Abstract
Activation of the B-cell antigen receptor (BCR) signaling pathway contributes to the initiation and maintenance of B-cell malignancies and autoimmune diseases. The Bruton tyrosine kinase (Btk) is specifically required for BCR signaling as demonstrated by human and mouse mutations that disrupt Btk function and prevent B-cell maturation at steps that require a functional BCR pathway. Herein we describe a selective and irreversible Btk inhibitor, PCI-32765, that is currently under clinical development in patients with B-cell non-Hodgkin lymphoma. We have used this inhibitor to investigate the biologic effects of Btk inhibition on mature B-cell function and the progression of B cell-associated diseases in vivo. PCI-32765 blocked BCR signaling in human peripheral B cells at concentrations that did not affect T cell receptor signaling. In mice with collagen-induced arthritis, orally administered PCI-32765 reduced the level of circulating autoantibodies and completely suppressed disease. PCI-32765 also inhibited autoantibody production and the development of kidney disease in the MRL-Fas(lpr) lupus model. Occupancy of the Btk active site by PCI-32765 was monitored in vitro and in vivo using a fluorescent affinity probe for Btk. Active site occupancy of Btk was tightly correlated with the blockade of BCR signaling and in vivo efficacy. Finally, PCI-32765 induced objective clinical responses in dogs with spontaneous B-cell non-Hodgkin lymphoma. These findings support Btk inhibition as a therapeutic approach for the treatment of human diseases associated with activation of the BCR pathway.
Keywords: lymphoma, X-linked agammaglobulinemia
Bruton tyrosine kinase (Btk) is a Tec family kinase with a well-defined role in B-cell antigen receptor (BCR) signaling (1, 2). Btk is activated by the upstream Src-family kinases Blk, Lyn, and Fyn (3, 4), and Btk in turn phosphorylates and activates phospholipase-Cγ (PLCγ) (5), leading to Ca2+ mobilization and activation of NF-κB and MAP kinase pathways. Btk mutations in humans cause the inherited disease X-linked agammaglobulinemia, characterized by a lack of peripheral B cells and low levels of serum Ig (6). In the mouse, point mutation or deletion of btk causes X-linked immunodeficiency (Xid), with approximately 50% fewer conventional B2 B cells, absent B1 B cells, and reduced serum Ig levels (7, 8). In transgenic mice in which Btk is expressed at approximately 25% of WT levels, development of conventional (i.e., B2) B cells is fully restored, but mature B cells are still deficient in responding to BCR stimulation. Thus, mature B cells may be particularly dependent on Btk for activation (9). Although Btk is also expressed in the myeloid lineage and there is some evidence that it contributes to other signaling pathways, the primary deficit in X-linked agammaglobulinemia is B cell-specific (8). Genetic ablation studies in the mouse of many other BCR-pathway kinases other than Btk have highlighted complex redundancies as well as pleiotropic effects on cell types other than B cells (10, 11); thus, Btk is a uniquely attractive kinase target for selective B-cell inhibition.
Clinical studies using the anti-CD20 antibody rituximab to deplete mature B cells have provided evidence for the role of B cells in the pathogenesis of rheumatoid arthritis (12), systemic lupus erythematosus (13), and multiple sclerosis (14). In addition, several lines of evidence suggest that the BCR pathway may provide a survival signal in tumor cells in non-Hodgkin lymphoma (NHL) (15, 16). In an unbiased screen, Btk was recently identified as an essential signaling kinase for survival of a subtype of diffuse large B-cell lymphoma (16). Thus, small molecule Btk inhibitors may provide therapeutic benefit in the treatment of lymphoma and autoimmune diseases.
Here we describe a potent irreversibly acting small molecule inhibitor of Btk, PCI-32765, that has demonstrated promising clinical activity in an ongoing phase I study in patients with B-cell NHL. We show that PCI-32765 inhibits BCR signaling downstream of Btk, selectively blocks B-cell activation, and is efficacious in animal models of arthritis, lupus, and B-cell lymphoma.
Results
PCI-32765 Is a Potent and Selective Inhibitor of Btk.
