Abstract
We demonstrate that single interneurons can toggle the output neurons of the cerebellar cortex (the Purkinje cells) between their two states. The firing of Purkinje cells has previously been shown to alternate between an “up” state in which the cell fires spontaneous action potentials and a silent “down” state. We show here that small hyperpolarizing currents in Purkinje cells can bidirectionally toggle Purkinje cells between down and up states and that blockade of the hyperpolarization-activated cation channels (H channels) with the specific antagonist ZD7288 (10 μM) blocks the transitions from down to up states. Likewise, hyperpolarizing inhibitory postsnyaptic potentials (IPSPs) produced by small bursts of action potentials (10 action potentials at 50 Hz) in molecular-layer interneurons induce these bidirectional transitions in Purkinje cells. Furthermore, single interneurons in paired interneuron → Purkinje cell recordings, produce bidirectional switches between the two states of Purkinje cells. The ability of molecular-layer interneurons to toggle Purkinje cells occurs when Purkinje cells are recorded under whole-cell patch-clamp conditions as well as when action potentials are recorded in an extracellular loose cell-attached configuration. The mode switch demonstrated here indicates that a single presynaptic interneuron can have opposite effects on the output of a given Purkinje cell, which introduces a unique type of synaptic interaction that may play an important role in cerebellar signaling.
Keywords: bistability, cerebellum, GABA
As the sole output of the cerebellar cortex, Purkinje cells are ultimately responsible for relaying the computation performed by the entire network. Even though membrane properties and synaptic currents have been extensively studied in the cerebellar circuit, it still remains unclear how Purkinje cells process information coming from the rest of the cerebellar cortex before passing it on to the deep cerebellar nuclei. Purkinje cells are known to intrinsically generate action potentials at high rates and also to have an intrinsic membrane bistability (1–4). The cell switches between hyperpolarized potentials during which it is silent and depolarized potentials during which it fires action potentials (1, 2, 5–11). The frequency of changes between the two states in awake versus anesthetized animals in vivo has been disputed, but it is agreed that the bistability exists in a fraction of Purkinje cells in both anesthetized and unanesthetized animals (5, 6). This unique characteristic of the output of Purkinje cells potentially plays a significant role in passing the computation of the cerebellar cortex onto the cerebellar nuclei. Therefore to understand how Purkinje cells process this information it is essential to understand how the cells within the cerebellar cortex influence this bistable output of Purkinje cells.
The climbing fibers, which provide glutamatergic inputs to Purkinje cells from outside the cerebellar cortex, have been shown to produce complex spikes in Purkinje cells (1). In experiments performed in vivo and in slices, complex spikes recorded from Purkinje cells have been correlated to state changes in Purkinje cells (5, 7, 8, 12). It has been shown that single stimuli to the parallel fibers, which originate from within the cerebellar cortex, can cause bursts of action potentials in Purkinje cells, which terminate with a brief pause in Purkinje cell firing, hypothesized to result from an increased Ca2+ sensitive K+ conductance (13, 14). Likewise, single action potentials from GABAergic interneurons in the molecular layer, have been shown to cause brief pauses in spontaneous firing of Purkinje cells (15). Single extracellular stimuli delivered to the molecular-layer interneurons have been shown to cause transitions to down states when hyperpolarization-activated cyclic nucleotide-gated (HCN) channels are blocked (9) and have been suggested to also be effective under control conditions (10, 16, 17). Likewise, evidence suggests that strong hyperpolarizing currents injected into Purkinje cells can bidirectionally switch Purkinje cells between states (10). Here we examine and quantify the effect of interneuron stimulation on these state transitions. Furthermore, under physiological conditions the inputs to Purkinje cells from within the cerebellar cortex seem to be activated by patterns that more closely resemble trains than single action potentials (18). Therefore, we investigate how these patterns of activity in molecular-layer interneurons affect the output of Purkinje cells.
Results
Purkinje Cells Are Spontaneously Bistable.
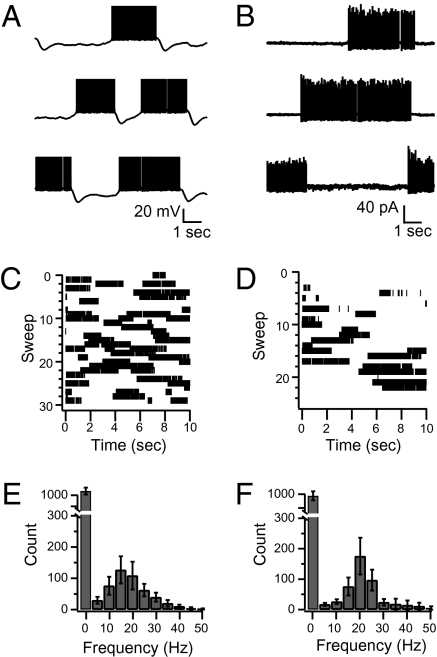
To identify their spontaneous spiking patterns we recorded from Purkinje cells in either whole-cell current clamp mode or in the loose, cell-attached configuration. Consistent with previous reports (1, 2), in whole-cell current clamp the membrane potential switched between depolarized, “up” states during which the cells fired spontaneous action potentials and hyperpolarized “down” states during which the cells were silent (Fig. 1 A and C). Also, as previously reported (1), in extracellular, cell-attached recordings spontaneous action potentials were often recorded during periods of presumed up states that were separated by prolonged periods of inactivity during presumed down states (Fig. 1 B and D). With the goal of quantifying the proportion of cells that showed this bistability versus the proportion of cells that did not, we developed the following analysis. After recording spontaneous spiking we made histograms of the number of action potentials recorded during 200-ms bins throughout the duration of the recording. Therefore any pause in action potential firing that lasted for more than 400 ms was guaranteed to be sampled by at least one of the 200-ms bins. Because synaptic activity occurs in small bursts of several action potentials (18) 400 ms should be sufficient to distinguish between silent periods resulting from short pauses caused by a change in conductance during spontaneous synaptic activity (13, 15) and transitions into down states. After making histograms of the bins that contained the various numbers of action potentials we could determine the average spontaneous firing frequency as well as the proportion of time the cell was silent for more than 400 ms. We classified cells as being bistable if such histograms contained two separate peaks, including a substantial component at 0 Hz. Using this analysis we found that when recorded under whole-cell conditions 11 of the 13 cells were bistable and all cells were bistable when recorded in the cell-attached configuration. When in the up state, cells fired with a stereotyped mean frequency close to 20 Hz (Fig. 1 E and F).
