significantly different from the telencephalon and cerebellum (Tables S1 and S2). This pattern of results is strikingly similar to that observed in mammals (8) (Fig. 2B). As in mammals, in chondrichthyans, as total brain size enlarges, the brain becomes disproportionately composed of telencephalon and cerebellum (Fig. 1B).
Grade shifts.
Fig. 1A shows that in chondrichthyans, some of the variation in brain component size relative to total brain size may be accounted for by taxon—notably the increased relative size of the telencephalon. The relationship between this variation and chondrichthyan phylogeny is complex and not immediately visually apparent, however. A different resolution of the phylogeny, currently a subject of debate (25–31), might simplify these observed scaling relationships; however, several clear grade shifts are apparent when chondrichthyans are compared with a sample of 112 nonprimate mammals. Despite conservation of the overall pattern of allometric scaling, substantial grade shifts across these two groups are seen when various structures are compared with the medulla. When matched on medulla size (Fig. 1B), mammals on average have a 4.18-fold larger telencephalon, a 3.47-fold larger diencephalon, and a 2.10-fold larger cerebellum compared with chondrichthyans.
Niche-specific brain alterations and factorial structure.
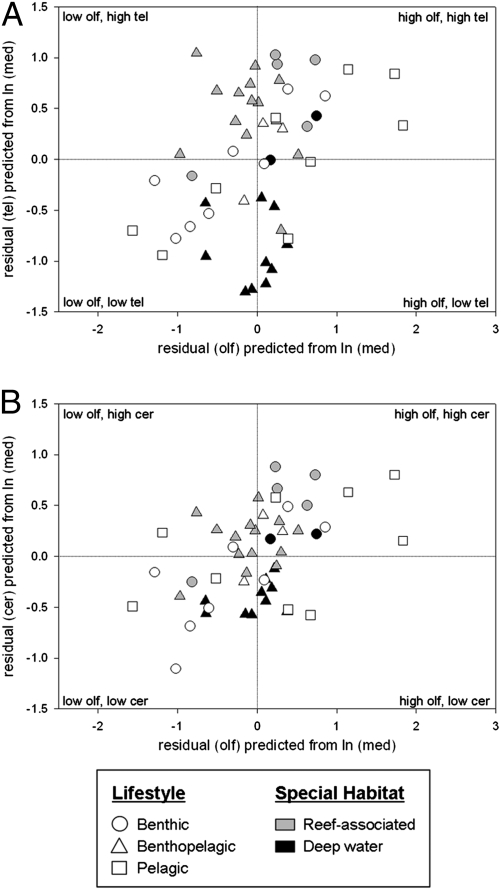
In mammals, the structures loading high on factor 1 versus those loading high on factor 2 also correlate negatively with each other when brain size is controlled (8). That is, primates and marine mammals have a relatively large neocortex and cerebellum and relatively small olfactory bulbs and associated limbic structures, whereas insectivorous mammals and anteaters exhibit an opposite pattern, which is thought to depend in part on each taxon's dependence on vision versus olfaction (1). The inverse relationship implies that these structures compete in some way for their proportion of total brain mass in evolutionary time. Fig. 3 plots the residual volumes of structures loading high on factor 1 (telencephalon and cerebellum) versus the structure loading high on factor 3 for chondrichthyans, contrasting those that live in association with reefs versus those living in deep water. Although it must be noted that squalomorph sharks do not inhabit reefs but galeomorph sharks do—leading to speculation as to whether the difference is phylogenetic, ecological, or in fact both—deep-water species are found across both major chondrichthyan groups, and clustering based on ecological parameters has been found despite phylogenetic dissimilarities (17). In general, the reef-associated species have a greater relative volume of telencephalon and cerebellum and the deep-water species have a greater volume of the olfactory bulbs—although, unlike mammals, these values are not inversely correlated but rather are independent. The reason why the relationship between the two major factors should be negative in mammals but additive in chondrichthyans is not obvious, but may well lie either in the different energetic trade-offs of the marine versus terrestrial environment (32) or in a pervasive life history distinction, such as the degree of maternal investment (33, 34).
Fig. 3.
The relationship between the olfactory bulbs and (A) the telencephalon and (B) the cerebellum across 51 chondrichthyans. Species are coded according to lifestyle and, where applicable, whether a species is reef-associated or dwells in deep water. Olf, olfactory bulbs; tel, telencephalon; cer, cerebellum; med, medulla oblongata.
