Abstract
G protein-coupled receptor-regulated PI3Kγ is abundantly expressed in myeloid cells and has been implicated as a promising drug target to treat various inflammatory diseases. However, its role in bone homeostasis has not been investigated, despite the fact that osteoclasts are derived from myeloid lineage. We therefore carried out thorough bone phenotypic characterization of a PI3Kγ-deficient mouse line and found that PI3Kγ-deficient mice had high bone mass. Our analyses further revealed that PI3Kγ deficiency did not affect bone formation because no significant changes in osteoblast number and bone formation rate were observed. Instead, the lack of PI3Kγ was associated with decreased bone resorption, as evidenced by decreased osteoclast number in vivo and impaired osteoclast formation in vitro. The decreased osteoclast formation was accompanied by down-regulated expression of osteoclastogenic genes, compromised chemokine receptor signaling, and an increase in apoptosis during osteoclast differentiation. Together, these data suggest that PI3Kγ regulates bone homeostasis by modulating osteoclastogenesis. Our study also suggests that inhibition of PI3Kγ, which is being considered as a potential therapeutic strategy for treating chronic inflammatory disorders, may result in an increase in bone mass.
Keywords: chemokine, signaling, apoptosis
Phosphoinositide 3-kinases (PI3Ks) are a family of lipid kinases that phosphorylate phosphoinositides at the 3′-OH position of the inositol ring to produce phosphatidylinositol 3-phosphate [PtdIns(3)P], phosphatidylinositol (3,4)-bisphosphate [PtdIns(3,4)P2], and phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5)P3]. These lipids, particularly PtdIns(3,4,5)P3, play a role as second messengers in activating downstream signaling cascades by providing a docking site for proteins with lipid-binding domains, such as protein kinase B (also known as Akt). Among three subfamilies (class I, II, and III), class I PI3Ks have been most extensively documented for their critical roles in a variety of cellular processes, including proliferation, growth, survival, differentiation, migration, and metabolism (1).
Class I PI3Ks are further divided into class IA and IB. Class IA PI3K catalytic subunits (p110α, p110β, and p110δ) form a heterodimeric complex with one of the regulatory subunits (p85α, p55α, p50α, p85β, and p55γ). Src homology 2 (SH2) domains in the regulatory subunits bridge class IA PI3Ks to tyrosine kinase receptors and cytoplasmic tyrosine kinases. In contrast, PI3Kγ (p110γ), the sole member of class IB PI3K, interacts with p101 regulatory subunit and is a key signaling player downstream of seven transmembrane G protein-coupled receptors (GPCRs). Stimulation of GPCRs triggers interaction of PI3Kγ and Gβγ subunits of heterotrimeric G proteins (2, 3). PI3Kγ was found at high levels in hematopoietic cells and also detected in the heart, endothelium, and brain. Studies with PI3Kγ knockout (KO) mice and kinase-dead knock-in mice have substantially advanced our understanding of the role of PI3Kγ-mediated signaling in leukocytes and cardiomyocytes and suggested PI3Kγ as a promising drug target for treatment of inflammatory diseases and cardioprotective therapy.
Given the recent development of selective PI3Kγ inhibitors, of which effectiveness was verified in experimental animal models of diseases (4, 5), the question arises as to whether PI3Kγ inhibition affects bone homeostasis. Bone homeostasis is maintained via the balance between bone formation by osteoblasts and bone resorption by osteoclasts. Osteoclasts are multinucleated cells specialized in bone resorption. Osteoclast precursors differentiate from monocyte–macrophage lineage cells of hematopoietic origin and undergo cell fusion process to become multinucleated mature osteoclasts. Receptor activator of NF-κB (RANK) signaling triggered by RANK ligand (RANKL) activates the downstream signaling pathways, including NF-κB, Erk, JNK, and p38, to induce expression of osteoclastogenesis-related genes, such as NFATc1, tartrate-resistant acid phosphatase (TRAP), cathepsin K, carbonic anhydrase II, osteoclast-associated receptor (OSCAR), dendritic cell–specific transmembrane protein (DC-STAMP), and calcitonin receptor (6). Although RANK signaling is the primary mediator of osteoclastogenesis and essential for the maintenance of functional mature osteoclasts, proper osteoclastogenesis also requires cooperation of costimulatory signals from macrophage colony-stimulating factor (M-CSF) receptor and Ig-like receptors (7). Moreover, RANK signaling induces osteoclast precursors to produce proinflammatory cytokines and chemokines that are known to stimulate osteoclast differentiation. Furthermore, a number of chemokines, including SDF-1 and MCP-1, have been found to play a role in bone homeostasis by regulating osteoclastogenesis.
