Abstract
Whereas humanized mouse models have contributed significantly to human immunology research, human T cells developing in mouse thymic environment fail to demonstrate HLA-restricted function. To achieve HLA-restricted human immune response, we created an immune-compromised non-obese diabetic/SCID/IL2rgnull strain (NSG) with homozygous expression of HLA class I heavy chain and light chain (NSG-HLA-A2/HHD). Transplantation of purified Lin−CD34+CD38− human hematopoietic stem cells into NSG-HLA-A2/HHD newborns resulted in the development of human CD4+ and CD8+ TCRαβ+ T cells and CD4−CD8− and CD8+ TCRγδ+ cells in recipient bone marrow and spleen. Human cytotoxic T lymphocytes (CTLs) become functionally mature, as evidenced by the production of granzyme corresponding to phenotypic transition from naïve to effector memory CTLs. In these recipients, human Th17 cells developed along with Th1 and Th2 cells. Epstein–Barr virus (EBV) infection in the humanized NSG-HLA-A2/HHD recipients resulted in the formation of lymphoproliferative lesions consisting mainly of human B cells with scattered human T cells. Human CTLs developing in the recipients recognized EBV-derived peptides in an HLA-restricted manner and exerted HLA-restricted cytotoxicity against EBV-infected human B cells. The HLA-expressing humanized mouse with functional HLA-restricted T cells and consistent representation of rare T-cell subsets overcomes a major constraint in human immunology, and serves as a useful model for investigation of human immune responses against pathogens and for the development of therapeutic strategies against human diseases.
Keywords: human immunology, T-cell development, HLA restriction, Epstein–Barr virus
The humanized mouse with reconstituted human hematopoietic and immune cells is a powerful tool for investigation of human biological systems and for translational research (1, 2). Humanized mice enable direct access into the dynamics of the human immunohematopoietic system in mice. The non-obese diabetic severe combined immunodeficiency (NOD/SCID) mouse repopulating assay has long been a gold standard for evaluating human hematopoiesis and immune reconstitution (3). However, this assay is associated with two major constraints. First, human hematopoietic engraftment is not sufficiently robust to evaluate in detail the development of a full spectrum of human immune subsets. Second, human T and B cells developing in NOD/SCID recipients do not become functionally mature. To overcome these limitations, two strains of NOD/SCID mice homozygous for targeted mutations at the Il2rg locus were generated: the NOD/SCID/IL2rγcnull (NOG) strain carrying a truncated mutation (4, 5) and the NOD/SCID/IL2rγnull (NSG) strain with a complete null mutation (6, 7). The transplantation of human hematopoietic stem cells (HSCs) into newborn NSG recipients greatly improved the engraftment efficiency of human hematopoietic cells (7). Humanized NSG and NOG recipients at least partially supported the maturation of human T and B cells, as evidenced by the development of Ig-producing human B cells as well as human CD4+ and CD8+ T cells in secondary lymphoid organs (5, 7, 8). NSG and NOG recipients also proved to be highly efficient in the engraftment and recapitulation of human diseases such as acute myeloid leukemia (9, 10). In addition, Manz and colleagues described the reconstitution of human acquired and innate immunity in Rag2−/−gc−/− mice (11). However, these mice do not express HLA molecules on thymic epithelial cells. Therefore, human T cells developing in NSG humanized mice lack the ability to recognize antigens in an HLA-restricted manner, precluding the investigation of human cytotoxic T lymphocyte (CTL) response against human infectious diseases and malignancies.
Here we report the development of the NSG-HLA-A2/HHD strain, an immunodeficient strain with humanized immune microenvironment expressing HLA class I heavy and light chains, that overcomes the lack of thymic human T-cell selection through interaction with HLA class I molecules. The reconstitution of human immunity in NSG-HLA-A2/HHD recipients through transplantation of purified human HSCs resulted in extensive development of human T cells including γδ T cells and Th17 cells in vivo. The human CD4+ and CD8+ T cells developing in NSG-HLA-A2/HHD recipients were functional, able to express cytotoxic molecules and generate cytokines in vivo. Most importantly, NSG-HLA-A2/HHD humanized mice demonstrated functional HLA-restricted CTLs in an in vivo Epstein–Barr virus (EBV) infection model.
Results
Transplantation of Purified Human HSCs into NSG-HLA-A2/HHD Newborns.