We have previously described the synthesis of a series of Btk inhibitors that bind covalently to a cysteine residue (Cys-481) in the active site leading to potent and irreversible inhibition of Btk enzymatic activity (17). One of these compounds, PCI-32765 (Fig. 1), was selected for the present studies because of its potency (IC50, 0.5 nM) and selectivity for Btk against a screening panel of kinase enzymes (Table S1). As only a small subset of kinases is predicted to contain a modifiable cysteine residue homologous to Cys-481 in Btk (17), we decided to explore the selectivity of PCI-32765 as an irreversible kinase inhibitor in cells. To this end, we created a unique cell permeable, fluorescently tagged derivative, PCI-33380 by attaching a Bodipy-FL fluorophore to PCI-32765 via a piperazine linker (Fig. 1). In cells transfected with Btk, PCI-33380 bound to Btk could be detected by denaturing gel electrophoresis and fluorescent gel scanning. As expected, Btk lacking Cys-481 (C481A) was not bound by PCI-33380. In addition PCI-33380 bound to a catalytically inactive mutant of Btk (K430A), suggesting that binding does not require catalytic activity (Fig. 2A). Identification of proteins capable of binding PCI-33380 was then evaluated in the B-cell lymphoma cell line DOHH2, a cell line that endogenously expresses the kinases that constitute the BCR signaling pathway, along with many other potentially reactive kinase and nonkinase proteins. Irreversibly bound proteins in the lysate were resolved by denaturing gel electrophoresis. Remarkably, PCI-33380 labeled a single predominant band; Western blotting of the gel indicated that the band labeled by PCI-33380 was likely to be Btk (Fig. 2B). To confirm that Btk was indeed the predominant band labeled by PCI-33380 in cells, Btk was immunoprecipitated from PCI-33380–labeled DOHH2 lysates. Btk immunoprecipitation captured PCI-33380–labeled protein and depleted the corresponding band from the lysate (Fig. 2C). Next, PCI-33380 labeling in DOHH2 cells was compared with PCI-33380 labeling in Jurkat, a T cell line that expresses several Tec family members but does not express Btk. As expected, the predominant labeled band (i.e., Btk) was found to be present in DOHH2, but not in Jurkat (Fig. 2D). Finally, pretreatment of cells with PCI-32765 completely prevented labeling of this single predominant band (Fig. 2 D and E). Taken together, these data indicate that both PCI-33380 and PCI-32765 are remarkably selective for irreversible binding to Btk in the context of a complex proteome.
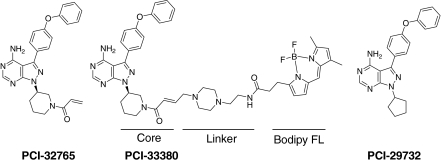
Fig. 1.
Chemical structure of Btk inhibitors. Chemical structures of the irreversible Btk inhibitor PCI-32765, the irreversible Btk inhibitor PCI-33380 (probe), and the reversible Btk inhibitor PCI-29732.
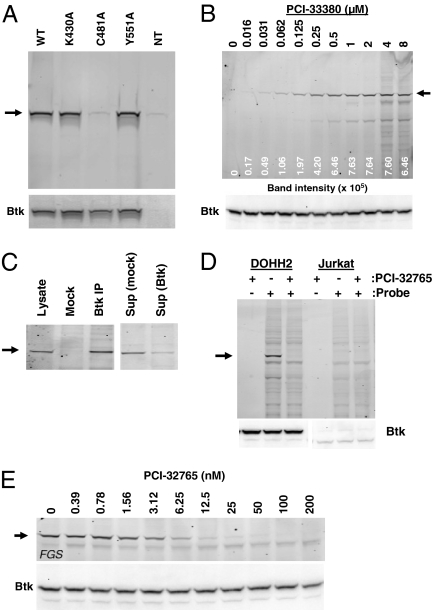
Fig. 2.
Selective irreversible targeting of Btk. (A–E) (Upper) Fluorescent gel scans of lysates from cells that were incubated with the affinity probe PCI-33380. Arrows indicate the predominant band labeled by the probe (approximately 76 kDa, the expected MW of Btk) and this band was confirmed to align with Btk as detected by Western blot of the same gel (Lower). (A) 293H cells transfected with WT Btk, K430A, C481A, or Y551A Btk mutants. Nontransfected (NT) 293H cells were included as a negative control. (B) DOHH2 cells incubated with increasing concentrations of PCI-33380 for 1 h. Densitometry values for the band corresponding to Btk are shown below the gel. (C) Btk immunoprecipitated (IP) from affinity probe labeled DOHH2 lysates. Total lysate (10 μg), and mock IP or Btk IP (from 50 μg of total lysate) were analyzed. A fluorescent gel scan of the immunodepleted supernatants (Sup) is also shown. (D) Affinity probe labeling of DOHH2 or Jurkat cells with and without 1 μM PCI-32765 pretreatment. (E) DOHH2 cells incubated with increasing concentrations of PCI-32765 for 1 h before labeling with affinity probe. By densitometry, the IC50 for active site occupancy by PCI-32765 is 4 nM.
PCI-32765 Selectively Inhibits B-cell Signaling and Activation.