Given the therapeutic potential of PI3Kγ inhibition and the role of PI3Kγ in chemokine signal transduction, it is important to investigate whether PI3Kγ plays a significant role in bone homeostasis. By using dual-energy x-ray absorptiometry (DXA), microcomputed tomography (μCT), and static and dynamic bone histomorphometry, we found that genetic ablation of PI3Kγ elevated bone mass. Our further analyses indicates that this increased bone mass was attributable to reduced bone resorption as the result of impaired osteoclast development.
Results
PI3Kγ-Deficient Mice Have Increased Bone Mass.
PI3Kγ KO mice are fertile and had normal skeletal morphology upon gross inspection. As an initial means to determine whether genetic absence of PI3Kγ has an impact on bone, a set of right femurs from 3-mo-old male PI3Kγ KO and WT littermate mice were subjected to DXA analysis. The lengths of these femurs were comparable between PI3Kγ KO and WT (Fig. S1). DXA analyses of these femoral bones revealed that PI3Kγ deficiency increased areal bone mineral density by 11% for whole femur (56.3 ± 1.8 mg/cm2 vs. 62.6 ± 1.5 mg/cm2, WT vs. KO; n = 12; P < 0.02) and by 10% for epiphysis/metaphysic area (63.7 ± 2 mg/cm2 vs. 70.2 ± 1.6 mg/cm2, WT vs. KO; n = 12; P < 0.03).
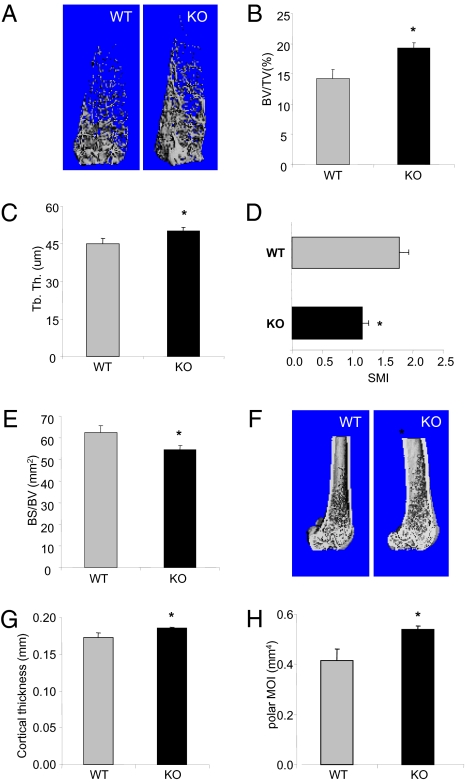
The same sets of femurs were subjected to μCT scans. Analyses of distal femoral metaphysis revealed that bone volume was significantly increased in cancellous bones (Fig. 1 A and B). PI3Kγ deficiency increased trabecular thickness by 11% compared with the WT controls (Fig. 1C). The loss of PI3Kγ also affected the trabecular bone architecture. Trabecular bones in PI3Kγ KO were denser and more “plate-like”, as revealed by the structure model index (8) (Fig. 1D). The reduced ratio of bone surface to bone volume further indicated that PI3Kγ-null bones have denser cancellous bone structures (Fig. 1E). The analysis of the middiaphysis showed that the PI3Kγ deficiency also affected cortical bones by increasing cortical thickness by 7% and bone rigidity (measured as polar moment of inertia) by 30% compared with the WT controls (Fig. 1 G and H). These results together indicated that genetic inactivation of PI3Kγ resulted in an augment in bone mass and stability.
Fig. 1.