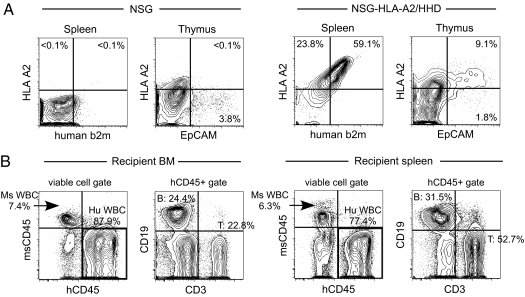
To achieve HLA-restricted human T-cell development in vivo, we created an immunodeficient strain by backcrossing the HLA class I transgene onto the NSG background. We chose the HHD construct designed for the expression of *A0201, one of the most prevalent HLA A genotypes, covalently bound to human β2-microglobulin (b2m), enabling the transgenic expression of both HLA heavy and light chains (12). The protein level expression of HLA-A2 and b2m on the surface of NSG-HLA-A2/HHD splenocytes was confirmed, whereas NSG splenocytes do not express either HLA-A2 or b2m (Fig. 1A). In addition, EpCAM+ thymic epithelial cells, responsible for the education and positive selection of thymocytes (13), expressed the HLA class I molecules in NSG-HLA-A2/HHD mice but not in NSG mice (Fig. 1A). Next, we examined the development of human immune cells in NSG-HLA-A2/HHD mice through i.v. transplantation of purified Lin−CD34+CD38− HSCs from HLA-A2+ cord blood (CB) (Fig. S1). Transplantation of purified human HSCs excludes the possibility of graft contamination by preformed human T cells, ensuring that all human T cells observed in the recipient arose within the recipient mouse microenvironment. The majority of hCD34+CD38− cells are Hoechst(low)PyroninY(−) cell-cycle quiescent HSCs (Fig. S1). Purified human HSCs were transplanted at 5 × 103–3 × 104 cells per recipient and achieved efficient long-term human hematopoietic reconstitution [%hCD45+ in recipient bone marrow (BM) 70.7 ± 8.5% and in recipient spleen 86.7 ± 3.7%; n = 8, each at 4–8 mo posttransplantation]. At the time of sacrifice, BM and spleen of NSG-HLA-A2/HHD recipients consistently showed human immunohematopoietic reconstitution with T cells, B cells, and myeloid cells (Fig. 1B). To determine the significance of HLA class I expression on in vivo T-cell development, we proceeded to characterize the phenotype and function of human T cells in NSG-HLA-A2/HHD recipients.
Fig. 1.
Purified human HSCs reconstitute human immune cells in NSG-HLA-A2/HHD mice. (A) Representative contour plots of 7AAD- splenocytes and CD45-7AAD-EpCAM+ thymic epithelial cells demonstrating surface HLA-A2/human b2m and HLA-A2/EpCAM coexpression in untransplanted NSG-HLA-A2/HHD but not untransplanted NSG mice. (B) Reconstitution of human immunity in NSG-HLA-A2/HHD recipients. High levels of human cell engraftment were found in BM and spleen. Engrafted human CD45+ cells contain CD3+ T cells, CD19+ B cells, and non-T non-B cells.
Functional Human T-Cell Subsets Develop in NSG-HLA-A2/HHD Recipients.
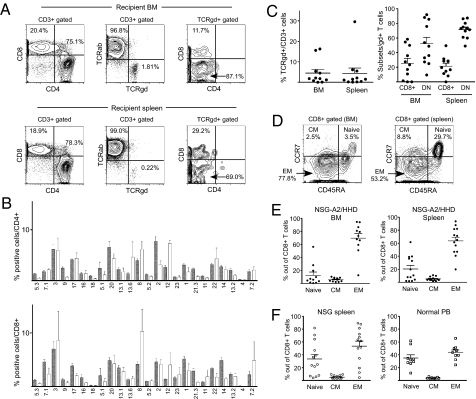
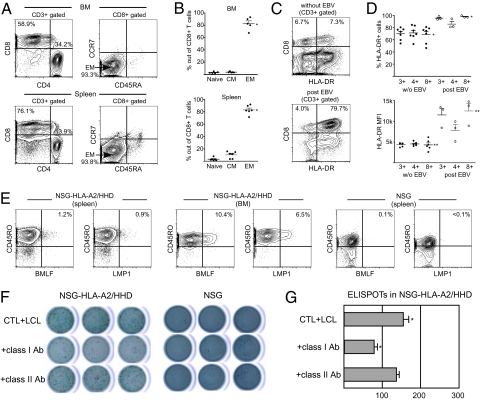
In NSG-HLA-A2/HHD recipient BM and spleen, we confirmed the differentiation of CD4+ and CD8+ human T cells at a 2–5:1 ratio (Fig. 2A). The CD4+ and CD8+ human T cells demonstrated diverse Vβ repertoire (Fig. 2B). Whereas the vast majority of human T cells in the recipient expressed TCRαβ, we consistently detected TCRγδ+ T cells (BM: 4.5 ± 1.8%; spleen: 4.4 ± 2.6%; n = 11 each) (Fig. 2 A and C). Human TCRγδ+ T cells developing in the recipient consisted of CD8+ single positive (SP) (BM: 23.4 ± 6.6%; spleen: 21.5 ± 4.1%; n = 11 each) and double negative (DN) T cells (BM: 52.3 ± 8.7%; spleen: 71.5 ± 3.5%; n = 11 each), consistent with physiological development in mammals (Fig. 2 A and C) (14). The characterization of CD8+ T-cell subsets revealed that in both NSG and NSG-HLA-A2/HHD humanized mice, CD45RA−CCR7− effector memory (EM) CD8+ T cells efficiently developed in the BM and spleen (Fig. 2 D–F).