Fig. 1.
Purkinje cells recorded in slices are bistable. (A) Three representative sweeps (10-s duration) from a Purkinje cell recorded under current clamp in control conditions. The cell spontaneously alternates between a depolarized membrane potential (up state) where it fires action potentials and a hyperpolarized membrane potential where it is silent (down state). Note the deep hyperpolarization following the transitions from up to down states. Action potentials are truncated to show membrane potential. (B) Three representative sweeps of a Purkinje cell recorded in cell-attached configuration under control conditions. Upward deflections from baseline are extracellularly recorded spontaneous action potentials and are presumed to occur during up states of the Purkinje cell. (C) Raster plot of intracellularly recorded action potentials from all of the sweeps recorded from the cell shown in A. (D) Raster plot of all of the extracellularly recorded action potentials from the cell shown in B. (E) Histograms of the action potential frequency per 200-ms bins throughout all of the recordings of all of the bistable cells recorded under whole-cell conditions (n = 11). Note the large peak representing the amount of time spent in the down state at 0 Hz and the mean firing frequency of around 15 Hz in the up state. (F) Same as E but for all bistable cells recorded in cell-attached recordings (n = 5).
Brief Hyperpolarizing Currents Toggle Purkinje Cells Between States.
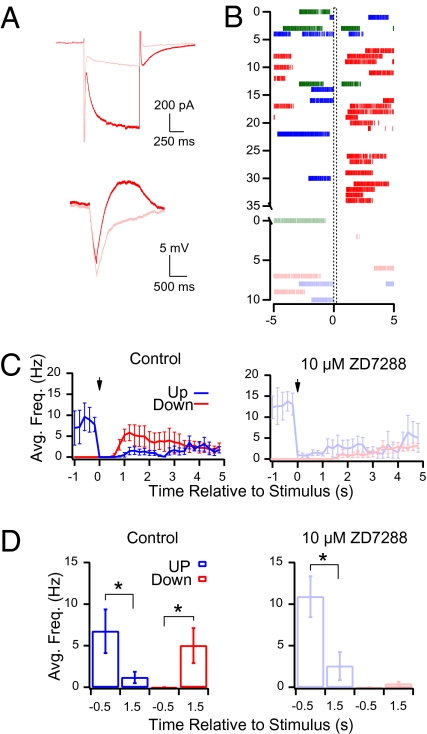
In response to hyperpolarizing current injections Purkinje cells exhibit an after depolarization (19) that is produced by the activation of an inward current (Ih (20); carried by the HCN channels (21, 22). Because this results in a biphasic hyperpolarization–depolarization sequence (Fig. 2A) we decided to investigate how brief hyperpolarizing currents affect Purkinje cells in both states. To determine this we made whole-cell current-clamp recordings and recorded spontaneous activity from Purkinje cells during 10-s-long sweeps and timed a current injection (−50 pA for 200 ms) to occur 5 s into every sweep. In this configuration the hyperpolarization occurred randomly with respect to the state of the Purkinje cell and could be classified as occurring while the Purkinje cell was in one of three distinct states. If the Purkinje cell fired at least one action potential between 4.85 and 5.0 s then the stimulation was considered to occur during an up state (blue traces in Fig. 2). If the Purkinje cell did not fire an action potential between 4.85 and 5.0 s but did fire an action potential between 4.0 and 4.85 s the stimulation was considered to occur in the first 1.0 second of a down state (green traces in Fig. 2). Finally, if the Purkinje cell did not fire an action potential between 4.0 and 5.0 s the stimulation was considered to occur more than 1.0 s after entry into a down state (red traces in Fig. 2).
Fig. 2.
