Abstract
Morphine-induced analgesia and antinociceptive tolerance are known to be modulated by interaction between δ-opioid receptors (DORs) and μ-opioid receptors (MORs) in the pain pathway. However, evidence for expression of DORs in nociceptive small-diameter neurons in dorsal root ganglia (DRG) and for coexistence of DORs with MORs and neuropeptides has recently been challenged. We now report, using in situ hybridization, single-cell PCR, and immunostaining, that DORs are widely expressed not only in large DRG neurons but in small ones and coexist with MORs in peptidergic small DRG neurons, with protachykinin-dependent localization in large dense-core vesicles. Importantly, both DOR and MOR agonists reduce depolarization-induced Ca2+ currents in single small DRG neurons and inhibit afferent C-fiber synaptic transmission in the dorsal spinal cord. Thus, coexistence of DORs and MORs in small DRG neurons is a basis for direct interaction of opioid receptors in modulation of nociceptive afferent transmission and opioid analgesia.
Keywords: dorsal root ganglion, spinal cord, peptides, pain, tolerance
The opioid system is critical for inhibitory modulation of pain transmission. Opioid analgesics (e.g., morphine), which mainly target μ-opioid receptors (MORs), remain the most powerful analgesics available for pain relief. However, their chronic use may lead to the development of antinociceptive tolerance. Blockade of δ-opioid receptors (DORs) results in enhanced morphine analgesia and reduced tolerance (1, 2), suggesting interaction between DORs and MORs in the pain pathway (3–6). This idea is further supported by findings that morphine tolerance can be reduced by preventing DOR phosphorylation (7) or by deleting exon 2 of the DOR1 gene (Oprd1) (8) or the preproenkephalin gene (Penk1) (9).Therefore, a further understanding of the interaction between the MOR and DOR systems is essential in attempts to improve pain treatment by means of opioid mechanisms.
Small-diameter neurons in dorsal root ganglia (DRG) convey nociceptive signals to the spinal cord through afferent Aδ- and C-fibers terminating in laminae I–II. DOR1 mRNA has been found in many DRG neurons, including large ones, and at lower levels in both isolectin B4-negative (IB4−, peptidergic) and IB4-positive (IB4+) subsets of small neurons (10–12). In neurons of the pain-modulating system, intracellular DORs are inserted into the plasma membrane following a variety of chemical and behavioral stimuli, including sustained pain conditions and chronic opioids (13–17). In peptidergic small neurons, DOR immunostaining is often associated with large peptide-storing dense-core vesicles (LDCVs), enabling stimulus-induced membrane insertion of DORs (13, 15, 16, 18–20). On the other hand, MORs are present on the cell surface of peptidergic small DRG neurons and the local neurons in spinal lamina II (21, 22). A few immunohistochemical studies suggest that DORs and MORs are coexpressed in some DRG neurons (23, 24). This distribution of opioid receptors is consistent with DOR- and MOR-mediated inhibition of Ca2+ currents in small DRG neurons (15, 25, 26). However, a recent study in the mouse expressing DOR1 with enhanced green fluorescent protein inserted at the C-terminus (DOR1-EGFP) (27) shows that only ∼17% DRG neurons express DOR1-EGFP predominantly in large neurons but not in MOR- or neuropeptide-expressing small DRG neurons (28), suggesting a distinct dissociation of DORs and MORs in primary sensory afferents and pain modulation.
Autoradiographic studies in the spinal cord have shown that the binding sites for DOR (10, 29) and MOR (29–31) agonists are mainly present in laminae I–II. Similarly, both DOR- and MOR-immunoreactive (ir) afferents are mostly distributed in spinal laminae I–II (18, 21, 32). The presence of DORs on nociceptive afferents is supported by findings that the release of the excitatory neurotransmitters glutamate, substance P (SP), and calcitonin gene-related peptide (CGRP) from afferent C- and Aδ-fibers can be inhibited by activating DORs (33, 34). DOR-mediated spinal analgesia is attenuated by the intrathecally applied antisense oligodeoxynucleotide of Oprd1 (35) and the deletion of Oprd1 (8) or Penk1 (9). However, this classic view of the presynaptic inhibitory mechanism of DORs has also been challenged by the above-mentioned study, which reported the absence of DOR1-EGFP in peptidergic afferents (28). Moreover, that study also proposes that mechanoreceptive afferent Aβ-fibers from DOR1-EGFP-expressing large neurons project into lamina II of the spinal cord and are involved in the inhibition of mechanical pain, whereas MORs in peptidergic afferents mediate inhibition of heat pain (28). Because this unique concept of separation of DOR and MOR systems and its functional consequences have important implications for the understanding of opioid analgesia, we undertook further studies to determine whether or not DORs are absent in MOR- and neuropeptide-expressing small DRG neurons. Our results provide evidence for coexistence of DORs and MORs in peptidergic small DRG neurons and their contribution to the presynaptic inhibition of nociceptive afferent transmission.
Results
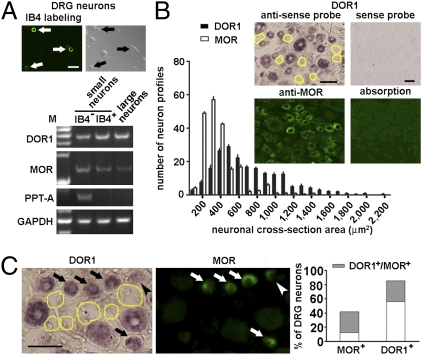
To evaluate the expression of DORs and MORs in subsets of DRG neurons, we collected 30 neurons of each subset from adult mice under a fluorescence microscope and performed RT-PCR to determine the presence of DOR and MOR mRNAs in IB4− or IB4+ small neurons (10–20 μm in diameter) and large neurons (35–50 μm in diameter) (Fig. 1A). DOR1 mRNA was found in all three subsets of DRG neurons (Fig. 1A), with the average level of DOR1 mRNA in IB4− small neurons being lower than in the other two subsets. Importantly, preprotachykinin (PPT)-A, a marker of peptidergic small neurons, was observed in IB4− neurons but not in the other two subsets. Interestingly, varying levels of MOR mRNA were also found in subsets of DRG neurons (Fig. 1A), with the highest level in IB4− small neurons. Thus, this supports coexpression of DOR and MOR in peptidergic small DRG neurons.
