Abstract
There is increasing concern that ocean acidification, caused by the uptake of additional CO2 at the ocean surface, could affect the functioning of marine ecosystems; however, the mechanisms by which population declines will occur have not been identified, especially for noncalcifying species such as fishes. Here, we use a combination of laboratory and field-based experiments to show that levels of dissolved CO2 predicted to occur in the ocean this century alter the behavior of larval fish and dramatically decrease their survival during recruitment to adult populations. Altered behavior of larvae was detected at 700 ppm CO2, with many individuals becoming attracted to the smell of predators. At 850 ppm CO2, the ability to sense predators was completely impaired. Larvae exposed to elevated CO2 were more active and exhibited riskier behavior in natural coral-reef habitat. As a result, they had 5–9 times higher mortality from predation than current-day controls, with mortality increasing with CO2 concentration. Our results show that additional CO2 absorbed into the ocean will reduce recruitment success and have far-reaching consequences for the sustainability of fish populations.
Keywords: animal behavior, mortality, recruitment success, coral reef fish, hypercapnia
As atmospheric CO2 increases, so does the amount of CO2 dissolved in the shallow ocean, which in turn causes ocean pH to decline—a process known as ocean acidification (1, 2). If the current trajectory of global emissions is maintained, atmospheric CO2 concentrations will exceed 500 ppm by midcentury and could reach between 730 and 1,020 ppm at the end of the century (3, 4). This would cause ocean pH to decline by 0.3–0.4 units compared with current-day levels (1, 4, 5), with a rate of change many times faster than at any time during the past 650,000 y (1, 2, 6). It is well-known that reduced carbonate-ion saturation states that accompany lower seawater pH can affect the ability of marine calcifiers to form shells and skeletons (6–12). In contrast, the likely impacts of ocean acidification on noncalcifying species, such as fish, are poorly understood (6, 8, 9, 13). There is increasing evidence from laboratory experiments that elevated levels of dissolved CO2 and reduced seawater pH can affect developmental (14–16), metabolic (17, 18), and behavioral processes (19) of some marine species, including some noncalcifying species. However, to establish whether ocean acidification threatens noncalcifying marine species, the CO2 concentrations at which population declines are likely to occur need to be determined.
Most benthic marine species have a planktonic larval phase that must transition to a benthic existence to join the adult population. This life-history transition is usually associated with high rates of mortality (20) and can be a period of strong selection (21, 22). Chemical cues are used by larvae of many marine species to locate suitable adult habitat and avoid predators during the settlement process (23–27). However, it was recently shown that larval clownfish lose their ability to distinguish chemical cues from preferred settlement habitat (19) and predators (28) when exposed to seawater that has been acidified with ≥1,000 ppm CO2. Impairment of the olfactory system by elevated CO2 would have far-reaching implications for marine diversity if other species are similarly affected and if it increases mortality of larvae during recruitment to adult populations.
We tested the levels of CO2 at which impairment of olfactory ability occurs in fish larvae and then tested if altered olfactory-mediated behavior leads to increased mortality in natural populations. First, we reared larval clownfishes (Amphiprion percula) under a range of CO2 environments (∼390 current-day control, 550, 700, and 850 ppm) and tested their behavioral responses to olfactory cues from predators throughout the larval phase (10–11 d). Second, we repeated the experiments with wild-caught damselfish larvae (Pomacentrus wardi) to determine if they were similarly affected. Finally, we transplanted settlement-stage damselfish to an array of natural reefs to test if exposure to elevated CO2 altered their behavior and increased the risk of mortality during recruitment to adult habitat.
Results
Behavioral responses were tested in a two-channel flume chamber (27) where larvae could chose between a stream of water containing the chemical cues of a common predator and a stream of water without that cue. Larval preference or avoidance of the predator cue was dependent on an interaction between CO2 treatment and days posthatching but was not dependent on clutch (best-fit log-linear model = clutch + preference × treatment × day; likelihood χ2 = 15.03189). Therefore, clutch was pooled in all further analyses.