Ih in Purkinje cells is essential for interneuron-induced transitions from down to up states but not for up to down states. (A Top) Response to a hyperpolarization from –74 to –134 mV for 1 s in voltage clamp with (pink) and without (red) ZD7288. (Bottom) Response of the same cell to 200 ms of 50 pA hyperpolarizing current injection in current clamp with (pink) and without (red) 10 μM ZD7288. (B) Raster plot of the response of the Purkinje cell in A to hyperpolarizing current injections (−50 pA for 200 ms) occurring during an up state (blue), more than 1.0 s after entry into a down state (red) and less than 1.0 s after entry into a down state (green). The Lower panel (lighter colors) depicts the response of each state 5 min after 10 μM ZD 7288 was washed into the recording chamber. (C Left) The average response of all cells recorded in control conditions (n = 4) to a −50 pA current injection for 200 ms. Blue line is the response of all sweeps in which the cell was in an up state at the time of current injection, whereas the red line is the response of cells that were in a down state. (Right) Same plot after the addition of 10 μM ZD7288. Note that when Ih is blocked in the presence of 10 μM ZD7288 the current injection continues to induce transitions from up to down states but is unable to induce the transition from down to up states. (D Left) Histogram of the average action potential frequency recorded for all cells (n = 4) 0.5 s before and 1.5 s after the current injection when the cells were in the up state (blue) or in the down state at the time of the hyperpolarization (red). (Right) Same as Left but in the presence of 10 μM ZD7288. Asterisks represent a significant difference before and after the current injections (P < 0.05; Student's t test).
When sweeps were sorted according to these criteria we found that hyperpolarizing current injections switched Purkinje cells bidirectionally between states. When Purkinje cells were in up states they switched to down states (blue traces is Fig. 2 C and D) and when they were in down states for more than 1.0 s they switched to up states (red traces in Fig. 2 C and D). However, when the cells were in down states for less than 1.0 s the current injection was ineffective at changing the state of the cell (see below). Consistent with previous reports (20, 21), the slow depolarization that followed hyperpolarizing current injections in down states, as well as the underlying inward current recorded in voltage clamp, was blocked by the HCN channel antagonist ZD7288 (10 μM; Fig. 2A), indicating that HCN channels were responsible for producing it. After recording the effect of current injections on Purkinje cell spiking in control conditions, we washed in 10 μM ZD7288 and tested the influence of the current injections in the absence of H currents. When ZD7288 was washed into the bath it consistently induced a substantial hyperpolarization of the Purkinje cell membrane potential which, in contrast to a previous report (9), completely silenced most cells. To test the effect on Purkinje cell spiking it was therefore necessary to offset this hyperpolarization by injecting positive current into the Purkinje cell to return the membrane potential near to control conditions. Nevertheless, despite this added depolarization, hyperpolarizing current injections occurring during down states were unable to switch Purkinje cells into up states (Fig. 2 C and D), indicating that HCN channels underlie the down- to up-state switch. As in the previous report, ZD7288 had no effect on state transitions caused by current injections occurring when Purkinje cells were in up states (9), nor on current injections occurring when Purkinje cells were in the first 1.0 s of a down state (Fig. 2 B, C, and D).
Physiological Patterns of Interneuron Activity Cause State Transitions.
Next we set out to determine whether activation of molecular-layer interneurons could reproduce the bidirectional state transitions in Purkinje cells caused by hyperpolarizing current injections. To assess this we first recorded from Purkinje cells in whole-cell voltage clamp in the presence of the AMPA receptor antagonist NBQX (10 μM) and positioned an extracellular stimulation electrode to evoke inhibitory postsynaptic currents (IPSCs) at two different holding potentials on either side of the measured GABA reversal potential of −82.6 ± 2.0 mV (Materials and Methods and ref. 23). We then used the ratio of the difference between the average peak amplitudes of these IPSCs to the difference in the holding potentials to determine the average peak synaptic conductance. We reduced the magnitude of the extracellular stimulation to generate the smallest average peak synaptic conductance possible, yielding a mean value of 9.4 ± 2.9 nS. This conductance was similar to, but slightly larger than, the synaptic conductance generated by the activation of single interneurons in paired recordings (see below and ref. 24). We then switched to current clamp mode and recorded the effect of interneuron stimulation on Purkinje cell output by repeating the protocol outlined above with interneuron stimulation (10 stimuli at 50 Hz) substituting for current injections. We then used the same criteria as described above and sorted sweeps according to whether they were in up or down states at the time of interneuron stimulation. To control for any natural pattern in spontaneous switching between states, interleaved control sweeps in which no stimulation was given were sorted according to the same criteria and the differences between the stimulated and unstimulated sweeps were evaluated to assess the effect of stimulation.
