Abstract
We examined whether maternal dietary choline modulates angiogenesis in fetal brain. Pregnant C57BL/6 mice were fed either a choline-deficient (CD), control (CT), or choline-supplemented diet (CS) from days 12 to 17 (E12-17) of pregnancy and then fetal brains were studied. In CD fetal hippocampus, proliferation of endothelial cells (EC) was decreased by 32% (p < 0.01 vs. CT or CS) while differentiated EC clusters (expressing factor VIII related antigen (RA)) increased by 25% (p < 0.01 vs. CT or CS). These changes were associated with > 25% decrease in the number of blood vessels in CD fetal hippocampus (p < 0.01 vs. CT and CS), with no change in total cross-sectional area of these blood vessels. Expression of genes for the angiogenic signals derived from both endothelial and neuronal progenitor cells (NPC) was increased in CD fetal hippocampus VEGF C (Vegfc), 2.0-fold, p < 0.01 vs. CT and angiopoietin 2 (Angpt2), 2.1-fold, (p < 0.01 vs. CT)). Similar increased expression was observed in NPC isolated from E14 fetal mouse brains and exposed to low (5 μM), CT (70 μM), or high choline (280 μM) media for 72 h (low choline caused a 9.7-fold increase in relative gene expression of Vegfc (p < 0.001 vs. CT and high) and a 3.4-fold increase in expression of Angpt2, (p < 0.05 vs. CT and high). ANGPT2 protein was increased 42.2% (p < 0.01). Cytosine-phosphate-guanine dinucleotide islands in the proximity of the promoter areas of Vegfc and Angpt2 were hypomethylated in low choline NPC compared to CT NPC (p < 0.01). We conclude that maternal dietary choline intake alters angiogenesis in the developing fetal hippocampus.
Keywords: brain, VEGFC, ANGPT2, endothelial cell, diet
Choline is an essential nutrient for mammalian cells (1), and it is needed for membrane formation, for methylation, and for acetylcholine biosynthesis (2). Pregnant women in the United States do not eat diets delivering the recommended intake of choline (3). Large amounts of choline are delivered to the fetus across the placenta (4), resulting in very high tissue choline concentrations in the fetus and newborn (5, 6). Maternal choline deficiency during pregnancy alters neurogenesis in fetal mouse hippocampus (7, 8), and this effect is likely mediated by alterations in gene-specific DNA methylation within neuronal progenitor cells (NPC) that will form the hippocampus (9, 10). Rodent dams fed choline-deficient (CD) diets during late pregnancy had offspring with diminished progenitor cell proliferation and increased apoptosis in fetal hippocampus (11, 12), accelerated differentiation of NPC (13–15), insensitivity to long-term potentiation (LTP) when they were adult animals (16), and decremented visuospatial and auditory memory as adults (17–20). In utero development of the nervous system requires coordination of neurogenesis with angiogenesis to assure that neurons are supplied with oxygen and that waste products are removed (21–23). Therefore, maternal dietary choline also might modulate angiogenesis in fetal brain.
The balance between angiogenesis and neurogenesis is carefully modulated by local cues (growth factors, extracelular matrix) in the mesoderm (21, 24–27). Endothelial cells (EC), derived from mesodermal cells, generate blood islands early in gestation (embryonic day 6.5; E6.5) and by E9-10 the primordial vasculature is formed (21, 28). Angiogenesis and vascular maturation are synergisticly regulated by vascular endothelial growth factors and their receptors and by angiopoietin (ANGPT)/endothelial receptor tyrosine kinase (Tie-2) signaling (29–31). EC in the perineural plexus of a ten-day-old mouse embryo express high levels of VEGF-receptor 1 (VEGF-R1), VEGF-receptor 2 (VEGF-R2), and tyrosine kinase with immunoglobulin-like and EGF-like domains 1 (TIE-1) and TIE-2, while their ligands are expressed in the surrounding tissues. This pattern of growth factors stimulates the invasion of neuroectoderm (21). VEGF synthesis by neurons and by NPC lining the ventricular zone of the hippocampus stimulates capillary ingrowth towards this region (22, 32). Also, binding of VEGF to its receptors activates the antiapoptotic kinase Akt PI3K that inhibits activation of caspase 9 and caspase 3, thereby increasing endothelial cell survival (23). Finally, VEGF mediates axonal guidance (23) and neural progenitor cell migration (33). Upon VEGF-R2 activation, this receptor is phosphorylated, activating ras, p38MAPK, and ERK, directly resulting in increased cell proliferation and migration (23). Vascular maturation and stabilization are required for functional angiogenesis (34) and this is mediated by ANGPT (35, 36).
We examined whether EC proliferation and differentiation in fetal hippocampus were affected by maternal dietary choline, and we determined whether NPC, when exposed to low choline, expressed angiogenic signals.
Results
Maternal Choline Deficiency Decreased Maternal Hepatic Phosphocholine.
Varying dietary choline intake for 5 d in pregnant dams reduced hepatic concentrations of phosphocholine as expected: CD, 201 nmol/g ± 26 SE (p < 0.05 different from control (CT)); CT, 464 nmol/g ± 51; and choline-supplemented (CS), 648 nmol/g ± 74 (n = 10/group). These findings match our previously published data on choline metabolites and maternal choline diet (8).
Choline Deficiency Increased mRNA Expression Encoding Angiogenic Signaling Molecules in Fetal Brain and in Cultured Neural Progenitor Cells.