Fig. 1.
Coexpression of DORs and MORs in small DRG neurons of mice. (A) IB4+ (arrows) and IB4− subsets of small neurons (30 cells for each subset) and large neurons (30 cells) were selected from dissociated DRG neurons and processed for RT-PCR. Varying levels of DOR1 and MOR mRNAs are present in three subsets of DRG neurons. M, marker. (Scale bar: 50 μm.) (B) In situ hybridization shows that DOR1 mRNA is present in both small (<800 μm2) and large NPs in the mouse DRG (200 DOR1+ NPs randomly selected from each DRG, n = 4 DRGs). A DOR1− neuron is indicated by a yellow outline. The size distribution of DOR1 mRNA+ NPs overlaps, in the range of small neurons, with that of MOR-ir ones. Hybridization with the sense probe for DOR1 and immunostaining with preabsorbed MOR antiserum were used as controls. (Scale bar: 50 μm.) (C) In situ hybridization combined with immunostaining shows that ∼73% of MOR-ir small DRG neurons also express DOR1 (arrows) (n = 600). A MOR-ir neuron contains only a very low level of DOR1 (arrowhead). (Scale bar: 50 μm.)
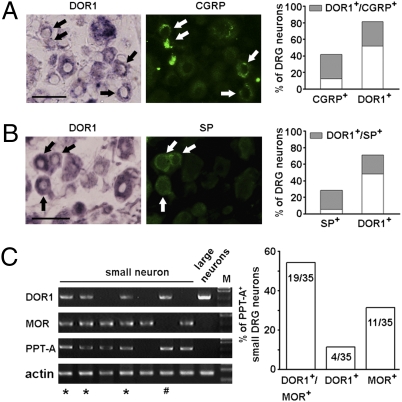
Furthermore, in situ hybridization showed that DOR1 mRNA was present in 71% of small neuron profiles (NPs, <800 μm2) and in 83% of large NPs in mouse DRGs (Fig. 1B). A similar expression pattern was found in the rat DRG (Fig. S1A). Immunostaining showed that the size distribution of MOR-ir cells partly overlapped with that of DOR-ir neurons (Fig. 1B). MOR antibodies recognized exogenous MORs, and the immunostaining in tissues could be abolished by antibody preabsorption (Fig. 1B and Fig. S1B). Importantly, in situ hybridization combined with immunostaining showed that a large fraction (73% in mice and 82% in rats) of MOR-ir small neurons contained DOR1 mRNA (Fig. 1C and Fig. S1C). About one-third of DOR1-expressing neurons were also CGRP-ir (Fig. 2A) or SP-ir (Fig. 2B).
Fig. 2.
Expression of DOR1 in peptidergic small DRG neurons of mice. In situ hybridization combined with immunostaining shows that DOR1 mRNA-containing small DRG neurons also express CGRP (A; ∼32% of DOR1+ neurons, n = 570) and SP (B; ∼30% of DOR1+ neurons, n = 550) (arrows). (Scale bar: 50 μm.) (C) Single-cell PCR shows coexistence of DOR1 and MOR mRNAs in PPT-A mRNA-containing small DRG neurons (*). Some PPT-A mRNA-containing small neurons express DOR1 but not MOR (#). Semiquantitative analysis of single-cell PCR shows that DOR1 is found in ∼66% of PPT-A–expressing small neurons (n = 35) and is coexpressed with MOR in ∼54% of PPT-A mRNA-containing small neurons.
Coexpression of DORs and MORs was further confirmed by performing single-cell PCR (36) in small neurons freshly dissociated from the mouse DRGs. Among 35 small DRG neurons containing PPT-A mRNA, 23 expressed DOR1 mRNA (Fig. 2C) and 19 coexpressed DOR1 and MOR mRNAs (Fig. 2C and Fig. S1D). Moreover, DOR1 was also expressed in 13 of 19 small DRG neurons that did not contain PPT-A mRNA, and some of them also contained MORs (Fig. S1D). Sequencing of the PCR products from randomly selected small neurons (n = 4) proved that they were indeed DOR1 and MOR mRNA, respectively. Taken together, DORs and MORs are coexpressed in a considerable fraction of peptidergic small DRG neurons.
