Abstract
Aire promotes the ectopic expression of a repertoire of peripheral-tissue antigens (PTAs) in thymic medullary epithelial cells (MECs) to mediate deletional tolerance and thereby prevent autoimmunity. Binding of hypomethylated histone 3 (H3)-tails by Aire's plant homeodomain (PHD) finger is essential for Aire function in cultured cell models, prompting speculation that Aire-PHD:H3-tail interactions underlie targeting of Aire to weakly transcribed loci. To evaluate the role of Aire's PHD finger in MECs on a global scale in vivo, we complemented Aire-deficient mice with a mutant of Aire that inhibits its binding to hypomethylated H3K4 residues. Although the range of Aire-targeted genes was largely unaffected in these mice, the D299A mutation caused a global dampening of Aire's transcriptional impact, resulting in an autoimmune disease similar in profile to that of their Aire-deficient counterparts. To test whether a low H3K4 methylation state is sufficient for Aire targeting, we overexpressed an H3K4-specific demethylase in an Aire-dependent cultured cell system, and determined its capacity to extend Aire's transcriptional footprint. The range and magnitude of Aire-regulated genes was largely unaffected, the only genes additionally induced by Aire in this context being those already accessed for repression. In short, Aire's H3-binding module is necessary for Aire-mediated regulation of gene expression and central tolerance induction, but this influence is unlikely to reflect a targeting mechanism solely based on the recognition of hypomethylated H3K4 residues.
Keywords: chromatin, epigenetic, T cells, thymus, immunological tolerance
To guard against pathogens vast in range and number, the adaptive immune system maximizes antigen-receptor diversity by using a set of random gene rearrangement processes. As reactivity to self is an unavoidable outcome of these events, mechanisms to impose immunological tolerance are needed to prevent autoimmune pathology. For αβ T cells, tolerance relies, in part, on thymic MECs, which ectopically express thousands of PTAs, the presentation of which purges differentiating thymocytes of corresponding specificities (1). Mutations in the transcriptional regulator Aire compromise this ectopic expression, resulting in multiorgan autoimmune disease both in humans and in mice (2).
Precisely how Aire regulates PTA expression is under active investigation. Of late, much attention has focused on a PHD finger located near the center of the amino acid sequence. It was recently demonstrated that this structural element is a histone-binding module, specific for H3-tails impoverished in posttranslational modifications (3, 4). The Aire PHD forms an anti-parallel β-strand–like conformation in conjunction with the amino terminus of H3, anchored by electrostatic interactions between its aspartate/glutamate residues and unmodified H3R2, H3T3, H3K4 and H3R8 amino acids (5, 6). Disruption of certain of these interactions either by posttranslational modification of an H3 residue or by mutation of an Aire residue compromised both Aire's binding to chromatin and its induction of PTA transcripts (at least the few tested) in cultured cell models (3, 4, 7).
Aire's specificity for relatively unmodified H3 tails is especially interesting in light of recent studies suggesting that the methyl zero state (me0) might bear epigenetic information that controls gene expression through multiple generations. For instance, the BRAF-histone deacetylase complex 80 (BHC80) subunit of the lysine-specific demethylase 1 (LSD1) corepressor complex binds the demethylation product H3K4me0 to maintain LSD1 at target loci to prevent derepression of target genes through remethylation of H3K4 (8). Similarly, DNA methyltransferase 3–like protein L (DNMT3L) recognition of H3K4me0 is required for de novo DNA methylation at imprinting control regions in female germ cells and of retro-transposons in male germ cells (9–12). Given that trimethylated (me3) H3K4 is a modification typically associated with active transcription (13–15), it was proposed that Aire-regulated genes in MECs are weakly transcribed loci marked by relatively unmodified H3 tails, which render them targets of Aire's PHD finger (2–4, 16). Such a targeting mechanism would be consistent with the breadth of Aire's transcriptional influence (2, 17) as well as its markedly different transcriptional footprints in different cell types (7, 18).
We have further explored the importance of the Aire-PHD:H3-tail interaction, in a broader perspective, by addressing two questions. What is the global impact of this interaction on Aire's transcriptional footprint in MECs in vivo and, as a consequence, on central tolerance induction? Can Aire's transcriptional footprint be extended by widespread reduction of the H3K4 methylation state?