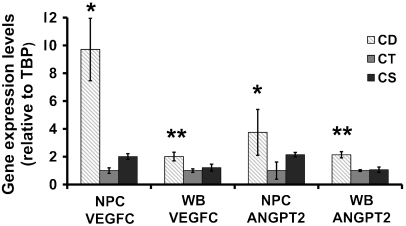
As discussed earlier, angiogenesis and vascular maturation are regulated by VEGF and its receptors as well as by ANGPT (29–31). Previous studies on gene expression profiling of NPCs indicated possible alterations in the mRNA levels of angiogenic related genes when exposed to choline deficiency (37). Progenitor cells isolated from E14 fetal mouse brains and grown in low choline (5 μM) medium as compared to CT medium (70 μM choline) had increased gene expression (relative to TATA box binding protein (Tbp) housekeeping gene) of Vegfc (9.7-fold, p < 0.001) and angiopoietin 2 (Angpt2) (3.4-fold, p < 0.05 vs. CT) (Fig. 1). The expression of these two genes also was increased in E17 fetal brain from CD as compared to CT and CS dams (Vegfc by 2-fold, p < 0.01; Angpt2 by 2.1-fold, p < 0.01).
Fig. 1.
Choline deficiency increases expression of Vegfc and Angpt2 in cultured neural progenitor cells and in E17 fetal brain. E14 mouse brain neural progenitor cells were grown in low choline (5 μM; CD), (70 μM; CT), or (280 μM; CS) medium for 72 h. Pregnant mice were fed a low, normal, or supplemented choline diet between days 12 to 17 of gestation and fetal hippocampi were collected at E17 (n = 5 pups from 5 dams/group). mRNA was isolated from NPC and whole brain (WB) and expression levels of Vegfc and Angpt2, relative to Tbp housekeeping gene, were assessed with qRTPCR using the 2-ΔΔCT method. n = 5 plates/group. Data are presented as mean ± SE. * = p < 0.05; ** = p < 0.01 different from CT and CS by REST analysis.
Choline Deficiency Altered the Protein Levels of ANGPT2 and VEGFC.
We measured ANGPT2 protein levels in NPC samples exposed to low, normal, and high choline levels by ELISA. Choline deficiency increased ANGPT2 protein levels by 42.2% (p < 0.01 vs. CT) (Fig. S1B). A similar pattern of changes was observed for VEGFC) (Fig. S1A).
Maternal Choline Deficiency Decreased Endothelial Cell Proliferation in the Fetal Hippocampus.
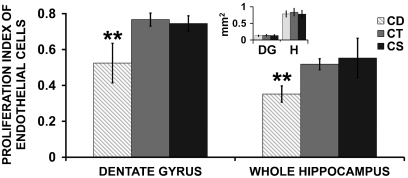
The proliferation index (ratio of BrdU-Isolectin cells/total DAPI-isolectin cells) in EC was decreased in the dentate gyrus of the hippocampus in the CD group relative to the CT (p < 0.0001) or CS group (p < 0.001). A similar decrease was observed when the proliferation index in whole hippocampus was analyzed in CD and compared to CT (p < 0.01) or supplemented groups (p < 0.01) (Fig. 2 A, B; Fig. 3). There were no differences between groups in the surface of the dentate gyrus or whole hippocampus (inset Fig. 3).
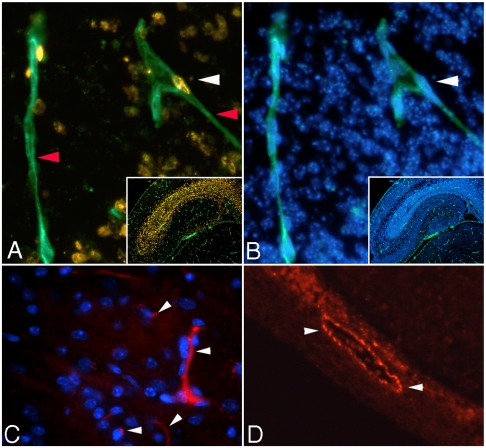
Fig. 2.
Immunohistochemistry studies in fetal brain. A and B: Representative 200X fluorescent images of the CORNUS AMMONI region 1 (CA1) of E17 hippocampus. A multiplex immunolabeling was performed for BrdU (golden staining—ALEXA 555, white arrowhead A), isolectin IB4 (green fluorescence—ALEXA 488, red arrowhead A) and DAPI (blue fluorescence when bound to genomic DNA, white arrowhead B). The proliferation index of EC was calculated as the ratio between the number of BrdU positive nuclei colocalized with isolectin (A) and DAPI positive nuclei colocalized with isolectin (B). Small insets are images of the entire hippocampus area captured with 50X magnification (A—isolectin-BrdU and B—DAPI-isolectin). C and D: Factor VIII RA staining of E17 fetal mouse hippocampus. Representative 200X fluorescent images of blood vessels expressing Factor VIII RA in a 5 μm hippocampal sections; image acquisition was performed with a rhodamine filter—blood cells fluoresce red (ALEXA 546 nm dye; white arrowheads) and nuclei fluoresce blue (DAPI staining). C shows small blood vessels with different trajectories and levels of Factor VIII as well as DAPI positive EC nuclei. D presents a more intense, Factor VIII positive, blood vessel with a bigger caliber situated in close proximity to the dentate gyrus.
Fig. 3.
Maternal choline deficiency decreases endothelial cell proliferation in E17 fetal hippocampus: Pregnant mice were fed CD, CT, or CS diet between days 12 to 17 of gestation and fetal brains were collected at E17. At E15, all dams received an intraperitoneal (i.p.) dose of BrdU. Brain sections were probed with isolectin IB4 and anti-BrdU antibodies. The proliferation index of EC was calculated as the ratio of colabeled BrdU-isolectin positive cells to DAPI-isolectin positive cells in both hippocampi (or both dentate gyri) of each brain in six consecutive 5 μm sections. The surface area of whole hippocampus (H) and dentate gyrus (DG) were measured for each sample and found to be constant between the treatments (small inset). Data are presented as mean ± SE. n = 6 pups from 6 dams/group. Groups were compared using one way ANOVA followed by Tukey-Kramer test. ** = p < 0.01
Maternal Choline Deficiency Decreased the Number of Blood Vessels Formed in the Fetal Hippocampus.