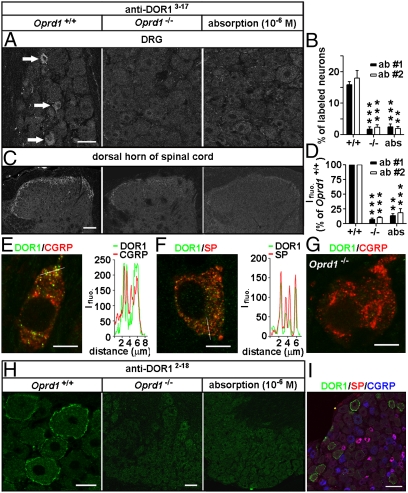
We next used two commercially available antibodies against DOR13–17 that recognized Myc-DOR1 expressed in HEK293 cells (Fig. S2A) to study the localization of DOR1. About 16% of mouse DRG neurons and their afferent fibers in laminae I–II of the spinal cord were immunostained for DORs (Fig. 3 A–D and Fig. S2B). The staining pattern was abolished in Oprd1 exon 1-deleted mice, which contain a truncated DOR1 mRNA (311–1,119 bp) and protein (Fig. S2 C–F), as well as after antiserum preabsorption with immunogenic peptide (10−6 M) when the antibodies were used at a dilution of 1:30,000–60,000 (Fig. 3 A–D) but was only partially reduced at a low dilution (e.g., 1:2,000). Therefore, the antiserum primarily recognizes DOR1, although an excessive amount of antiserum might generate nonspecific binding. Using the antibodies above at a dilution of 1:30,000, we found that DORs were present in peptidergic and vanilloid receptor type 1-ir small neurons (Fig. 3 E and F and Fig. S2G). The DOR labeling associated with vesicles in CGRP- and SP-containing small DRG neurons (Fig. 3 E and F) was also abolished by the deletion of Oprd1 exon 1 (Fig. 3G). Thus, endogenous DORs are localized in vesicles in peptidergic small DRG neurons.
Fig. 3.
Distinct distribution patterns of DORs in subsets of DRG neurons of mice. Immunostaining with antibodies against DOR13–17 [A: 1:30,000, antibody 1 (ab #1); DiaSorin and C: antibody 2 (ab #2); Neuromics] shows DORs in small DRG neurons and afferent fibers in spinal laminae I–II. This immunostaining pattern is abolished by the antiserum preabsorption or the deletion of Oprd1 exon 1. Reduction in immunostaining is quantitatively assayed by determining the percentage of positive DRG neurons (B; n = 6) and fluorescence intensity (Ifluo.) in the laminae I–II (D; n = 5). **P < 0.01; ***P < 0.001. (Scale bars: A and C, 40 μm.). DOR labeling (anti-DOR13–17, 1:30,000; DiaSorin) associated with vesicles in peptidergic small DRG neurons (E and F) is absent in Oprd1 exon 1-deleted mice (G). Colocalization of DORs and neuropeptides is shown by correlated peaks of Ifluo. measured along lines. (Scale bar: 8 μm.) (H) Immunostaining with antibodies against DOR12–18 (1:60,000; Alomone) shows the presence of DORs on the cell surface of large DRG neurons of mice. (Scale bar: 25 μm.) This staining pattern is abolished by preabsorption and is absent in Oprd1 exon 1-deleted mice. (Scale bar: 80 μm.) (I) Triple-immunostaining shows that DOR+ large DRG neurons contain neither SP nor CGRP. (Scale bar: 80 μm.)
Interestingly, we found the presence of DORs on the cell surface in ∼14% of DRG neurons when we tested another antibody, now against DOR12–18 (1:60,000–120,000; Alomone) that also recognized Myc-DOR1 expressed in HEK293 cells (Fig. 3 H and I and Fig. S2A). About 97% of these neurons were large ones (cross-sectional area >800 μm2, n = 224), and most of them expressed neurofilament 200 (Fig. 3I and Fig. S3 A and B). This immunostaining pattern of DORs was abolished by preabsorption and was absent in the Oprd1 exon 1-deleted mouse (Fig. 3H). We did not found any convincing immunostaining of afferent fibers in the dorsal spinal cord with this antiserum. Thus, immunostaining reveals two distinct distribution patterns of endogenous DORs in DRG neurons, namely, in LDCVs, in peptidergic small neurons, and on the cell surface in large neurons.
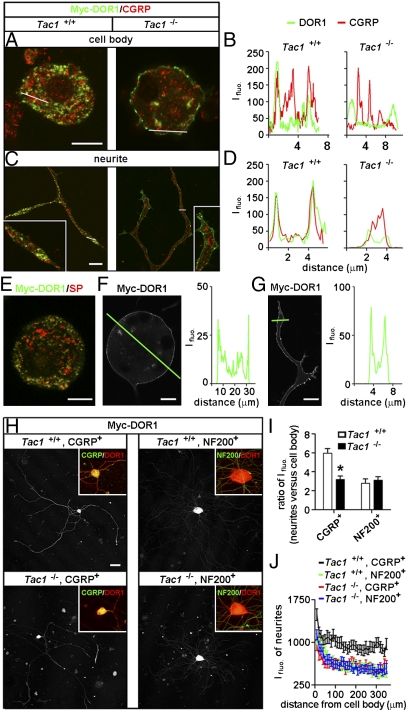
The subcellular localization of DORs was further evaluated by transfecting Myc-DOR1 into DRG neurons. Immunostaining with antibodies against Myc showed that the Myc-DOR1 was mainly associated with CGRP- and SP-containing vesicles in peptidergic small DRG neurons (Fig. 4 A–E), consistent with the distribution pattern of endogenous DORs. The Myc-DOR1 was further found to be localized on the cell surface but absent in CGRP-ir vesicles in small DRG neurons cultured from PPT-A gene-deleted (Tac1−/−) mice (37) (Fig. 4 A–D). A similar immunostaining pattern was also shown by DOR antibodies (Fig. S3C). These results support the notion that protachykinin is required for sorting DORs into LDCVs (19), indicating that the protachykinin-dependent LDCV localization represents a specific distribution pattern of DORs in peptidergic small DRG neurons.
Fig. 4.