PCI-32765 was examined in a series of cellular signaling assays. First, in DOHH2, a cell line in which the BCR pathway can be activated by stimulation with anti-IgG, we confirmed that PCI-32765 inhibits autophosphorylation of Btk (IC50, 11 nM), phosphorylation of Btk's physiological substrate PLCγ (IC50, 29 nM), and phosphorylation of a further downstream kinase, ERK (IC50, 13 nM; Fig. 3A). In this experiment, PCI-32765 was washed out before stimulation, so the results are consistent with irreversible inhibition of these phosphorylation events. As expected, phosphorylation of Syk, which functions upstream or in parallel to Btk, was not affected. To model therapeutic applications, we evaluated PCI-32765 in primary cultures of human peripheral B cells. To distinguish reversible from irreversible effects of PCI-32765 we directly compared continuous versus pulse exposure. We also included a structurally similar but reversibly acting Btk inhibitor PCI-29732 (17, 18) (IC50, 0.3 nM; Fig. 1) as a control. In human CD20+ B cells stimulated at the BCR, both PCI-32765 and PCI-29732 blocked the transcriptional up-regulation of a panel of B-cell activation genes that occurs within 6 h of stimulation (Fig. 3B). A 1-h pulse exposure of PCI-32765 followed by washout was sufficient to prevent up-regulation of these genes, indicating that PCI-32765 acts as an irreversible inhibitor of the BCR pathway. In contrast, pulse exposure to the reversible inhibitor PCI-29732 did not result in BCR inhibition. As further confirmation that PCI-32765 inhibits BCR signaling, we found that continuous exposure to 10 nM PCI-32765 for 18 h completely prevented up-regulation of the B-cell activation marker CD69 (Fig. 3C). A 1-h pulse exposure to 10 nM PCI-32765 resulted in a similar level of CD69 inhibition in B cells, consistent with the effect being primarily caused by an irreversible inhibition of Btk. By using the fluorescently tagged derivative PCI-33380, we found that 10 nM of PCI-32765 was sufficient to fully occupy the active site of Btk in primary B cells in culture. Thus, the concentration of PCI-32765 required to covalently bind Btk was well correlated with the concentration required to inhibit B-cell activation (Fig. 3D). In primary cultures of T cells (which do not express Btk), up-regulation of CD69 induced by T cell receptor stimulus was blocked only by continuous exposure to PCI-32765 at 10 μM (Fig. 3C). These results indicate that PCI-32765 is more than 1,000-fold selective for inhibition of antigen receptor signaling in B cells over T cells, and that only B-cell inhibition is sustained following short duration treatment. It is noteworthy that the mean terminal plasma half-life of PCI-32765 following oral dosing in mice is 1.7 to 3.1 h (Fig. S1). By combining fast irreversible binding to Btk with rapid in vivo elimination, PCI-32765 defines a unique approach to improve selectivity for Btk in vivo relative to reversibly inhibited off-target kinases.
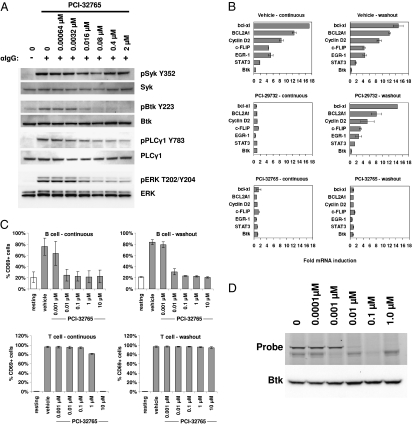
Fig. 3.
Inhibition of B-cell receptor signaling. (A) Concentration-dependent inhibition of BCR stimulation-induced phosphorylation events in DOHH2 cells. Cells were exposed to PCI-32765 and then drug was washed out before stimulation with anti-IgG. Blots were probed with the indicated antibodies. (B) Comparison of the reversible Btk inhibitor PCI-29732 to the irreversible Btk inhibitor PCI-32765 on the transcriptional response of six genes induced by BCR stimulation. Purified human peripheral B cells were treated with vehicle or 1 μM inhibitor for 1 h and then stimulated for 6 h with anti-IgM. In the washout condition, inhibitor-containing cell media was replaced with fresh media before addition of anti-IgM. Gene expression levels (mean ± SD) were measured by TaqMan RT-PCR and normalized to unstimulated cells. Btk expression is not affected by BCR stimulation or drug treatment. (C) Concentration-dependent inhibition of antigen receptor stimulation induced cell surface expression of the lymphocyte activation marker CD69 (mean ± SD). Purified human B or T cells were treated with PCI-32765 for 1 h and then stimulated for 18 h. In the washout condition, inhibitor-containing cell media was replaced with fresh media before stimulation. (D) Concentration-dependent covalent binding of PCI-32765 to Btk in purified human B cells as measured by the ability of Btk to bind to the fluorescent affinity probe PCI-33380. Total Btk levels are measured by Western blot (Bottom).
Btk Inhibition by PCI-32765 Is Efficacious in Mouse Models of Autoimmune Disease.
To test the effects of PCI-32765 in vivo, we focused initially on two autoimmune models in which Btk or B-cell function has been previously implicated. xid mice have been shown to be resistant to the induction of collagen-induced arthritis and to partially suppress disease in the MRL-Fas(lpr) lupus model (19, 20). To evaluate the consequences of inhibiting Btk activity during the establishment of arthritis, arthritic DBA/1 mice were assigned to treatment groups when their disease had partially progressed as measured by a mean clinical arthritis score between 1.0 and 1.5. PCI-32765 was administered orally for 11 consecutive d at dosages of 3.125, 12.5, or 50 mg/kg per day and clinical arthritis scores, reflecting paw swelling and joint inflammation, were measured daily. As shown in Fig. 4A, marked inhibition of clinical arthritis scores was seen in mice treated at all doses. A partial and a nearly complete elimination of clinical signs of disease occurred after 9 to 11 d of treatment at dosages of 3.125 and 12.5 mg/kg per day, respectively. Consistent with in vivo inhibition of B-cell activation, there was a significant reduction in the production of anticollagen autoantibodies, and a modest reduction of total IgG levels (Fig. 4 B and C). A oral single dose of PCI-32765 at 3.125 mg/kg per day resulted in partial Btk occupancy in splenocytes, and the maximally efficacious dose (12.5 mg/kg per day) was sufficient to fully occupy Btk for 12 h (Fig. 4D). Thus, the level of Btk inhibition in vivo by PCI-32765 is well correlated with the degree of efficacy in the collagen-induced arthritis model.