We explored the effect of decreased EC proliferation on capillary number and cross-sectional area. The blood vessels were counted per dentate gyrus and per whole hippocampus. The number of isolectin-labeled blood vessels in fetal hippocampus was decreased by > 25% in CD as compared to CT or CS (p < 0.01) (Table 1). There were no significant changes in the average cross-sectional area of individual blood vessels or in the combined cross-sectional area of all the microvessels from one hippocampus.
Table 1.
Maternal dietary choline deficiency decreases the number of blood vessels in fetal hippocampus
| Angiometric analysis | CD | CT | CS |
| Number of blood vessels per hippocampus | 342 ± 19** | 473 ± 21 | 455 ± 26 |
| Combined cross-sectional area (μm2) | 29,507 ± 3,638 | 38,631 ± 3,734 | 39,193 ± 1,421 |
| Individual microvessel area (μm2) | 101 ± 8 | 106 ± 7 | 121 ± 9 |
Pregnant mouse dams were fed a CD, CT, or CS diet from E12 to E17 when angiogenesis in fetal hippocampi was evaluated. Blood vasculature was fluorescent labeled using isolectin-ALEXA488 conjugated (green fluorescence) as described in Materials and Methods section. The number, total surface area, and the diameter of microvessels that were isolectin GS-IB4 positive were measured using the ImageJ Software in both hippocampi of one fetal brain and the average numbers were reported. Six consecutive sections (5 μm) of medial hippocampal region from each sample (30 μm total) were analyzed. Statistical analysis was performed by ANOVA and Tukey-Kramer. Data is presented as mean ± SE, n = 6 pups from 6 different dams/group, ** = p < 0.01 different from CT and CS groups.
Though we observed changes in the hippocampus region, there were no differences observed in the cingulate and midposterior neocortex (Table S1). We suggest that choline acts by changing epigenetic marks and that this may occur in progenitor cells only at a specific period when they are dividing and differentiating. Neurogenesis and angiogenesis in the cortical areas start before embryonic day 12, the beginning of our dietary manipulation. The regional specificity likely reflects these differences.
Maternal Choline Deficiency Increased the Number of Endothelial Cells Expressing Von Willebrand factor-Factor VIII Complex in the Fetal Hippocampus.
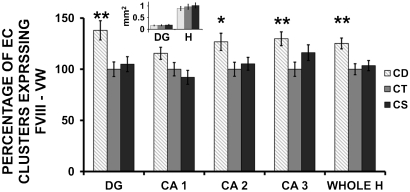
As they differentiate, endothelial cell clusters synthesize Von Willebrand (VW) factor and Factor VIII in the Weibel-Palade bodies within the cells (38). The number of EC clusters expressing VW factor in fetal hippocampus was increased by 25% in the CD group as compared to CT and CS (p < 0.01) (Fig. 2 C, D, Fig. 4). A similar pattern was observed in the CA2, CA3 regions and dentate gyrus of the fetal hippocampus.
Fig. 4.
Maternal choline deficiency increases the number of endothelial cells expressing Factor VIII and Von Willebrand factor in E17 fetal hippocampus. Pregnant mice were fed CD, CT, or CS diet between days 12 to 17 of gestation and fetal brains were collected at E17. Consecutive fetal brain sections were immunolabeled with anti-VW Factor VIII antibodies conjugated with ALEXA 546 nm. Clusters of endothelial cells expressing VW Factor were counted in E17 hippocampal sections (DG = dentate gyrus; CA1 = cornu ammonis 1; CA2 = cornu ammonis 2; CA3 = cornu ammonis 3; and Whole H = whole hippocampus. The surface area of whole hippocampus (H) and dentate gyrus (DG) were measured for each sample and found to be constant between the treatments (small inset). Data are presented as mean ± SE. n = 7 pups from 7 dams/group. * = p < 0.05, ** = p < 0.01, as compared to CT and CS groups by ANOVA with Tukey-Kramer tests.
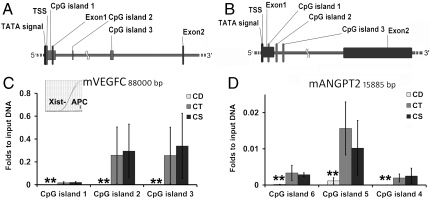
Choline Deficiency Altered Methylation of Several CpG (Cytosine-Phosphate-Guanine Dinucleotide) Islands Downstream of the Transcription Starting Site (TSS) of Vegfc and Angpt2.
Cytosine methylation modulates gene expression (39). We examined the methylation status of the first three CpG islands from Vegfc and Angpt2 because of their close proximity to the TSS for each gene. Decreased methylated DNA in both Vegfc and Angpt2 was detected (despite low signal compared to the positive CT (Xist)) in the low choline NPC for all islands (p < 0.01 vs. CT) (Fig. 5).
Fig. 5.