Protachykinin-dependent LDCV localization and transport of DORs. (A and B) Double-immunostaining with antibodies against Myc or CGRP shows that CGRP and exogenously expressed Myc-DOR1 are colocalized in LDCVs in the cell body of small DRG neurons cultured from WT mice, whereas Myc-DOR1 is localized on the cell surface of small DRG neurons of Tac1−/− mice (A). (B) Dissociation of DORs from CGRP-containing LDCVs is indicated by loss of correlated peaks of Ifluo. measured along the line. (Scale bar: 8 μm.) (C and D) Immunostaining of Myc-DOR1 is associated with CGRP-containing LDCVs in neurites of small DRG neurons cultured from WT mice. However, in neurites of small DRG neurons of Tac1−/− mice, Myc-DOR1 is present in the plasma membrane but not in CGRP+ LDCVs. (Scale bar: 8 μm.) (E) Immunostaining of Myc-DOR1 is associated with SP+ LDCVs in small DRG neurons. Exogenously expressed Myc-DOR1 is localized on the surface of the cell body (F) and neurites (G) of large DRG neurons. (Scale bar: 8 μm.) (H and I) Ifluo. of Myc-DOR1 in neurites of peptidergic small DRG neurons of WT mice is ∼6-fold higher than that in cell bodies (n = 25), whereas Ifluo. in neurites of large neurons is <3-fold higher than that in cell bodies (n = 11). (H and J) Ifluo. along neurites of peptidergic small DRG neurons of Tac1−/− mice is reduced to a level similar to that of large neurons (n = 25). *P < 0.05 compared with Tac1+/+. (Scale bar: 80 μm.)
In Myc-DOR1–expressing large DRG neurons, the Myc-DOR1 was localized on the surface of cell bodies and neurites (Fig. 4 F and G). Additionally, we found that DOR1 tagged with EGFP appeared on the cell surface of both small and large DRG neurons (Fig. S3D), suggesting that the localization of DOR1-EGFP is a consequence of constitutive delivery of DORs. Immunostaining with DOR antibodies showed both vesicle-associated endogenous DORs and cell surface-localized DOR1-EGFP in small DRG neurons (Fig. S3E). These results indicate that DOR1-EGFP distribution is distinct from endogenous DORs. Furthermore, the protachykinin-dependent LDCV localization of DORs in small neurons enabled efficient transport of DORs into neurites. In contrast, in large neurons, the levels of surface DORs gradually decreased from cell bodies to neurite endings (Fig. 4 H–J). Therefore, the protachykinin-dependent LDCV localization is a key mechanism for regulating centrifugal transport and the intracellular pool of DORs in afferent terminals.
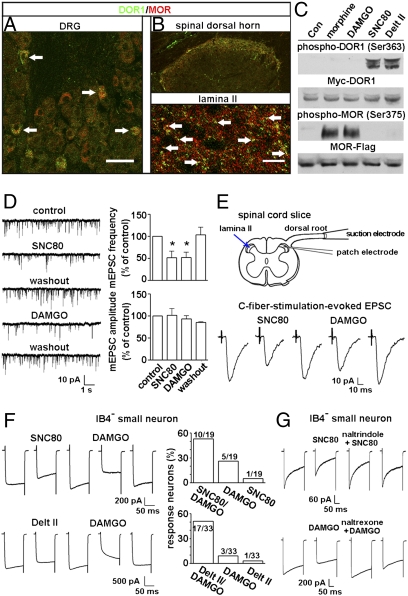
Immunostaining showed coexistence of DORs and MORs in small DRG neurons of mice and in their afferent terminals in laminae I–II of the spinal cord (Fig. 5 A and B). To examine the functions of DORs and MORs, we first proved that the DOR agonists (+)-4-[(αR)-α-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide (SNC80) and deltorphin (Delt) II selectively phosphorylate DORs in HEK293 cells expressing Myc-DOR1, whereas the MOR agonist [D-Ala2, N-Me-Phe4, Gly-ol5]-enkephalin (DAMGO) and morphine phosphorylate MORs in the cells expressing MOR-Flag (Fig. 5C). Recording of the synaptic transmission between the C-fiber terminal and the second-order neuron was carried out in lamina II, a translucent easily identified band in the superficial dorsal horn of spinal cord (38). We measured the miniature excitatory postsynaptic currents (mEPSCs) in the presence of tetrodotoxin (0.5 μM) and found that SNC80 and DAMGO reduced the frequency but not the amplitude of mEPSCs (Fig. 5D), suggesting presynaptic suppression of glutamate release by activating, respectively, DORs and MORs. Furthermore, C-fibers with a threshold of 0.12–0.25 mA (duration of 0.1 ms) were identified with six pulses of electrical stimulation of the dorsal root. The C-fiber stimulation–evoked EPSC could be inhibited by sequentially applied SNC80 and DAMGO in 62% (5 of 8) of recorded neurons (Fig. 5E). Thus, both DORs and MORs in the C-fiber terminal mediate presynaptic inhibition of excitatory synaptic transmission.
Fig. 5.
DOR- and MOR-mediated inhibitory effects. Double-immunostaining shows that DORs (anti-DOR13–17, 1:30,000; DiaSorin) and MORs are colocalized in small DRG neurons (A, arrows) and in afferent terminals in laminae I–II of the spinal cord (B, arrows) of mice. (Scale bars: A, 40 μm; B, 80 μm.) (C) Immunoblotting of extracts from HEK293 cells transfected with plasmids expressing Myc-DOR1 or MOR-Flag shows that DORs are phosphorylated by SNC80 (10 μM) and Delt II (10 μM) but by neither DAMGO (1 μM) nor morphine (1 μM), whereas MOR phosphorylation is induced only by DAMGO and morphine. Con, control. (D) Whole-cell recording in lamina II of the spinal cord slice of rats shows that sequential application of SNC80 (10 μM) and DAMGO (1 μM) reduces the frequency but not the amplitude of mEPSCs (n = 4). *P < 0.05. (E) Whole-cell recording of the synaptic transmission between a C-fiber and a lamina II neuron of the rat spinal cord slice shows that the C-fiber stimulation–evoked EPSC is inhibited by sequentially applied SNC80 (10 μM) and DAMGO (1 μM) (n = 5). (F) Whole-cell recording in IB4− small DRG neurons of mice shows that the Ca2+ current in a single neuron is inhibited by both SNC80 (10 μM) or Delt II (10 μM) and DAMGO (1 μM). (G) DOR agonist-induced effect is blocked by naltrindole (n = 5), whereas the DAMGO-induced effect is attenuated by naltrexone (n = 5).