Cerebellar foliation.
To explore the determinants of cerebellar foliation, we used a dataset of 47 contrasts from 48 chondrichthyan species, with contrast values on foliation based on a five-point scale of corpus complexity (16), plus logged sizes of the telencephalon, diencephalon, mesencephalon, cerebellum, medulla oblongata, olfactory bulbs, and body. We ran a backward stepwise regression in which foliation is predicted first from all of these other variables. We then dropped out, one at a time, the variables contributing least to the regression, provided that their contributions were nonsignificant. Following that rule, each variable dropped out except the cerebellum itself. Its simple correlation with foliation was 0.7149 across the 47 contrasts (t = 6.8579; two-tailed P = 1.66 × 10−8). Thus, the simplest well-confirmed rule is that the larger the cerebellum, the greater its foliation.
Thus far, we have considered only the scaling of brain components with respect to absolute brain size and have not examined brain size relative to body size. Alterations in internal brain organization associated with relative brain size are of particular interest, because relative brain size is more strongly associated with behavioral complexity than is absolute brain size (1, 6). In mammals, there has been an interesting suggestion that the number of cortical areas and cortical folding are somewhat better predicted by relative brain size than by absolute brain size (35, 36). In light of this, we ran a parallel analysis similar to our first one, but with foliation corrected for body size rather than simple foliation as the dependent variable. In this analysis, the stepwise regression ended up with two predictors; cerebellum size had a positive weight, and body size had a negative weight. Thus, the predictive value is cerebellum size corrected for body size. This basically confirms the results of our initial analysis; when the dependent variable switches from foliation to foliation corrected for body size, the best prediction changes in parallel from cerebellum size itself to cerebellum corrected for body size.
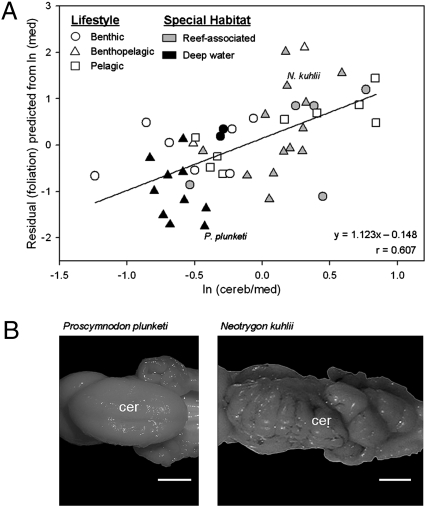
Several authors have suggested that conclusions are most firmly established if similar results appear both in contrast analyses and in analyses using species as data points (37–39). When results are presented graphically, using species has another advantage of allowing the graph to show species characteristics, such as lifestyle. This is done in Fig. 4, which uses medulla size as a proxy for body size as the control variable. The two are very highly correlated (y = 1.123x − 0.148; r = 0.607; P < 0.001); thus, Fig. 4 corroborates the results of previous analyses. In summary, the number of cerebellar folia increases with cerebellar size, and if foliation is controlled for body size, then foliation is highest when the cerebellum is relatively large with respect to the body.
Fig. 4.
Foliation of the chondrichthyan cerebellum. (A) Regression of foliation index score, controlling for total brain size, as a function of cerebellar volume. (B) Dorsal views of the cerebellum of Proscymnodon plunketi (plunket shark; photo: P. Brown) and Neotrygon kuhlii (bluespotted maskray; photo: T. Lisney), species with similar absolute medulla mass, but which diverge maximally on the cerebellum to medulla ratio. Cer, cerebellum; med, medulla oblongata. (Scale bar: 0.5 cm.)
Discussion
Possible Sources and Significance of Increased Foliation.
One paradox about brain allometry is that whereas the relative slopes of various structures are a function of absolute brain size, for the most part it is brain size with respect to body size, or “encephalization,” that is most reliably associated with behavioral complexity across multiple taxa (6). Can a morphological signature of relative brain size across brain sizes be found? In mammals, “gyrification” of the neocortex might be such a case. Considering only primates (35) or a smaller but diverse set of mammals (36), both absolute and relative brain sizes contribute to gyrification. In birds, “foliation,” or the amount of folding in the cerebellum, is similarly related to both the absolute size of the cerebellum and its relative size compared with the medulla (40).