Choline deficiency alters the methylation status of CpG islands within Vegfc and Angpt2. E14 NPC were grown in low, control of CS medium as described in Fig. legend 1 (n = 4/group). Enzymatically sheared DNA samples were enriched in methylated cystosine following the MIRA protocol and analyzed by real time PCR as described in Materials and Methods section. A and C: Diagram (to scale) of Vegfc (80 Kb) and Angpt2 (16 Kb), respectively, containing the first three CpG islands analyzed and the gene features in close proximity to these CpGs: exons 1 and 2, TSS. B: Methylation (folds to input DNA) of CpG islands within Vegfc. Inset shows the amplification curves of positive control Xist gene and no amplification for the negative control APC gene detected by real time PCR; D: Methylation (folds to input DNA) of CpG islands within Angpt2. Values are reported as mean ± SE. Statistic analysis was perfomed with REST (rest pair wise reallocation randomisation test). ** = p < 0.01.
Discussion
We show, using a rodent model, that maternal dietary choline intake modulates angiogenesis in fetal brain. Maternal diets low in choline were associated with diminished proliferation of EC in fetal hippocampus. We suggest the following hypotheses: Choline is an important methyl-donor (9) and a diet low in choline in the pregnant dam results in decreased DNA methylation in fetal brain within the promoter of two genes that are important regulators of angiogenesis (Vegfc and Angpt2). Hypomethylation of genes is usually associated with increased gene expression (9), and Vegfc and Angpt2 are over expressed in fetal brain. This change increases VEGFC and ANGPT2 angiogenic signaling, causing EC to differentiate more rapidly (and to secrete the proteins VW factor and Factor VIII). EC differentiation ends cell proliferation and this results in decreased numbers of blood vessels in the hippocampus. Previously published studies established that maternal dietary intake of choline during sensitive periods of neurodevelopment in the fetus altered hippocampal neurogenesis (8); in our current study, we learned that these effects are not limited to neuronal cells but extend to endothelial cells and blood vessel formation in the fetal hippocampus.
As discussed earlier, angiogenesis and vascular maturation are regulated by VEGF and ANGPT-TIE2. Though CD was associated with increased VEGFC, it was paradoxically associated with decreased, not increased endothelial cell proliferation. This modification occurred because differentiation of EC was accelerated, and differentiated cells divide less frequently, if at all. We assessed endothelial cell differentiation using several proteins (and glycoproteins) that are specifically made by mature endothelial cells. One such protein is von Willebrand factor (vWf) which is stored in the Weibel-Palade bodies (25). This protein stabilizes Factor VIII, a protein involved in the clotting cascade (40). Expression of VW factor is higher in mature EC, and is minimal in early endothelial progenitor cells (38). This expression can be influenced by a number of factors including epigenetic mechanisms (41), hormones (17 β-estradiol) (42), and homocysteine (43) (note that choline availability is an important modulator of homocysteine (44)).
Modifications in the proliferation of EC can be associated with structural differences in the vascular tree that are reflected in capillary morphometry and number of blood vessels (21). The number of blood vessels in fetal brain was decreased in CD, but the total cross-sectional area was not significantly altered. This modification may be explained by increased levels of VEGFR ligands in the CD group, as this signal encourages formation of wide lumina with thin walls (21, 45). Studies on pupillary membrane capillaries show that increased ANGPT2 in the absence of VEGF increases EC death. Disruption of VE-cadherin junctions by ANGPT2 increases the response to VEGF stimulation in EC as a survival mechanism (46). ANGPT2 potentiates the effects of VEGF and therefore, the combination of VEGF and ANGPT2 promotes a rapid increase in capillary diameter and remodeling of basal lamina (46). VEGF also stimulates nitric oxide synthase (eNOS) in EC (47). Both eNOS and VEGF can promote microvasculature maturation in combination with increased ANGPT2 levels (48). Indeed, alterations in the choline levels induce high calretinin expression (15), a known marker of differentiation. VEGFC binds to VEGF-R2 and 3 and enhances lymphangiogenesis, angiogenesis, and neurogenesis (49). By activating VEGF-R2, VEGFC stimulates endothelial cell migration via P38MAPK and focal adhesion kinase (FAK) (23). It is possible that NPC lining the periventriculi areas secrete VEGFC and ANGPT2 as survival factors (46) that also happen to stimulate angiogenesis.
Choline is an essential nutrient and a major source of methyl groups used to synthesize S-adenosylmethionine. Low choline levels alter global and gene-specific DNA and histone methylation (9, 10, 50). Therefore, we suggest that the gene expression changes in Vegfc and Angpt2 are the result of epigenetic modifications. We analyzed the gene sequences of Vegfc and Angpt2 and found several CpG islands downstream from the promoter area of these genes, but also at the TSS. These CpGs were hypomethylated in the low choline group; epigenetic modifications occurring near the promoter area, sometimes many kilobases from the TSS can alter gene expression by modulating the binding of enhancers/insulators to specific loci (51, 52). DNA hypomethylation decreases the recruitment of methyl binding domain (MBD) families of proteins such as MeCP2. Once bound to methylated CpG sites, MeCP2 silences transcription of downstream genes by virtue of its interaction with a histone deacetylase (HDAC)/Sin3 complex (53). This mechanism modifies chromatin and alters binding of transcription factors (53).