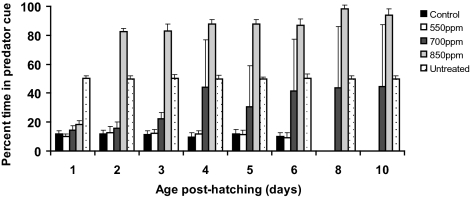
Clownfish larvae reared in control water exhibited a strong avoidance to predator odor at all stages of development (Fig. 1) (P < 0.001 for all comparisons with controls exposed to untreated water). By 8 d posthatching, control larvae exhibited complete avoidance of the predator cue. Larvae reared in 550 ppm CO2 also exhibited a similar, strong avoidance of the predator cue that increased to complete avoidance by 8 d posthatching (Fig. 1) (P < 0.001 for all comparisons). There was no difference in the response between control and 550-ppm CO2-treated fish (P > 0.05 for all comparisons), indicating that this level of CO2 had no effect on behavioral responses.
Fig. 1.
Effects of elevated CO2 concentrations on olfactory ability of clownfish larvae. Clownfish were exposed to 390 (control), 550, 700, or 850 ppm CO2 from hatching, and their behavioral responses were assessed at regular ontogenetic stages using a flume chamber where they could freely move between a stream of water containing the predator cue and a stream of water without the cue. Shown is mean time (±SD) that clownfish larvae spent in the stream of water containing the predator odor. The untreated category shows the time larvae spent on one side of the chamber when neither water stream contained the predator cue.
Clownfish reared in the 700-ppm CO2 treatment initially avoided the predator cue, but after 4 d, their behavioral response was markedly different from larvae reared in the control and 550-ppm CO2 treatments (P < 0.001), with larvae spending ∼30–45% of their time in the water stream containing the predator cue (Fig. 1). However, there were distinct behavioral differences among individuals. Approximately one-half of the larvae continued to avoid the water stream containing the predator cue, with the strength of avoidance increasing with age (Table 1) (χ2 test of independence, P < 0.001). In contrast, other individuals exhibited a strong preference for the predator cue, spending 74–88% of their time in the water stream containing the predator cue.
Table 1.
Behavioral responses of clownfish larvae reared at 700 ppm CO2 when exposed to the chemical cue of a common predator
| Avoidance of predator cue |
Attraction to predator cue |
|||
| Age posthatch (d) | Time in water stream with cue (%) | No. of individuals | Time in water stream with cue (%) | No. of individuals |
| 1 | 18.3 | 22 | — | 0 |
| 2 | 15.9 | 22 | — | 0 |
| 3 | 22.3 | 22 | — | 0 |
| 4 | 16.3 | 11 | 77.8 | 9 |
| 5 | 15.8 | 15 | 74.2 | 5 |
| 6 | 13.5 | 14 | 84.9 | 8 |
| 8 | 6.8 | 12 | 88.3 | 10 |
| 10 | 0.7 | 12 | 84.6 | 13 |
Behavioral responses were tested in a two-channel flume chamber, where one stream of water contained the chemical cue of the predator and the other stream of water did not contain the cue. Responses are split into individuals that avoided the predator cue (<50% of time spent in the water stream containing the cue) and individuals that were attracted to the predator cue (>50% of time spent in the water stream containing the cue).
Clownfish reared in the 850-ppm CO2 treatment avoided the predator cue 1 d after hatching (P < 0.001) but exhibited a strong attraction to the predator cue on all subsequent days (Fig. 1) (P < 0.001 for all comparisons). After 8 d, the larvae spent over 94% of their time in the water stream containing the predator cue, with all individuals exhibiting a similar level of attraction (coefficient of variation = 2.4–4.6%).
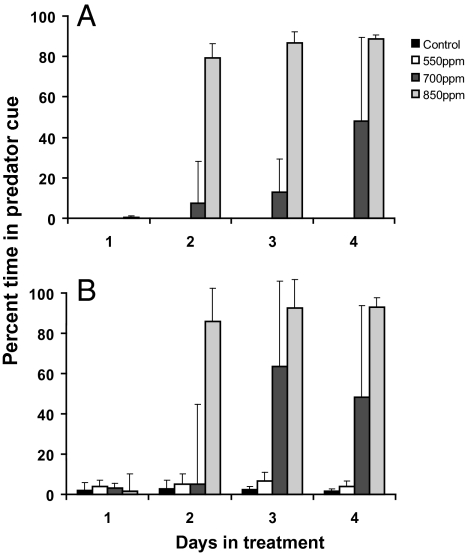
Identical results were observed for clownfish larvae that had been reared in control water for 10 d and were then transferred to the different CO2 treatments, showing that behavioral responses to elevated CO2 are associated with the concentration of CO2 and the duration of exposure, not the fish's ontogenetic stage of development. Settlement-stage clownfish larvae kept in control water, or transferred to 550 ppm CO2, completely avoided the predator cue at all times (Fig. 2A). Fish transferred to 700 ppm CO2 initially avoided the predator cue, but after 4-d exposure, they spent 45% of their time in the water stream containing the predator odor. Fish transferred to 850 ppm CO2 developed a strong attraction to the predator cue within 2 d (Fig. 2A).