Like in birds but unlike in mammals, in some chondrichthyans, the cerebellum, but not the telencephalon, has a cortical, layered structure (19, 20). There is some evidence that the size and complexity of the chondrichthyan cerebellum are correlated with ecological and behavioral parameters, such as habitat dimensionality, activity levels, and agile prey capture (16–20, 41, 42). Therefore, as in the bird cerebellum and the mammalian cortex, the degree of folding in the shark cerebellum appears to be a morphological signature of relative brain size and is associated with greater behavioral complexity.
In the mammalian cortex, the organizational feature associated with a gyrus is the cortical “area,” a highly interconnected region, often representing a sensory surface. These areas tend to be represented on the convexities of folds, and divisions between areas are found at concave flexures, where connection density drops (43). A developmental mechanism has been proposed to produce folding of this kind in an initially uniform sheet, wherein the denser connections between a single processing region pull the sheet into a fold (44). This tethering effect of axons ultimately “saves wire” by reducing the lengths of connections in the sheet overall. Vertebrates, and indeed chondrichthyans, appear to represent sensory surfaces topographically and repeatedly (45–47). The same mechanism is likely to apply in the cerebellum (although all empirical links in this argument have not yet been demonstrated). If the input region (i.e., the medulla and the associated body) is small compared with the cerebellum, representations of the sensory surfaces may “fission” into separate sensory, motor, and “computed” representations of increased specificity, as the somatosensory modality maps do in the cortex (48). High numbers of gyri (in the mammalian cortex) or folia (in the chondrichthyan and avian cerebellum) might be an identifying feature of highly encephalized brains.
A Conserved Pattern and Its Significance.
The allometric scaling of the major brain components in chondrichthyans is startlingly similar to that in mammals, especially given their phylogenetic distance and the many reasons to expect them to be dissimilar. In both, the fundamental structures of correlation show the very high correlation of major brain components with one another, along with hyperallometry of the telencephalon and cerebellum with respect to the rest of the brain. The factorial structure is similar, with the olfactory bulbs in part statistically independent from the rest of the brain and the factorial structure related to niche in both sharks and mammals; a similar factorial structure was recently described in a set of teleost fishes (49). Why the olfactory brain should retain a substantial degree of allometric independence from the rest of the brain throughout vertebrate history is unknown. Finally, the internal structure of a cortical brain region, such as the cerebellum in chondrichthyans and the neocortex in mammals, varies with respect to relative brain size.
The relative scaling of brain components is noteworthy. In both chondrichthyans and mammals, the volume of the cerebellum and telencephalon (or the subcomponent of the telencephalon, the neocortex, in mammals) increases regularly and disproportionately with brain size, such that brains large in absolute size become more and more composed of these elements, reaching >90% of total mass in mammals. Furthermore, positive grade shifts between taxa concentrate in just those structures that enlarge disproportionately with total brain size. In mammals, these disproportionately enlarging brain regions are those that continue neurogenesis longest in early development. It would be interesting to examine whether some corresponding gradient of cell proliferation can be determined in sharks, which might be the proximate mechanism of this pattern. Possibly, a conserved pattern of neurogenesis could be a “developmental constraint” locked in early vertebrate evolution and maintained thereafter for no important functional reason. More interesting (and more likely), however, is that this pattern of allometric scaling might serve as an important permissive innovation, giving the brain an “evolvable” architecture that combines well with new niche opportunities afforded by the evolution of jaws, permitting the extensive radiation of fish in the Devonian.
In current computing research, scalable computer architectures, or “subsumption architectures,” allow for the graceful addition and subtraction of components and add computational power while maintaining fundamental functionality, and are of much practical and theoretical interest (50, 51). One insight arising from this literature is that locating new computational power directly in command lines executing central functions is ill-advised, and locating new computational power as ancillary loops modifying basic functions is preferable. Note that in both sharks and mammals, those structures that increase relatively little with brain size are those that contain primary sensory and motor neurons (i.e., the mesencephalon and medulla—and, of course, the spinal cord), whereas those structures without a primary sensory or motor component (i.e., the telencephalon and cerebellum) undergo disproportionate expansion. A factor allowing the extensive radiation of vertebrates might have been an evolutionary innovation of a developmental plan for the brain generating an evolvable subsumption architecture.
