Abstract
Stress affects the hippocampus, a brain region crucial for memory. In rodents, acute stress may reduce density of dendritic spines, the location of postsynaptic elements of excitatory synapses, and impair long-term potentiation and memory. Steroid stress hormones and neurotransmitters have been implicated in the underlying mechanisms, but the role of corticotropin-releasing hormone (CRH), a hypothalamic hormone also released during stress within hippocampus, has not been elucidated. In addition, the causal relationship of spine loss and memory defects after acute stress is unclear. We used transgenic mice that expressed YFP in hippocampal neurons and found that a 5-h stress resulted in profound loss of learning and memory. This deficit was associated with selective disruption of long-term potentiation and of dendritic spine integrity in commissural/associational pathways of hippocampal area CA3. The degree of memory deficit in individual mice correlated significantly with the reduced density of area CA3 apical dendritic spines in the same mice. Moreover, administration of the CRH receptor type 1 (CRFR1) blocker NBI 30775 directly into the brain prevented the stress-induced spine loss and restored the stress-impaired cognitive functions. We conclude that acute, hours-long stress impairs learning and memory via mechanisms that disrupt the integrity of hippocampal dendritic spines. In addition, establishing the contribution of hippocampal CRH–CRFR1 signaling to these processes highlights the complexity of the orchestrated mechanisms by which stress impacts hippocampal structure and function.
Keywords: corticotropin-releasing factor, long-term potentiation, memory, synaptic plasticity, hippocampus
The hippocampal formation is involved in a circuit that is required for several types of memory in both humans and rodents (1–4). At the physiological/cellular level, memory processes generally are believed to involve long-term potentiation (LTP) of synaptic function (5–8). This potentiation, in turn, is associated with an increase in the size (9, 10) and altered composition (11–13) of dendritic spines of hippocampal principal cells that carry excitatory synapses (14, 15).
Stress affects both the function and the structure of the hippocampus (16–25). A large body of work has demonstrated that chronic stress may result in memory deficits (26–31) and abnormal LTP (32–34), and these functional deficits often are accompanied by diminished dendritic arborization (35–42). Short-term or acute stress, lasting minutes to hours, also has been found to affect memory (43–47). In parallel, short-term stress has been reported to influence LTP (48–52) and reduce the density of dendritic spines in area CA1 (44, 53) or area CA3 (51, 54). Whereas hippocampus-mediated memory deficits commonly were associated with—and perhaps result from—loss of synapse-bearing dendrites and dendritic spines, this association has not been universal (37, 46, 55), so that the structure–function relationship underlying the effects of stress on hippocampal neurons has not been resolved.
Because of the prevalence and pervasiveness of stress in modern life, the mechanisms by which acute and chronic stress impact the hippocampus have received significant attention. Stress involves activation of the hypothalamic-pituitary-adrenal axis (16, 56). This activation consists of release of the stress neuropeptide corticotropin-releasing hormone (CRH) from hypothalamic neurons and activation of CRH receptors (CRHRs) within the pituitary. The resulting release of adrenocorticotropic hormone leads to secretion of corticosteroids from the adrenal gland into the circulation. Indeed, a large number of seminal studies have elucidated the roles of steroid hormones in the effects of both chronic (16, 19, 35) and acute stress (52, 57, 58). However, stress initiates a protean response involving, in addition to corticosteroids, the activation of classical neurotransmitters including monoamines and of neuropeptides (24, 56, 59, 60). Contributions of serotonin (27, 42) and glutamate receptor (GR) activation (43, 49) to the effects of stress on dendritic structure and LTP, often in concert with glucocorticoids (24), have been reported. Interestingly, many of the structural and functional consequences of stress on hippocampus have been apparent in area CA3, where there is a paucity of GRs, the receptor type activated by stress levels of corticosteroids (61, 62). These observations have raised the possibility that the mechanisms by which stress influences hippocampal neurons might be complex, involving a broad repertoire of stress mediators and receptors (16, 24, 63).