Fig. 2.
T-cell differentiation in NSG-HLA-A2/HHD recipients recapitulates normal human T-cell development in vivo. (A) Representative contour plots showing CD4, CD8, and TCR expression in NSG-HLA-A2/HHD recipient BM (upper panels) and spleen (lower panels) cells. (B) Diversity of Vβ TCR repertoire was similar between NSG-HLA-A2/HHD recipient CD4+ (n = 7, open bars, upper panel) and CD8+ (n = 7, open bars, lower panel) splenocytes and CB CD4+ (n = 6, hatched bars, upper panel) and CD8+ (n = 6, hatched bars, lower panel) cells. (C) (Left) TCRγδ+ CD3+ T cells were detected in recipient BM and spleen (n = 11 each). (Right) CD8+ and DN T cells were present in TCRγδ+ T-cell populations in recipient BM and spleen (n = 11 each). (D) Representative contour plots showing the development of naïve, central memory, and effector memory CD8+ T cells in NSG-HLA-A2/HHD recipient BM (left) and spleen (right) based on CD45RA and CCR7 expression. (E) The frequencies of CD8+ memory T-cell subsets in NSG-HLA-A2/HHD recipient BM (left) and spleen (right). BM naïve memory 12.4 ± 5.1%, CM 5.7 ± 0.9%, EM 69.5 ± 7.0%, n = 11; spleen naïve memory 20.4 ± 5.4%, CM 5.1 ± 0.8%, EM 63.6 ± 5.9%, n = 13. (F) The frequencies of CD8+ memory T-cell subsets in NSG recipient spleen (left) and normal adult PB (right). NSG spleen naïve memory 33.4 ± 7.2%, CM 5.1 ± 0.8%, EM 53.5 ± 7.8%, n = 13; normal PB naïve memory 34.8 ± 5.2%, CM 3.8 ± 0.4%, EM 43.57 ± 4.0%, n = 9. Horizontal bars indicate means ± SEM.
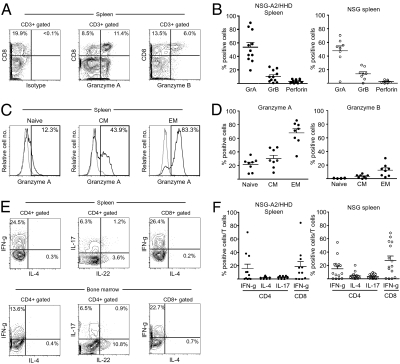
We then examined T-cell receptor (TCR)-mediated functional maturation of human T cells developing in NSG-HLA-A2/HHD recipients. At baseline, without stimulation via the TCR, CD8+ human T cells expressed cytotoxic molecules granzyme A, granzyme B, and perforin in their cytoplasm (Fig. 3 A and B). Among these, granzyme A was most frequently expressed, reflecting that the majority of human CD8+ T cells are early EM cells. The expression of these cytotoxic molecules is comparable between humanized NSG recipients and humanized NSG-HLA-A2/HHD recipients (Fig. 3B). Consistent with phenotypic maturation from naïve to memory as determined by the expression of CD45RA and CCR7, human CD45RA−CCR7− EM CTLs expressed higher levels of granzyme A and granzyme B compared with CD45RA+CCR7+ (naïve) or CD45RA-CCR7+ central memory (CM) CTLs (Fig. 3 C and D). These human CTLs in the recipient spleen produced IFN-γ after nonspecific TCR stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin (18.7 ± 7.5%, n = 11) (Fig. 3E). Finally, an intracellular cytokine production assay demonstrated that human CD4+ T cells preferentially developed into Th1 cells in both NSG-HLA-A2/HHD and NSG recipients (Fig. 3 E and F). Although there was considerable individual variability among recipients, Th17+ cells were also detected both in the BM and spleen of NSG-HLA-A2/HHD humanized mice. Taken together, HLA class I-expressing immune microenvironment within NSG-HLA-A2/HHD mice supports the in vivo differentiation of functionally mature human CTLs associated with a wide spectrum of functional human T-cell subsets from the purified human HSCs.
Fig. 3.