Materials and Methods
Data Compilation and Brain Organization.
Data on absolute brain mass and the relative mass of five individual brain components (telencephalon, diencephalon, mesencephalon, cerebellum, and medulla oblongata) as a proportion of total brain size in chondrichthyans were compiled from reports of Northcutt (20), Yopak et al. (16), Yopak and Montgomery (17), and Lisney et al. (18). Mass data were compiled for 51 chondrichthyan species across 22 families, where data on brain size and brain organization was available in the literature that overlapped with species for which we had collected mass data on the olfactory bulbs. Telencephalic ventricles had been drained before weighing. Brain masses were not corrected for shrinkage due to fixation, and body mass information was recorded on fresh, unfixed samples.
Brain mass data and brain structure mass for 160 mammalian species were compiled from reports of Stephen et al. (2), Baron et al. (12), and Reep et al. (8). See SI Materials and Methods for detailed descriptions of brain structure boundaries of both datasets.
Cerebellar foliation.
Foliation of the cerebellum varies widely between chondrichthyan species. A visual grading method developed by Yopak et al. (16) was used to quantify this morphological variation on a scale of 1–5. A foliation grade of 1 corresponded to a smooth cerebellar surface; increasing grades reflected increased surface complexity of the corpus up to grade 5, which described an extremely foliated cerebellar surface with deep, branched grooves (16). Data on varying foliation of the cerebellar corpus in chondrichthyans were compiled from reports of Yopak et al. (16), Yopak and Montgomery (17), and Lisney et al. (18).
Olfactory bulbs.
Previously unpublished data on olfactory bulbs (including the olfactory peduncles) were collected along with brain mass and brain organization data from 45 of the 76 shark, batoid, and holocephalan species reported by Yopak et al. (16), Yopak and Montgomery (17), and Lisney et al. (18). These specimens were obtained from various localities in Australasia in accordance with the ethical guidelines of the National Health and Medical Research Council of Australia and/or the University of Auckland. Adult individuals were sampled whenever possible to limit allometric bias (52–54). The brain was excised from each specimen and preserved in an aldehyde-based fixative [(10% formalin in 0.1 M phosphate buffer or Karnovsky's buffer (2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M cacodylate buffer)]. Postfixation, the olfactory bulbs, not including the sensory lamellae but including the olfactory peduncles, were dissected from the telencephalon, blotted, and weighed to the nearest 0.1 g (Table S4). For species for which more than one individual was available, mean values were used. Additional data on the olfactory bulbs for six species were obtained from Northcutt (19, 20). This resulted in a dataset on the mass of the olfactory bulbs from 51 chondrichthyans.
Statistical Analysis.
Allometry.
We plotted the brain mass and brain structure mass data for 51 chondrichthyans on logarithmic coordinates, and calculated the regression line describing the allometric relationship by generalized least squares regression (55) using the equation log(y) = α + β*log(x). Each brain structure (telencephalon, diencephalon, mesencephalon, cerebellum, and medulla oblongata) was plotted as a function of total brain size. Arbitrary constants were added to separate the plots in Fig. 1, ordered from highest slope to lowest; slopes and intercepts are presented in Tables 1 and 2.
Table 1.
Regression equation values for change in brain structure sizes across chondrichthyans (Fig. 1A)
| Brain structure | Slope (α) | Intercept (β) |
| Telencephalon | 1.14 | 6.81 |
| Cerebellum | 1.02 | 0.21 |
| Diencephalon | 0.92 | 4.22 |
| Mesencephalon | 0.86 | 2.09 |
| Medulla | 0.78 | −1.15 |
Table 2.
Regression equation values for the size of individual brain structures plotted against medulla size (Fig. 1B) in chondrichthyans and mammals
| Mammals |
Chondrichthyans |
|||
| Brain structure | Slope (α) | Intercept (β) | Slope (α) | Intercept (β) |
| Telencephalon | 1.45 | −0.34 | 1.27 | −0.71 |
| Cerebellum | 1.32 | −1.01 | 1.21 | −1.21 |
| Diencephalon | 1.26 | −1.40 | 1.09 | −1.82 |
| Mesencephalon | 1.05 | −0.65 | 1.05 | −0.86 |
Principal components analysis and independent contrasts.