Focusing on the potential involvement of CRH, we previously have shown that short-term stress releases this neuropeptide not only from hypothalamic neurons but also within the hippocampus (64, 65). In addition, selective loss of dendritic spines in the stratum radiatum of area CA3, provoked by acute stress, was abrogated by an antagonist of the CRH receptor type 1 (CRFR1) (54). However, the functional significance of the loss of spines and the relationship of the loss of spines to both cellular and functional measures of learning and memory have remained unclear. Here we report that acute stress-induced spine loss is associated with attenuation of LTP in the corresponding synapses but not in the mossy fiber–CA3 synapses. In addition, using transgenic mice with visible YFP-expressing neurons, we find that the degree of stress-provoked spine loss correlates significantly with the memory impairment in individual mice. Finally, we discover here that elimination of spine loss by blocking CRHR signaling rescues memory function. These data illustrate a mechanistic structure–function relationship of stress-vulnerable memory processes and highlight the complexity of the orchestrated mechanisms by which severe stress impacts hippocampal structure and function.
Results
Hours-Long Multimodal Stress Impairs Learning/Memory and Attenuates LTP in Select Synapses.
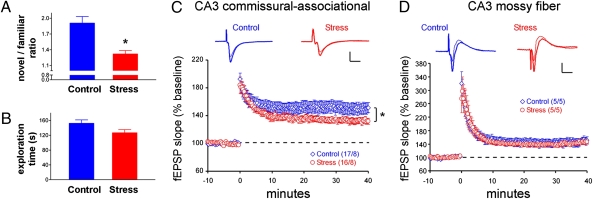
Simulating intense, real-life stress, we exposed mice to 5 h of restraint, bright light, unpredictable loud noise, awareness of peer discomfort, and jostling (54, 65). This multimodal stress resulted in defective learning and memory functions evident as an inability to remember a previously seen object (1, 66). Specifically, at 90 min after the stress, both control and stressed mice were capable of exploring two objects, and both groups explored the two presented objects for the same duration (Fig.1). However, when tested for memory of these objects 6 h later, stressed mice failed to distinguish a previously encountered object from a novel one (Fig. 1A).
Fig. 1.
Short multimodal stress impairs learning and memory and area CA3 synaptic plasticity in select dendritic lamina. (A) Mice experiencing a 5-h combined psychological/physical stress failed to distinguish an object that they had seen before from a novel one. Control mice explored the novel object preferentially, whereas stressed mice spent almost equal time exploring an object they had encountered 6 h earlier and a novel one, suggesting they did not distinguish between them. *P < 0.05. (B) Stressed mice that were allowed a 90-min recovery period explored two objects for the same duration as control mice, eliminating the possibility that their apparent memory defects were a result of freezing or poor motivation. n = 10 mice per group. (C) Slices prepared from mice < 1 h after multimodal stress (red) exhibited deficient LTP magnitude at area CA3 C/A synapses as compared with controls (blue). Representative traces from baseline (thin lines) and 30-min postinduction (thick lines) are shown. Values in parentheses indicate numbers of slices. *P = 0.01 for minutes 30–40. (D) Mossy fiber potentiation tested in slices from the same animals as in C showed no effect of stress. Results are shown as means ± SEM. Values in parentheses indicate number of mice. [Scale bars in C and D: 1 mV (vertical bar) and 10 ms (horizontal bar).]
Although novel object recognition probably involves a wide range of neuronal populations (1, 66, 67), impairment of synaptic function in hippocampal field CA3 was apparent in the stressed mice when LTP was examined in acute slices. Field excitatory postsynaptic potentials (fEPSPs) in area CA3 stratum radiatum were assessed after single stimulation pulses delivered to commissural/associational (C/A) afferents; LTP was evaluated in response to a single train of theta-burst stimulation (TBS) (Fig. 1C). The magnitude of the LTP (fEPSP slope; means ± SEM) was reduced significantly in slices from eight stressed mice (132 ± 4% of baseline) as compared with controls (150 ± 8; P = 0.01; Fig. 1C). In contrast, mossy fiber potentiation in the adjacent stratum lucidum of area CA3 was not impaired in stressed animals (Fig.1D). No effect of stress was observed on input (stimulus duration)/output (fEPSP amplitude) relationships in either lamina. These results indicate that the acute stress provoked a region- and circuit-specific deficit of synaptic plasticity and provide a plausible mechanism for the observed behavioral memory deficits (5–8).
Stress-Induced Reduction of Spine Density Is Selective and Corresponds to LTP Deficits.