Results
We focused on Aire-PHD's recognition of hypomethylated H3K4 for several reasons. First, as mentioned above, unmethylated H3K4 has been associated with reduced levels of transcription (8, 9), while trimethylation at this site is a mark of active transcriptional initiation (13–15). Second, structural studies revealed key electrostatic interactions between H3K4 and the D299 residue of murine Aire, which were blocked by heavy methylation of H3K4 (4, 6). Finally, in cultured cell models, D299 was important for both Aire's interaction with chromatin and its induction of some diagnostic PTA transcripts (3, 4). It remained to be determined just how important the Aire-PHD:H3K4 interaction is for Aire function at a global level and in vivo. As one approach, we engineered transgenic mice expressing the Aire D299A mutation in thymic epithelial cells, and compared its impact on the repertoire of MEC transcripts and on peripheral autoimmunity with that of WT Aire in analogous transgenic mice. Isothermal titration calorimetry had shown the D299A mutation to decrease the affinity of Aire-PHD binding to the H3-tail by 15- to 30-fold (3, 4).
Construction of Transgenic Mice Expressing PHD-Mutated Aire.
We used a previously described inducible-Aire (iA) double-transgenic mouse system: the driver transgene directs expression of a tetracycline (tet)–responsive transactivator to thymic epithelial cells, mostly MECs (mouse line, TA); the reporter transgene includes a transactivator-dependent Aire cDNA, which is expressed in the absence, but not the presence, of tet (mouse line, TOA) (19). In a previous study, we determined that double-transgenic mice expressing WT Aire on an Aire-deficient NOD genetic background in the absence of tet were completely protected from the devastating multiorgan autoimmunity characteristic of NOD mice devoid of Aire (19). For the present study, we generated analogous double-transgenic mice expressing the D299A mutation of Aire on an Aire-negative NOD background (iA-D299A) for comparison with their WT counterparts (iA-WT).
We first examined Aire expression in the various transgenic mice, directly comparing those expressing PHD-mutated and WT Aire (Fig. 1). Aire transcripts were found predominantly in the thymus in both cases, at similar levels (Fig. 1A, Left and Center). Owing to leakiness of the Aire-D299A transgene reporter (Fig. 1A, Right), Aire transcripts were also detected, at lower levels, in the spleen of iA-D299A mice; however, we have demonstrated that such spurious transcripts are not relevant in complementation of Aire deficiency (19).
Fig. 1.
Expression of Aire in iA-WT vs. iA-D299A transgenic mice. (A) Real-time PCR analysis of Aire transcripts templated from the TOA transgene in whole thymi and peripheral organs of TA+ TOA+ double-transgenic mice. Liv, liver; LN, lymph node; Spl, spleen; Thy, thymus. Values are expressed relative to iA-WT thymus as a standard, n = 3 (B) Representative flow-cytometric analysis of CD45-negative thymic stromal cells from three independent experiments. Ly51-/LO MHC classIIHI medullary epithelial cells (MECHI), Ly51-/LO MHC classIILO (MECLO), and Ly51+ cortical epithelial cells (CECs) are gated, with values denoting percentage of cells in each gated region. (C) Percentage of MECHI in the total thymus, gated on CD45-negative cells. Numbers represent P values from Student t test, n = 3. (D) Percentage of Aire+ cells within MECHI, MECLO, CEC, and CD45+ compartments, n = 3 (E) Mean fluorescence intensity (MFI) of Aire in MECHI cells, n = 3 (F) Aire staining (green) in 5-μM frozen sections of thymi from 4-wk-old iA-WT and iA-D299A mice counterstained with anti–keratin-5 (orange). No Aire staining was detectable in samples from Aire-deficient mice (100× objective).
Flow-cytometric analyses revealed similar stromal cell compartments in iA-WT and iA-D299A mice (Figs. 1 B and C). There were more MHC class IIHI cells (MECHI) in the latter, but the increase was not as great as in Aire-deficient animals. As expected, Aire was mostly expressed in the MECHI stromal cell compartment (Fig. 1D and Fig.S1), and the fraction of Aire-positive cells and level of their Aire expression were similar for the WT and mutant transgenic mice (Fig. 1 D and E). Furthermore, Aire was localized in iA-D299A MECs within punctate nuclear structures, as was also observed in their iA-WT counterparts (Fig. 1F).