Because species cannot be considered statistically independent of one another, we also performed an independent contrasts analysis (56), in which the numbers analyzed were not the logged species values themselves, but rather the difference between the two logged species values at a branch of the phylogenetic tree. These differences, or contrast values, can be considered statistically independent of one another. We calculated independent contrasts using our own custom-written software and the phylogenies of Shirai (27, 28) and McEachran and Aschliman (30); see Figs. S1 and S2 for more information. Because the branch lengths for many taxa are unknown, arbitrary branch lengths were assigned (57). The trees had several nodes with three or four branches. These were arbitrarily broken into binary nodes. The total number of nodes in the reduced tree was 71 (Table S5).
We compiled qualitative ecological information on lifestyle for each of the 51 species (14, 16–18). Species were categorized as being benthic, benthopelagic, or pelagic. Two additional habitats within those lifestyle categories, deep-water and reef-associated, were also assigned.
Factor analysis of data on brains of sharks and their relatives.
We used data on the sizes of six parts of the brains of sharks, batoids, and holocephalans: telencephalon, diencephalon, mesencephalon, cerebellum, medulla oblongata, and olfactory bulbs. We used 51 species for which we had data on all six of these structures. Independent contrasts analysis was applied to the logged sizes of these six structures, yielding a contrast matrix with 50 rows and 6 columns. Ordinary factor analysis programs take the sample mean as the “center” of each variable, whereas contrast data should be centered at 0. To make the factor analyses work properly, we expressed each of the 50 contrasts as two contrasts, one with the original contrast values and one with these values multiplied by −1. Thus, our data matrix had 100 rows instead of 50, and the mean of each of the six columns was exactly 0. We factor-analyzed the covariance matrix computed from this score matrix, not the correlation matrix. The covariance between two variables is the correlation times the two SDs; this gave more weight to variables with large SDs. We used an Oblimin oblique rotation with three factors (Table S6).
Testing for hyperallometry.
In neuroscience, hyperallometry typically refers to the tendency of one brain part to expand more rapidly than some other part as the brain as a whole expands across species, due to either increased body size or increased brain size relative to body size. Neuroscientists usually measure hyperallometry in terms of the ratio of two regression slopes; for instance, we might predict both telencephalon size and medulla size across species from total brain size, and observe that the former regression slope is higher.
This hypothesis about regression slopes is one of three hypotheses that are not exactly equivalent mathematically but are very similar conceptually. If two structure sizes are both highly related to brain size but structure X has a higher regression slope than structure Y, then it follows that across species, X has a higher SD than Y. But how does evolution produce a higher SD in X than in Y? This must occur at splits in the phylogenetic tree. On average, the two new species created at each split must differ more on X than on Y. Thus, three hypotheses are very similar; X has a higher slope than Y, X has a higher SD than Y, and the absolute contrast values on X are on average higher than those on Y.
The second and third of these hypotheses are actually easier to assess than the first, because they require no measure of the “comparison” variable, such as brain size; the only measures needed are X and Y. Moreover, the third hypothesis is susceptible to a statistical test, because it is phrased in terms of contrast values, which are statistically independent. We would argue that the third hypothesis is also the most interesting of the three scientifically, because it is a statement about evolution itself rather than about the outcome of evolution.
Abouheif and Fairbairn (58) have suggested testing the third hypothesis with a simple matched-pairs t test, applied to the absolute values of the contrast values of X and Y. This test has been applied in many areas of biology, including testing whether male body size is hyperallometric relative to female body size (58). Note the complete lack of the need to measure a third variable comparable to brain size. We used a small modification of their approach to test differences between pairs of brain structures. Because contrast values typically are not normally distributed, we prefer to use the nonparametric Wilcoxon signed-rank test rather than the matched-pairs t test. This approach generates both descriptive statistics and significance levels. The descriptive statistics are presented in Table S1, and the significance levels are given in Table S2.
For more detailed discussion of and rationale for these methodologies, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank those who assisted in the acquisition of data and/or specimen photography, particularly C. Duffy, N. Bagley, and P. Brown. K.E.Y. thanks members of the Leigh Marine Laboratory and Center for Scientific Computation in Imaging. The acquisition of data on the olfactory bulbs was funded by a University of Auckland doctoral fees scholarship (to K.E.Y.) and a University of Queensland postgraduate scholarship (to T.J.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002195107/-/DCSupplemental.