Computational analysis of CpG islands in Vegfc and Angpt2 (Fig. S2) revealed several CpGs located near putative binding sites for a number of transcription factors (for Vegfc: AP2, Sp1, PAX4, bHLH, NF-kB, and NRSF; for Angpt2: HMG, bHLH, ETS1, ARNT and PPAR). Epigenetic modulation of transcription factor binding at one or all of these sites could alter angiogenesis. For example, Sp1 and AP2 enhancers increase Vegf expression during developmental angiogenesis and this is critical for vasculature formation (54, 55). In pathological situations, Vegfc and d expression can be modulated by NF-Kappa B activation (56). There is a NRSF binding site located in the third CpG island of the Vegfc gene; modifications of epigenetic marks at the NRSF binding site in Calb1 gene in fetal brain were associated with altered gene expression (50). Similar mechanisms could alter VEGF signaling. Angpt2 is up-regulated in endothelial cells by a hypoxia induced factor binding site located in its first intron and by Ets-1 (57). SOX18 is a member of the Sry-related HMG box-containing family of transcription factors and is expressed transiently in endothelial cells during the development of blood vessels. Mutations resulting in expression of dominant negative SOX18 severely impair vascular development (58). DNA bending induced by the HMG domain can facilitate the formation of higher-order nucleoprotein complexes altering H3K4 (59) methylation, suggesting that HMG domain proteins may have an architectural role in assembling such complexes (60). The aryl hydrocarbon receptor nuclear translocator (ARNT) serves as the obligate heterodimeric partner for bHLH-PAS proteins involved in sensing and coordinating transcriptional responses to hypoxia and developmental pathways (61). Lastly, PPARs upregulate angiogenic factors in other model systems (62).
The effects of maternal dietary intake of choline on angiogenesis in fetal brain could have great importance for humans. Only 14% of pregnant women in the United States consume adequate amounts of choline in their diets (and less than 5% of 18–30 young women who are not pregnant consume the recommended intake) (3). Approximately half of young women have a single nucleotide polymorphism in the PEMT gene and inefficiently form new choline moiety and therefore must eat choline or they develop organ dysfunction (63–65). These women are likely to need more choline during pregnancy to sustain normal fetal development (66, 67). In the United States, pregnant women in the lowest quartile for choline intake have a 4-fold increased risk of having a baby with a birth defect compared with women in the highest quartile for choline intake (68, 69). Thus, humans eat marginal amounts of choline and a large portion of the population has increased dietary requirement for choline, making the effects of choline on fetal brain angiogenesis of potential public health importance.
Materials and Methods
Additional information in SI Text)
Animals.
Timed-pregnant C57BL/6 mice were used in all experiments according to a protocol described elsewhere (8). At the end of E11 they were randomly assigned to one of three feeding groups: CD (CD; AIN-76A diet with no choline), CT, or CS (CS; AIN-76A diet with 4.95 g/kg choline chloride) and fed these diets until they were killed on E17. On E15, a single intraperitoneal injection of 50 mg/kg body weight 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich, Inc.) was administered to the pregnant dams.
Fetal Brain Collection.
On E17 the fetal brains from two male pups from each litter were collected using methods we previously published (8, 70). 5 μm hippocampal sections were cut in coronal orientation. The remaining brains were frozen in liquid nitrogen and used for gene expression determinations.
Fetal Mouse NPC in Culture.
E14 NPC from C57BL/6 mice were obtained from Lonza and plated according to the manufacturer’s protocol. Small tertiary neurospheres were generated as previously described (50) and suspended in custom Neurobasal medium (D700SA, Atlanta Biologicals) containing 5 μM choline chloride (CD), 70 μM choline chloride (CT) or 280 μM choline chloride (CS) with the ingredients previously described (50).
Immunohistochemistry in Paraffin Embedded Sections.
To assess EC proliferation, a double immunolabeling technique using both BrdU (S-phase marker) (71) and isolectin IB4 (vasculature marker) (28, 72) was used. Developmental angiogenesis occurs in waves (73); therefore we evaluated EC proliferation over a period of 48 h (E15-E17). Similarly, equivalent sections were probed for Von Willebrand factor (Factor VIII related antigen, VW factor)—Factor VIII complex. In all cases, 4′, 6-diamidino-2-phenylindole (DAPI, Sigma) 0.1 μg/mL for 20 min was used for staining the nuclear DNA (full details provided in SI Text).
Image Analysis.
Fluorescent images were collected with a Nikon FXA microscope (Nikon) equipped with an Optronics TEC-470 CCD Video Camera System (Optronics Engineering). The number of blood vessels and the average cross-sectional area of isolectin positive vessels were counted using the ImageJ Software version 1.37v (http://rsb.info.nih.gov/ij, NIH) with an integrated macro for analyzing particles. The proliferation index of EC was calculated as the ratio of colabeled BrdU-isolectin positive cells to DAPI-isolectin positive cells in both hippocampi of each brain in six consecutive 5 μm sections. For analysis of capillary numbers and cross-sectional volume, we used consecutive sections totaling 30 μm of thickness from the medial-frontal region of the hippocampus from six fetuses from different dams. To assess the number of EC clusters that were VW Factor positive, the fluorescent structures were counted per entire hippocampus as well as for selected hippocampal regions: CA1 (Cornus Ammoni region 1), CA2, CA3 and DG (Dentate Gyrus).
Real-Time RT-PCR.
RNA was extracted from cultured NPC or from whole brain samples using the RNeasy Mini Kit (Qiagen) transcribed to cDNA and 2 ng per PCR was amplified in the second step. TBP mRNA levels do not vary with choline availability in the central nervous system, as shown in published microarray studies (37). Primers were designed for Angpt2 and for Vegfc and synthesized as described in SI Text. The conditions for the amplification reactions were: 95 °C for 10 min; 40 cycles of 94 °C for 15 sec, 56 °C for 30 sec, and 72 °C for 50 sec. The relative quantification method (2ΔΔCT) was used to analyze gene expression changes as described elsewhere (74).
Methylated CpG Island Recovery Assay (MIRA).