PI3Kγ KO mice show increased bone mass and rigidity. (A–E) μCT analysis of cancellous bone in the distal metaphysic of femurs. (A) 3D μCT reconstruction of representative femurs from PI3Kγ-null and WT mice (4 mo old, male). Trabecular bone parameters shown are (B) bone volume fraction (BV/TV, %), (C) trabecular thickness (μm), (D) structural model index (SMI, quantifies the characteristic form of the cancellous bone in terms of plate-like to rod-like; for an ideal plate and rod structure, the SMI value is 0 and 3, respectively), and (E) bone surface/bone volume (BS/BV). (F–H) μCT analyses of cortical bone in the middiaphysis of the femurs. (F) Representative images of 3-D μCT, (G) cortical thickness, and (H) polar moment of inertia (pMOI). Data are presented as means ± SEM (n = 12). *P < 0.05 vs. WT.
PI3Kγ Deficiency Does Not Affect Bone Formation.
Consistent with the observation from the DXA and μCT analyses, quantitative histomorphometry also showed that PI3Kγ-null bone samples had greater bone volume fraction than the WT controls (Fig. 2 A and B). This apparent increase in bone mass associated with PI3Kγ deficiency could be due to either increased bone formation or reduced bone resorption. To evaluate the effect of PI3Kγ deficiency on bone formation, the number and activity of osteoblasts were examined. Osteoblast numbers on trabecular bone surface were comparable between PI3Kγ KO and WT (Fig. 2C). Dynamic histomorphometry of calcein double-labeled bone sections revealed that bone formation parameters (mineral apposition rate, mineralizing surface, and bone formation rate) were not significantly affected by PI3Kγ deficiency (Fig. 2 D–G). These results were supported by the equivalent level of serum osteocalcin, a bone formation marker, between the two genotype groups (Fig. 2H). Moreover, examination of mineralization in the bone marrow stromal cell-derived osteoblast culture showed no significant difference between WT and KO (Fig. S2), suggesting that PI3Kγ may not affect differentiation and function of osteoblasts in vitro. Therefore, the bone phenotype seen in the PI3Kγ KO mice is unlikely to be caused by an effect of PI3Kγ deficiency on bone formation.
Fig. 2.
Deletion of PI3Kγ has no impact on bone formation. (A–C) Static histomorphometry analysis of cancellous bone in metaphysis of tibiae from PI3Kγ WT and KO mice (4 mo old, male). (A) Representative images of toluidine blue–stained sections of tibiae from PI3Kγ WT and KO mice. (B) Bone volume fraction (BV/TV, %). (C) Number of osteoblasts per bone perimeter (N.Ob/B.Pm.). (D–G) Dynamic histomorphometry of tibiae from PI3Kγ WT and KO mice. (D) Representative epifluorescence images of endosteal surface of middiaphysis of tibiae. (E) Mineral apposition rate (MAR). (F) Mineralizing surface (MS/BS). (G) Bone formation rate (BFR/BS). (H) Osteocalcin level in serum was measured by RIA. Data are plotted as mean ± SEM (n = 12). *P < 0.05 vs. WT. NS, not statistically significant.
PI3Kγ Deficiency Reduces Osteoclast Number in Vivo and Impairs Osteoclast Formation in Vitro.
Knowing that osteoblast-mediated bone formation is unaffected by PI3Kγ deficiency, we next determined whether the increased bone mass in PI3Kγ-null mice was associated with impaired bone resorption. TRAP staining and histomorphometric analysis of sections of tibia bones revealed that there was substantial reduction in both osteoclast surface and osteoclast number in PI3Kγ-null bone samples in comparison with those of WT (Fig. 3 A–C).
Fig. 3.
Impaired osteoclast development in PI3Kγ-deficient bones. Histomorphometric analysis of TRAP-stained tibiae from PI3Kγ-null and WT mice (4 mo old, male). (A) Representative images of TRAP-stained tibia sections. (B) Osteoclast surface per bone surface (Oc.S/BS). (C) Number of osteoclasts per bone perimeter (N.Oc/B.Pm.). *P < 0.05 vs. WT. Data are shown as mean ± SEM, n = 12.
We next investigated the effect of PI3Kγ deficiency on osteoclastogenesis in vitro. Bone marrow monocytes/macrophages (BMMs) from WT and PI3Kγ-null mice were primed with M-CSF and RANKL to generate osteoclasts. After 6 d in culture, the number and size of TRAP-positive multinucleated cells (number of nuclei >3) were determined. PI3Kγ deficiency significantly impaired the formation of TRAP-positive multinucleated osteoclasts (Fig. 4A). The reduction occurred in both number and size (Fig. 4 B–D).