Fig. 2.
Duration of exposure to elevated CO2 required to cause olfactory impairment in (A) laboratory-reared settlement-stage clownfish and (B) wild-caught settlement-stage damselfish. Settlement-stage clownfish and damselfish were transferred from untreated water to water treated with 390 (control), 550, 700, or 850 ppm CO2, and their behavioral responses to predator odor were assessed at 24-h intervals. Shown is mean time (±SD) that fish spent in the stream of water containing the chemical cues from a common predator when presented in a two-channel flume chamber.
Wild-caught damselfish larvae exhibited similar responses to the clownfish, with preference or avoidance of the predator cue dependent on an interaction between CO2 treatment and days posthatching (best-fit log-linear model = preference × treatment × day). Damselfish larvae kept in control water, or in 550 ppm CO2, maintained a strong avoidance of the predator cue at all times (Fig. 2B). Fish kept at 700 ppm CO2 initially avoided the predator cue, but after 4-d exposure, they spent 48% of their time in the predator cue. As observed for the clownfish, approximately one-half (49%; n = 109) of the fish exhibited a strong attraction to the predator cue, spending 93% of their time in the water stream with the cue. The other fish continued to avoid the predator cue, spending <2% of their time in the water stream with the cue. A subset of 700-ppm CO2-exposed larvae (n = 30) was retested after 6 and 12 h, and all exhibited the same response as the original test. Larvae kept at 850 ppm CO2 developed a strong attraction to the predator cue after 2 d, spending 93% of their time in the water stream containing the cue (Fig. 2B).
Settlement-stage damselfish larvae exposed to control, 700, or 850 ppm CO2 for 4 d were transplanted to an array of patch reefs exposed to natural predators. Larvae treated with 850 ppm CO2 exhibited riskier behaviors than control larvae transplanted to the same reef (n = 56 pairs); they were more active (distance moved was 23.0 vs. 17.4 cm; P = 0.002), stayed farther away from the reef on average (1.8 vs. 1.0 cm; P < 0.001), ventured a greater maximum distance from the reef (4.5 vs. 2.8 cm; P < 0.001), and had bolder behavior (boldness index was 2.1 vs. 1.6; P < 0.001). Larvae treated with 700 ppm CO2 that exhibited a positive response to the predator cue in the flume stayed farther away from the reef on average (3.4 vs. 1.8 cm; P = 0.044) compared with 700-ppm CO2-treated larvae that avoided the predator cue in the flume (n = 16 pairs). The two 700-ppm groups did not differ in the other behavioral attributes.
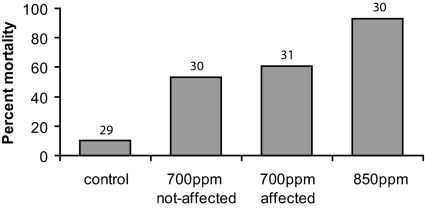
Larvae exposed to elevated CO2 had 9-fold (850 ppm = 28/30) and 5-fold (700 ppm = 35/61) higher mortality rates compared with control larvae (390 ppm = 3/29) in the first 30 h of residence on the reef (Fig. 3) (P < 0.001). In the 700-ppm treatment, there was no difference in mortality rate between larvae that exhibited a positive response to the predator cue in the flume (19/31) compared with larvae that exhibited a negative response to the predator cue (16/30); 100% of fish from all treatments survived when protected from predators by fine-mesh cages (n = 24 distributed among treatments), indicating that mortality was caused by predation.
Fig. 3.
Percent mortality of settlement-stage damselfish on patch reefs in nature after exposure to 390 (control), 700, or 850 ppm CO2. N shown above bars.
Discussion
Our results show that CO2 concentrations predicted to occur in the ocean by the end of this century may have dramatic effects on the behavior of fish larvae, with highly significant consequences for population replenishment and sustainability. Average CO2 concentrations in the atmosphere and shallow ocean could reach 850 ppm by the end of the century (3, 4). Levels of dissolved CO2 in this range (700–850 ppm) impaired the ability of larvae to respond to predator odors and caused them to exhibit riskier behavior in natural coral-reef habitat. Altered behavior was associated with dramatically higher mortality during a life-history stage when individuals are inherently vulnerable to predation, with the effects 50% greater at 850 ppm compared with 700 ppm CO2. Increased recruitment mortality, of the scale detected here, would seriously threaten population sustainability. If other marine species exhibit similar behavioral responses, with corresponding impacts on survival, the effects of rising atmospheric CO2 on marine biodiversity will be profound.