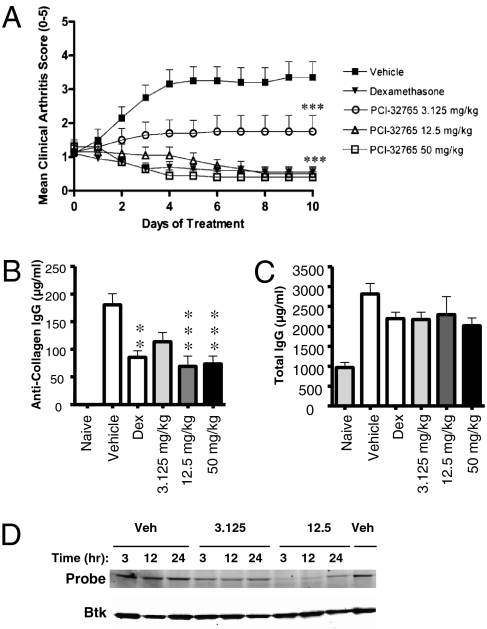
Fig. 4.
Btk inhibition by PCI-32765 inhibits collagen-induced arthritis in mice. (A) Mean clinical arthritis scores ± SEM (n = 5) from daily oral treatment for 11 d with different doses of PCI-32765 or dexamethasone as indicated. Disease control mice received vehicle only. Mean clinical scores from dexamethasone or PCI-32765 treatments at 3.125, 12.5, and 50 mg/kg were significantly different when compared with vehicle treatment (***P < 0.001, repeated-measures ANOVA). (B and C) Antibody levels from experiment shown in A. Data are mean ± SEM (**P < 0.01 and ***P < 0.001, ANOVA). (D) PCI-32765 occupancy of splenocyte Btk following a single oral dose in DBA/1 mice. Binding of PCI-32765 to Btk prevents binding of PCI-33380, detected by fluorescent gel scanning of PCI-33380-labeled lysates (Upper). Subsequent Western blotting confirms the presence of Btk (Lower).
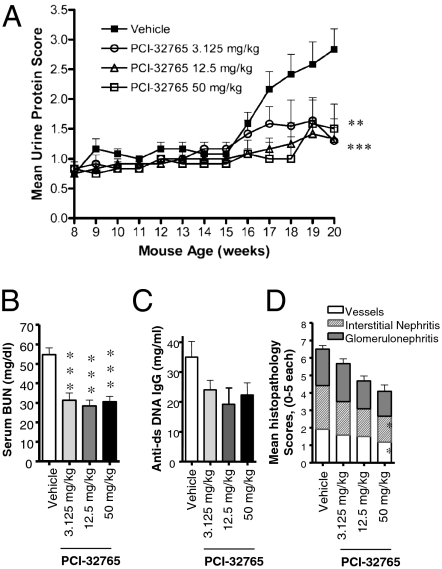
We next tested PCI-32765 in the MRL-Fas(lpr) lupus model, in which a mutation in the Fas receptor leads to survival of autoreactive cells, production of autoantibodies and progressive glomerulonephritis. Eight-week-old MRL-Fas(lpr) mice were treated for 12 wk with daily oral doses of PCI-32765. Treatment with PCI-32765 reduced proteinuria, a measure of glomerular dysfunction, and reduced blood urea nitrogen (BUN), a general measure of renal impairment (Fig. 5 A and B). Consistent with inhibition of B-cell activation, serum anti-dsDNA levels were reduced at all dose levels compared with vehicle-treated controls (Fig. 5C). Histopathological evaluation of the kidneys revealed a significant reduction in interstitial nephritis, reduced perivascular inflammation, and a nonsignificant trend toward reduction of glomerulonephritis at dosages of 12.5 and 50 mg/kg per day (Fig. 5D).
Fig. 5.
Inhibition of Btk reduces renal disease and autoantibody production in MRL-Fas(lpr) mice. (A) Eight-week-old MRL-Fas(lpr) mice (n = 12) were randomized and treated orally with PCI-32765 or vehicle once daily for 12 wk at different concentrations as indicated. Range of urine protein concentration is calculated as a proteinurea score. Proteinurea scores from PCI-32765 treatments were significantly lower than vehicle group (**P < 0.01 for 3.125 mg/kg and ***P < 0.001 for 12.5 and 50 mg/kg treatments; repeated-measure ANOVA). (B) Serum BUN in PCI-32765–treated and vehicle-treated mice (***P < 0.001). (C) dsDNA-specific IgG in PCI-32765 treated and vehicle treated mice. (D) Histopathology scores at week 20 from the animals shown in A. Scores are mean ± SEM (n = 12; *P < 0.05).
Btk inhibition by PCI-32765 Leads to Objective Clinical Responses in Spontaneous Canine B-cell Lymphomas.