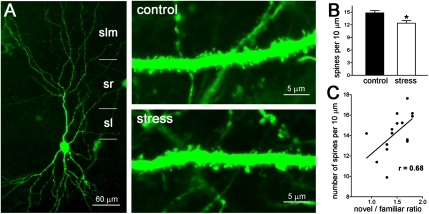
The density of spines in apical dendrites of stress-sensitive area CA3 pyramidal cells (35, 36) in stressed and unstressed mice was compared. Spine density in proximal stratum radiatum, the portion of the apical dendrite innervated by the C/A pathways, was diminished in mice that experienced the short multimodal stress (Fig. 2 A and B), in line with our previous study (54). Spine densities in the distal apical dendrites of the same neurons were not altered.
Fig. 2.
Reduced spine density after short multimodal stress and its correlation to cognitive function. (A) (Left) An area CA3 hippocampal neuron expressing YFP. The apical dendritic arbor is delineated, showing its span within stratum lucidum (sl), stratum radiatum (sr), and stratum lacunosum-moleculare (slm). Stress-vulnerable dendritic spines are located in the stratum radiatum. (Right) Segments of apical dendrites (within the stratum radiatum) from a control mouse (Upper) and from a mouse killed after a 5-h multimodal stress (Lower) are shown. (B) The reduced spine density is quantified; *P < 0.05. (C) Correlation between spine density in apical dendrites (third and fourth branches in the stratum radiatum) and the ratio of time spent exploring a novel vs. a familiar object in individual mice. r = 0.68 (Spearman's correlation coefficient); P = 0.0055.
Correlation of Stress-Induced Spine Loss and Memory Impairment in Individual Mice.
If the stress-induced reduction of spine density (and associated loss of postsynaptic elements of excitatory synapses) contributed to the learning and memory impairment provoked by the stress, then spine density and memory performance should correlate in individual mice. To test this possibility, we examined the effects of stress on novel object recognition and the density of apical dendrite spines in area CA3 in the same mice. We first queried if the reduction in dendritic spine density was still apparent at the end of the memory-testing paradigm (7.5 h after the termination of the stress). Dendritic spine density in the area CA3 stratum radiatum of stressed mice still was significantly lower than that of unstressed mice at this time point (12.39 ± 0.62 spines/10 μm dendrite, n = 6 mice vs.14.83 ± 0.56 spines/10 μm dendrite, n = 7 mice; P = 0.0013). We then plotted memory function for objects, expressed as the ratio of the time spent exploring the novel object to the time spent exploring the familiar object (novel/familiar) versus spine density in area CA3 stratum radiatum (Fig. 2C). The resulting correlation (r = 0.68; P = 0.0055) was highly significant, uncovering the relationship of spine density and cognitive function.
Prevention of Stress-Induced Spine Loss Rescues Memory Function.
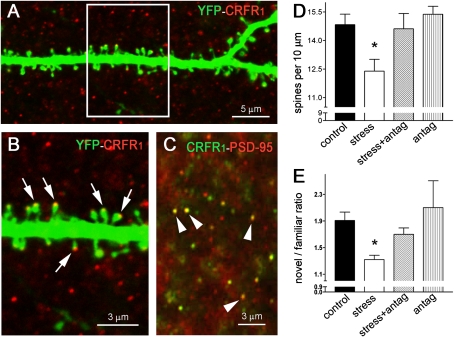
We reasoned that if reduced dendritic spine density in hippocampal area CA3 pyramidal cells contributed to the mechanisms of memory impairment provoked by stress, then prevention of the spine loss should abrogate the cognitive defects. In hippocampal area CA3 neurons, CRFR1 resided on dendritic spine heads (Fig. 3 A and B), where it colocalized with the postsynaptic density protein-95 (PSD-95) (Fig. 3C). Based on our previous work, we attempted to prevent stress-induced spine loss by blocking the ability of CRH to bind its receptor, CRFR1, within the hippocampus (54). The selective blocker of CRFR1, NBI 30775, was infused directly into the brains of stressed and control mice at doses that do not leak to the peripheral blood and thus do not prevent activation of pituitary CRFR1 and the stress-induced increase of plasma glucocorticoids (65). This procedure, selectively blocking central CRH−CRFR1 signaling, prevented the reduction of spine density provoked by the 5-h stress (Fig. 3D). Notably, the antagonist rescued the novel object recognition in stressed mice (Fig. 3E).
Fig. 3.