We then inquired whether coexpressed DORs and MORs could regulate neuronal excitation by examining the agonist-induced effect on depolarization-induced Ca2+ currents in small neurons freshly dissociated from DRGs of mice and rats (25). Whole-cell recording showed that both SNC80 and Delt II inhibited Ca2+ currents in small (IB4−) DRG neurons (Fig. 5F and Fig. S4 A and B). The DOR agonist-induced effect could be blocked by the DOR antagonist naltrindole (Fig. 5G and Fig. S4C). The number of IB4− small neurons responding to DAMGO stimulation was generally greater than that the number responding to DOR agonists (Fig. 5F and Fig. S4B), consistent with the result of in situ hybridization showing a greater number of small DRG neurons expressing MORs than DORs and a previous report of the effect of DAMGO on Ca2+ currents in small DRG neurons (26). Moreover, the inhibitory effect of DOR agonists could be enhanced by 10-Hz electrical stimulation, which induced membrane depolarization and cell surface expression of intracellular DORs (Fig. S4D). Importantly, we found that Ca2+ currents in a single small neuron could be inhibited by a DOR agonist (SNC80 or Delt II) as well as by the MOR agonist DAMGO (Fig. 5F and Fig. S4B). These results suggest that coexpressed DORs and MORs in small DRG neurons mediate inhibitory effects on voltage-gated Ca2+ channels.
Discussion
The present study provides evidence for the coexistence of DORs and MORs in peptidergic nociceptors. First, RT-PCR shows the expression of DORs in both IB4− and IB4+ subsets of small DRG neurons as well as in large DRG neurons. Second, single-cell PCR directly exhibits the presence of DORs in a considerable population of PPT-A-expressing small DRG neurons, and most of them also contain MORs. Third, both in situ hybridization and immunostaining illustrate colocalization of DORs, MORs, and neuropeptides in small DRG neurons. Finally, electrophysiological recording shows DOR- and MOR-mediated inhibitory effects on a single IB4− small DRG neuron. Thus, DORs and MORs are coexpressed in a considerable population of nociceptive afferent neurons.
To evaluate the subcellular localization, various tags can be fused at either the N or C terminus of receptors. In PC12 cells, the exogenously expressed DOR1-EGFP is distributed on the cell surface, whereas HA- or Myc-DOR1 is associated with LDCVs (39). We found here that the pattern of subcellular distribution of Myc-DOR1 in DRG neurons is similar to the endogenous distribution pattern shown with DOR antibodies. However, DOR1-EGFP appears on the cell surface of peptidergic small DRG neurons, suggesting that GFP might interfere with recognition of sorting signals for the LDCV localization. This is not the only example, because the subcellular localization of GFP-tagged granulysins is distinct from native granulysins (40). The difference between the EGFP molecule and the small Myc tag may be attributable to the large size and rigidity of EGFP more easily generating steric hindrance or causing artificial interactions with endogenous proteins. In fact, the absence of DOR1-EGFP in small DRG neurons of knockin mice (28) might be attributable to reduced expression or degradation of the fusion protein in these neurons. Thus, Myc and HA seem to be better tags than GFP for the study of receptor trafficking.
The present study shows two distinct subcellular localizations of DORs: in LDCVs in peptidergic small DRG neurons and on the cell surface in large DRG neurons. These patterns are shown by immunostaining of both exogenously expressed Myc-DOR1 and endogenous DORs using antibodies against DOR13–17 or DOR12–18. Thus, these antibodies against different N-terminal regions of DOR1 might recognize DORs in different states of activation, conformation, glycosylation, and/or palmitoylation (41–44). Moreover, the translocation of exogenously expressed Myc-DOR1 from LDCVs to the cell surface in peptidergic small DRG neurons of Tac1−/− mice supports an essential role of the DOR/protachykinin interaction in sorting DORs into LDCVs (19). Our results suggest that the receptor trafficking can be neuron-specifically regulated, enabling differential modulation of the sensitivity to opioids in somatic sensory pathways.
The LDCV localization of DORs in small neurons contributes to the dense distribution of DORs found in afferents in spinal laminae I–II (19). In view of the presence of DORs also in large neurons (refs. 27, 28; the present study; and other studies), this receptor would be expected to localize also in nerve terminal in deeper layers. However, neither receptor radiography nor immunostaining shows a correspondingly extensive distribution of DORs in Aβ-fibers in spinal laminae III–V. The present finding of a gradual reduction in Myc-DOR1 labeling intensity from cell bodies to neurite endings of large neurons may result in low levels of DORs in afferent Aβ-fibers undetectable with immunostaining. The strong expression of DORs on the surface of cell bodies of large DRG neurons suggests that somatic DORs primarily mediate effects of circulating DOR agonists on mechanoreceptive sensation.