Genomic DNA was isolated from NPC samples using Qiagen DNAeasy minicolumns (Qiagen) following manufacturer’s protocol (75), quantified with a Nanodrop 8000 spectrophotometer (Nanodrop) and stored in elution buffer at -80 °C. In preparation for the enrichment step, 4,000 ng of gDNA from each sample were digested with MseI restriction enzyme for 2 h at 37 °C followed by heat-inactivation. Input DNA aliquots were incubated with recombinant His-MBD2b/MBD3L1 protein complex followed by capture with protein G coated magnetic beads. Vegfc and Angpt2 genomic sequences were retrieved from the NIH mouse genome database (http://www.ncbi.nlm.nih.gov/nuccore) including the gene features (CpG islands and exons/introns locations). For each CpG island several primer pairs were designed and synthesized, and PCR amplification performed as described in detail in SI Text.
Maternal Hepatic Phosphocholine.
Phosphocholine is the labile storage form for choline in liver and is an excellent indicator of dietary choline status (76). It was assayed using liquid chromatography—electrospray ionization—isotope dilution mass spectrometry as previously described (77).
Statistical Analysis.
For gene expression the analysis was performed using the Relative Expression Software Tool—Multiple Condition Solver REST-MCS version 2 (free from the website http://www.gene-quantification.de/rest.html) which uses a pair wise fixed reallocation randomization test. All statistical analyses of epitope levels and vascular tree determinations were performed using JMP software (V 2; SAS Institute, Cary, NPC) with ANOVA and Tukey-Kramer tests. Data are presented as mean ± SE.
Supplementary Material
Acknowledgments.
We thank Dr. Robert Bagnell, Jr., for his assistance with microscopy. We also thank Dr. Scott Olenych for his assistance with microscopy. This work was supported by Grants from the National Institutes of Health AG09525, DK55865, and DK56350 (to S.H.Z.) and Pilot Grant ES010126-08 (to M.G.M.)
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914328107/-/DCSupplemental.
References
- 1.Institute of Medicine & National Academy of Sciences USA. Dietary reference intakes for folate, thiamin, riboflavin, niacin, vitamin B12, panthothenic acid, biotin, and choline. Washington D.C.: National Academy Press; 1998. pp. 390–422. [PubMed] [Google Scholar]
- 2.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jensen HH, Batres-Marquez SP, Carriquiry A, Schalinske KL. Choline in the diets of the U.S. population: NHANES, 2003–2004. Faseb J. 2007;21:lb219. [Google Scholar]
- 4.Sweiry JH, Page KR, Dacke CG, Abramovich DR, Yudilevich DL. Evidence of saturable uptake mechanisms at maternal and fetal sides of the perfused human placenta by rapid paired-tracer dilution: studies with calcium and choline. J Dev Physiol. 1986;8:435–445. [PubMed] [Google Scholar]
- 5.Zeisel SH, Wurtman RJ. Developmental changes in rat blood choline concentration. Biochem J. 1981;198:565–570. doi: 10.1042/bj1980565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozarda Ilcol Y, Uncu G, Ulus IH. Free and phospholipid-bound choline concentrations in serum during pregnancy, after delivery and in newborns. Arch Physiol Biochem. 2002;110:393–399. doi: 10.1076/apab.110.5.393.11832. [DOI] [PubMed] [Google Scholar]
- 7.Albright CD, et al. Regulation of choline deficiency apoptosis by epidermal growth factor in CWSV-1 rat hepatocytes. Cell Physiol Biochem. 2005;15:59–68. doi: 10.1159/000083653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J Nutr. 2003;133:3614–3618. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. Faseb J. 2006;20:43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niculescu MD, Yamamuro Y, Zeisel SH. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J Neurochem. 2004;89:1252–1259. doi: 10.1111/j.1471-4159.2004.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Research. 1999;113:13–20. doi: 10.1016/s0165-3806(98)00183-7. [DOI] [PubMed] [Google Scholar]
- 12.Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Research. 1999;115:123–129. doi: 10.1016/s0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 13.Albright CD, Mar MH, Craciunescu CN, Song J, Zeisel SH. Maternal dietary choline availability alters the balance of netrin-1 and DCC neuronal migration proteins in fetal mouse brain hippocampus. Brain Research. 2005;159:149–154. doi: 10.1016/j.devbrainres.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albright CD, Mar MH, Friedrich CB, Brown EC, Zeisel SH. Maternal choline availability alters the localization of p15Ink4B and p27Kip1 cyclin-dependent kinase inhibitors in the developing fetal rat brain hippocampus. Dev Neurosci. 2001;23:100–106. doi: 10.1159/000048701. [DOI] [PubMed] [Google Scholar]
- 15.Albright CD, et al. Choline availability during embryonic development alters the localization of calretinin in developing and aging mouse hippocampus. Nutr Neurosci. 2003;6:129–134. doi: 10.1080/1028415031000084418. [DOI] [PubMed] [Google Scholar]
- 16.Jones JP, Meck W, Williams CL, Wilson WA, Swartzwelder HS. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Brain Research. 1999;118:159–167. doi: 10.1016/s0165-3806(99)00103-0. [DOI] [PubMed] [Google Scholar]
- 17.Cheng RK, MacDonald CJ, Williams CL, Meck WH. Learning & memory. Vol. 15. Cold Spring Harbor, N.Y: Cold Spring Harbor; 2008. Prenatal choline supplementation alters the timing, emotion, and memory performance (TEMP) of adult male and female rats as indexed by differential reinforcement of low-rate schedule behavior; pp. 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meck W, Williams C. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport. 1997;8:3053–3059. doi: 10.1097/00001756-199709290-00010. [DOI] [PubMed] [Google Scholar]