Together, these results indicate, first, that the D299A mutation did not grossly compromise Aire folding/stability in the in vivo context; and second, that the WT and mutant transgenic systems had similar characteristics.
Compromising Aire-PHD:H3K4 Interactions Inhibited Most, but Not All, of Aire's Transcriptional Footprint in MECs.
Having validated iA-D299A transgenic mice as a model system, we addressed whether and how this mutation of Aire's H3-binding module influences its transcriptional footprint in MECs. MECHI cells were sorted as previously described (17), in triplicate, from iA-D299A and iA-WT mice, as well as from reference WT and KO animals, and microarray-based gene-expression profiling was performed.
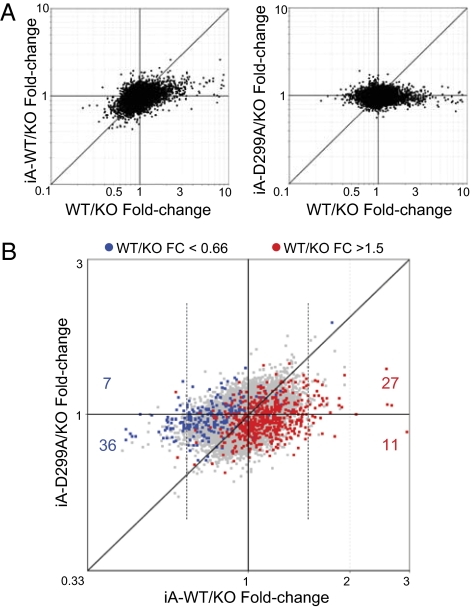
We first compared the ability of Aire or Aire-D299A to influence MEC gene expression, using the fold-change/fold-change plots of Fig. 2A. In both panels, the horizontal axis portrays Aire's normal footprint, as the ratio of expression in MECs from Aire WT vs. KO mice. For MECs from iA-WT mice (Fig. 2, Left), there was a strong correlation between the impact of the iA-WT transgene and Aire's normal influence. The off-diagonal distribution of points denoted a dampening of the transcriptional footprint imposed by the transgene-encoded Aire compared with endogenous Aire, as previously reported (19). In contrast, the distribution of points flattened almost completely for the iA-D299A transgene (Fig. 2, Right), indicating a strong reduction in transcriptional activity.
Fig. 2.
Aire-dependent transcripts in iA-WT vs. iA-D299A MECs (A) Scatterplots of gene fold-changes (FCs) induced by iA-WT (y axis) vs. WT Aire (x axis) (Left), or iA-D299A vs. WT Aire (Right) in MECs. (B) Scatterplots of gene FCs induced by iA-D299A (y axis) vs. iA-WT (x axis) in MECs. Red and blue dots indicate Aire-induced or -repressed genes, respectively at a 1.5-FC cutoff from WT/KO MECs. Red dots which also make the 1.5-FC cutoff in iA-WT/KO MECs (vertical dotted line) are enumerated for genes that exhibit iA-D299A/KO FC >1 (red number above x axis), or <1 (red number below x axis). Blue dots that also make the 0.66-FC cutoff in iA-WT/KO MECs are enumerated for genes that exhibit iA-D299A/KO >1 (blue number above x axis), or <1 (blue number below x axis).
For a more direct comparison of the ability of WT and PHD-mutated Aire to influence MEC gene expression, we plotted the fold-change elicited by iA-WT and iA-D299A vs. KO values, with red and blue highlights for transcripts belonging to known Aire–up-regulated and Aire–down-regulated signatures, respectively (Fig. 2B). Here, the distribution of points showed a subtle but distinct bias, reflecting an attenuation, but not a complete abrogation, of the Aire transcriptional activity in iA-D299A MECs. At an arbitrary 1.5-fold cutoff for expression in iA-WT MECs relative to fully deficient KO MECs, 27/38 transcripts were correspondingly up-regulated in iA-D299A MECs, and 36/43 down-regulated (P = 0.002, Fisher's exact). Thus, many Aire-regulated genes were still affected by PHD-mutated Aire, although the magnitude of the effect was strongly reduced.
Aire's Interaction with Hypomethylated H3K4 Is Necessary for Central Tolerance Induction.