Blocking the effects of short multimodal stress on dendritic spines abrogates cognitive deficits. (A) The CRH receptor CRFR1 resides on dendritic spine heads, as shown by confocal microscopy of dendrites from YFP-expressing mice. (B) Boxed area in A; arrows denote spine heads expression the CRH receptor. (C) Confocal microscopic image obtained after dual immunohistochemistry for the receptor and PSD-95 (arrowheads point to dual-labeled puncta typical of spine heads). The receptor colocalizes with PSD-95. (D) Mean spine density in area CA3 stratum radiatum segments of YFP-labeled pyramidal neurons in control mice, mice subjected to stress, mice subjected to stress with intracerebral pretreatment with the CRFR1 antagonist NBI 30775 (15 μg in 1 μL) (stress + antag), and control mice administered an equal dose of NBI 30775 (antag). All mice had indwelling cannulae and received vehicle or NBI 30775 to eliminate potential confounders of the surgical or infusion procedures. n = 6–7 mice per group. (E) Novel object recognition ratio in mice subjected to the four treatments described in D. Stressed mice explored the novel object significantly less than the other groups. n = 6–7 mice per group. Asterisks in D and E indicate significant difference from control values.
Discussion
Stress is a biologically important and ubiquitous circumstance that can influence brain function. Because of the importance of both the beneficial and deleterious effects of acute and chronic stress on cognitive function, they have been a subject of numerous studies during the past 8 decades. Focusing on the effects of stress on the hippocampus, a large body of work has uncovered effects of chronic and short-term stress on learning and memory (17, 19, 22, 23). These effects have been accompanied by morphological changes: Specifically, reduced dendritic arbors after chronic stress (35, 36, 68) and reduced spine density after acute stress (39, 43, 54) have been described. Interestingly, diverse locations of stress-induced structural changes have been reported. For example, chronic stress reportedly reduced dendritic complexity in area CA1 (39), in area CA3 (35, 37, 42), or in both fields (38). After short-term stress, altered spine density has been found in basal dendrites of area CA1 (46) or in apical dendrites of area CA3 (35, 36, 44, 54). Even within field CA3, investigators found alterations of synapses in stratum lucidum (44), in stratum radiatum (51, 54), throughout the apical dendrites (35), or in locations that varied depending on the type of the area CA3 pyramidal cells (long vs. short shaft) that were examined (38, 51).
These diverse morphological effects of stress are not surprising, given the wide array of stress types and contexts (24) coupled with the biological variability of the hippocampus that is being stressed, including gender (53, 69, 70) and age (41, 71). Therefore it is reasonable to propose that the plethora of structural and functional changes provoked by stress might be a result of a broad repertoire of stress-activated mediators acting via numerous mechanisms. Among these mediators are corticosteroids that are released from the adrenal gland, enter the brain, and activate GRs and mineralocorticoid receptors within hippocampus (16, 61, 72). In addition, stress activates the autonomic nervous system and a variety of monoamines and neuropeptides, including hypothalamic and hippocampal CRH (22, 24, 56). The crucial role of glucocorticoids in structural and functional consequences of acute stress on hippocampus, the focus of the current study, has been widely documented (73). For example, Alfarez et al. (74) modulated the effects of acute psychosocial stress on LTP in area CA1 by manipulating glucocorticoid levels. Cazakoff and Howland (52) found memory deficits and attenuated LTP in area CA1 in a model of acute psychological stress, and these effects were prevented by a GR antagonist, as reported also by others (75). Area CA1 is rich in GRs (61, 62, 76), further validating the important role of these hormones in stress-induced modulation of synaptic plasticity in this region.
A number of studies have supported the involvement of additional mechanisms in the detrimental effects of stress on hippocampal structure and function (68, 77), including activation of glutamate (43, 45, 49, 63), serotonin (27, 37, 42, 47), and GABA (78) receptors. The resulting downstream processes might be reversed by antidepressants including lithium (55, 79), tianeptine, and agomelatine (28, 63), potentially via cellular cascades including neural cell adhesion molecules (47, 79).