Functional maturation accompanies phenotypic differentiation of human T cells in NSG-HLA-A2/HHD recipients. (A) Representative contour plots and (B) summary of cytotoxic molecule production in CD8+ splenocytes. NSG-HLA-A2/HHD recipients: granzyme A 53.7 ± 6.8%, granzyme B 10.5 ± 2.6%, perforin 3.1 ± 0.8%, n = 11. NSG recipients: granzyme A 47.6 ± 7.3%, granzyme B 13.9 ± 3.1%, perforin 2.3 ± 0.7%, n = 8. (C) Representative histograms and (D) summary of cytoplasmic expression of granzyme A associated with maturation of CTLs from naïve to effector memory phenotype in NSG-HLA-A2/HHD recipient spleen (n = 8). Gray lines indicate isotype control. Granzyme A production in: naïve CTLs 21.6 ± 3.3%, central memory CTLs 30.3 ± 4.9%, effector memory CTLs 68.0 ± 6.0%. Granzyme B production in: naïve CTLs 0.6 ± 0.2%, central memory CTLs 3.4 ± 0.9%, effector memory CTLs 12.7 ± 3.2%. (E) Representative contour demonstrating production of IFN-γ, IL-4, IL-17, and IL-22 by CD4+ and IFN-γ by CD8+ T cells in BM and spleen of NSG-HLA-A2/HHD recipients following 4-h stimulation with PMA, ionomycin, and anti-CD28 mAb. (F) Percentages of IFN-γ–, IL-4–, and IL-17–producing splenocytes from NSG-HLA-A2/HHD (n = 11) and NSG (n = 15) recipients. NSG-HLA-A2/HHD: IFN-γ+ in CD4+ T cells 15.7 ± 6.8%, IL-4+ in CD4+ T cells 1.7 ± 0.5%, IL-17+ in CD4+ T cells 2.4 ± 0.6%, IFN-γ+ in CD8+ T cells 18.7 ± 7.5%. NSG: IFN-γ+ in CD4+ T cells 14.9 ± 4.0%, IL4+ in CD4+ T cells 4.2 ± 1.4%, IL17+ in CD4+ T cells 3.5 ± 0.8%, IFN-γ+ in CD8+ T cells 27.7 ± 6.3%. Horizontal bars represent means ± SEM.
EBV-Associated B-Cell Proliferation Occurs in EBV-Infected NSG-HLA-A2/HHD Humanized Mice.
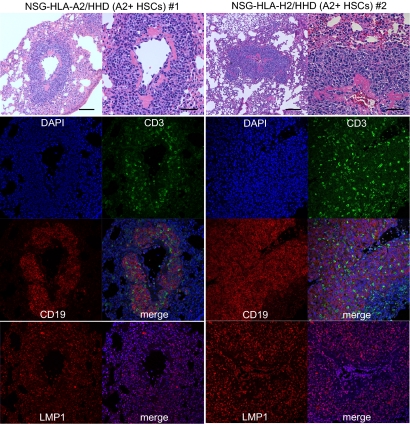
To evaluate whether HLA class I expression in the recipient mouse leads to HLA-restricted, virus-specific human CTL development in vivo, we infected humanized NSG and NSG-HLA-A2/HHD mice with EBV at 4–6 mo posttransplantation. At 4–6 wk postinfection, we examined specific T-cell responses against EBV-infected cells and EBV-derived peptides in recipient tissues. EBV-infected NSG recipients developed disseminated lymphoproliferative disease (LPD) in multiple organs including the liver and kidney (Fig. S2). In contrast, in EBV-infected NSG-HLA-A2/HHD mice, lymphoid infiltrates were reproducibly identified in the lung, a preferential site for EBV-related LPD (Fig. 4) (15, 16). Immunofluorescence studies demonstrated that the majority of infiltrated lymphocytes were human CD19+ B cells. The human B cells in the infiltrative lesions contained intracellular LMP1, consistent with EBV-related LPD. Within EBV-LPD lesions forming in HLA-A2 HSC-engrafted NSG-HLA-A2/HHD mice, CD3+ human T cells were also consistently detected (Fig. 4). The observation that human T cells localize to EBV-associated lesions in the HLA-matched (A2+ HSCs into A2+ microenvironment) transplantation setting suggests that a functional in vivo HLA-restricted antiviral T-cell response is present in NSG-HLA-A2/HHD recipients.
Fig. 4.
EBV-infected human B cells and human T cells form LMP1+ focal infiltrative lesions in the recipient lung. Hematoxylin/eosin (top panels, low- and high-magnification) and immunofluorescence [middle panel, CD3 (green), CD19 (red), DAPI (blue), and merged images; bottom panel, LMP1 (red) and merged with DAPI (blue) images] -labeled lung sections from two EBV-infected NSG-HLA-A2/HHD humanized mice engrafted with HLA-A2–positive human T cells.
HLA-A2–Restricted EBV-Specific Human T Cells Develop in EBV-Infected Humanized NSG-HLA-A2/HHD Mice.