Fig. 4.
Loss of PI3Kγ impairs osteoclastogenesis in vitro. (A) TRAP-stained multinucleated osteoclasts generated from BMMs of PI3Kγ WT and KO mice (4 mo old, male). BMMs were cultured in the presence of M-CSF (25 ng/mL) and RANKL (100 ng/mL) for 6 d, with refreshing media every other day. Quantification of (B) total surface area occupied by multinucleated TRAP-positive osteoclasts, (C) cell number per well, and (D) mean osteoclast surface. The experiment was performed in triplicate, and representative data from three experiments are shown. Data are presented as mean ± SEM. *P < 0.05 vs. WT, n = 12.
To substantiate the effect of PI3Kγ deficiency on osteoclastogenesis, we examined the expression of osteoclast marker genes. BMMs were cultured in the presence of M-CSF, and relative quantity of mRNAs encoding osteoclast-related genes was determined at 0, 3, and 6 d after osteoclastogenic induction by RANKL by real-time RT-PCR. Under the culture condition used in this study, multinucleated cells usually start to appear as early as day 4, and osteoclast formation peaks around day 6. The results showed that osteoclast-associated genes, including NFATc1, TRAP, OSCAR, Atp6v0d2, DC-STAMP, cathepsin K, Fra-2, and calcitonin receptor (9–16) were significantly down-regulated in PI3Kγ-null cells compared with WT (Fig. 5). However, the expression of transcription factors required for commitment of hematopoietic precursors to osteoclast progenitors, including MitF and c-fos (17), were not significantly altered by PI3Kγ deficiency (Fig. 5 I and J).
Fig. 5.
Down-regulated expression of osteoclast-associated genes in PI3Kγ-null cells. Real-time quantitative RT-PCR analysis for relative expression of (A) nuclear factor of activated T cells c1 (NFATc1), (B) TRAP (Acp5), (C) d2 isoform of vacuolar [H(+)] ATPase (v-ATPase) V(0) domain (Atp6v0d2), (D) DC-STAMP, (E) cathepsin K, (F) OSCAR, (G) calcitonin receptor, (H) fos-related antigen-2 (Fra-2, fosl2), (I) c-fos, and (J) microphthalmia-associated transcription factor (MitF). Expression level of WT at day 0 was set to 1. Graphs are plotted with mean values of triplicates in a representative experiment. Error bars present SEM. *P < 0.02 vs. WT at each time point.
PI3Kγ Deficiency Affects Specifically Chemokine-Induced Akt Activation and Increases Apoptosis During Osteoclastogenesis.
To understand the molecular mechanism that underlies the impaired osteoclastogenesis of PI3Kγ-null cells, we examined Akt phosphorylation at both Thr-308 and Ser-473. Both phosphorylation events are dependent on PtdIns(3,4,5)P3 (18, 19). Although PI3Kγ deficiency did not affect M-CSF–induced Akt phosphorylation, it abrogated SDF-1–induced phosphorylation (Fig. 6 A and B). Because M-CSF signals through its tyrosine kinase receptor, whereas SDF-1 signals through its GPCR, this result is consistent with the knowledge that PI3Kγ is primarily regulated by GPCRs (20).
Fig. 6.
Effect of PI3Kγ deficiency on Akt phosphorylation and apoptosis in BMM. (A) PI3Kγ deficiency does not affect Akt phosphorylation by M-CSF. BMM cells from PI3Kγ WT and KO mice were serum-starved for 3 h and treated with M-CSF (25 ng/mL), RANKL (100 ng/mL), or both for durations as indicated. (B) PI3Kγ deficiency abrogated Akt phosphorylation by SDF-1α. BMM cells were serum-starved for 3 h and treated with SDF-1α (200 ng/mL) for durations as indicated. (C) PI3Kγ deficiency increases caspase activity. BMM cells were cultured in M-CSF (25 ng/mL), RANKL (100 ng/mL) or M-CSF plus RANKL for 2 d. Caspase activity was determined by a homogeneous caspase assay kit. (D) PI3Kγ deficiency does not influence cell proliferation. BMMs were labeled with 10 μM BrdU for 4 h, and the incorporated BrdU was detected by chemiluminescent ELISA.