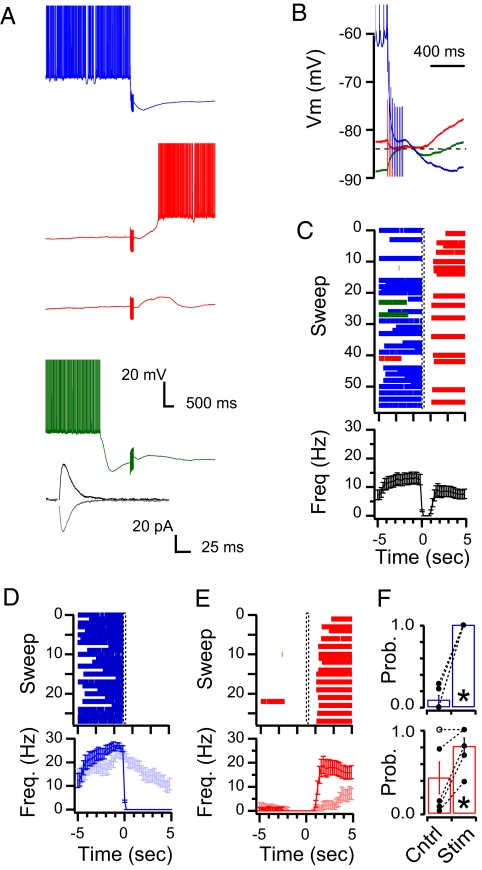
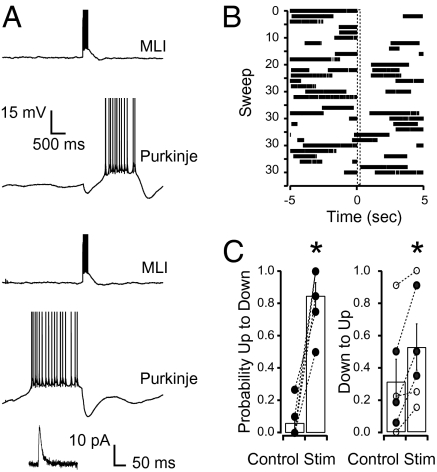
Interneuron stimulation that occurred when the Purkinje cell was in an up state produced a hyperpolarization in the Purkinje cell (blue traces in Fig. 3 A and B). The average data across all cells found to be bistable using the analysis described above show that this hyperpolarization caused a uniform switch from an up state to a down state, whereas interleaved unstimulated control sweeps showed a spontaneous switch at the same time point with a probability of only 0.08 ± 0.05 (P < 0.05; n = 5 cells; Fig. 3F). In all five bistable cells there were significantly more up- to down-state transitions compared with interleaved control sweeps. Stimulation that occurred when the Purkinje cell had been in a down state for more than 1.0 s also resulted in a hyperpolarizing potential (red trace of Fig. 3B; in this trace the hyperpolarization amounts to 2.1 mV). However, like in the current injection experiments (Fig. 2), these IPSPs were followed by a rebound depolarization that switched the cell into an up state with a probability of 0.80 ± 0.10, whereas interleaved unstimulated control sweeps showed a spontaneous switch during the same time window with a probability of 0.42 ± 0.19 (n = 5 cells; P < 0.05; Fig. 3 C, E, and F). Four of the five cells found to be bistable were significantly more likely to switch from down to up states in response to stimulation, compared with unstimulated interleaved control sweeps. Finally, immediately following transitions from up to down states the membrane potential would hyperpolarize beyond the measured GABA reversal potential, and even though interneuron stimulation that occurred during this time period produced a depolarization in the Purkinje cell (green traces in Fig. 3 A and B), it was unable to significantly affect the firing frequency in any of the experiments. Thus the outcome of stimulation for cells in a down state depended on the time spent from the previous up- to down-state transition. It should be noted that this was not the case for transitions from up to down states that occurred even if the previous switch to an up state was recent (e.g., sweep 22 in Fig. 3C). All three effects were blocked by the specific GABAAR antagonist SR95531 (10 μM; n = 3).
Fig. 3.
Purkinje cells recorded under whole-cell current clamp conditions are switched between states by extracellular stimulation of interneurons. (A) Representative recordings showing the response of one Purkinje cell to interneuron stimulation (10 stimuli at 50 Hz) occurring during an up state (blue), more than 1.0 s after entry into a down state (red) and less than 1.0 s after entry into a down state (green). Note that although they cannot be seen in the raster plots some stimuli do not switch the cell from a down to an up state (second red trace). Action potentials are truncated to show membrane potential. Inset displays averages of single IPSCs recorded at −74 (black) and −89 mV (gray) from the same cell. (B) Time expansion of traces in A to show the polarity of the PSPs in the three conditions: hyperpolarizing in blue and red traces and depolarizing in the green trace. Also note the rebound depolarization in the red trace (dashed line represents EGABA). (C) Raster plot of the action potentials from all traces recorded in the cell shown in A and B. (Lower) Average frequency of action potentials across all traces of the raster plot (stimulation starting at time 0). (D) Raster plot of the action potentials from sweeps in which the cell was in an up state at the time of interneuron stimulation. The average action potential frequency is plotted in the Lower panels with the light blue trace representing the action potential frequency recorded from interleaved controls in which no stimulation was given but the cell was in an up state at time = 0 s. (E) Same as D but for sweeps in which the cell was in a down state at the time of interneuron stimulation. Light red trace represents interleaved controls. (F) The average probability across all cells (n = 7) of a state change occurring at the time of stimulation (time = 0 s) is significantly higher for stimulated sweeps than for interleaved control sweeps in which no stimulation was applied. Upper graph (blue) depicts the probability of up-to-down switches and Lower graph (red) shows the down-to-up transitions.
State Transitions in Cell-Attached Recordings.