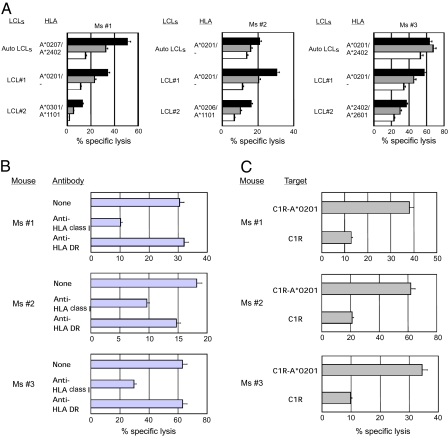
Based on the finding that human T cells accumulate within the EBV-LPD lesions in humanized NSG-HLA-A2/HHD recipients, we evaluated the in vivo development of human CTLs recognizing virus-derived peptides in NSG-HLA-A2/HHD humanized mice (Fig. 5). In all five recipients examined, numbers of CD8+ T cells increased and the CD4:CD8 ratio was inverted at 4–6 wk postinfection (Fig. 5A). The proportion of effector memory T cells within CD8+ T cells significantly increased postinfection (Fig. 5B). Elevated HLA-DR expression and increased frequency of HLA-DR+ cells within CD45RO+CD8+ memory T cells as compared with the steady-state CD8+ T cells (Fig. S3) suggest the activated status of human T cells postinfection (Fig. 5 C and D). To evaluate HLA-restricted and EBV-specific recognition by human T cells in vivo, we next performed the tetramer assay. HLA-restricted anti-BMLF and anti-LMP1 human CTLs were detected specifically in CD45RO+ memory CTL fraction in NSG-HLA-A2/HHD recipients but not in NSG recipients or in uninfected NSG-HLA-A2/HHD recipients (Fig. 5E and Fig. S4). We then enriched human CD8+ T cells from the recipient spleen and performed an enzyme-linked immunospot (ELISPOT) assay to measure IFN-γ production by human CTLs recognizing autologous EBV-infected B-lymphoblastoid cell line cells (LCLs) in an HLA-restricted manner. Human CTLs derived from NSG-HLA-A2/HHD recipients, but not those derived from NSG recipients or uninfected NSG-HLA-A2/HHD recipients, produced IFN-γ in the presence of target LCLs (Fig. 5 F and G and Fig. S5). The addition of anti-HLA class I antibody specifically inhibited IFN-γ production (Fig. 5 F and G). Finally, we established human CTL lines from the spleen cells of three humanized NSG-HLA-A2/HHD mice infected with EBV and examined their cytotoxic function (Fig. 6). All human CTL lines established from these three humanized NSG-HLA-A2/HHD mice exerted strong cytotoxicity against autologous LCLs and HLA-A2–positive allogeneic LCLs, but not against HLA-A2–negative allogeneic LCLs (Fig. 6A). Cytotoxicity against HLA-A2–positive LCLs mediated by the human CTLs was significantly inhibited by adding anti-HLA class I framework monoclonal antibody but not anti-HLA-DR antibody (Fig. 6B). In addition, the human CTLs derived from the humanized NSG-HLA-A2/HHD mice exhibited strong cytotoxicity against HLA-A*0201 gene-transduced EBV-expressing C1R (C1R-A*0201) cells but not against HLA-A2–negative C1R cells (Fig. 6C). These findings clearly indicate that cytotoxicity against LCLs mediated by human CTLs developing in EBV-infected humanized NSG-HLA-A2/HHD mice is EBV-specific and HLA-A2–restricted. Altogether, humanizing the immune microenvironment by the transgenic expression of HLA class I molecules especially in the thymus resulted in the development of HLA-restricted functionally mature human T cells.
Fig. 5.
Human T cells demonstrate functional maturation and HLA-restricted recognition of EBV-specific peptides in EBV-infected NSG-HLA-A2/HHD recipients. (A) Representative contour plots of BM (upper panels) and spleen (lower panels) T-cell subsets in NSG-HLA-A2/HHD recipients 4–6 wk after EBV infection. (B) CTL memory subsets in NSG-HLA-A2/HHD recipient BM (upper panel; n = 5) and spleen (lower panel; n = 6) demonstrate significantly increased EM CTL frequencies compared with uninfected recipients (Fig. 2E). *P = 0.0103 and **P = 0.0352, two-tailed t test. (C) Representative contour plots of HLA-DR expression in CD8+ T cells in NSG-HLA-A2/HHD recipient spleen pre- (upper panel) and post- (lower panel) EBV infection. (D) Frequency of HLA-DR-positive cells and mean fluorescence intensity (MFI) of HLA-DR within CD8+ T cells were significantly higher in EBV-infected NSG-HLA-A2/HHD recipients (n = 3) than in recipients without EBV infection (n = 7). *P = 0.0063 and **P = 0.0006, two-tailed t test. (E) Representative contour plots showing the recognition of EBV-specific CD45RO+ human CTLs in EBV-infected NSG-HLA-A2/HHD recipient BM and spleen but not in EBV-infected NSG recipient spleen by HLA-A*0201/BMLF1280–288 and LMP1159–167 tetramers. (F and G) EBV-specific T-cell responses were quantified by the IFN-γ ELISPOT assay in triplicate. (F) Stimulation with autologous LCL promoted IFN-γ secretion by CD8+ splenocytes from an NSG-HLA-A2/HHD recipient but not those from an NSG recipient (top row). The addition of anti-HLA class I (middle row) but not anti-HLA class II (bottom row) antibody inhibited IFN-γ secretion. (G) The inhibitory effect of anti-HLA class I antibody was statistically significant (*P = 0.0074, two-tailed t test). Horizontal bars represent means ± SEM.