Exposure to 550 ppm CO2, a much more conservative concentration of atmospheric CO2 that will be reached by 2050 on the current emissions trajectory (3, 4), did not have any detectable effect on the olfactory responses of larval clownfish and damselfish. Larval fishes exposed to 550 ppm CO2 responded in a similar manner to current-day controls when presented with the odor of reef-based predators. Concentrations of dissolved CO2 in the vicinity of coral reefs can exceed atmospheric levels because of CO2 released by calcification and respiration. For example, CO2 concentrations in surface waters within several kilometers of Kaneohe Bay (Hawaii) average ∼400–500 ppm (29). Consequently, larvae may be adapted to CO2 concentrations in this range, because they are already encountered in the environments inhabited immediately before and after settling to reef habitat.
Settlement-stage larvae that had been exposed to elevated CO2 (700 and 850 ppm) were more active, ventured farther away from shelter, and were less responsive to threats (i.e., were behaviorally bolder) than controls. This decrease in sensitivity to risk might be directly related to the impaired olfactory ability detected in the flume experiments. Specifically, the dramatic switch from avoidance to preference of the chemical cues from predators might cause larvae exposed to elevated CO2 to engage in riskier behavior, because they no longer perceive predator odor as a threat. Alternatively, the increase in activity and reduction in risk sensitivity might indicate that elevated CO2 has a more general effect on physiological processes that mediate a range of behavioral traits in fish larvae. For example, disrupted internal acid–base balance caused by exposure of elevated CO2 might potentially affect neuronal pathways (30, 31) that could mediate a range of functions, including olfactory discrimination, activity levels, and risk perception. Detailed investigations of the physiological processes responsible for the behavioral changes we observed would be required to separate these alternatives. Nevertheless, our results show that a range of behavioral traits critical to survival of newly settled fish larvae are affected by ocean acidification.
Assessing the capacity for biological adaptation is important for predicting the impacts of rapid climate change on ecological communities (32–34), although rarely is this possible. Our results suggest that 700 ppm CO2 is close to the threshold at which adaptation of behavioral responses might be possible in reef fishes, provided that the variation in sensitivity to elevated CO2 we observed between individuals at this concentration has a genetic basis. The olfactory capacity of approximately one-half of the larvae was unaffected by exposure to 700 ppm CO2, and these individuals exhibited less risky behavior in the field (remained closer to shelter) compared with affected individuals. Even a slight survival advantage could lead to rapid selection given that a large proportion of all fish larvae that settle to coral reefs are consumed by predators within the first few days (35). In contrast, there seems to be little scope for adaptation at 850 ppm CO2, with the behavior of all individuals strongly affected and over 90% mortality within the first 30 h on the reef.
On the current emissions trajectory, atmospheric CO2 concentrations will exceed 500 ppm by 2050 and could reach 850 ppm by 2100 (3, 4). There seems to be little potential for adaptation of behavioral responses should 850 ppm CO2 be reached, with serious, irreversible consequences for marine biodiversity. Although adaptation of behavioral responses might be possible at 700 ppm CO2, significant population declines are still likely because of markedly higher rates of mortality in newly settled larvae compared with larvae exposed to current-day conditions. Furthermore, it is possible that other species will be affected by lower levels of CO2 than the species tested here. Our results show that even moderate increases in CO2 concentrations dissolved in the ocean are likely to have significant impacts on the sustainability of fish populations by altering individual behavior and increasing mortality at critical life-history transitions.
Materials and Methods
Larval Rearing.
Clownfish.
A. percula were reared at James Cook University, Townsville, Australia, using established techniques (36). Brood stock was wild-caught pairs of A. percula from the Great Barrier Reef, Australia. Breeding pairs laid eggs on a terracotta pot in their aquarium. Exposure of eggs to elevated CO2 does not affect the behavior of newly hatched larvae (28); therefore, egg clutches were left in the parental aquarium where they were tended by the parents. Larvae from a total of four clutches from three different parental pairs were used in the experiment. Immediately after hatching, the larvae from each clutch were divided into four equal-sized groups (∼50–100 individuals per group) and transferred to 4 × 60-L rearing aquariums, each treated with a different CO2 concentration (see below). A different block of four aquariums was used for each clutch of fish, giving a total of 16 replicate aquariums. Larval clownfishes were reared for 10 d, at which stage they were competent to settle, as evidenced by attraction to the wall of the aquarium. Larvae were subsampled without replacement from the aquariums as required. Larvae were fed rotifers (Brachionus sp.) and Artemia nauplii daily (36). Larvae were sampled each day (or as required) without replacement for use in the flume experiments.