References
- 1.Jerison HJ. Evolution of the Brain and Intelligence. New York: Academic; 1973. [Google Scholar]
- 2.Stephan H, Frahm H, Baron G. New and revised data on volumes of brain structures in insectivores and primates. Folia Primatol (Basel) 1981;35:1–29. doi: 10.1159/000155963. [DOI] [PubMed] [Google Scholar]
- 3.Gould SJ. Allometry in primates, with emphasis on scaling and the evolution of the brain. In: Szalay FS, editor. Approaches to Primate Paleobiology. Vol. 5. Basel: Karger; 1975. pp. 244–292. [PubMed] [Google Scholar]
- 4.Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- 5.Barton RA, Harvey PH. Mosaic evolution of brain structure in mammals. Nature. 2000;405:1055–1058. doi: 10.1038/35016580. [DOI] [PubMed] [Google Scholar]
- 6.Lefebvre L, Reader SM, Sol D. Brains, innovations and evolution in birds and primates. Brain Behav Evol. 2004;63:233–246. doi: 10.1159/000076784. [DOI] [PubMed] [Google Scholar]
- 7.Clark DA, Mitra PP, Wang SSH. Scalable architecture in mammalian brains. Nature. 2001;411:189–193. doi: 10.1038/35075564. [DOI] [PubMed] [Google Scholar]
- 8.Reep RL, Finlay BL, Darlington RB. The limbic system in mammalian brain evolution. Brain Behav Evol. 2007;70:57–70. doi: 10.1159/000101491. [DOI] [PubMed] [Google Scholar]
- 9.Finlay BL, Darlington RB, Nicastro N. Developmental structure in brain evolution. Behav Brain Sci. 2001;24:263–278. [PubMed] [Google Scholar]
- 10.Herculano-Houzel S, Collins CE, Wong P, Kaas J. Cellular scaling rules for primate brains. Proc Natl Acad Sci USA. 2007;104:3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey PH, Rambaut A. Comparative analyses for adaptive radiations. Philos Trans R Soc Lond B Biol Sci. 2000;355:1599–1605. doi: 10.1098/rstb.2000.0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baron G, Stephan H, Frahm HD. Comparative Neurobiology in Chiroptera. Basel: Birkhäuser; 1996. [Google Scholar]
- 13.Northcutt RG. Evolution of the telencephalon in nonmammals. Annu Rev Neurosci. 1981;4:301–350. doi: 10.1146/annurev.ne.04.030181.001505. [DOI] [PubMed] [Google Scholar]
- 14.Musick JA, Harbin MM, Compagno LJV. Historical zoogeography of the Selachii. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of Sharks and Their Relatives. London: CRC; 2004. pp. 33–78. [Google Scholar]
- 15.Cailliet GM, Goldman KJ. Age determination and validation in chondricthyan fishes. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of Sharks and Their Relatives. London: CRC; 2004. pp. 399–446. [Google Scholar]
- 16.Yopak KE, Lisney TJ, Collin SP, Montgomery JC. Variation in brain organization and cerebellar foliation in chondrichthyans: Sharks and holocephalans. Brain Behav Evol. 2007;69:280–300. doi: 10.1159/000100037. [DOI] [PubMed] [Google Scholar]
- 17.Yopak KE, Montgomery JC. Brain organization and specialization in deep-sea chondrichthyans. Brain Behav Evol. 2008;71:287–304. doi: 10.1159/000127048. [DOI] [PubMed] [Google Scholar]
- 18.Lisney TJ, Yopak KE, Montgomery JC, Collin SP. Variation in brain organization and cerebellar foliation in chondrichthyans: Batoids. Brain Behav Evol. 2008;72:262–282. doi: 10.1159/000171489. [DOI] [PubMed] [Google Scholar]
- 19.Northcutt RG. Elasmobranch central nervous system organization and its possible evolutionary significance. Am Zool. 1977;17:411–429. [Google Scholar]
- 20.Northcutt RG. Brain organization in the cartilaginous fishes. In: Hodgson ES, Mathewson RF, editors. Sensory Biology of Sharks, Skates, and Rays. Arlington, VA: Office of Naval Research; 1978. pp. 117–194. [Google Scholar]