- 19.Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol. 1988;21:339–353. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- 20.Meck WH, Williams CL. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Brain Research. 1999;118:51–59. doi: 10.1016/s0165-3806(99)00105-4. [DOI] [PubMed] [Google Scholar]
- 21.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 23.Brockington A, Lewis C, Wharton S, Shaw PJ. Vascular endothelial growth factor and the nervous system. Neuropathol Appl Neurobiol. 2004;30:427–446. doi: 10.1111/j.1365-2990.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- 24.Eichmann A, Corbel C, Le Douarin NM. Segregation of the embryonic vascular and hemopoietic systems. Biochem Cell Biol. 1998;76:939–946. [PubMed] [Google Scholar]
- 25.Giblin JP, Hewlett LJ, Hannah MJ. Basal secretion of von Willebrand factor from human endothelial cells. Blood. 2008;112:957–964. doi: 10.1182/blood-2007-12-130740. [DOI] [PubMed] [Google Scholar]
- 26.Munoz-Chapuli R, et al. Differentiation of hemangioblasts from embryonic mesothelial cells? A model on the origin of the vertebrate cardiovascular system. Differentiation. 1999;64:133–141. doi: 10.1046/j.1432-0436.1999.6430133.x. [DOI] [PubMed] [Google Scholar]
- 27.Kinder SJ, Loebel DA, Tam PP. Allocation and early differentiation of cardiovascular progenitors in the mouse embryo. Trends Cardiovasc Med. 2001;11:177–184. doi: 10.1016/s1050-1738(01)00091-3. [DOI] [PubMed] [Google Scholar]
- 28.Coffin JD, Harrison J, Schwartz S, Heimark R. Angioblast differentiation and morphogenesis of the vascular endothelium in the mouse embryo. Dev Biol. 1991;148:51–62. doi: 10.1016/0012-1606(91)90316-u. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y, et al. Effects of angiopoietin-1 on vascular endothelial growth factor-induced angiogenesis in the mouse brain. Acta Neurochirurgica Supplement. 2006;96:438–443. doi: 10.1007/3-211-30714-1_90. [DOI] [PubMed] [Google Scholar]
- 30.Zacharek A, et al. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27:1684–1691. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patan S. Vasculogenesis and angiogenesis. Cancer Treatments. 2004;117:3–32. doi: 10.1007/978-1-4419-8871-3_1. [DOI] [PubMed] [Google Scholar]
- 32.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Vutskits L, Pepper MS, Kiss JZ. VEGF is a chemoattractant for FGF-2-stimulated neural progenitors. J Cell Biol. 2003;163:1375–1384. doi: 10.1083/jcb.200308040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirschi KK, Rohovsky SA, D’Amore PA. Cell-cell interactions in vessel assembly: a model for the fundamentals of vascular remodelling. Transpl Immunol. 1997;5:177–178. doi: 10.1016/s0966-3274(97)80034-2. [DOI] [PubMed] [Google Scholar]
- 35.Pfaff D, Fiedler U, Augustin HG. Emerging roles of the Angiopoietin-Tie and the ephrin-Eph systems as regulators of cell trafficking. J Leukocyte Biol. 2006;80:719–726. doi: 10.1189/jlb.1105652. [DOI] [PubMed] [Google Scholar]
- 36.Suri C, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 37.Niculescu MD, Craciunescu CN, Zeisel SH. Gene expression profiling of choline-deprived neural precursor cells isolated from mouse brain. Brain Research. 2005;134:309–322. doi: 10.1016/j.molbrainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 39.Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev. 2005;15:490–495. doi: 10.1016/j.gde.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Wang YJ, Kelner DN. Coagulation factor VIII: structure and stability. Int J Pharm. 2003;259:1–15. doi: 10.1016/s0378-5173(03)00227-8. [DOI] [PubMed] [Google Scholar]
- 41.Peng Y, Jahroudi N. The NFY transcription factor inhibits von Willebrand factor promoter activation in non-endothelial cells through recruitment of histone deacetylases. J Biol Chem. 2003;278:8385–8394. doi: 10.1074/jbc.M213156200. [DOI] [PubMed] [Google Scholar]
- 42.Brussaard HE, Leuven JA, Krans HM, Kluft C. The effect of 17 beta-oestradiol on variables of coagulation and fibrinolysis in postmenopausal women with type 2 diabetes mellitus. Vascul Pharmacol. 2002;39:141–147. doi: 10.1016/s1537-1891(02)00303-8. [DOI] [PubMed] [Google Scholar]
- 43.Lentz SR, Sadler JE. Homocysteine inhibits von Willebrand factor processing and secretion by preventing transport from the endoplasmic reticulum. Blood. 1993;81:683–689. [PubMed] [Google Scholar]
- 44.Shin OH, et al. Methyl-group donors cannot prevent apoptotic death of rat hepatocytes induced by choline-deficiency. J Cell Biochem. 1997;64:196–208. doi: 10.1002/(sici)1097-4644(199702)64:2<196::aid-jcb3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 45.Chang PY, et al. Homocysteine inhibits arterial endothelial cell growth through transcriptional downregulation of fibroblast growth factor-2 involving G protein and DNA methylation. Circ Res. 2008;102:933–941. doi: 10.1161/CIRCRESAHA.108.171082. [DOI] [PubMed] [Google Scholar]
- 46.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gelinas DS, Bernatchez PN, Rollin S, Bazan NG, Sirois MG. Immediate and delayed VEGF-mediated NO synthesis in endothelial cells: role of PI3K, PKC, and PLC pathways. Br J Pharmacol. 2002;137:1021–1030. doi: 10.1038/sj.bjp.0704956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanazawa H. VEGF, angiopoietin-1 and -2 in bronchial asthma: new molecular targets in airway angiogenesis and microvascular remodeling. Recent Patents in Inflammation, Allergy, and Drug Discovery. 2007;1:1–8. doi: 10.2174/187221307779815066. [DOI] [PubMed] [Google Scholar]
- 49.Le Bras B, et al. VEGFC is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nature neurosci. 2006;9:340–348. doi: 10.1038/nn1646. [DOI] [PubMed] [Google Scholar]