In the current study, a 5-h period of psychological/physical stress reduced spine density in hippocampal area CA3 and prominently in the lamina where C/A fibers form synapses on dendritic spines of area CA3 pyramidal cells. The spine loss was associated with the attenuation of LTP selectively in the C/A pathway and required CRH–CRFR1 signaling. CRH is released in hippocampus during stress and at modest levels contributes to priming of LTP (80). At higher levels the peptide may injure hippocampal neurons directly (81, 82) via CRFR1 (54). Together with the current finding that blocking CRH–CRFR1 signaling abolished the stress-induced spine loss, these observations suggest that acute stress may release endogenous CRH within the hippocampus in sufficient amounts to impact the integrity of dendritic spines in hippocampal area CA3 apical dendrites. The mechanisms by which CRH results in rapid loss of spines are not fully understood. Although the final step includes disintegration of spine cytoskeleton (54), upstream mechanisms might involve cellular adhesion molecules (79) and/or tissue-plasminogen activator (40). In addition, the basis for the region specificity of the CRH-dependent stress-induced changes found here remains unclear. We speculate that the elimination of spines and loss of synaptic plasticity might require interaction of CRH signaling with other stress mediators acting on the same neurons and subcellular domains (24). Thus, although blocking of CRH–CRFR1 signaling prevented the stress-induced spine loss and overt memory defects, it is likely that CRH is only one of several effectors that interact coordinately to impact synapse and spine integrity.
The current results also further our understanding of the relationship of stress-induced spine and dendritic loss and cognitive deficits. Significant correlation of dendritic atrophy and memory defects has been found in chronic stress, as well as correlations between recovery of dendritic trees and spatial memory loss induced by chronic stress or corticosterone (16–18, 34). Others have discussed the relationship between area CA3 spine loss in acute stress and LTP (83). However, several groups found a dissociation between the effects of therapeutic interventions such as lithium or tianeptine on hippocampal structure and function (37, 46, 55), suggesting that the causal relationship between dendrite, spine, and synapse loss and impaired memory might be complex. Here we found congruent lamina- and circuit-specific effects of acute stress on hippocampal structure and function. Loss of spines was apparent in area CA3 pyramidal cell apical dendrites in proximal stratum radiatum, a region where spines provide the postsynaptic targets for C/A axons, but not in distal dendritic branches which are innervated predominantly by entorhinal (temporoammonic) afferents. This lamina specificity was in accord with the impairment of LTP in C/A afferents but not in the mossy fiber pathway that terminates in the more proximal apical dendrites (stratum lucidum). Importantly, the significant correlation of the degree of spine loss and the degree of cognitive impairment in individual mice supports a causal relationship, a conclusion strengthened by our finding that blocking stress-induced spine loss abrogated the memory impairments in the same mice. Together, and as proposed in the prefrontal cortex after chronic stress (84, 85), these data suggest that stress causes loss of spines and thus reduction in the number of postsynaptic elements harboring glutamatergic receptors crucial for LTP, learning, and memory. These structural changes, in turn, underlie the poor cognitive function of the stressed mice.
In summary we find that a combined psychological/physical stress for several hours impairs memory and leads to a selective, congruent loss of LTP and dendritic spines in hippocampus. The degree of spine loss correlates significantly with the memory defects in individual mice, and preventing spine loss using a CRHR blocker improves memory function. These findings support selective spine loss, with resulting loss of excitatory synapses, as a basis of LTP and cognitive defects. The data further highlight a role for hippocampal CRH in the complex stress-activated machinery that is involved in stress-induced hippocampal dysfunction, suggesting potential therapeutic strategies.
Methods
Stress Paradigms.
The multimodal, combined physical/psychological 5-h stress was carried out as described (54). Briefly, 3- to 4-mo-old male mice expressing YFP under the Thy-1 promoter (B6.Cg-TgN Thy1-YFP; Jackson Laboratories) were restrained in 50-mL tubes, and put six in a cage that was placed on a rapid laboratory shaker in a brightly lit room bathed in loud rap music for 5 h. The CRFR1 antagonist NBI 30775 (15 μg/μL) was infused into the lateral ventricle of mice 30 min before the stress. Infusion was accomplished via cannulae implanted 6–7 d earlier as described (54, 65).
The novel object recognition test was performed as described (41) and modified for mice (86). Initial studies were carried out to determine the duration required for stressed mice to recover, enabling them to explore objects to the same extent as nonstressed cohorts. We found that a recovery period of 90 min after the end of stress sufficed to engender equal object exploration by control and stressed mice. Mice then were presented with two objects in a dimly lit, quiet room and permitted 10 min of exploration. Six hours later, mice were presented with a replica of one of the encountered objects and a novel one, and the duration of exploration of each object was quantified (41, 86). The ratio of time (in seconds) spent exploring the novel vs. the previously encountered object was considered a measure of recognition memory.