We noticed that a significant amount of cell death occurred at the early stage of osteoclastogenesis (i.e., a couple of days after RANKL induction). Our microarray analysis of gene expression of osteoclast cultures revealed that a number of chemokines, including SDF-1, CCL9, CXCL4, and CXCL16, were expressed at high levels. CCL9 and SDF-1 were also previously found in osteoclast cultures (21–23) and activate PI3Kγ and subsequently Akt via their GPCRs (Fig. 6B) (24). Knowing that Akt activation can protect cells from apoptosis, we hypothesized that these chemokines may protect the cells from apoptosis during osteoclast differentiation upon RANKL induction. To test the hypothesis, we examined the effects of PI3Kγ deficiency on caspase-3 activity, which elevates during cell apoptosis (25). We found that addition of RANKL led to an increase in caspase activity by 60% in WT cultures (Fig. 6C). The withdrawal of M-CSF led to a further increase (≈4-fold) in caspase activity (Fig. 6C), suggesting that M-CSF protects the cells from RANKL-induced apoptosis (26). Supporting our hypothesis, PI3Kγ deficiency significantly increased caspase activity in cells treated with RANKL compared with WT controls, regardless of the presence of M-CSF. There was an 80% increase in caspase activity in the samples treated with both M-CSF and RANKL and a 120% increase in those treated with RANKL alone (Fig. 6C). TUNEL staining was also used to assess the apoptosis. There were 80% more TUNEL-positive cells in PI3Kγ-null differentiating osteoclasts than in WT cells (Fig. S3). The PI3K pathways are also known to regulate cell proliferation. To test whether PI3Kγ has an influence on proliferation of osteoclast precursor cells, we performed BrdU incorporation assay. There was no significant difference in the cell proliferation between WT and PI3Kγ KO (Fig. 6D). Therefore, we conclude that GPCR-regulated PI3Kγ complements M-CSF–activated signaling, primarily contributing to cell survival regulation during osteoclast differentiation.
Discussion
In this report, we investigated a G protein–regulated PI3K isoform, PI3Kγ, for its role in regulating bone homeostasis. Our results indicate that genetic inactivation of this gene resulted in an increase in bone mass, likely due to impairment in osteoclastogenesis. Osteoclastogenesis is a multistep process comprising of hematopoietic precursor commitment, differentiation, multinucleation, maturation, and apoptosis (27). Two factors, M-CSF and RANKL, have been shown to be essential for the process. M-CSF supports osteoblast precursor proliferation and survival, whereas RANKL promotes osteoclast differentiation. In addition, osteoclast progenitors produce a number of cytokines, including chemokines (28). Although chemokine receptor signaling alone is not sufficient for formation of functional osteoclast, chemokines have a costimulatory effect on RANKL-stimulated osteoclastogenesis (29).
The PI3K pathway has been implicated in having important roles in different phases of osteoclastogenesis. In addition to a large body of studies using pharmacological inhibitors of PI3K, two genetic studies using mouse lines lacking inositol 5-phosphatase and PI3K p85α provide both in vitro and in vivo evidence for the importance of this pathway in osteoclastogenesis and bone homeostasis. M-CSF signals through its cell surface tyrosine kinase receptor, which primarily regulates Class IA of PI3K, including PI3Kα, -β, and -δ. Significant attenuation of PtdIns(3,4,5)P3 levels and Akt activation by M-CSF in BMMs lacking PI3K p85α, a regulatory subunit for Class IA PI3K, supports this idea. Our result showing the lack of an effect of PI3Kγ deficiency on M-CSF–induced Akt phosphorylation is also consistent with the idea that PI3Kγ is primarily regulated by GPCRs rather than tyrosine kinase receptors. Knowing that PI3Kγ deficiency abrogated Akt phosphorylation by SDF-1, a chemokine, and that chemokines including SDF-1, abundantly detected in the osteoclast cultures, can facilitate osteoclastogenesis, we conclude that PI3Kγ is likely to mediate the effects of chemokines on osteoclastogenesis.