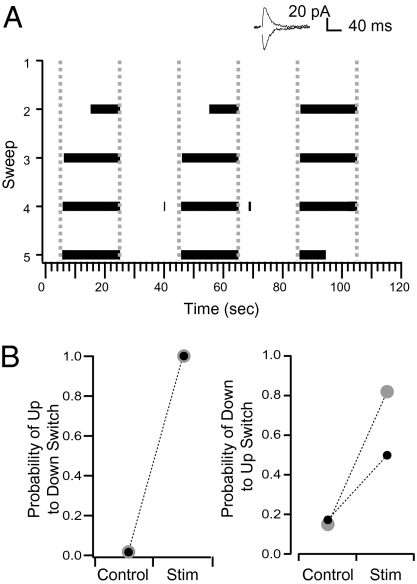
To determine whether the ability of interneuron activity to toggle Purkinje cells between states was dependent upon the conditions that were imposed on the Purkinje cell with the whole-cell pipette (including the values of the holding current and the GABA reversal potential) we performed similar experiments to those described above in cell-attached configuration. For these experiments we first made whole-cell recordings from the Purkinje cells and, after placing the stimulation electrode and decreasing the stimulation to produce an IPSC with an average conductance of 7.5 ± 3.8 nS, we retracted the whole-cell pipette from the Purkinje cell to form an outside-out patch (25). After subsequently rupturing the patch on the tip of the pipette, the pipette was repositioned onto the Purkinje cell and the spontaneous spiking activity was recorded extracellularly in a loose, cell-attached configuration. As we did in the whole-cell configuration, we sorted sweeps into the same categories on the basis of the state of the cell at the time of interneuron stimulation, and found that stimulation of interneurons in these conditions could also toggle Purkinje cells between states (Fig. 4 A and B). As in whole-cell conditions, stimulation that occurred during periods when the Purkinje cell was spiking (presumed to be equivalent to up states in whole-cell conditions) systematically caused a transition to down states in all cells. Analysis of average data showed that these transitions were significantly more likely than the probability of spontaneous up to down transitions occurring at the same time point in the interleaved control sweeps (0.08 ± 0.08; n = 6 cells; P < 0.05). In all six of the cells considered to be bistable, state transitions were significantly more likely in stimulated sweeps than in the unstimulated control sweeps (Materials and Methods). Similar to whole-cell recording conditions, stimuli that occurred when the Purkinje cell was in a down state for less than 1 s were ineffective at changing the state of the cell when recorded in cell-attached mode. In two of the six cells considered to be bistable, the interneuron stimulation caused a significant switch to up states in stimulated sweeps compared with the unstimulated controls (Fig. 4 A and B). These experiments show that IPSP-induced bidirectional switches can be obtained not only under whole-cell recordings but also when recorded in the loose cell-attached recording. They also suggest that the ability of IPSPs to induce a down- to up-state switch depends on the state of the recorded cell.
Fig. 4.
Purkinje cells recorded under cell-attached conditions are switched between states by extracellular stimulation of interneurons. (A) Raster plot of action potentials from one cell in which interneuron stimulation switched the Purkinje cell between states bidirectionally. Dashed lines represent the interneuron stimulation (10 stimuli at 50 Hz). Inset is the IPSCs recorded during whole-cell conditions before establishing the cell-attached configuration. IPSCs were evoked by single simulations and were recorded at −74 and −89 mV. (B) The average probability in cells that showed bidirectional state changes in response to interneuron stimulation in both sweeps with stimulation and interleaved control sweeps. The gray points are from the cell in A.
Single Interneurons Are Capable of Switching Purkinje Cells Between States.
Because the extracellular stimulation used in the above experiments produced a synaptic conductance that was on average slightly larger than the average synaptic conductance reported for paired interneuron to Purkinje cell recordings (24), we set out to determine whether activation of individual interneurons in paired recordings could also switch Purkinje cells between states. We performed the same experimental protocol as above while activating the presynaptic interneuron to produce 10 action potentials at 50 Hz in place of the extracellular stimulation and recorded the resulting effect on Purkinje cell spiking (Fig. 5A). All six of the postsynaptic Purkinje cells were found to be bistable and overall when Purkinje cells were in up states before interneuron activation, these short bursts of activity from single interneurons caused a switch to a down state with a probability of 0.85 ± 0.08 (compared with a probability of 0.12 ± 0.04 that a spontaneous transition occurred in interleave controls; n = 6 cells, P < 0.05; Fig. 5C). In four of these six cells the probability of the cell switching from up to down states was significantly greater in the stimulated than in the unstimulated control sweeps. When the Purkinje cell was in a down state for more than 1.0 s the interneuron activation caused a transition to an up state with an overall probability of 0.53 ± 0.14 (compared with a probability of 0.31 ± 0.14 that a spontaneous transition occurred in interleave controls; n = 6, P < 0.05; Fig. 5C). In three of the six pairs the stimulation caused a significantly greater probability of switching the cell from down to up states. However, when the Purkinje cell had been in a down state for less than 1.0 s the interneuron activation did not affect Purkinje cell firing in any of the cells. Finally, when interneurons were activated to produce single action potentials in place of the standard bursts of 10 action potentials at 50 Hz, the stimulation failed to switch Purkinje cells in either direction, similar to previous reports (9, 15).
Fig. 5.
Stimulation of single interneurons causes Purkinje cells to switch between states in paired interneuron to Purkinje cell recordings. (A) Example traces from one pair in which the Purkinje cell was either in a down state (Upper traces) or an up state (Lower traces) at the time that the presynaptic interneuron was stimulated (10 action potentials at 50 Hz). Action potentials are truncated to show membrane potential. Inset shows the IPSC that was recorded in the Purkinje cell in voltage clamp, whereas the presynaptic interneuron fired one action potential in current clamp (holding potential, −74 mV). (B) Raster plot of action potentials of all traces from the cell in A. (C) The average probability across all cells (n = 6) of a state change occurring at the time of stimulation in both the stimulated sweeps and interleaved control sweeps in which no stimulation was applied. MLI, molecular-layer interneurons.