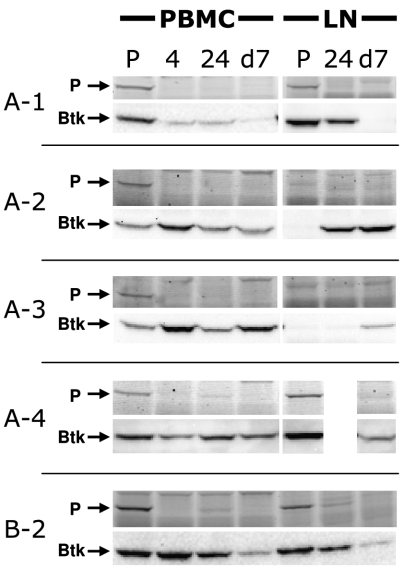
To determine if blocking BCR signaling by inhibiting Btk would affect the progression of lymphoma, we initiated a trial of PCI-32765 in naturally occurring B-cell NHL in companion dogs. Canine NHL shares many characteristics with human NHL, including diagnostic classifications and response to CHOP-based chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisone/prednisolone) (21). In the study, both treatment-naive and relapsed dogs were enrolled and PCI-32765 was dosed orally once per day using the capsule formulation prepared for human clinical trials. Inhibition of Btk was monitored in vivo by labeling peripheral blood mononuclear cell (PBMC) and tumor lysates ex vivo with PCI-33380 and labeled Btk was visualized by fluorescent gel scanning. In five dogs in which tissue samples were analyzed, a single administration of PCI-32765 at dosage levels ranging from 2.5 to 20 mg/kg per day was sufficient to fully occupy Btk in peripheral blood and tumor tissue for 24 h (Fig. 6). Total Btk levels varied significantly across samples, which may reflect heterogeneity in biopsy sampling as well as potential drug-induced changes in peripheral blood Btk expression levels. To date, eight dogs have been treated. We have observed three partial responses per Response Evaluation Criteria In Solid Tumors (RECIST), including one dog in which measurable tumor burden was reduced 77%, and three instances of stable disease (Table 1).
Fig. 6.
Orally-dosed PCI-32765 leads to sustained occupancy of Btk in dogs with lymphoma. PBMCs and biopsy specimens from affected lymph nodes (LN) were collected from dogs (Table 1) treated with PCI-32765 (oral capsule formulation). Tissue samples were then treated with PCI-33380 to determine Btk occupancy by PCI-32765. Shown are predose (P), 4 h, 24 h, and predose d 7 occupancy data for all available week 1 samples in the study. Arrow marked “P” indicates fluorescent probe (PCI-33380) signal, arrow marked “Btk” indicates Btk protein level by Western blot. Full occupancy was achieved in all dogs. Western blots indicate that some LN biopsies did not contain Btk, presumably as a result of sample collection heterogeneity.
Table 1.
Study summary of the effect of Btk inhibitor PCI-32765 in naturally occurring canine lymphomas
| Dog | Stage | Histology | PARR (monoclonal BCR) | Previous Treatment | Dose, mg/kg | Outcome (RECIST) | Progression free interval, d | Decrease in tumor sums, % |
| A1 | IIIa | NA | — | — | 20 | SD | 28 | 10 |
| A2 | Va | NA | + | COP | 20 | PD | 0 | — |
| A3 | IIIa | Follicular large cell | — | CHOP | 20 | SD | 14 | — |
| A4 | IIIa | Diffuse immunoblastic | + | COP | 20 | PR | 35 | 77 |
| B1 | IIIa | Diffuse immunoblastic | + | — | 2.5/5.0/7.5 | SD | 21 | — |
| B2 | IIIa | Follicular large cell | + | — | 2.5/5.0 | PR | 70 | 31 |
| B3 | IIIa | Diffuse immunoblastic | + | — | 2.5 | PD | 0 | — |
| B4 | Va | Follicular large cell | + | — | 2.5/5.0 | PR | 63 | 62 |
CR, complete response; NA, not applicable; PARR, PCR of antigen receptor rearrangement; PD, progressive disease; PR, partial response; SD, stable disease.
Discussion
We have described a selective and irreversible Btk inhibitor and its efficacy in models of autoimmune disease and spontaneous B-cell lymphoma. The use of irreversible inhibitors has previously been shown to be a viable method to achieve potent and selective inhibition of kinase enzymes (22, 23). We previously reported the discovery and characterization of a series of Btk-selective irreversible inhibitors that bind covalently to a noncatalytic Cys (Cys-481) residue in Btk (17). Structural alignments revealed that only 10 kinases have a Cys at this position (17), representing a significant selectivity filter. Indeed, a fluorescently tagged derivative of PCI-32765, PCI-33380, was shown to bind predominantly to a single protein, Btk, in B-cell lysates. Cellular selectivity of PCI-32765 was also demonstrated by the observation that PCI-32765 inhibits antigen receptor signaling in B cells but not in T cells.
We used the fluorescently tagged affinity probe PCI-33380 as a tool to directly monitor the degree of Btk occupancy by PCI-32765 in cells or target tissues. Validation of this probe in dogs with spontaneous NHL and the agreement between Btk occupancy in PBMC and tumor biopsies supports the current use of PCI-33380 as a pharmacodynamic marker in PBMCs in ongoing human clinical trials. This approach to direct measurement of kinase occupancy in a clinical setting enables the determination of a minimum dosage level that leads to full inhibition of Btk in specific populations of patients with cancer.