Larval-rearing aquariums were aerated with air (390 ppm CO2 current-day control) or one of three CO2-enriched air treatments: 538 ± 54 [standard deviation (SD)] ppm, 744 ± 47 ppm, or 1,023 ± 64 ppm CO2 (36). The concentration of CO2-enriched air was controlled by a scientific-grade pressure regulator and precision needle valve and measured continuously with an infrared CO2 probe (Vaisala GMT222) linked to a computer. Aeration with CO2-enriched air produced dissolved CO2 levels of 541 ± 36 (SD) ppm, 699 ± 30 ppm, and 863 ± 64 ppm, respectively. Therefore, we report values of ∼550, 700, and 850 ppm CO2. Dissolved CO2 was measured directly in the aquariums using a submerged CO2-permeable membrane connected to an aspirated CO2 probe (Vaisala GM70) in a closed loop (37). Each 60-L rearing tank, which had no water flow during the day, was flushed at night with filtered seawater that had been aerated all day with the same concentration of CO2-enriched air. Water temperature was 30 °C ± 0.5, and oxygen levels were always above 90% saturation (WTW Oxi340i electrode).
Damselfish.
Settlement-stage P. wardi (16–21 d old) larvae were caught overnight in light traps (38) moored at Lizard Island, Great Barrier Reef, Australia, during November and December 2009. Each morning, P. wardi collected in the traps were divided into equal-sized groups and transferred to 35-L rearing aquariums aerated with 390 ppm (current-day control), 544 ± 33 (SD) ppm, 728 ± 88 ppm, or 1,008 ± 74 ppm CO2 in air. Group size depended on the number of larvae caught in the light traps. A different block of aquariums was used for each day's catch. Larvae were reared for 4 d and sampled each day (or as required) without replacement for use in the flume and field experiments. The rearing system was similar to that described above, except that rearing tanks received a continuous flow of seawater aerated with control or CO2-enriched air. Seawater for the system was pumped directly from the ocean into 70-L sumps, where it was aerated with the same concentration of CO2-enriched air as the rearing aquariums. Rearing aquariums received a continuous flow of water from the relevant sump at ∼225–250 mL/min. Water temperature averaged 27.6 ± 1.3 °C (SD), and oxygen levels were always above 90% saturation. Larval damselfishes were fed freshly hatched Artemia nauplii three times daily.
Behavioral Responses to Predator Odor.
A two-channel choice flume (27) was used to test the behavioral responses of larvae. One channel received seawater containing the chemical cues of a common predator of newly settled fish larvae. The other channel received seawater with no additional chemical cues. Water from the two different sources was gravity fed into the flume at 100 mL/min using flow meters. Fish were released at the downstream end of the flume where they were free to move to either side or swim to the preferred water source. For each trial, a single fish was placed into the center of the downstream end of the choice flume and acclimated to the two water choices for 2 min. After the acclimation period, the position of the fish was recorded at 5-s intervals for a 2-min period. This was followed by a 1-min rest period, during which the water sources were switched. The test was then repeated. Each fish was tested only one time, and different individuals were tested each day. For each set of trials, an untreated control was also used, where both channels of the flume received seawater without the predator odor. This provided a control distribution against which to test the response of fish when a cue was present in one channel.
For the larval clownfish, ∼20 larvae coming from four different clutches (from three different parental pairs) were tested on each day posthatching for each CO2 treatment (Table S1). Clownfish larvae were tested using predator cues from the rockcod Cephalopholis cyanostigma kept in 70-L aquariums and fed commercial fish food (Fish Dinner; Fish Fuel Co.) every second day. Chemical cues from predators were collected by turning off the water flow to their aquariums for 2 h and then removing water directly from the aquarium.
For the damselfish, a total of at least 10 wild-caught larvae were tested for each CO2 treatment on each of the 4 d (Table S1). A larger number of trials (n = 109) were conducted for the 700-ppm CO2-treated fish on day 4, because the olfactory preference of fish from this treatment was tested before being placed on patch reefs to assess behavior and mortality (see below). Damselfish larvae were tested using predator cues from the dottyback Pseudochromis fuscus kept in a 70-L aquarium and fed every day with reef fish larvae.