Discussion
This is a unique analysis of the ability of GABAergic activity from molecular-layer interneurons to switch Purkinje cells between states under control conditions. Short bursts from single interneurons at a physiologically relevant frequency and duration (18) are sufficient to induce the mode switch. Surprisingly not only did interneurons cause a switch from up to down states but also from down to up states. When Purkinje cells were in the down state, activation of interneurons opened a GABAA conductance that caused a hyperpolarizing IPSP in the Purkinje cell and brought the cell membrane toward EGABA (−82.6 ± 2.0 mV in whole-cell experiments; see also ref. 23). Because EGABA was close to the half maximal activation voltage for HCN channels of −76 mV (9), the hyperpolarization potently activated an inward HCN conductance. After the cessation of the GABAA conductance, the HCN conductance remained and caused a rebound depolarization of the Purkinje cell membrane that brought the cell to the action potential threshold, as shown previously for hyperpolarizing current injections (20, 19); and eventually to an up state (Fig. 3 A and B). A similar phenomenon has previously been reported at the synapse formed between Purkinje cells and deep cerebellar nuclei (26–28). However, if the Purkinje cell was already in an up state when the GABAA conductance occurred, the resulting IPSP caused a deep hyperpolarization of the cell membrane beyond the reversal potential for GABA.
Previous studies examining the trigger responsible for initiating the transition between states in Purkinje cells have focused on the complex spikes generated by climbing fiber inputs (5–7). The present results introduce unique options concerning the origin and physiological role of state transitions and raise the possibility that state changes could result from interneuron activation. In this scenario state changes would correlate with complex spikes due to concurrent interneuron activation produced during spillover of glutamate from climbing fibers (29) or by simultaneous off-beam interneuron activity resulting from the stimulus.
The molecular-layer interneurons are organized with respect to the parallel fibers in such a way that they provide both on- and off-beam inhibition to the Purkinje cells (30). It is not clear how information originating from the parallel fibers will interact with on-beam (feed forward) and off-beam (surround) inhibition provided by the molecular-layer interneurons to affect the downstream Purkinje cells. In a possible scenario repetitive action potentials from the parallel fibers, like those that occur in vivo (18), could activate glutamatergic synapses onto Purkinje cells to produce a short excitation that would put the on-beam Purkinje cells in an up state regardless of their previous state. The feed-forward inhibition that would then follow (31, 32) would switch these on-beam Purkinje cells into a down state, whereas off-beam Purkinje cells, that do not receive the short excitation provided by parallel fibers, would switch states. Our experiments using cell-attached recording techniques suggest that although the switch from up to down states would occur in nearly every cell, approximately a third of the off-beam Purkinje cells would switch to an up state.
This study shows that, contrary to presently accepted views, the net effect of molecular-layer interneurons on Purkinje cells is not necessarily inhibitory. Whereas the traditional view of an inhibitory action was established when applying single stimuli, we find a much more complex outcome when using repetitive stimuli that approach those presumably provided to the cells in vivo (18). With short bursting interneuron inputs the net effect is almost neutral when averaging across trials (Fig. 3C); however, the input does exert a powerful action in individual trials depending on the state of the Purkinje cell at the time of the stimulus. When the Purkinje cell is in an up state the effect of interneuron activity is inhibition, whereas when the Purkinje cell is in a down state the effect is excitation. Thus, the interneuron–Purkinje network does not function in the same manner in response to single stimuli and to short bursts. The next question will obviously be how this conclusion can be integrated into the entire cerebellar network. Purkinje cells make GABAergic synapses onto deep cerebellar nuclei, which themselves make GABAergic synapses onto the principal neurons of the inferior olive, and the latter neurons send excitatory projections back to Purkinje cells (and via climbing fiber spill-over, to molecular-layer interneurons), as well as to deep cerebellar neurons (33). Studying the consequences of potential GABAAR-induced sign switches should help elucidate this vast feedback loop.
Materials and Methods
Slice Preparation.
Eleven- to 18-d-old Wistar rats were anesthetized with isoflurane and decapitated before the vermis of the cerebellar cortex was removed. Sagittal slices of the vermis, 200 μm thick, were cut using a Leica VT 1000S vibratome (Nussloch) in ice-cold artificial cerebrospinal fluid (ACSF) bubbled with 95% oxygen and 5% carbon dioxide. ACSF contained the following (in mM): 130 NaCl, 2.4 KCl, 1.3 NaH2PO4, 1 MgCl2, 2 CaCl2, 10 glucose, 26 NaHCO3, and 1–3 kynurenic acid. Slices were incubated for 1 h at 34 °C in ACSF (without the kynurenic acid) and then kept at room temperature until transferred to the electrophysiological recording chamber.
Electrophysiological Recordings.