- 21.Yopak KE, Frank LR. Brain size and brain organization of the whale shark, Rhincodon typus, using magnetic resonance imaging. Brain Behav Evol. 2009;74:121–142. doi: 10.1159/000235962. [DOI] [PubMed] [Google Scholar]
- 22.Birse SC, Leonard RB, Coggeshall RE. Neuronal increase in various areas of the nervous system of the guppy, Lebistes. J Comp Neurol. 1980;194:291–301. doi: 10.1002/cne.901940202. [DOI] [PubMed] [Google Scholar]
- 23.Marcus RC, Delaney CL, Easter SS., Jr Neurogenesis in the visual system of embryonic and adult zebrafish (Danio rerio) Vis Neurosci. 1999;16:417–424. doi: 10.1017/s095252389916303x. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann HA, Benson ME, Fernald RD. Social status regulates growth rate: Consequences for life-history strategies. Proc Natl Acad Sci USA. 1999;96:14171–14176. doi: 10.1073/pnas.96.24.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Compagno LJV. Interrelationships of living elasmobranchs. In: Greenwood PH, Miles RS, Patterson C, editors. Interrelationships of Fishes. London: Academic; 1973. pp. 15–61. [Google Scholar]
- 26.Maisey JG. Higher elasmobranch phylogeny and biostratigraphy. J Linn Soc. 1984;82:33–54. [Google Scholar]
- 27.Shirai S. Phylogenetic relationships of the angel sharks, with comments on elasmobranch phylogeny (Chondrichthyes, Squatinidae) Copeia. 1992;2:505–518. [Google Scholar]
- 28.Shirai S. Phylogenetic interrelationships of neoselachains (Chondrichthyes: Euselachii) In: Stiassny MLJ, Parenti LR, Johnson GD, editors. Interrelationships of Fishes. San Diego, CA: Academic; 1996. pp. 9–34. [Google Scholar]
- 29.de Carvalho MR. Higher-level elasmobranch phylogeny, basal squaleans, and paraphyly. In: Stiassny MLJ, Parenti LR, Johnson GD, editors. Interrelationships of Fishes. San Diego, CA: Academic; 1996. pp. 35–62. [Google Scholar]
- 30.McEachran JD, Aschliman N. Phylogeny of Batoidea. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of Sharks and Their Relatives. London: CRC; 2004. pp. 79–113. [Google Scholar]
- 31.Douady CJ, Dosay M, Shivji MS, Stanhope MJ. Molecular phylogenetic evidence refuting the hypothesis of Batoidea (rays and skates) as derived sharks. Mol Phylogenet Evol. 2003;26:215–221. doi: 10.1016/s1055-7903(02)00333-0. [DOI] [PubMed] [Google Scholar]
- 32.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- 33.Isler K, Van Schaik CP. Why are there so few smart mammals (but so many smart birds)? Biol Lett. 2009;5:125–129. doi: 10.1098/rsbl.2008.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones KE, MacLarnon AM. Affording larger brains: Testing hypotheses of mammalian brain evolution on bats. Am Nat. 2004;164:E20–E31. doi: 10.1086/421334. [DOI] [PubMed] [Google Scholar]
- 35.Zilles K, Armstrong E, Moser KH, Schleicher A, Stephan H. Gyrification in the cerebral cortex of primates. Brain Behav Evol. 1989;34:143–150. doi: 10.1159/000116500. [DOI] [PubMed] [Google Scholar]
- 36.Finlay BL, Cheung D, Darlington RB. In: Developmental Constraints on or Developmental Structure in Brain Evolution? Processes of Change in Brain and Cognitive Development. Munakata Y, Johnson M, editors. XXI. New York: Oxford Univ. Press; 2005. [Google Scholar]
- 37.Price T. Correlated evolution and independent contrasts. Philos Trans R Soc Lond B Biol Sci. 1997;352:519–529. doi: 10.1098/rstb.1997.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillooly JF, Ophir AG. The energetic basis of acoustic communication. Proc R Soc Lond B. 2010;277:1325–1331. doi: 10.1098/rspb.2009.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garland T, Jr, Midford PE, Ives AR. An introduction to phylogenetically based statistical methods, with a new method for confidence intervals on ancestral values. Am Zool. 1999;39:374–388. [Google Scholar]