There was no difference in behavioral responses of the larvae when the predator cue was presented using control water in the flume compared with water aerated with 850 ppm CO2. This applied to both clownfish and damselfish larvae reared in control water and larvae reared in 850 ppm CO2 (Fig. S1) (P > 0.1 for both comparisons). Consequently, control water was used for all flume trials.
Field Assessment of Behavior.
Damselfish larvae treated with 700 or 850 ppm CO2 retained their behavioral responses for over 24 h when transferred back into control water (Fig. S2). This provided the opportunity to experimentally examine behavior and mortality rate of damselfish larvae in nature. Size-matched pairs of P. wardi larvae were placed on an array of small-patch reefs at Lizard Island, Great Barrier Reef, Australia (14°41′S, 145°27′E), and their behavior was compared in situ. Two treatments were used: (i) a fish exposed to 850 ppm CO2 was paired with a fish exposed to control CO2 (n = 56 pairs) and (ii) a fish exposed to 700 ppm CO2 that was attracted to the predator odor in the flume was paired with a fish exposed to 700 ppm CO2 that avoided the predator odor in the flume (n = 16 pairs). After 4 d in elevated or control CO2 treatments, fish were individually measured (standard length to nearest 0.1 mm), sorted into pairs, and tagged with a small colored elastomer tag (Northwest Technologies) injected under the skin. Pairs were released onto an array of small reefs (18 × 12 × 12 cm) made from live and dead Pocillopora damicornis (a common bushy hard coral). Reefs were cleared of any other fishes or invertebrates before release using a hand net. One pair was placed on each reef, and a wire cage (30 × 30 × 30 cm; 12-mm mesh size) was placed over the reef for 30–60 min to allow fish to acclimate to their new surroundings while being protected from predators.
Behavior of the fish was assessed over a 3-min period shortly after the cage was removed (22). Five aspects of activity and behavior were estimated: total distance moved (cm) during the observation period, mean distance ventured (cm) from the reef, maximum distance ventured (cm), height above substratum (categorized as percent of the time spent within the bottom, middle, or top one-third of the patch), and boldness (recorded on a scale from 0 to 3 at 0.5 increments where 0 is hiding in hole and seldom emerging, 1 is retreating to hole when scared and taking more than 5 s to reemerge, weakly or tentatively striking at food, 2 is shying to shelter of the reef when scared but quickly emerging, purposefully striking at food, and 3 is readily venturing away from the reef, exploring with no hiding and striking aggressively at food. At the end of the 3-min observation period, the fish was approached with a pencil, and the fish's reaction and latency to emerge from shelter was taken into account in the assessment of boldness.
Field Assessment of Mortality.
Newly settled fish larvae are vulnerable to an array of resident and transient predators. At Lizard Island, predators include the moonwrasse Thalassoma lunare, the dottyback Pseudochromis fuscus, and lizardfishes Synodus variegatus and S. dermatogenys (39, 40). These species can be seen striking at and occasionally capturing recently settled and juvenile reef fishes. To compare mortality rates among newly settled P. wardi, fish that had been exposed to each of the CO2 treatments were placed singly on the reefs described above between 1000 and 1100 hours, and their survival was monitored at 1600 hours the same day and then, 1200 and 1600 hours the following day. Approximately 30 fish from each treatment were placed on the reefs over 3 successive d. For the 700-ppm treatment, 30 fish that were attracted to the predator odor in the flume and 30 fish that avoided the predator odor in the flume were used. To confirm that larvae could survive on the patch reefs and that any loss observed was most likely because of predation, another sample of fish (n = 24) was placed on reefs protected by fine-mesh cages.
Analysis.