- 50.Mehedint MG, Niculescu MD, Craciunescu CN, Zeisel SH. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. Faseb J. 2010;24:184–195. doi: 10.1096/fj.09-140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurukuti S, et al. CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc Natl Acad Sci USA. 2006;103:10684–10689. doi: 10.1073/pnas.0600326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Nan X, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 54.Song Y, et al. Sp-1 and c-Myc mediate lysophosphatidic acid-induced expression of vascular endothelial growth factor in ovarian cancer cells via a hypoxia-inducible factor-1-independent mechanism. Clin Cancer Res. 2009;15:492–501. doi: 10.1158/1078-0432.CCR-08-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin Y, et al. JNK/AP-1 pathway is involved in tumor necrosis factor-alpha induced expression of vascular endothelial growth factor in MCF7 cells. Biomed Pharmacother. 2009;63:429–435. doi: 10.1016/j.biopha.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watari K, et al. Role of macrophages in inflammatory lymphangiogenesis: Enhanced production of vascular endothelial growth factor C and D through NF-kappaB activation. Biochem Biophys Res Commun. 2008;377:826–831. doi: 10.1016/j.bbrc.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 57.Simon MP, Tournaire R, Pouyssegur J. The angiopoietin-2 gene of endothelial cells is up-regulated in hypoxia by a HIF binding site located in its first intron and by the central factors GATA-2 and Ets-1. J Cell Physiol. 2008;217:809–818. doi: 10.1002/jcp.21558. [DOI] [PubMed] [Google Scholar]
- 58.Luo M, et al. Inhibition of tumor angiogenesis by cell-permeable dominant negative SOX18 mutants. Med Hypotheses. 2008;70:880–882. doi: 10.1016/j.mehy.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 59.Wynder C, Hakimi MA, Epstein JA, Shilatifard A, Shiekhattar R. Recruitment of MLL by HMG-domain protein iBRAF promotes neural differentiation. Cell Bio. 2005;7:1113–1117. doi: 10.1038/ncb1312. [DOI] [PubMed] [Google Scholar]
- 60.Grosschedl R, Giese K, Pagel J. HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet. 1994;10:94–100. doi: 10.1016/0168-9525(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 61.Partch CL, Card PB, Amezcua CA, Gardner KH. Molecular basis of coiled coil coactivator recruitment by the aryl hydrocarbon receptor nuclear translocator (ARNT) J Biol Chem. 2009;284:15184–15192. doi: 10.1074/jbc.M808479200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chintalgattu V, Harris GS, Akula SM, Katwa C. PPAR-gamma agonists induce the expression of VEGF and its receptors in cultured cardiac myofibroblasts. Cardiovasc Res. 2007;74:140–150. doi: 10.1016/j.cardiores.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 63.da Costa KA, et al. Common genetic polymorphisms affect the human requirement for the nutrient choline. Faseb J. 2006;20:1336–1344. doi: 10.1096/fj.06-5734com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fischer LM, et al. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr. 2007;85:1275–1285. doi: 10.1093/ajcn/85.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Resseguie M, et al. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. Faseb J. 2007;21:2622–2632. doi: 10.1096/fj.07-8227com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McMahon KE, Farrell PM. Measurement of free choline concentrations in maternal and neonatal blood by micropyrolysis gas chromatography. Clin Chim Acta. 1985;149:1–12. doi: 10.1016/0009-8981(85)90267-0. [DOI] [PubMed] [Google Scholar]
- 67.Zeisel SH, Mar M-H, Zhou Z-W, da Costa K-A. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J Nutr. 1995;125:3049–3054. doi: 10.1093/jn/125.12.3049. [DOI] [PubMed] [Google Scholar]
- 68.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160:102–109. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- 69.Shaw GM, Carmichael SL, Laurent C, Rasmussen SA. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology. 2006;17:285–291. doi: 10.1097/01.ede.0000208348.30012.35. [DOI] [PubMed] [Google Scholar]
- 70.Park JJ, Baum MJ, Paredes RG, Tobet SA. Neurogenesis and cell migration into the sexually dimorphic preoptic area/anterior hypothalamus of the fetal ferret. J Neurobiol. 1996;30:315–328. doi: 10.1002/(SICI)1097-4695(199607)30:3<315::AID-NEU1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 71.Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: a new reagent for detection of DNA replication. Science. 1982;218:474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- 72.Laitinen L. Griffonia simplicifolia lectins bind specifically to endothelial cells and some epithelial cells in mouse tissues. Histochem J. 1987;19:225–234. doi: 10.1007/BF01680633. [DOI] [PubMed] [Google Scholar]
- 73.Furuta C, et al. Discordant developmental waves of angioblasts and hemangioblasts in the early gastrulating mouse embryo. Development. 2006;133:2771–2779. doi: 10.1242/dev.02440. [DOI] [PubMed] [Google Scholar]
- 74.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 75.Rauch T, Pfeifer GP. Methylated-CpG island recovery assay: a new technique for the rapid detection of methylated-CpG islands in cancer. Lab Invest. 2005;85:1172–1180. doi: 10.1038/labinvest.3700311. [DOI] [PubMed] [Google Scholar]
- 76.Pomfret EA, da Costa K, Zeisel SH. Effects of choline deficiency and methotrexate treatment upon rat liver. J Nutr Biochem. 1990;1:533–541. doi: 10.1016/0955-2863(90)90039-n. [DOI] [PubMed] [Google Scholar]
- 77.Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Analytical Chemistry. 2002;74:4734–4740. doi: 10.1021/ac025624x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.