B-cell activation is a complex process involving Btk-dependent signal transduction initiated by stimulation of the BCR. BCR stimulation recruits Btk to the cell membrane via interactions between the N-terminal PH domain and cell membrane phosphoinositides and membrane-associated Btk is then phosphorylated at Tyr-551 in the activation loop by Src family kinases (3). Subsequent Btk autophosphorylation at Tyr-223 stabilizes the active conformation and fully activates Btk kinase activity (18). Activated Btk phosphorylates PLCγ (5), initiating calcium mobilization and generating diacylglycerol as secondary signals, eventually leading to NF-κB transcriptional activation and amplification of BCR stimulation. PCI-32765 was shown to block signaling downstream of Btk, and completely and irreversibly inhibit B-cell activation as assayed by either gene expression or the cell surface marker CD69.
As an orally bioavailable, selective, and irreversible inhibitor, PCI-32765 is an appropriate tool to examine the effects of Btk inhibition in animal models of disease. The xid mutation in mice has been shown to suppress or partially suppresses disease in models of autoimmune disease (19, 20, 24), but these genetic experiments cannot distinguish Btk's developmental role from its role in mature B lymphocyte function in a disease caused by sustained generation of autoimmunity. PCI-32765 treatment led to a dose-dependent decrease in disease manifestation in both the collagen-induced arthritis and MRL-Fas(lpr) lupus models. The level of Btk inhibition in vivo by PCI-32765 was shown to correlate with the degree of efficacy in the collagen-induced arthritis model, consistent with Btk as the relevant target in vivo; however, a role for other cysteine-containing kinases that may also be inhibited by PCI-32765 (e.g., Blk, Bmx) cannot be ruled out. Inhibition of Btk in myeloid lineage cells such as mast cells and macrophages may also have some contribution to efficacy in these disease models, although in both models efficacy was associated with decreases in circulating autoantibody levels, consistent with a mechanism of action involving inhibition of B-cell activation in vivo.
Chronic activation of the BCR pathway has been demonstrated in some B-cell lymphomas, and this activation has been shown to be required for tumor cell survival (16, 25). Traditional human xenograft studies have limited relevance to the study of BCR dependent lymphoma growth in vivo as, in these systems, tumor growth is not thought to be driven by BCR signaling. To evaluate a general antitumor effect of PCI-32765 in a true B-cell lymphoma, we studied dogs with spontaneously occurring disease. In these dogs, treatment with PCI-32765 at well-tolerated doses led to complete active site occupancy of Btk in both peripheral blood and on tumor biopsies. Disease stabilization and objective clinical responses were observed in dogs diagnosed with rapidly progressive disease. Although it is not possible to assess the significance of disease stabilization in this study without a randomized controlled trial, the objective responses argue that PCI-32765 is an active drug in this setting, supporting a role for BTK in the maintenance of tumor growth.
PCI-32765 is currently undergoing human clinical development in patients with B-cell malignancies and has shown promising clinical activity. Here we have shown that this compound is a potent, selective, and irreversible Btk inhibitor that blocks signaling downstream of the BCR in mature human B cells, inhibits disease progression in a collagen-induced arthritis model, and can treat the progressive lupus-like autoimmunity of the MRL-Fas(lpr) model. The degree of active site occupancy of Btk by PCI-32765 was tightly correlated with the efficacy response, both in vitro and in vivo, indicating that the probe assay will have utility as a tool for assessing pharmacodynamics in human clinical trials. Potent and highly selective Btk inhibitors such as PCI-32765 represent a unique therapeutic approach for the treatment of diseases driven by inappropriate activation of the BCR pathway.
Materials and Methods
B and T Cells.
CD20+ B and CD3+ T cells were purified by negative selection (RosetteSep, >90% purity) from buffy coat PBMCs and viably frozen in 10% DMSO. Cells were thawed at 37 °C and maintained in growth media (RPMI media containing 10% FCS). B cells were stimulated with goat antihuman IgM F(ab′)2 (10 μg/mL; Invitrogen) and T cells were stimulated with anti-CD3/CD28 coated beads (Dynabeads) at a 1:1 bead/cell ratio. Cells were stained with PE-CD69 (BD Biosciences) and analyzed by flow cytometry, gating on viable lymphocytes. PCI-32765 at concentrations lower than 10 μM did not decrease B- or T-cell viability during the course of the experiment, although PCI-32765 did block the modest survival benefit of anti-IgM stimulation in B cells. For washout experiments, cells were rinsed three times in 10 volumes of growth media, a protocol that was confirmed to completely wash away inhibition of BCR signaling by PCI-29732, a reversible Btk inhibitor.
Real-Time RT-PCR.
B cells were stimulated as described earlier and mRNA prepared by RNeasy 96 (Qiagen). Real-time RT-PCR was performed using standard cycling conditions (ABI 7300) and predesigned assay reagents for the indicated genes (Applied Biosystems). Expression levels were normalized (in arbitrary units) to total RNA amount as determined by Ribogreen (Invitrogen).
Phospho-Blots.
DOHH2 cells were preincubated with compound for 1 h, washed three times in 10 volumes of PBS solution, and then stimulated with anti-IgG F(ab′)2 (30 μg/mL; Invitrogen) for 2 min. Cells were lysed in LDS sample buffer containing sample reducing agent (Invitrogen) and analyzed using phospho-specific antibodies Syk pY352 (cat. no. 2701; Cell Signaling), Btk pY223 (EP420Y; Epitomics), PLCγ1 pY783 (cat. no. 2821; Cell Signaling), and ERK1/2 pT202/pY204 (197G2; Cell Signaling); chemiluminescent detection (Pierce Dura) was then performed. Blots were then stripped and reprobed with antibodies Syk (cat. no. 2712; Cell Signaling), Btk (53/Btk; BD Biosciences), PLCγ1 (cat. no. 2822; Cell Signaling), and ERK1/2 (3A7; Cell Signaling) to detect total protein levels.