Field electrophysiology was tested in300-μm hippocampal slices prepared from adult male mice 20–45 min after 5 h of multimodal stress and maintained in an interface recording chamber as described (87). Baseline potentials were set at 30–40% of the maximum spike-free fEPSP. For area CA3 C/A recordings, stimulation was delivered to area CA3c stratum radiatum and recorded in area CA3b stratum radiatum. LTP was induced using TBS (10 bursts, each containing four 100-Hz pulses, with 200-ms interburst intervals). For mossy fiber recordings, stimulation was delivered to the hilus near the inner blade of the dentate gyrus, and recordings were made from the stratum lucidum. Mossy fiber potentiation was induced by tetanic stimulation (100 Hz, 1 s); presynaptic potentiation was confirmed using paired test pulses with 100-ms interpulse intervals (88). Input (stimulus duration)–output (fEPSP amplitude) relationships were tested as described (87). LTP magnitude was determined by comparisons of fEPSP slopes at 35–40 min after TBS by two-way repeated-measures ANOVA and Tukey's post hoc test. One statistical outlier was removed from analysis in the area CA3 comparisons.
Immunohistochemistry was carried out on 20-μm free-floating sections from perfused, fixed brains as described previously (89). Antisera used included an anti-CRH receptor, CRFR1, directed against the N terminus (1:2,000; Everest), and anti-PSD-95 (1:2,000; Affinity BioReagents). Sections from all experimental groups were run concurrently in the same solutions and conditions, and all sections were processed and analyzed without knowledge of treatment group.
Spine density analyses were performed as described (54). Briefly, dendrites and spines were traced using Neurolucida (Neurolucida, Inc.) and counted per branch and per unit distance without knowledge of treatment. Differences among groups were analyzed using two-way ANOVA and post hoc Bonferroni test.
Acknowledgments
This work was supported by National Institutes of Health Grants NS28912 and MH73136 (to T.Z.B.), NS45540 (to C.S.R.), NS37799 (to C.M.G.), and NS45260 (to C.M.G, G.L., and T.Z.B.) and by a grant from the George E. Hewitt Foundation for Medical Research (to C.J.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: A new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen P, Moser E, Moser MB, Trommald M. Cellular correlates to spatial learning in the rat hippocampus. J Physiol Paris. 1996;90:349. doi: 10.1016/s0928-4257(97)87917-x. [DOI] [PubMed] [Google Scholar]
- 4.Morris RG, et al. Elements of a neurobiological theory of the hippocampus: The role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:773–786. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larson J, Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986;232:985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- 6.Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 7.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 8.Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: A neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 9.Hering H, Sheng M. Dendritic spines: Structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- 10.Park M, et al. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J Neurosci. 2007;27:5363–5372. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch G, Rex CS, Chen LY, Gall CM. The substrates of memory: Defects, treatments, and enhancement. Eur J Pharmacol. 2008;585:2–13. doi: 10.1016/j.ejphar.2007.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukazawa Y, et al. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- 14.Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- 15.Förster E, Zhao S, Frotscher M. Laminating the hippocampus. Nat Rev Neurosci. 2006;7:259–267. doi: 10.1038/nrn1882. [DOI] [PubMed] [Google Scholar]
- 16.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 17.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 18.Fenoglio KA, Brunson KL, Baram TZ. Hippocampal neuroplasticity induced by early-life stress: Functional and molecular aspects. Front Neuroendocrinol. 2006;27:180–192. doi: 10.1016/j.yfrne.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 20.Kim JJ, Song EY, Kosten TA. Stress effects in the hippocampus: Synaptic plasticity and memory. Stress. 2006;9:1–11. doi: 10.1080/10253890600678004. [DOI] [PubMed] [Google Scholar]
- 21.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 22.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 23.Howland JG, Wang YT. Synaptic plasticity in learning and memory: Stress effects in the hippocampus. Prog Brain Res. 2008;169:145–158. doi: 10.1016/S0079-6123(07)00008-8. [DOI] [PubMed] [Google Scholar]