Our electrophysiological analyses show that both DOR and MOR agonists induce inhibitory effects on depolarization-induced Ca2+ currents in the same IB4− small DRG neuron, indicating that coexpressed DORs and MORs function through reducing Ca2+ influx. Moreover, DORs and MORs were found to be transported to afferent fibers in laminae I–II of the spinal cord and to mediate presynaptic inhibition of glutamate release. This is consistent with early reports of DOR- and MOR-mediated analgesic effects on thermal nociceptive responses (45, 46). Interaction between DORs and MORs has been considered to be an important mechanism for modulating opioid analgesia. Gomes et al. (6) reported that DORs interact with MORs in membranes purified from the spinal cord, negatively regulating MOR activity. Moreover, antinociceptive tolerance to morphine can be reduced by pharmacological blockade (1, 2) or genetic interruption of the DOR system (8, 9, 19). Therefore, further study of the mechanism underlying DOR-mediated regulation of MOR activity could clarify the role of DORs in the modulation of morphine-induced antinociception and tolerance and may help to improve opioid analgesia.
Methods
Cell Preparation and RT-PCR.
Neurons were dissociated from DRGs of adult male mice or rats (13). Neurons were treated with fluorescein-labeled griffonia simplicifolia lectin I-isolectin B4 (GSL I-IB4) (1:500; Vector Lab) for 10 min at room temperature and divided into three groups: IB4− and IB4+ small neurons and large ones. The RNA was reverse-transcribed. The resulting cDNA was used as templates for RT-PCR using specific primers (Table S1).
For single-cell PCR (36), neurons were dissociated from mouse DRGs and diluted with medium containing 10% (vol/vol) FBS in DMEM (Invitrogen). Single neurons were aspirated by a Quixell Automated Cell Selection and Transfer System (Stoelting) and processed for RT-PCR with the HotStarTaq DNA polymerase (Qiagen) and primers (Table S1) within 3 h. The PCR products of receptors were sequenced.
The RNA from the spinal cord of WT mice or Oprd1 exon 1-deleted mice (Jackson Lab) was reverse-transcribed, and the full-length and partial DNA of Oprd1 were amplified.
Gene Transfection.
HEK293 cells were transfected with plasmid pCMV-Myc-DOR1 or pCMV-HA-MOR or pcDNA3-MOR-Flag or pCMV vector (19). Dissociated DRG neurons were transfected with pCMV-Myc-DOR1 by electroporation (SI Text).
In Situ Hybridization.
Antisense probes were amplified with PCR primers for mouse DOR1 (NM013622) (Table S1). Probes were labeled with digoxigenin. Sections of L4 and L5 DRGs of adult mice and rats were treated with 10 μg/mL proteinase K for 30 min and then hybridized with probes for 18 h at 67 °C. The hybridization signal was detected with alkaline phosphatase-coupled antibody against digoxigenin (1:2,000; Roche) and nitroblue tetrazolium / 5-bromo-4-chloro-3-indolyl phosphate as color reaction substrates. Some sections were also immunostained with rabbit (Rb) anti-MOR (1:1,000; Neuromics), Rb anti-SP (1:1,000; DiaSorin), or Rb anti-CGRP (1:1,000; DiaSorin) antibody.
Immunostaining.
Adult rats, mice, and Oprd1 exon 1-deleted mice were fixed. Cryostat sections of L4 and L5 DRGs and spinal cord segments were processed for immunofluorescence staining (13) with Rb anti-DOR13–17 (1:2,000–1:60,000; DiaSorin and 1:4,000–1:60,000; Neuromics), Rb anti-DOR12–18 (1:30,000–1:120,000; Alomone), Rb anti-DOR1358–372 (1:1,000–1:2,000; Lifespan Biosciences), Rb anti-MOR (1:1,000; Neuromics); guinea pig anti-SP (1:500; Neuromics), and mouse anti-CGRP (1:1,000; Biogenesis) antibodies. IB4-labeling was carried out with fluorescein-labeled GSL I-IB4 (1:200). The Myc-DOR1–transfected HEK293 cells and neurons were fixed and processed with mouse anti-Myc antibodies (1:500; DSHB). Nuclear DAPI staining was used to indicate HEK293 cells in control experiments.
Drug Treatment.
DAMGO, naltrindole, and naltrexone (Tocris) were dissolved in distilled water, and Delt II (GL Biochem) and SNC80 (Tocris) were dissolved in DMSO. The final concentration of DMSO was 0.1%, which did not affect voltage-gated Ca2+ channels. HEK293 cells expressing Myc-DOR1 or MOR-Flag were treated with morphine, DAMGO, SNC80, or Delt II for 30 min at 37 °C.
Immunoblotting.
The samples were processed for SDS/PAGE, transferred, probed with Rb antibodies against MOR (1:500; Neuromics), phospho-DOR1 (1:1,000; Neuromics), phospho-MOR (1:1,000; Neuromics), Myc (1:500; DSHB), Flag (1:1,000; Sigma), or actin (1:50,000; Chemicon) and visualized with enhanced chemiluminescence (19).
Whole-Cell Patch-Clamp Recording.
Whole-cell recordings were made within 10 h after dissociation of DRG neurons. Barium currents flowing through Ca2+ channels were recorded with the whole-cell configuration of the patch-clamp technique (25) (SI Text).
Neurons freshly dissociated from DRGs of mice were first stimulated by repetitive electrical current (0.5-ms width, 8–10 V) at 10 Hz for 1 min with platinum wires contacting the culture medium. IB4− small neurons were then used to examine the effect of SNC80 on Ca2+ currents.
Statistical Analysis.
All data are shown as mean ± SEM. Statistical analysis was performed using PRISM (GraphPad Software). Paired data were evaluated by Student's t test, and the difference was considered significant at P < 0.05.