Although PI3Kγ deficiency does not affect the expression of MitF and c-fos, the transcription factors required for commitment of hematopoietic precursors to osteoclast progenitors (17), PI3Kγ deficiency reduced a panel of osteoclast marker gene expression (Fig. 5). The reduction in the expression of these osteoclast marker genes provides an explanation for the impairment of osteoclastogenesis by the lack of PI3Kγ. It is possible that the PI3Kγ-linked pathway directly regulates the expression of these genes, but we reasoned that the reduction in the expression of these osteoclast marker genes might be, at least in part, the result of the increase in apoptosis of osteoclast precursors during the early stage of their differentiation upon RANKL induction.
The PI3K–Akt pathway is known to have an important role in cell proliferation, survival, and apoptosis. The suppressive effect of a phosphatase and tensin homolog (PTEN) inhibitor on differentiating osteoclast apoptosis suggests that PIP3 has an important role in osteoclast survival (Fig. S4). Although M-CSF can protect osteoclast precursors from apoptosis via PIP3 during the early stage of RANKL-induced differentiation, PI3Kγ, which is presumably activated by autocrine chemokines, also makes a significant contribution to cell survival as demonstrated by the data shown in Fig. 6C. On the other hand, the PI3Kγ pathway does not seem to contribute significantly to osteoclast precursor proliferation (Fig. 6D), in which the M-CSF–PI3K pathway had an important role (30). These results together suggest that the PI3Kγ pathway may not be a rate-limiting step in proliferation regulation. In other words, M-CSF–mediated PI3K activation may be sufficient for supporting proliferation, whereas GPCR-mediated PI3K activation provides a biologically significant supplementation to the M-CSF–mediated one in protecting cells from apoptosis.
Previous studies showed that P3Kγ-deficient BMMs had reduced migration efficiency (31). Because migration of mononuclear osteoclast progenitor cells into close proximity is also an important event that precedes cell fusion during multinuclear osteoclast formation, another possible mechanism by which PI3Kγ deficiency affects osteoclastogenesis is the defective migration of monocytic precursors. Although this mechanism may occur in vivo, we do not think that it would contribute significantly to the impairment observed in the cultures because cells were seeded at high densities, and there were few gaps between the cells (Fig. 4A).
Osteoclasts are unique cells that resorb bones and are involved in not only bone remodeling but also pathological bone loss, such as osteoporosis and rheumatoid arthritis. Therefore, blockade of PI3Kγ with selective inhibitors may have a potential application for osteoporosis, as well as for treatment of chronic inflammatory diseases with bone-loss issues. Thus, future studies with emphasis on the disease models will determine whether inhibition of PI3Kγ would be an effective therapeutic strategy for any pathological conditions. Nevertheless, the present study indicates that long-term inhibition of PI3Kγ would alter bone homeostasis by increasing bone mass through a decrease in osteoclastogenesis.
Materials and Methods
Experimental Animals.
All mice were housed in a temperature-controlled room with a light/dark cycle of 12 h. All mice had free access to sterilized water and were fed standard laboratory chow ad libitum at the Yale University Animal Care Facilities in accordance with approved procedures. PI3Kγ KO mice were generated as described previously (32) and maintained on a C57BL/6 background. Wild-type and PI3Kγ KO mice were generated from the intercross between PI3Kγ heterozygous mice. Genotyping of the mice was performed at 3 wk of age by PCR with tail genomic DNA. All experiments were performed according to the protocol approved by Yale University's Institutional Animal Care and Use Committee.
Statistical Analyses.
Data were analyzed for statistical significance using Student's t test. Results are presented as mean and SEM. The difference between the experimental groups (e.g., WT vs. KO) was considered to be statistically significant if P < 0.05.
Methods for bone sample preparation and characterization, cell culture, proliferation, and caspase assays are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Michelle Orsulak for technical assistance and the Yale Core Center for Musculoskeletal Diseases for bone analyses. This work is supported by National Institutes of Health Grant AR051476 (to D.W.) and Pilot Grant P30 AR04603211 from the Yale Core Center for Musculoskeletal Diseases.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001499107/-/DCSupplemental.