- 24.Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010 doi: 10.1016/j.pnpbp.2009.11.003. E-pub Ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Wilson RS, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68:2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- 27.Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 28.Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 29.Krugers HJ, et al. Exposure to chronic psychosocial stress and corticosterone in the rat: Effects on spatial discrimination learning and hippocampal protein kinase Cgamma immunoreactivity. Hippocampus. 1997;7:427–436. doi: 10.1002/(SICI)1098-1063(1997)7:4<427::AID-HIPO8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 30.Park CR, Campbell AM, Diamond DM. Chronic psychosocial stress impairs learning and memory and increases sensitivity to yohimbine in adult rats. Biol Psychiatry. 2001;50:994–1004. doi: 10.1016/s0006-3223(01)01255-0. [DOI] [PubMed] [Google Scholar]
- 31.Sunanda Rao BSS, Raju TR. Chronic restraint stress impairs acquisition and retention of spatial memory task in rats. Curr Sci. 2000;79:1581–1584. [Google Scholar]
- 32.Pavlides C, Nivón LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–257. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- 33.Alfarez DN, Joëls M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- 34.Joëls M, et al. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–231. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- 35.Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- 36.Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conrad CD, LeDoux JE, Magariños AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 38.Lambert KG, et al. Activity-stress induces atrophy of apical dendrites of hippocampal pyramidal neurons in male rats. Physiol Behav. 1998;65:43–49. doi: 10.1016/s0031-9384(98)00114-0. [DOI] [PubMed] [Google Scholar]
- 39.Donohue HS, et al. Chronic restraint stress induces changes in synapse morphology in stratum lacunosum-moleculare CA1 rat hippocampus: A stereological and three-dimensional ultrastructural study. Neuroscience. 2006;140:597–606. doi: 10.1016/j.neuroscience.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 40.Pawlak R, et al. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci USA. 2005;102:18201–18206. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunson KL, et al. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKittrick CR, et al. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 43.Shors TJ, Falduto J, Leuner B. The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur J Neurosci. 2004;19:145–150. doi: 10.1046/j.1460-9568.2003.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart MG, et al. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: A three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005;131:43–54. doi: 10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 45.Bremner JD, Elzinga B, Schmahl C, Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diamond DM, et al. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16:571–576. doi: 10.1002/hipo.20188. [DOI] [PubMed] [Google Scholar]
- 47.Conboy L, et al. The antidepressant agomelatine blocks the adverse effects of stress on memory and enables spatial learning to rapidly increase neural cell adhesion molecule (NCAM) expression in the hippocampus of rats. Int J Neuropsychopharmacol. 2009;12:329–341. doi: 10.1017/S1461145708009255. [DOI] [PubMed] [Google Scholar]
- 48.Diamond DM, Rose GM. Stress impairs LTP and hippocampal-dependent memory. Ann N Y Acad Sci. 1994;746:411–414. doi: 10.1111/j.1749-6632.1994.tb39271.x. [DOI] [PubMed] [Google Scholar]
- 49.Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proc Natl Acad Sci USA. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamada K, McEwen BS, Pavlides C. Site and time dependent effects of acute stress on hippocampal long-term potentiation in freely behaving rats. Exp Brain Res. 2003;152:52–59. doi: 10.1007/s00221-003-1519-0. [DOI] [PubMed] [Google Scholar]
- 51.Kole MH, Costoli T, Koolhaas JM, Fuchs E. Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neuroscience. 2004;125:337–347. doi: 10.1016/j.neuroscience.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 52.Cazakoff BN, Howland JG. Acute stress disrupts paired pulse facilitation and long-term potentiation in rat dorsal hippocampus through activation of glucocorticoid receptors. Hippocampus. 2010 doi: 10.1002/hipo.20738. In press. [DOI] [PubMed] [Google Scholar]
- 53.Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y, Dubé CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: Prevention by lithium treatment. Proc Natl Acad Sci USA. 2004;101:3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 58.Krugers HJ, et al. Corticosterone shifts different forms of synaptic potentiation in opposite directions. Hippocampus. 2005;15:697–703. doi: 10.1002/hipo.20092. [DOI] [PubMed] [Google Scholar]
- 59.Neumann ID. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc Trans. 2007;35:1252–1257. doi: 10.1042/BST0351252. [DOI] [PubMed] [Google Scholar]