Supplementary Material
Acknowledgments
We thank Drs. M.-M. Poo, L.-P. Cheng, R. Elde, R.-R. Ji, and A. Zimmer for discussions. This work was supported by the National Natural Science Foundation of China (Grants 30621062, 30630029, and 30623003), Ministry of Science and Technology 973 project (Grants 2006CB806600, 2007CB914501, and 2009CB522005), and Chinese Academy of Sciences (Grants KSCX2-YW-R-31 and GJHZ06).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008382107/-/DCSupplemental.
References
- 1.Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- 2.Schiller PW. Bi- or multifunctional opioid peptide drugs. Life Sci. 2010;86:598–603. doi: 10.1016/j.lfs.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George SR, et al. Oligomerization of μ- and δ-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 4.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Law PY, et al. Heterodimerization of μ- and δ-opioid receptors occurs at the cell surface only and requires receptor-G protein interactions. J Biol Chem. 2005;280:11152–11164. doi: 10.1074/jbc.M500171200. [DOI] [PubMed] [Google Scholar]
- 6.Gomes I, et al. A role for heterodimerization of μ and δ opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci USA. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie WY, et al. Disruption of Cdk5-associated phosphorylation of residue threonine-161 of the δ-opioid receptor: Impaired receptor function and attenuated morphine antinociceptive tolerance. J Neurosci. 2009;29:3551–3564. doi: 10.1523/JNEUROSCI.0415-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Y, et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in δ opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 9.Nitsche JF, et al. Genetic dissociation of opiate tolerance and physical dependence in δ-opioid receptor-1 and preproenkephalin knock-out mice. J Neurosci. 2002;22:10906–10913. doi: 10.1523/JNEUROSCI.22-24-10906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mennicken F, et al. Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. J Comp Neurol. 2003;465:349–360. doi: 10.1002/cne.10839. [DOI] [PubMed] [Google Scholar]
- 11.Minami M, Maekawa K, Yabuuchi K, Satoh M. Double in situ hybridization study on coexistence of μ-, δ- and κ-opioid receptor mRNAs with preprotachykinin A mRNA in the rat dorsal root ganglia. Brain Res Mol Brain Res. 1995;30:203–210. doi: 10.1016/0169-328x(94)00290-u. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Wessendorf MW. Equal proportions of small and large DRG neurons express opioid receptor mRNAs. J Comp Neurol. 2001;429:590–600. doi: 10.1002/1096-9861(20010122)429:4<590::aid-cne6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Bao L, et al. Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron. 2003;37:121–133. doi: 10.1016/s0896-6273(02)01103-0. [DOI] [PubMed] [Google Scholar]
- 14.Ma J, Zhang Y, Kalyuzhny AE, Pan ZZ. Emergence of functional delta-opioid receptors induced by long-term treatment with morphine. Mol Pharmacol. 2006;69:1137–1145. doi: 10.1124/mol.105.019109. [DOI] [PubMed] [Google Scholar]
- 15.Walwyn W, et al. Induction of δ opioid receptor function by up-regulation of membrane receptors in mouse primary afferent neurons. Mol Pharmacol. 2005;68:1688–1698. doi: 10.1124/mol.105.014829. [DOI] [PubMed] [Google Scholar]
- 16.Patwardhan AM, et al. Bradykinin-induced functional competence and trafficking of the δ-opioid receptor in trigeminal nociceptors. J Neurosci. 2005;25:8825–8832. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cahill CM, et al. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001;21:7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Bao L, Arvidsson U, Elde R, Hökfelt T. Localization and regulation of the delta-opioid receptor in dorsal root ganglia and spinal cord of the rat and monkey: Evidence for association with the membrane of large dense-core vesicles. Neuroscience. 1998a;82:1225–1242. doi: 10.1016/s0306-4522(97)00341-2. [DOI] [PubMed] [Google Scholar]
- 19.Guan JS, et al. Interaction with vesicle luminal protachykinin regulates surface expression of δ-opioid receptors and opioid analgesia. Cell. 2005;122:619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Cheng PY, et al. Ultrastructural immunolabeling shows prominent presynaptic vesicular localization of δ-opioid receptor within both enkephalin- and nonenkephalin-containing axon terminals in the superficial layers of the rat cervical spinal cord. J Neurosci. 1995;15:5976–5988. doi: 10.1523/JNEUROSCI.15-09-05976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, et al. Down-regulation of mu-opioid receptors in rat and monkey dorsal root ganglion neurons and spinal cord after peripheral axotomy. Neuroscience. 1998b;82:223–240. doi: 10.1016/s0306-4522(97)00240-6. [DOI] [PubMed] [Google Scholar]
- 22.Spike RC, et al. MOR-1-immunoreactive neurons in the dorsal horn of the rat spinal cord: evidence for nonsynaptic innervation by substance P-containing primary afferents and for selective activation by noxious thermal stimuli. Eur J Neurosci. 2002;15:1306–1316. doi: 10.1046/j.1460-9568.2002.01969.x. [DOI] [PubMed] [Google Scholar]