First, log-linear models were used to determine if attraction or repulsion of clownfish to the predator odor in the flume was associated with CO2 treatment (four levels), ontogenetic stage (eight levels), or clutch (four levels). For this analysis, each fish was scored as being attracted to the predator odor if it spent >50% of its time in the stream with the predator odor and repelled from the predator odor if it spent <50% of its time in the stream with the predator odor. Starting from the saturated model, log-linear models were then compared in decreasing order of complexity until removal of further terms resulted in a significant increase in deviance from one model to the next. Clutch had no effect on larval preference or avoidance of the predator cues (Results), and therefore, clutch was pooled in all further analysis. Kolmogorov–Smirnov tests were then used to compare the proportion of time that individuals spent in the stream of water containing the predator cue in any given set of trials with the proportion of time that individuals spent on one side of the chamber when there was no cue presented in either water stream (i.e., a null distribution). A separate test was conducted for each CO2 concentration at each ontogenetic stage. Kolmogorov–Smirnov tests were also used to compare the proportion of time that individuals spent in the stream of water containing the predator cue between CO2 treatments. Although larvae were reared in small groups, they were considered as independent replicates. It was not possible to rear fish larvae individually, and survival is enhanced in small groups. Furthermore, there were no tank effects that might bias the results, because clutches were blocked in rows of rearing tanks and the initial log-linear analysis showed that clutch identity did not affect larval preference or avoidance of the predator cue.
For the damselfish larvae, log-linear analysis was used to compare preference or avoidance of the predator cue in the flume, as described above for the clownfish. Paired t tests were used to compare behavioral attributes of size-matched pairs of fish placed on reefs. Finally, a Pearson's χ2 test was used to compare the frequency of mortality in single fish from the different CO2 treatments placed on patch reefs.
Supplementary Material
Acknowledgments
We thank Geoff Endo for technical assistance, the staff at the James Cook University Marine Research Aquarium Facility and Lizard Island Research Station for logistical support, and Geoff Jones and Terry Hughes for helpful comments on the manuscript. This project was supported by funding from the Australian Research Council (P.L.M. and M.I.M.), the Australian Research Council Centre of Excellence for Coral Reef Studies (P.L.M. and M.I.M.), and the Great Barrier Reef Marine Park Authority (D.L.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004519107/-/DCSupplemental.
References
- 1.Raven J, et al. Ocean Acidification due to Increasing Atmospheric Carbon Dioxide. London: The Royal Society; 2005. [Google Scholar]
- 2.Hoegh-Guldberg O, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 3.Raupach MR, et al. Global and regional drivers of accelerating CO2 emissions. Proc Natl Acad Sci USA. 2007;104:10288–10293. doi: 10.1073/pnas.0700609104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meehl GA, et al. In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group 1 to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon SD, et al., editors. Cambridge, UK: Cambridge University Press; 2007. pp. 747–845. [Google Scholar]
- 5.Caldiera K, Wickett ME. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J Geophys Res. 2005 10.1029/2004JC002671. [Google Scholar]
- 6.Fabry VJ, Seibel BA, Feely RA, Orr JC. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci. 2008;65:414–432. [Google Scholar]
- 7.Orr JC, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437:681–686. doi: 10.1038/nature04095. [DOI] [PubMed] [Google Scholar]
- 8.Kleypas JA, Yates KK. Coral reefs and ocean acidification. Oceanography (Wash DC) 2009;22:108–117. [Google Scholar]
- 9.Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: The other CO2 problem. Annu Rev Marine Sci. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- 10.Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci USA. 2008;105:17442–17446. doi: 10.1073/pnas.0804478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall-Spencer JM, et al. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature. 2008;454:96–99. doi: 10.1038/nature07051. [DOI] [PubMed] [Google Scholar]
- 12.Moy AD, Howard WR, Bray SG, Trull TW. Reduced calcification in modern Southern Ocean planktonic foraminifera. Nature Geosci. 2009;2:276–280. [Google Scholar]
- 13.Ishimatsu A, Hayashi M, Kikkawa T. Fishes in high-CO2, acidified oceans. Mar Ecol Prog Ser. 2008;373:295–302. [Google Scholar]
- 14.Kurihara H. Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar Ecol Prog Ser. 2008;373:275–284. [Google Scholar]