- 40.Iwaniuk AN, Hurd PL, Wylie DR. Comparative morphology of the avian cerebellum, I: Degree of foliation. Brain Behav Evol. 2006;68:45–62. doi: 10.1159/000093530. [DOI] [PubMed] [Google Scholar]
- 41.Yopak KE, Frank LR. Variation in cerebellar foliation in cartilaginous fishes: Ecological and behavioral considerations. Brain Behav Evol. 2007;70:210–225. [Google Scholar]
- 42.New JG. Comparative neurobiology of the elasmobranch cerebellum: Theme and variations on a sensorimotor interface. Environ Biol Fish. 2001;60:93–108. [Google Scholar]
- 43.Welker W. Why does cerebral cortex fissure and fold? A review of determinants of gyri and sulci. In: Jones EG, Peters A, editors. Cerebral Cortex: Comparative Structure and Evolution of Cerebral Cortex, Part II. New York: Plenum; 1990. pp. 3–136. [Google Scholar]
- 44.Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- 45.Bodznick D, Schmidt AW. Somatotopy within the medullary electrosensory nucleus of the little skate, Raja erinacea. J Comp Neurol. 1984;225:581–590. doi: 10.1002/cne.902250408. [DOI] [PubMed] [Google Scholar]
- 46.Bodznick D. Elasmobranch vision: Multimodal integration in the brain. J Exp Zool Suppl. 1990;5(Suppl 5):108–116. doi: 10.1002/jez.1402560515. [DOI] [PubMed] [Google Scholar]
- 47.Bell CC. Evolution of cerebellum-like structures. Brain Behav Evol. 2002;59:312–326. doi: 10.1159/000063567. [DOI] [PubMed] [Google Scholar]
- 48.Carlson M. Ontogenetic and phylogenetic perspectives on somatic sensory cortex and tactile function. In: Franzen O, Westman J, editors. Information Processing in the Somatosensory System. Hampshire, UK: Macmillan; 1991. [Google Scholar]
- 49.Gonzalez-Voyer A, Winberg S, Kolm N. Brain structure evolution in a basal vertebrate clade: Evidence from phylogenetic comparative analysis of cichlid fishes. BMC Evol Biol. 2009 doi: 10.1186/1471-2148-9-238. 10.1186/1471-2148-9-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brooks R. A robust layered control system for a mobile robot. IEEE J Robot Autom. 1986;2:14–23. [Google Scholar]
- 51.Hawes N, et al. Proceedings of the Twenty-Second AAAI Conference on Artificial Intelligence. Menlo Park, CA: AAAI Press; 2007. Towards an integrated robot with multiple cognitive functions; pp. 1–6. [Google Scholar]
- 52.Brandstätter R, Kotrschal K. Brain growth patterns in four European cyprinid fish species (Cyprinidae, Teleostei): roach (Rutilus rutilus), bream (Abramis brama), common carp (Cyprinus carpio) and sabre carp (Pelecus cultratus) Brain Behav Evol. 1990;35:195–211. doi: 10.1159/000115867. [DOI] [PubMed] [Google Scholar]
- 53.Kotrschal K, van Staaden MJ, Huber R. Fish brains: Evolution and environmental relationships. Rev Fish Biol Fish. 1998;8:373–408. [Google Scholar]
- 54.Lisney TJ, Bennett MB, Collin SP. Volumetric analysis of sensory brain areas indicates ontogenetic shifts in the relative importance of sensory systems in elasmobranchs. Raffles Bull Zool. 2007;14:7–15. [Google Scholar]
- 55.Garland T, Jr, Ives AR. Using the past to predict the present: Confidence intervals for regression equations in phylogenetic comparative methods. Am Nat. 2000;155:346–364. doi: 10.1086/303327. [DOI] [PubMed] [Google Scholar]
- 56.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- 57.Pagel MD. A method for the analysis of comparative data. J Theor Biol. 1992;156:431–442. [Google Scholar]
- 58.Abouheif E, Fairbairn DJ. A comparative analysis of allometry for sexual size dimorphism: Assessing Rensch's rule. Am Nat. 1997;149:540–562. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.