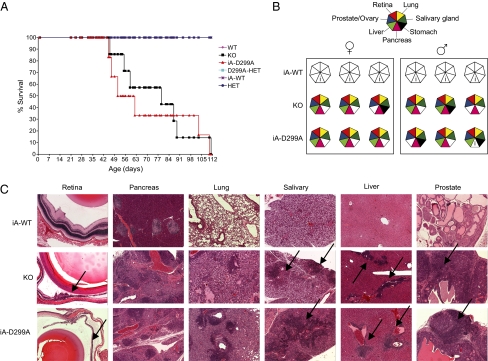
We wondered whether the quantitative effects of the D299A mutation on Aire's transcriptional footprint in MECs would impinge on central tolerance induction. The survival rate of iA-D299A transgenic mice was reduced as compared with the survival rates of both iA-WT transgenics and straight WT mice, dropping to the level of Aire-deficient NOD mice (Fig. 3A). NOD animals carrying an Aire-null allele and a PHD-mutated Aire transgene showed very similar profiles of autoimmune attack on the organs, in particular the severe exocrine pancreatitis typical of the NOD genetic background (Fig. 3 B and C). Thus Aire binding to hypomethylated H3K4 via residue 299 in the PHD is critical for establishing central tolerance. This finding indicates that the PHD mutation's global dampening of Aire's transcriptional impact was immunologically relevant but that the residual Aire-dependent transcripts could not stave off autoimmunity.
Fig. 3.
Aire's recognition of hypomethylated H3K4 is necessary for central tolerance induction (A) Kaplan–Meier plot of Aire-WT(n = 8), -KO(n = 7), -HET(n = 11), -HET/D299A (n = 5), iA-D299A (n = 6), and iA-WT(n = 7) littermates. Mice were killed upon 15–20% loss of body weight. iA-D299A vs. iA-WT, P = 0.00011; KO vs. iA-WT, P = 0.00036 by log test. (B) Histological analysis of iA-WT, iA-D299A, and KO mice; analysis was via H&E staining of fixed tissues. (▲) Diseased tissues, as indicated in key. i, Insulitis typical of Aire-positive NOD mice. (C) Representative histopathology of H&E-stained tissues affected in Aire-deficient and iA-D299A mice performed at 12–14 wk, or earlier for mice that were killed as a result of wasting disease. Black arrows point at areas of infiltration or retinal degeneration (5× objective).
Generation of Cultured Cells Overexpressing an H3K4-Specific Demethylase.
If binding to hypomethylated H3K4 is sufficient for Aire's targeting of particular genes, one might expect the repertoire of Aire-dependent loci to expand if more unmodified H3K4 residues were to be generated. To test this notion, we modified the global H3K4 landscape in cultured cells by transfecting an expression plasmid encoding the histone demethylase, PLU-1 (JARID1B), and assessed whether new Aire target genes emerged.
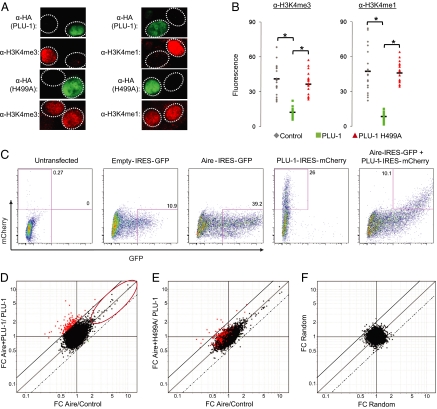
We chose the U2OS human osteosarcoma cells for these experiments because overexpression of PLU-1 in these cells has been shown to globally reduce levels of tri, di-, and monomethylated H3K4 (20, 21). Transfected Aire localized in punctate nuclear structures in U2OS cells and induced thousands of genes, preferentially those encoding PTAs, indicating that Aire is able to function in this cell line. U2OS cells cotransfected with PLU-1 plus Aire had Aire expression levels comparable with those of cells transfected with Aire alone (Fig. S2), and exhibited a specific and robust global H3K4 demethylase activity as compared with cells transfected with the PLU-1 catalytic mutant H499A (Fig. 4 A and B). We conclude that U2OS is an appropriate system to test whether demethylation suffices for Aire targeting. If H3K4 hypomethylation is sufficient, then substantial depletion of overall H3K4 methylation is expected to create new Aire target sites somewhere in the genome, altering Aire's transcriptional footprint in U2OS cells.
Fig. 4.