PCI-33380 Labeling.
For PCI-33380 labeling of human B cells, 16 cells were treated with PCI-33380 at 2 μM for 1 h, washed, lysed in LDS sample buffer containing sample reducing agent (Invitrogen), and analyzed by SDS/PAGE and fluorescent gel scanning using a Typhoon scanner (Ex, 532 nm; Em, 555 nm). The gel was then blotted and total Btk levels detected by standard Western blot. Btk occupancy in cellular assays was assessed by preincubation with PCI-32765 for 1 h before labeling with PCI-33380. For analysis of Btk occupancy in vivo following oral dosing of PCI-32765, spleens were processed to splenocytes followed by 5 min incubation in red blood cell lysing buffer (Sigma-Aldrich). Cells were then PCI-33380–labeled and lysates analyzed by fluorescent gel scanning as described earlier. For immunoprecipitation of Btk, DOHH2 cells were lysed and labeled with PCI-33380 (2 μM) before immunoprecipitation. Lysates were then incubated at room temperature for 1 h with Btk antibody (53/Btk; BD Biosciences) or mouse IgG2a isotype control antibody. Immune complexes were then incubated for 1 h at 4 °C with immobilized Protein A (Thermo Fisher Scientific). Immune complexes were washed with immunoprecipitation buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl) four times and eluted with LDS sample buffer and sample reducing agent (Invitrogen) and analyzed by Western blot as described earlier.
Arthritis and Lupus Models.
Male DBA/1 mice were immunized with type II collagen plus Freund adjuvant and boosted 21 d later. On a rolling basis, as significant swelling appeared in at least one paw, mice were enrolled and randomized. PCI-32765 or dexamethasone (0.2 mg/kg) were administered orally once per day for 11 d. Arthritis scores (0–5) were assigned to the mice based on the degree and extent of paw swelling. Mouse anti–type II collagen antibody and total IgG levels were measured by ELISA (Chondrex and Bethyl). Female MRL/MpJ-Faslpr mice (stock no. 000485; Jackson Labs) received PCI-32765 by oral gavage once per day from week 8 through week 20. Proteinuria was monitored weekly (Multistick; Clinitech). At week 20, serum was collected and analyzed for BUN (IDEXX) and mouse anti-dsDNA antibody levels (ELISA; Alpha Diagnostic). Kidney histology was scored according to established criteria (26). No drug-induced weight loss was observed at any of the dose levels tested. These studies were carried out at Boulder Biopath according to approved animal care protocols. Results are presented as the mean ± SEM. Statistical significance between groups were evaluated with repeated measures one-way ANOVA or one-way ANOVA using GraphPad Prism with Tukey or Bonferroni multicomparison posttest.
Spontaneous Canine Lymphoma.
Spontaneous canine lymphoma studies were conducted with approval from the Colorado State University Institutional Animal Care and Use Committee and the Colorado State University Veterinary Medical Teaching Hospital Clinical Review Board. Client-owned dogs presenting as patients to the Colorado State University Animal Cancer Center were enrolled with the following inclusion criteria: (i) confirmed histologic or cytologic diagnosis of B-cell lymphoma (immunohistochemistry or flow cytometry for CD21 and CD79a or PCR for monoclonal Ig gene rearrangement), (ii) adequate organ function as indicated by standard laboratory tests, and (iii) modified Eastern Cooperative Oncology Group performance status of 0 or 1 on d 0 (27). Exclusion criteria were (i) T cell or null-cell immunophenotype, (ii) chemotherapy within 3 wk, (iii) radiation therapy within 6 wk, and (iv) corticosteroids within 72 h. Signed informed consent was obtained from all owners before study entry. PCI-32765 was administered daily until disease progression with 40 mg and 200 mg hard gelatin capsules prepared using standard pharmaceutically acceptable excipients. Animals were rechecked weekly for 4 wk and then biweekly thereafter. Tumor burden was defined as the sum of the longest diameters of all target lesions. Response (complete response/partial response/stable disease/progressive disease) was evaluated according to Veterinary Cooperative Oncology Group criteria for assessment of response in peripheral nodal lymphoma in dogs, an adaptation of published RECIST criteria (28). Adverse events were recorded and prospectively graded according to the Veterinary Cooperative Oncology Group Common Terminology for Adverse Events, version 1.0 (27). For pharmacodynamic analysis, blood was collected in CPT tubes (BD Biosciences) and PBMCs purified using standard techniques. PBMC pellets were snap-frozen and stored at −80 °C. Tumor biopsies were stored at −80 °C and subsequently pulverized in PBS solution before analysis. PBMCs or tumor cells were lysed and 50 μg of soluble protein was labeled with PCI-33880 as described earlier.
Supplementary Material
Acknowledgments
We thank Patti Theimann, Purvi Jejurkar, and Nadia Moore (Pharmacyclics) for their technical assistance and Dr. Debra Kamstock for histopathologic review of the canine samples.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004594107/-/DCSupplemental.