The recording chamber was continuously perfused at a rate of 1–2 mL/min with the same ACSF used for slicing without the kynurenic acid. The Purkinje cell layer and the molecular layer of the cerebellar cortex were visualized using an Axioskop microscope (Zeiss) equipped with a 63× water immersion objective. Experiments used for display and analysis were performed at room temperature, although interneuron-induced bidirectional state transitions were reproduced in whole-cell conditions when recordings were made at 34.5 ± 0.5° C and 10 μM cAMP (34) was added to the internal solution (P < 0.05; n = 5) to replace any cAMP that is potentially degraded at higher temperatures. Under these conditions, interneuron stimulation systematically induced up- to down-state transitions (transition probability of 1.00 ± 0.00 compared with 0.07 ± 0.36 in interleaved control sweeps; n = 5 cells; P < 0.05) and down- to up-state transitions with a probability of 0.66 ± 0.10 (compared with 0.37 ± 0.09 in interleaved controls; n = 5 cells; P < 0.05). Signals were recorded with an EPC-10 amplifier (HEKA Elektronik) with Patchmaster software v2.32. Recording pipette solutions contained (in mM) 150 K-gluconate, 2.4 MgCl2, 10 hepes, 1 EGTA, 0.1 CaCl2, 2 Na-ATP, 0.4 Na-GTP, and the pH was adjusted to 7.3 with KOH. The same internal solution was used for patching Purkinje cells and interneurons. It had a calculated Cl− reversal potential of −84 mV, close to the reversal potential of GABAARs for Purkinje cells (23). Borosilicate glass pipettes were pulled using a HEKA Elektronik pipette puller and had an open tip resistance of ~3 MΩ for Purkinje cell recordings and ~8 MΩ for interneuron recordings. The concentrations of the drugs used were (in μM): 2 NBQX, 10 ZD7288, 10 Gabazine. Pukinje cell somas were targeted in the Purkinje cell layer and interneurons were selected in the molecular layer following morphological and size (>5 μm soma) criteria. Their identity was further confirmed by their electrophysiological properties. To perform extracellular stimulations, electrodes were constructed from theta glass and opposite poles of the stimulator were connected to each barrel of the pipette. The stimulating electrode was filled with ACSF and was connected to an isolated pulse stimulator (model 2100; A-M Systems) and was set to produce a single pulse 200 μs in duration. After establishing whole-cell voltage clamp conditions on a Purkinje cell the stimulation pipette was then positioned in the molecular layer and moved until an IPSC was produced in the Purkinje cell. The voltage from the stimulator was then reduced to produce a minimal IPSC in the Purkinje cell (0 to ~50 V). After the connection was established we then either adjusted the stimulator to produce a 200-ms train of pulses at 50 Hz (10 pulses) or began to change the configuration to loose cell attached. The bistability was recorded in cell-attached recordings that were derived from this prior whole-cell recording as well as when naïve cells were recorded directly in the cell-attached configuration with ACSF in the pipette (n = 5 cells).
Data Analysis.
Data were analyzed using Igor Pro software (version 6.0). IPSC and spike analysis were performed using Neuromatic software version 2.0 (thinkrandom.com). Statistical comparisons were done using a Student's t test and the significance level was set at a value of 0.05.
Raster sweeps were sorted into categories of up or down for both the stimulated sweeps as well as interleaved control sweeps on the basis of whether there was a spike in the 150 ms immediately before stimulation (Results). For each cell the probability that stimulation induced a state switch was calculated for up- to down-state transitions by selecting raster sweeps in which there was a spike in the 150 ms immediately before stimulation and then finding the percentage of these sweeps in which there were no spikes for 400 ms after the stimulation. Whereas the probability that stimulation induced a switch from down to up states was calculated by selecting the sweeps in which there were no spikes in the 1.0 s immediately before stimulation and then finding the percentage of these sweeps in which there was a spike in the 2.0 s that followed the stimulation. Different time windows were used for the up- to down-state transitions and the down- to up-state transitions because the underlying mechanisms responsible for these two different switches occur on different timescales. Therefore identical analysis was performed on the interleaved control sweeps and two sets of statistical analysis were performed between the stimulated and interleaved control traces: one on individual experiments and the other on average data. To determine whether interneuron activity significantly increased the probability of a state switch in an individual cell, a G test (35) was performed on the fraction of sweeps that switched states compared with the fraction of sweeps that did not switch states in control and stimulated sweeps. To determine whether there was a significant difference between the stimulated and interleaved control traces across groups of cells, a paired Student's t test was performed to compare the probability of a state switch in control and stimulated sweeps across all bistable cells in a given recording condition.
Acknowledgments
We thank P. Ascher and I. Llano for critical comments on the manuscript. Preliminary experiments were performed during the Neurobiology course at the Marine Biological Laboratory, Woods Hole, MA. This work was supported by a grant from the Fondation pour la Recherche Médicale (to A.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tank DW, Sugimori M, Connor JA, Llinás RR. Spatially resolved calcium dynamics of mammalian Purkinje cells in cerebellar slice. Science. 1988;242:773–777. doi: 10.1126/science.2847315. [DOI] [PubMed] [Google Scholar]
- 3.Gähwiler BH, Llano I. Sodium and potassium conductances in somatic membranes of rat Purkinje cells from organotypic cerebellar cultures. J Physiol. 1989;417:105–122. doi: 10.1113/jphysiol.1989.sp017793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci. 1999;19:1663–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loewenstein Y, et al. Bistability of cerebellar Purkinje cells modulated by sensory stimulation. Nat Neurosci. 2005;8:202–211. doi: 10.1038/nn1393. [DOI] [PubMed] [Google Scholar]