Fig. 6.
EBV-infected NSG-HLA-A2/HHD humanized mice exhibit HLA-restricted CTL cytotoxicity. (A) Five-hour 51Cr-release assays at effector:target (E:T) ratios of 10:1 (black), 5:1 (gray), and 2.5:1 (white) showing that the cytotoxicity of CTL lines established from EBV-infected NSG-HLA-A2/HHD mice against autologous and HLA-A2–positive allogeneic LCLs was higher than that against HLA-A2–negative allogeneic LCLs. (B) Five-hour 51Cr-release assays at an E:T ratio of 10:1 in the presence or absence of anti-HLA class I monoclonal antibody or anti-HLA-DR monoclonal antibody showing that the cytotoxicity of CTL lines against autologous LCLs was significantly inhibited by adding anti-HLA class I monoclonal antibody but not anti-HLA DR monoclonal antibody. (C) Five-hour 51Cr-release assays at an E:T ratio of 5:1 showed that the CTL lines established from EBV-infected NSG-HLA-A2/HHD recipients exerted cytotoxicity against HLA-A*0201 gene-transfected C1R-A*0201 cells but not against C1R cells lacking the expression of HLA-A2.
Discussion
Studies using mouse models have led to significant advances in understanding the mammalian immune system, motivating many investigators to translate the findings into therapeutic applications in humans. The creation of an in vivo model that allows the development of a full spectrum of functional human T cells including HLA-restricted antigen-specific CTLs greatly facilitates the examination of in vivo human cellular immunity against human diseases such as infection and malignancy. In this study, we report the development of functional human T cells and the establishment of a viral infection model using the HLA class I-expressing NSG-HLA-A2/HHD strain.
The humanization of recipient mouse thymic microenvironment is required for in vivo thymic education and development of HLA-restricted human CD8+ T cells (1, 2). NOD/SCID/BLT system supports functional human T-cell development by meeting this requirement through implantation of fetal thymic and liver tissues into NOD/SCID mice (17). Although this approach successfully provides human immune microenvironment in recipients, it necessitates the procurement of human fetal tissues. Therefore, we established an immunodeficient mouse strain, NSG-HLA-A2/HHD, with humanized microenvironment expressing both HLA class I heavy and light chains, by backcrossing HLA class I transgenic mice (12) onto the NSG background to homozygosity. Transplantation of fluorescence-activated cell sorter-purified human HSCs assured that the human T cells detected were those that developed within the recipients and not mature human T cells introduced unintentionally.
The development of human T cells in mice using adult NOG, NOD/SCID/BLT, Rag2−/−gc−/−, and NSG repopulating assays has been reported to date (5, 7, 8, 11, 17, 18). In these studies, CD4 SP and CD8 SP human T cells were observed in the recipient secondary lymphoid organs. In the NSG-HLA-A2/HHD humanized mouse, we demonstrated the presence of human TCR+ T cells among the more predominant TCR+ CD4 SP and CD8 SP human T cells. Through further detailed analyses, we identified CD8 SP TCR+ and DN TCR+ T cells, each at a frequency similar to healthy human subjects (14). Various reports suggest the important role of TCR+ T cells for immune surveillance in virus infection and cancer (19). The humanized mouse may be an ideal system for examining in vivo functional biology of human TCR+ T cells, including investigation into distinct roles of each TCR+ T cell subset and preclinical testing of cellular therapy against malignancies.
We also detected human T cells at various stages of differentiation, as documented by the expression of cell surface molecules including CD45RA, CD45RO, and CCR7. This phenotypic transition from naïve to central memory to effector memory T cells was accompanied by functional transition, as evidenced by increasing intracellular granzyme A expression in human CD8+ T cells (20). Although the frequency of EM CTLs in the humanized recipients at baseline is higher than that in normal human peripheral blood (PB), the recipients did not exhibit any signs (weight loss, diarrhea, alopecia) or pathological findings (infiltration of activated human lymphocytes in liver, skin, or intestine) compatible with graft-versus-host disease.
Th17 cells seem to play key roles in normal and pathological immune responses, reportedly responsible for host protection against extracellular pathogens and for induction and maintenance of chronic inflammatory diseases (21). However, several crucial differences between murine and human Th17 cell function have been reported (22). Human Th17 cells were consistently detected in humanized mice and will enable direct investigation into the function of human Th17 cells in vivo. In addition, we detected CD4+ T cells producing IL-22 in NSG-HLA-A2/HHD humanized mice. The IL-22–producing human CD4+ cells were reported to play crucial roles in normal and pathological cutaneous immunity (23). In vivo development of Th17- and IL-22–producing human helper T-cell subsets in humanized mice suggests that this model is useful in investigating as yet uncharacterized roles of novel and rare T-cell subsets in human physiology and pathophysiology in vivo.