References
- 1.Satterthwaite AB, Witte ON. The role of Bruton's tyrosine kinase in B-cell development and function: A genetic perspective. Immunol Rev. 2000;175:120–127. [PubMed] [Google Scholar]
- 2.Khan WN. Regulation of B lymphocyte development and activation by Bruton's tyrosine kinase. Immunol Res. 2001;23:147–156. doi: 10.1385/IR:23:2-3:147. [DOI] [PubMed] [Google Scholar]
- 3.Afar DE, et al. Regulation of Btk by Src family tyrosine kinases. Mol Cell Biol. 1996;16:3465–3471. doi: 10.1128/mcb.16.7.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng G, Ye ZS. Baltimore D binding of Bruton's tyrosine kinase to Fyn, Lyn, or Hck through a Src homology 3 domain-mediated interaction. Proc Natl Acad Sci USA. 1994;91:8152–8155. doi: 10.1073/pnas.91.17.8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphries LA, et al. Tec kinases mediate sustained calcium influx via site-specific tyrosine phosphorylation of the phospholipase Cgamma Src homology 2-Src homology 3 linker. J Biol Chem. 2004;279:37651–37661. doi: 10.1074/jbc.M311985200. [DOI] [PubMed] [Google Scholar]
- 6.Conley ME, et al. Primary B cell immunodeficiencies: Comparisons and contrasts. Annu Rev Immunol. 2009;27:199–227. doi: 10.1146/annurev.immunol.021908.132649. [DOI] [PubMed] [Google Scholar]
- 7.Tsukada S, Rawlings DJ, Witte ON. Role of Bruton's tyrosine kinase in immunodeficiency. Curr Opin Immunol. 1994;6:623–630. doi: 10.1016/0952-7915(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 8.Rawlings DJ, et al. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993;261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 9.Satterthwaite AB, Cheroutre H, Khan WN, Sideras P, Witte ON. Btk dosage determines sensitivity to B cell antigen receptor cross-linking. Proc Natl Acad Sci USA. 1997;94:13152–13157. doi: 10.1073/pnas.94.24.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Texido G, et al. The B-cell-specific Src-family kinase Blk is dispensable for B-cell development and activation. Mol Cell Biol. 2000;20:1227–1233. doi: 10.1128/mcb.20.4.1227-1233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowell CA. Src-family kinases: Rheostats of immune cell signaling. Mol Immunol. 2004;41:631–643. doi: 10.1016/j.molimm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Edwards JC, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 13.Favas C, Isenberg DA. B-cell-depletion therapy in SLE—what are the current prospects for its acceptance? Nat Rev Rheumatol. 2009;5:711–716. doi: 10.1038/nrrheum.2009.218. [DOI] [PubMed] [Google Scholar]
- 14.Hauser SL, et al. HERMES Trial Group B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 15.Küppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5:251–262. doi: 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- 16.Davis RE, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan Z, et al. Discovery of selective irreversible inhibitors for Bruton's tyrosine kinase. ChemMedChem. 2007;2:58–61. doi: 10.1002/cmdc.200600221. [DOI] [PubMed] [Google Scholar]
- 18.Marcotte DJ, et al. Structures of human Bruton's tyrosine kinase in active and inactive conformations suggest a mechanism of activation for TEC family kinases. Protein Sci. 2010;19:429–439. doi: 10.1002/pro.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansson L, Holmdahl R. Genes on the X chromosome affect development of collagen-induced arthritis in mice. Clin Exp Immunol. 1993;94:459–465. doi: 10.1111/j.1365-2249.1993.tb08218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180:1295–1306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breen M, Modiano JF. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans—man and his best friend share more than companionship. Chromosome Res. 2008;16:145–154. doi: 10.1007/s10577-007-1212-4. [DOI] [PubMed] [Google Scholar]
- 22.Fry DW, et al. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc Natl Acad Sci USA. 1998;95:12022–12027. doi: 10.1073/pnas.95.20.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen MS, Zhang C, Shokat KM, Taunton J. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science. 2005;308:1318–1321. doi: 10.1126/science1108367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanaba K, et al. B cell depletion delays collagen-induced arthritis in mice: Arthritis induction requires synergy between humoral and cell-mediated immunity. J Immunol. 2007;179:1369–1380. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood. 2008;111:2230–2237. doi: 10.1182/blood-2007-07-100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan O, Madaio MP, Shlomchik MJ. The roles of B cells in MRL/lpr murine lupus. Ann N Y Acad Sci. 1997;815:75–87. doi: 10.1111/j.1749-6632.1997.tb52046.x. [DOI] [PubMed] [Google Scholar]
- 27.Vail DM. Veterinary Co-operative Oncology Group - Common Terminology Criteria for Adverse Events following chemotherapy or biological antineoplastic therapy in dogs and cats. Vet Comp Oncol. 2004;2:194–213. doi: 10.1111/j.1476-5810.2004.0053b.x. [DOI] [PubMed] [Google Scholar]
- 28.Vail DM, Michels GM, Khanna C, Selting KA, London CA. Veterinary Cooperative Oncology Group Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0)—a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. 2010;8:28–37. doi: 10.1111/j.1476-5829.2009.00200.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.