- 6.Schonewille M, et al. Purkinje cells in awake behaving animals operate at the upstate membrane potential. Nat Neurosci. 2006;9:459–461. doi: 10.1038/nn0406-459. author reply 461. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong DM, Rawson JA. Activity patterns of cerebellar cortical neurones and climbing fibre afferents in the awake cat. J Physiol. 1979;289:425–448. doi: 10.1113/jphysiol.1979.sp012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yartsev MM, Givon-Mayo R, Maller M, Donchin O. Pausing purkinje cells in the cerebellum of the awake cat. Front Syst Neurosci. 2009;3:2. doi: 10.3389/neuro.06.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams SR, Christensen SR, Stuart GJ, Häusser M. Membrane potential bistability is controlled by the hyperpolarization-activated current I(H) in rat cerebellar Purkinje neurons in vitro. J Physiol. 2002;539:469–483. doi: 10.1113/jphysiol.2001.013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rokni D, Tal Z, Byk H, Yarom Y. Regularity, variability and bi-stability in the activity of cerebellar purkinje cells. Front Cell Neurosci. 2009;3:12. doi: 10.3389/neuro.03.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Womack M, Khodakhah K. Active contribution of dendrites to the tonic and trimodal patterns of activity in cerebellar Purkinje neurons. J Neurosci. 2002;22:10603–10612. doi: 10.1523/JNEUROSCI.22-24-10603.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hounsgaard J, Midtgaard J. Synaptic control of excitability in turtle cerebellar Purkinje cells. J Physiol. 1989;409:157–170. doi: 10.1113/jphysiol.1989.sp017490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steuber V, et al. Cerebellar LTD and pattern recognition by Purkinje cells. Neuron. 2007;54:121–136. doi: 10.1016/j.neuron.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Womack MD, Chevez C, Khodakhah K. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J Neurosci. 2004;24:8818–8822. doi: 10.1523/JNEUROSCI.2915-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Häusser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–678. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- 16.Midtgaard J. Stellate cell inhibition of Purkinje cells in the turtle cerebellum in vitro. J Physiol. 1992;457:355–367. doi: 10.1113/jphysiol.1992.sp019382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson GA, Rokni D, Yarom Y. A model of the olivo-cerebellar system as a temporal pattern generator. Trends Neurosci. 2008;31:617–625. doi: 10.1016/j.tins.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Chadderton P, Margrie TW, Häusser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- 19.Kapoor R, Jaeger CB, Llinás R. Electrophysiology of the mammalian cerebellar cortex in organ culture. Neuroscience. 1988;26:493–507. doi: 10.1016/0306-4522(88)90164-9. [DOI] [PubMed] [Google Scholar]
- 20.Crepel F, Penit-Soria J. Inward rectification and low threshold calcium conductance in rat cerebellar Purkinje cells: An in vitro study. J Physiol. 1986;372:1–23. doi: 10.1113/jphysiol.1986.sp015993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santoro B, et al. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nolan MF, et al. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell. 2003;115:551–564. doi: 10.1016/s0092-8674(03)00884-5. [DOI] [PubMed] [Google Scholar]
- 23.Eilers J, Plant TD, Marandi N, Konnerth A. GABA-mediated Ca2+ signalling in developing rat cerebellar Purkinje neurones. J Physiol. 2001;536:429–437. doi: 10.1111/j.1469-7793.2001.0429c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent P, Marty A. Fluctuations of inhibitory postsynaptic currents in Purkinje cells from rat cerebellar slices. J Physiol. 1996;494:183–199. doi: 10.1113/jphysiol.1996.sp021484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conti R, Tan YP, Llano I. Action potential-evoked and ryanodine-sensitive spontaneous Ca2+ transients at the presynaptic terminal of a developing CNS inhibitory synapse. J Neurosci. 2004;24:6946–6957. doi: 10.1523/JNEUROSCI.1397-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol. 1999;82:1697–1709. doi: 10.1152/jn.1999.82.4.1697. [DOI] [PubMed] [Google Scholar]
- 27.Gardette R, Debono M, Dupont JL, Crepel F. Electrophysiological studies on the postnatal development of intracerebellar nuclei neurons in rat cerebellar slices maintained in vitro. II. Membrane conductances. Brain Res. 1985;352:97–106. doi: 10.1016/0165-3806(85)90091-4. [DOI] [PubMed] [Google Scholar]
- 28.Llinás R, Mühlethaler M. Electrophysiology of guinea-pig cerebellar nuclear cells in the in vitro brain stem-cerebellar preparation. J Physiol. 1988;404:241–258. doi: 10.1113/jphysiol.1988.sp017288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szapiro G, Barbour B. Multiple climbing fibers signal to molecular layer interneurons exclusively via glutamate spillover. Nat Neurosci. 2007;10:735–742. doi: 10.1038/nn1907. [DOI] [PubMed] [Google Scholar]
- 30.Palay SL, Chan-Palay V. Cerebellar Cortex: Cytology and Organization. New York: Springer; 1974. [Google Scholar]
- 31.Mittmann W, Koch U, Häusser M. Feed-forward inhibition shapes the spike output of cerebellar Purkinje cells. J Physiol. 2005;563:369–378. doi: 10.1113/jphysiol.2004.075028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunel N, Hakim V, Isope P, Nadal JP, Barbour B. Optimal information storage and the distribution of synaptic weights: Perceptron versus Purkinje cell. Neuron. 2004;43:745–757. doi: 10.1016/j.neuron.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 33.Llinás R, Walton K, Lang E. In: The Synaptic Organization of the Brain. 5th Ed. Shepherd G, editor. Oxford: Oxford Univ Press; 2004. pp. 271–311. [Google Scholar]
- 34.Wang J, Chen S, Nolan MF, Siegelbaum SA. Activity-dependent regulation of HCN pacemaker channels by cyclic AMP: Signaling through dynamic allosteric coupling. Neuron. 2002;36:451–461. doi: 10.1016/s0896-6273(02)00968-6. [DOI] [PubMed] [Google Scholar]
- 35.Sokal RR, Rohlf FJ. Introduction to Biostatistics. 2nd Ed. New York: Freeman; 1987. [Google Scholar]