- 60.Caldwell HK, Lee HJ, Macbeth AH, Young WS., 3rd Vasopressin: Behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 62.Van Eekelen JA, De Kloet ER. Co-localization of brain corticosteroid receptors in the rat hippocampus. Prog Histochem Cytochem. 1992;26:250–258. doi: 10.1016/s0079-6336(11)80102-6. [DOI] [PubMed] [Google Scholar]
- 63.Zoladz PR, Park CR, Muñoz C, Fleshner M, Diamond DM. Tianeptine: An antidepressant with memory-protective properties. Curr Neuropharmacol. 2008;6:311–321. doi: 10.2174/157015908787386096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y, et al. Hippocampal corticotropin releasing hormone: Pre- and postsynaptic location and release by stress. Neuroscience. 2004;126:533–540. doi: 10.1016/j.neuroscience.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y, Fenoglio KA, Dubé CM, Grigoriadis DE, Baram TZ. Cellular and molecular mechanisms of hippocampal activation by acute stress are age-dependent. Mol Psychiatry. 2006;11:992–1002. doi: 10.1038/sj.mp.4001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2010;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winters BD, Saksida LM, Bussey TJ. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 68.Alfarez DN, Karst H, Velzing EH, Joëls M, Krugers HJ. Opposite effects of glucocorticoid receptor activation on hippocampal CA1 dendritic complexity in chronically stressed and handled animals. Hippocampus. 2008;18:20–28. doi: 10.1002/hipo.20360. [DOI] [PubMed] [Google Scholar]
- 69.Dalla C, Whetstone AS, Hodes GE, Shors TJ. Stressful experience has opposite effects on dendritic spines in the hippocampus of cycling versus masculinized females. Neurosci Lett. 2009;449:52–56. doi: 10.1016/j.neulet.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luine V. Sex differences in chronic stress effects on memory in rats. Stress. 2002;5:205–216. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- 71.Brunson KL, Avishai-Eliner S, Hatalski CG, Baram TZ. Neurobiology of the stress response early in life: Evolution of a concept and the role of corticotropin releasing hormone. Mol Psychiatry. 2001;6:647–656. doi: 10.1038/sj.mp.4000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joëls M, Karst H, DeRijk R, de Kloet ER. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 2008;31:1–7. doi: 10.1016/j.tins.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 73.de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol. 2009;30:358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 74.Alfarez DN, et al. Corticosterone reduces dendritic complexity in developing hippocampal CA1 neurons. Hippocampus. 2009;19:828–836. doi: 10.1002/hipo.20566. [DOI] [PubMed] [Google Scholar]
- 75.Xu L, Holscher C, Anwyl R, Rowan MJ. Glucocorticoid receptor and protein/RNA synthesis-dependent mechanisms underlie the control of synaptic plasticity by stress. Proc Natl Acad Sci USA. 1998;95:3204–3208. doi: 10.1073/pnas.95.6.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yi SJ, Masters JN, Baram TZ. Glucocorticoid receptor mRNA ontogeny in the fetal and postnatal rat forebrain. Mol Cell Neurosci. 1994;5:385–393. doi: 10.1006/mcne.1994.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Magariños AM, Orchinik M, McEwen BS. Morphological changes in the hippocampal CA3 region induced by non-invasive glucocorticoid administration: A paradox. Brain Res. 1998;809:314–318. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- 78.Magariños AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- 79.Sandi C, et al. Acute stress-induced impairment of spatial memory is associated with decreased expression of neural cell adhesion molecule in the hippocampus and prefrontal cortex. Biol Psychiatry. 2005;57:856–864. doi: 10.1016/j.biopsych.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 80.Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: Implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baram TZ, Ribak CE. Peptide-induced infant status epilepticus causes neuronal death and synaptic reorganization. Neuroreport. 1995;6:277–280. doi: 10.1097/00001756-199501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Y, et al. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci USA. 2004;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 84.Goldwater DS, et al. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hains AB, et al. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci USA. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lauterborn JC, et al. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J Neurosci. 2007;27:10685–10694. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Staubli U, Larson J, Lynch G. Mossy fiber potentiation and long-term potentiation involve different expression mechanisms. Synapse. 1990;5:333–335. doi: 10.1002/syn.890050410. [DOI] [PubMed] [Google Scholar]
- 89.Chen Y, Bender RA, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: A quantitative spatiotemporal analysis. J Neurosci. 2001;21:7171–7181. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]