EBV is a γ herpes virus that causes a variety of human diseases including infectious mononucleosis, Burkitt lymphoma, Hodgkin's disease, and posttransplant LPD (24). In immune-competent individuals, EBV-infected B cells and antigen presenting cells cross-present virus-associated peptides to CTLs, suppressing EBV-associated oncogenic events and leading to latency (25). To demonstrate the significance of HLA class I expression within the mouse microenvironment on antigen recognition by human CD8+ T cells in vivo, we infected humanized NSG and NSG-HLA-A2/HHD mice with EBV. At 4–6 wk after infection, the expansion of an EM CTL subset was observed with a concurrent increase in HLA-DR expression, indicating the activation of human T cells in response to EBV infection in vivo. The severity of EBV infection as evidenced by lymphocyte infiltration and LPD development in various organs was distinct between NSG-HLA-A2/HHD and NSG humanized mice. The differences may be accounted for by the HLA-restricted EBV-specific recognition and cytotoxicity against EBV-infected cells by human CTLs developing in EBV-infected NSG-HLA-A2/HHD humanized mice. Although long-term observation is required to clarify the role of human CTLs in immune surveillance, distinct lesions were found between EBV-infected NSG and NSG-HLA-A2/HHD recipients in our study. Our study supports a recent report by Münz and colleagues (26) showing that human fetal liver CD34+ cells generate HLA-restricted, cytokine-producing CTLs against EBV-associated peptides at a higher frequency in HLA-expressing humanized recipients compared with humanized recipients without HLA expression. Jaiswal et al. (27) reported using the NSG-HLA-A2/HHD strain in detecting cytokine-producing human T cells in a model of Dengue virus infection. We demonstrate here dose-dependent cytotoxic function against autologous and HLA-matched allogeneic virus-infected cells by human CTLs developing in NSG-HLA-A2/HHD mice homozygously expressing both heavy and light chains of HLA class I molecules. However, the lack of HLA class II molecules in the NSG-HLA-A2/HHD mouse likely results in less than optimal T-cell help, contributing to inefficient production of antigen-specific IgG. The coexpression of both HLA class I and class II molecules on recipient thymic epithelial cells would enable the evaluation of T-helper function and antigen production in the next generation of humanized mice.
The newborn NSG-HLA-A2/HHD xenotransplantation system will open the possibility for a wide range of biomedical applications by enabling the investigation of not only normal human immunohematopoietic development but also human disease pathogenesis. By allowing in vivo HLA-restricted human immune responses, the NSG-HLA-A2/HHD humanized mouse model may facilitate in vivo examination of human immune responses against pathogens and malignant cells as well as preclinical testing for vaccines against infectious diseases and cancer.
Methods
Mice.
NOD.Cg-PrkdcscidIL2rgtmlWjl/Sz (NSG) mice were developed at The Jackson Laboratory by backcrossing a complete null mutation at the Il2rg locus onto the NOD.Cg-Prkdcscid (NOD/SCID) strain (6). NOD.Cg-PrkdcscidIl2rgtm1WjlTg (HLA-A2/H2-D/B2M) 1Dvs/Sz (NSG-HLA-A2/HHD) mice were generated by backcrossing the (HLA-A/H2-D/B2M) transgene (12) from the NOD/ShiLtDvs-Tg(HLA-A/H2-D/B2M)1Dvs/J strain (28) onto the NSG background. Mice were bred and maintained under defined flora with irradiated food and acidified water at the animal facility of RIKEN and at The Jackson Laboratory according to guidelines established by the Institutional Animal Committee at each institution.
Purification of Human HSCs and Xenogeneic Transplantation.
All experiments were performed with authorization from the Institutional Review Board for Human Research at RIKEN Research Center for Allergy and Immunology. HLA-A2+ CB samples were enriched for hCD34+ cells by using anti-hCD34 microbeads (Miltenyi Biotec) and sorted for 7AAD-lineage (hCD3/hCD4/hCD8/hCD19/hCD56)-CD34+CD38− HSCs. The purity of HSCs was higher than 98% after sorting (Fig. S1). Newborn (within 2 d of birth) recipients received 150 cGy total body irradiation using a 137Cs-source irradiator, followed by i.v. injection of sorted HSCs via the facial vein.
Cell Preparation, Flow Cytometry, and Histological Analysis.
For a full description of procedures and reagents used for cell preparation, flow cytometry, and histological analysis, see SI Methods.
EBV Infection.
Humanized mice were inoculated at 12–20 wk of age by i.p. injection of 500 μL B95-8 supernatant, containing 0.45 log10 (TCD50/mL) of EBV (29). EBV-infected recipients were analyzed at 4–6 wk after injection.