- 23.Ji RR, et al. Expression of μ-, δ-, and κ-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci. 1995;15:8156–8166. doi: 10.1523/JNEUROSCI.15-12-08156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rau KK, Caudle RM, Cooper BY, Johnson RD. Diverse immunocytochemical expression of opioid receptors in electrophysiologically defined cells of rat dorsal root ganglia. J Chem Neuroanat. 2005;29:255–264. doi: 10.1016/j.jchemneu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Acosta CG, López HS. δ opioid receptor modulation of several voltage-dependent Ca2+ currents in rat sensory neurons. J Neurosci. 1999;19:8337–8348. doi: 10.1523/JNEUROSCI.19-19-08337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu ZZ, Chen SR, Pan HL. Differential sensitivity of N- and P/Q-type Ca2+ channel currents to a μ opioid in isolectin B4-positive and -negative dorsal root ganglion neurons. J Pharmacol Exp Ther. 2004;311:939–947. doi: 10.1124/jpet.104.073429. [DOI] [PubMed] [Google Scholar]
- 27.Scherrer G, et al. Knockin mice expressing fluorescent δ-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci USA. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherrer G, et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Besse D, Lombard MC, Besson JM. Time-related decreases in μ and δ opioid receptors in the superficial dorsal horn of the rat spinal cord following a large unilateral dorsal rhizotomy. Brain Res. 1992;578:115–127. doi: 10.1016/0006-8993(92)90237-4. [DOI] [PubMed] [Google Scholar]
- 30.Moskowitz AS, Goodman RR. Light microscopic autoradiographic localization of μ and δ opioid binding sites in the mouse central nervous system. J Neurosci. 1984;4:1331–1342. doi: 10.1523/JNEUROSCI.04-05-01331.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gouardères C, Beaudet A, Zajac JM, Cros J, Quirion R. High resolution radioautographic localization of [125I]FK-33-824-labelled mu opioid receptors in the spinal cord of normal and deafferented rats. Neuroscience. 1991;43:197–209. doi: 10.1016/0306-4522(91)90427-p. [DOI] [PubMed] [Google Scholar]
- 32.Arvidsson U, et al. δ-opioid receptor immunoreactivity: Distribution in brainstem and spinal cord, and relationship to biogenic amines and enkephalin. J Neurosci. 1995;15:1215–1235. doi: 10.1523/JNEUROSCI.15-02-01215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda M, Sugimoto K, Oyama T, Kuraishi Y, Satoh M. Opioidergic inhibition of capsaicin-evoked release of glutamate from rat spinal dorsal horn slices. Neuropharmacology. 1995;34:303–308. doi: 10.1016/0028-3908(94)00160-t. [DOI] [PubMed] [Google Scholar]
- 34.Zachariou V, Goldstein BD. δ-Opioid receptor modulation of the release of substance P-like immunoreactivity in the dorsal horn of the rat following mechanical or thermal noxious stimulation. Brain Res. 1996;736:305–314. doi: 10.1016/0006-8993(96)00718-4. [DOI] [PubMed] [Google Scholar]
- 35.Standifer KM, Chien CC, Wahlestedt C, Brown GP, Pasternak GW. Selective loss of delta opioid analgesia and binding by antisense oligodeoxynucleotides to a delta opioid receptor. Neuron. 1994;12:805–810. doi: 10.1016/0896-6273(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 36.Wu SX, et al. Pyramidal neurons of upper cortical layers generated by NEX-positive progenitor cells in the subventricular zone. Proc Natl Acad Sci USA. 2005;102:17172–17177. doi: 10.1073/pnas.0508560102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmer A, et al. Hypoalgesia in mice with a targeted deletion of the tachykinin 1 gene. Proc Natl Acad Sci USA. 1998;95:2630–2635. doi: 10.1073/pnas.95.5.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakatsuka T, Park JS, Kumamoto E, Tamaki T, Yoshimura M. Plastic changes in sensory inputs to rat substantia gelatinosa neurons following peripheral inflammation. Pain. 1999;82:39–47. doi: 10.1016/S0304-3959(99)00037-8. [DOI] [PubMed] [Google Scholar]
- 39.Wang HB, Guan JS, Bao L, Zhang X. Distinct subcellular distribution of δ-opioid receptor fused with various tags in PC12 cells. Neurochem Res. 2008;33:2028–2034. doi: 10.1007/s11064-008-9678-9. [DOI] [PubMed] [Google Scholar]
- 40.Hanson DA, Ziegler SF. Fusion of green fluorescent protein to the C-terminus of granulysin alters its intracellular localization in comparison to the native molecule. J Negat Results Biomed. 2004;3:2. doi: 10.1186/1477-5751-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta A, et al. Conformation state-sensitive antibodies to G-protein-coupled receptors. J Biol Chem. 2007;282:5116–5124. doi: 10.1074/jbc.M609254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta A, et al. Post-activation-mediated changes in opioid receptors detected by N-terminal antibodies. J Biol Chem. 2008;283:10735–10744. doi: 10.1074/jbc.M709454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Micovic V, Ivanovic MD, Dosen-Micovic L. Docking studies suggest ligand-specific delta-opioid receptor conformations. J Mol Model. 2009;15:267–280. doi: 10.1007/s00894-008-0396-7. [DOI] [PubMed] [Google Scholar]
- 44.Petäjä-Repo UE, et al. Distinct subcellular localization for constitutive and agonist-modulated palmitoylation of the human δ opioid receptor. J Biol Chem. 2006;281:15780–15789. doi: 10.1074/jbc.M602267200. [DOI] [PubMed] [Google Scholar]
- 45.Takemori AE, Portoghese PS. Enkephalin antinociception in mice is mediated by δ1- and δ2-opioid receptors in the brain and spinal cord, respectively. Eur J Pharmacol. 1993;242:145–150. doi: 10.1016/0014-2999(93)90074-r. [DOI] [PubMed] [Google Scholar]
- 46.Fraser GL, Gaudreau GA, Clarke PB, Ménard DP, Perkins MN. Antihyperalgesic effects of δ opioid agonists in a rat model of chronic inflammation. Br J Pharmacol. 2000;129:1668–1672. doi: 10.1038/sj.bjp.0703248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.