References
- 1.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch E, et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 3.Laffargue M, et al. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity. 2002;16:441–451. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 4.Barber DF, et al. PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med. 2005;11:933–935. doi: 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- 5.Camps M, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 6.Roodman GD. Regulation of osteoclast differentiation. Ann N Y Acad Sci. 2006;1068:100–109. doi: 10.1196/annals.1346.013. [DOI] [PubMed] [Google Scholar]
- 7.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Hildebrand T, Rüegsegger P. Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Engin. 1997;1:15–23. doi: 10.1080/01495739708936692. [DOI] [PubMed] [Google Scholar]
- 9.Takayanagi H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 10.Oddie GW, et al. Structure, function, and regulation of tartrate-resistant acid phosphatase. Bone. 2000;27:575–584. doi: 10.1016/s8756-3282(00)00368-9. [DOI] [PubMed] [Google Scholar]
- 11.Kim N, Takami M, Rho J, Josien R, Choi Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med. 2002;195:201–209. doi: 10.1084/jem.20011681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SH, et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med. 2006;12:1403–1409. doi: 10.1038/nm1514. [DOI] [PubMed] [Google Scholar]
- 13.Martin TJ, et al. Heterogeneity of the calcitonin receptor: functional aspects in osteoclasts and other sites. J Nutr. 1995;125(7 Suppl):2009S–2014S. doi: 10.1093/jn/125.suppl_7.2009S. [DOI] [PubMed] [Google Scholar]
- 14.Suda T, Nakamura I, Jimi E, Takahashi N. Regulation of osteoclast function. J Bone Miner Res. 1997;12:869–879. doi: 10.1359/jbmr.1997.12.6.869. [DOI] [PubMed] [Google Scholar]
- 15.Yagi M, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202:345–351. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozec A, et al. Osteoclast size is controlled by Fra-2 through LIF/LIF-receptor signalling and hypoxia. Nature. 2008;454:221–225. doi: 10.1038/nature07019. [DOI] [PubMed] [Google Scholar]
- 17.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 18.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 19.Stokoe D, et al. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 20.Brock C, et al. Roles of G beta gamma in membrane recruitment and activation of p110 gamma/p101 phosphoinositide 3-kinase gamma. J Cell Biol. 2003;160:89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu X, Huang Y, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J Bone Miner Res. 2003;18:1404–1418. doi: 10.1359/jbmr.2003.18.8.1404. [DOI] [PubMed] [Google Scholar]
- 22.Okamatsu Y, et al. MIP-1 gamma promotes receptor-activator-of-NF-kappa-B-ligand-induced osteoclast formation and survival. J Immunol. 2004;173:2084–2090. doi: 10.4049/jimmunol.173.3.2084. [DOI] [PubMed] [Google Scholar]
- 23.Yang M, et al. Chemokine and chemokine receptor expression during colony stimulating factor-1-induced osteoclast differentiation in the toothless osteopetrotic rat: A key role for CCL9 (MIP-1gamma) in osteoclastogenesis in vivo and in vitro. Blood. 2006;107:2262–2270. doi: 10.1182/blood-2005-08-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravindran C, Cheng YC, Liang SM. CpG-ODNs induces up-regulated expression of chemokine CCL9 in mouse macrophages and microglia. Cell Immunol. 2010;260:113–118. doi: 10.1016/j.cellimm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson DW, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 26.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 27.Yavropoulou MP, Yovos JG. Osteoclastogenesis—current knowledge and future perspectives. J Musculoskelet Neuronal Interact. 2008;8:204–216. [PubMed] [Google Scholar]
- 28.Kim MS, Day CJ, Morrison NA. MCP-1 is induced by receptor activator of nuclear factor-kappaB ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. J Biol Chem. 2005;280:16163–16169. doi: 10.1074/jbc.M412713200. [DOI] [PubMed] [Google Scholar]
- 29.Kim MS, et al. MCP-1-induced human osteoclast-like cells are tartrate-resistant acid phosphatase, NFATc1, and calcitonin receptor-positive but require receptor activator of NFkappaB ligand for bone resorption. J Biol Chem. 2006;281:1274–1285. doi: 10.1074/jbc.M510156200. [DOI] [PubMed] [Google Scholar]
- 30.Munugalavadla V, et al. The p85alpha subunit of class IA phosphatidylinositol 3-kinase regulates the expression of multiple genes involved in osteoclast maturation and migration. Mol Cell Biol. 2008;28:7182–7198. doi: 10.1128/MCB.00920-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones GE, et al. Requirement for PI 3-kinase gamma in macrophage migration to MCP-1 and CSF-1. Exp Cell Res. 2003;290:120–131. doi: 10.1016/s0014-4827(03)00318-5. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, et al. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.