Tetramer Assay, Generation of B-Lymphoblastoid Cell Lines, and IFN-γ ELISPOT Assay.
For a full description of procedures and reagents used for tetramer assay, LCL generation, and ELISPOT assay, see SI Methods.
Statistical Analysis.
The numerical data are presented as means ± SEM unless otherwise noted. Where noted, two-tailed t tests were performed and differences with the P < 0.05 were deemed statistically significant (GraphPad Prism; GraphPad).
Supplementary Material
Acknowledgments
We thank Dr. A. John Barrett (National Heart, Lung, and Blood Institute, National Institutes of Health) for providing the C1R-A*0201 cell line. We also thank Dr. Hiroo Saji, HLA Laboratory, Japan, for HLA typing. This work was supported by National Institutes of Health Grants DK072473, CA34196, and AI46629 (to L.D.S.), the Biology of the Beta Cell Consortium (L.D.S.), the Juvenile Diabetes Foundation International (L.D.S.), the Helmsley Foundation (L.D.S.), Takeda Science Foundation (F.I.), Uehara Memorial Foundation (F.I.), and Ministry of Education, Culture, Sports, Science and Technology, Japan (F.I.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.E.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000475107/-/DCSupplemental.
References
- 1.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 2.Manz MG. Human-hemato-lymphoid-system mice: Opportunities and challenges. Immunity. 2007;26:537–541. doi: 10.1016/j.immuni.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Shultz LD, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- 4.Ito M, et al. NOD/SCID/γ(c)(null) mouse: An excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 5.Hiramatsu H, et al. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/γcnull mice model. Blood. 2003;102:873–880. doi: 10.1182/blood-2002-09-2755. [DOI] [PubMed] [Google Scholar]
- 6.Shultz LD, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R γ null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa F, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yahata T, et al. Functional human T lymphocyte development from cord blood CD34+ cells in nonobese diabetic/Shi-scid, IL-2 receptor γ null mice. J Immunol. 2002;169:204–209. doi: 10.4049/jimmunol.169.1.204. [DOI] [PubMed] [Google Scholar]
- 9.Ninomiya M, et al. Homing, proliferation and survival sites of human leukemia cells in vivo in immunodeficient mice. Leukemia. 2007;21:136–142. doi: 10.1038/sj.leu.2404432. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa F, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 11.Traggiai E, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 12.Pascolo S, et al. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from β2 microglobulin (β2m) HLA-A2.1 monochain transgenic H-2Db β2m double knockout mice. J Exp Med. 1997;185:2043–2051. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladi E, Yin X, Chtanova T, Robey EA. Thymic microenvironments for T cell differentiation and selection. Nat Immunol. 2006;7:338–343. doi: 10.1038/ni1323. [DOI] [PubMed] [Google Scholar]
- 14.Borst J, et al. Distinct molecular forms of human T cell receptor γ/δ detected on viable T cells by a monoclonal antibody. J Exp Med. 1988;167:1625–1644. doi: 10.1084/jem.167.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zutter MM, et al. Epstein-Barr virus lymphoproliferation after bone marrow transplantation. Blood. 1988;72:520–529. [PubMed] [Google Scholar]
- 16.Shapiro RS, et al. Epstein-Barr virus associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood. 1988;71:1234–1243. [PubMed] [Google Scholar]
- 17.Denton PW, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008;5:e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melkus MW, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 19.Girardi M, et al. Regulation of cutaneous malignancy by γδ T cells. Science. 2001;294:605–609. [PubMed] [Google Scholar]
- 20.Takata H, Takiguchi M. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J Immunol. 2006;177:4330–4340. doi: 10.4049/jimmunol.177.7.4330. [DOI] [PubMed] [Google Scholar]
- 21.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 22.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 23.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 24.Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343:481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 25.Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: Lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 26.Strowig T, et al. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med. 2009;206:1423–1434. doi: 10.1084/jem.20081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaiswal S, et al. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rγnull mice. PLoS One. 2009;4:e7251. doi: 10.1371/journal.pone.0007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takaki T, et al. HLA-A*0201-restricted T cells from humanized NOD mice recognize autoantigens of potential clinical relevance to type 1 diabetes. J Immunol. 2006;176:3257–3265. doi: 10.4049/jimmunol.176.5.3257. [DOI] [PubMed] [Google Scholar]
- 29.Moss DJ, Pope JH. Assay of the infectivity of Epstein-Barr virus by transformation of human leucocytes in vitro. J Gen Virol. 1972;17:233–236. doi: 10.1099/0022-1317-17-2-233. [DOI] [PubMed] [Google Scholar]
- 30.Yasukawa M, et al. Expression of perforin and membrane-bound lymphotoxin (tumor necrosis factor-β) in virus-specific CD4+ human cytotoxic T-cell clones. Blood. 1993;81:1527–1534. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.