- 15.Havenhand JN, Buttler FR, Thorndyke MC, Williamson JE. Near-future levels of ocean acidification reduce fertilization success in a sea urchin. Curr Biol. 2008;18:R651–R652. doi: 10.1016/j.cub.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Ellis RP, Bersey J, Rundle SD, Hall-Spencer JM, Spicer JI. Subtle but significant effects of CO2 acidified seawater on embryos of the intertidal snail, Littorina obtusata. Aquat Biol. 2009;5:41–48. [Google Scholar]
- 17.Rosa R, Seibel BA. Synergistic effects of climate-related variables suggest future physiological impairment in a top oceanic predator. Proc Natl Acad Sci USA. 2008;105:20776–20780. doi: 10.1073/pnas.0806886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munday PL, Crawley NE, Nilsson GE. Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes. Mar Ecol Prog Ser. 2009;388:235–242. [Google Scholar]
- 19.Munday PL, et al. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc Natl Acad Sci USA. 2009;106:1848–1852. doi: 10.1073/pnas.0809996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caley MJ, et al. Recruitment and the local dynamics of open marine populations. Annu Rev Ecol Syst. 1996;27:477–500. [Google Scholar]
- 21.Hamilton SL, Regetz J, Warner RR. Postsettlement survival linked to larval life in a marine fish. Proc Natl Acad Sci USA. 2008;105:1561–1566. doi: 10.1073/pnas.0707676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormick MI. Behaviourally mediated phenotypic selection in a disturbed coral reef environment. PLoS One. 2009;4:e7096. doi: 10.1371/journal.pone.0007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wisenden BD. Olfactory assessment of predation risk in the aquatic environment. Philos Trans R Soc Lond B Biol Sci. 2000;355:1205–1208. doi: 10.1098/rstb.2000.0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hay ME. Marine chemical ecology: Chemical signals and cues structure marine populations, communities, and ecosystems. Annu Rev Marine Sci. 2009;1:193–212. doi: 10.1146/annurev.marine.010908.163708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kingsford MJ, et al. Sensory environments, larval abilities and local self-recruitment. Bull Mar Sci. 2002;70:309–340. [Google Scholar]
- 26.Brown GE. Learning about danger: Chemical alarm cues and local risk assessment in prey fishes. Fish Fish. 2003;4:227–234. [Google Scholar]
- 27.Gerlach G, Atema J, Kingsford MJ, Black KP, Miller-Sims V. Smelling home can prevent dispersal of reef fish larvae. Proc Natl Acad Sci USA. 2007;104:858–863. doi: 10.1073/pnas.0606777104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dixson DL, Munday PL, Jones GP. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol Lett. 2010;13:68–75. doi: 10.1111/j.1461-0248.2009.01400.x. [DOI] [PubMed] [Google Scholar]
- 29.Fagan KE, Mackenzie FT. Air-sea CO2 exchange in a subtropical estuarine-coral reef system, Kaneohe Bay, Oahu, Hawaii. Mar Chem. 2007;106:174–191. [Google Scholar]
- 30.Wang W, Bradley SR, Richerson GB. Quantification of the response of rat medullary raphe neurones to independent changes in pH(o) and P(CO2) J Physiol. 2002;540:951–970. doi: 10.1113/jphysiol.2001.013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pörtner HO, Langenbuch M, Michaelidis B. Synergistic effects of temperature extremes, hypoxia, and increases in CO2 on marine animals: From Earth history to global change. J Geophys Res. 2005 10.1029/2004JC002561. [Google Scholar]
- 32.Hughes TP, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- 33.Bradshaw WE, Holzapfel CM. Climate change. Evolutionary response to rapid climate change. Science. 2006;312:1477–1478. doi: 10.1126/science.1127000. [DOI] [PubMed] [Google Scholar]
- 34.Skelly DK, et al. Evolutionary responses to climate change. Conserv Biol. 2007;21:1353–1355. doi: 10.1111/j.1523-1739.2007.00764.x. [DOI] [PubMed] [Google Scholar]
- 35.Almany GR, Webster MS. The predation gauntlet: Early post-settlement mortality in reef fishes. Coral Reefs. 2006;25:19–22. [Google Scholar]
- 36.Munday PL, Donelson JM, Dixson DL, Endo GGK. Effects of ocean acidification on the early life history of a tropical marine fish. Proc R Soc Lond B Biol Sci. 2009;276:3275–3283. doi: 10.1098/rspb.2009.0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hari P, et al. High-frequency measurements of productivity of planktonic algae using rugged nondispersive infrared carbon dioxide probes. Limnol Oceanogr Methods. 2008;6:347–354. [Google Scholar]
- 38.Meekan MG, Wilson SG, Halford A, Retzel A. A comparison of catches of fishes and invertebrates by two light trap designs, in tropical NW Australia. Mar Biol. 2001;139:373–381. [Google Scholar]
- 39.Beukers JS, Jones GP. Habitat complexity modifies the impact of piscivores on a coral reef fish population. Oecologia. 1998;114:50–59. doi: 10.1007/s004420050419. [DOI] [PubMed] [Google Scholar]
- 40.Holmes TH, McCormick MI. Influence of prey body characteristics and performance on predator selection. Oecologia. 2009;159:401–413. doi: 10.1007/s00442-008-1220-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.