Broad H3K4 hypomethylation does not have a major effect on the range of Aire-targeted genes (A) Global H3K4 hypomethylation: Expression plasmids encoding HA-tagged PLU-1 or PLU-1 H499A were cotransfected with Aire on U2OS cells, and H3K4me1, H3K4m3 (both red), and HA (green) levels were analyzed via immunofluorescence. (B) Quantification for n = 20 cells by calculating mean fluorescence intensity of an invariant area. Filled bars indicate average mean intensity. *χ2 P value <10−4. (C) Bicistronic vectors were constructed with PLU-1, PLU-1H499A and/or Aire amino-terminal to an internal ribosome entry site driving mCherry or GFP. U2OS cells were transfected with an empty IRES-GFP vector, PLU-1-IRES-mCherry, Aire-IRES-GFP, or were cotransfected with Aire-IRES-GFP and PLU-1- or PLU-1H499A-IRES mCherry and sorted via a FACSAria. (D) FC scatterplots comparing effect of Aire in presence (y axis) and absence (x axis) of PLU-1. Red dots indicate genes differentially induced by Aire in the presence of PLU-1 vs. Aire alone. Red ellipse indicates genes induced by Aire alone (FC > 2). Solid diagonal above x = y indicates FC ratio of 2. Dotted diagonal below x = y indicates FC ratio of 0.5. (E) Same as D, but with PLU-1-H499A. (F) Same as D, but drawn from a random dataset.
Global H3K4 Hypomethylation Did Not Have a Major Effect on the Repertoire of Aire-Targeted Genes.
Gene-expression profiles were generated from U2OS cells transfected with Aire, PLU-1 or both, sorted as shown in Fig. 4C. First, we plotted the changes in gene expression induced by Aire within cells overexpressing PLU-1, vs. by Aire alone (y- and x-axes in Fig. 4D). Two observations emerged. First, Aire's usual induced and repressed targets in U2OS cells were similarly affected in cells expressing PLU-1 as well, as indicated by their diagonal disposition in Fig. 4D (ellipse). All Aire-induced transcripts were still induced, and to the same extent. Thus, enforced demethylation did not enhance Aire's activity on targets that it was affecting already.
Second, a new set of genes was influenced by Aire in the presence of PLU-1 (highlighted in red in Fig. 4D). Surprisingly, most of these transcripts were actually repressed slightly by Aire alone in U2OS cells. This bias suggests that these genes were not loci newly accessed by Aire but, rather, genes normally repressed by Aire but induced in the additional presence of PLU-1. Two elements argued for the significance of this gene set: (i) when a catalytically inactive mutant of PLU-1 was used, the induction by Aire vanished (Fig. 4E); and (ii) with randomized datasets (Fig. 4F), hardly any transcripts were found in the same quadrant (13 vs. 180; P < 10−10).
Thus, widespread hypomethylation of H3K4 residues did not appear to alter the range of Aire's target genes, but it did convert a number of Aire-repressed loci into Aire-induced loci.
Discussion
Putting together the findings from the two experimental approaches used in this study, we would like to suggest that Aire-PHD binding to H3-tails is globally important for Aire-mediated regulation of gene expression, but that this critical influence is unlikely to reflect a targeting mechanism solely keyed on the recognition of hypomethylated H3K4 residues.
The data on transgenic mice expressing PHD-mutated Aire showed a strong reduction in the expression of most, although not all, Aire-regulated MEC genes. One interpretation of this result is that Aire-PHD is not required for targeting of particular loci, rather exerting an essentially quantitative influence. Indeed, Ruthenburg et al. (15) have argued that patterns of histone modifications, H3K4 methylation and other, are not primary determinants of targeting, but are instead stabilizing elements that operate to increase the dwell time of transcriptional complexes subsequent to recruitment. However, another possible interpretation is that the D299A mutation does not entirely abrogate Aire-PHD:H3K4 interactions in the context of the whole proteins in vivo, so that downstream events are permitted to proceed a certain fraction of the time. Indeed, the D299A mutation did not totally abolish binding to the H3 tail (3, 4). It should also be kept in mind that these were in vitro determinations entailing an isolated recombinant Aire-PHD and a synthetic H3 amino-terminal peptide. Residual binding of the D299A mutant to H3-tails is now not so surprising, given recently published 3D solution structures of WT Aire-PHD with the H3-tail, demonstrating additional contributions by other residues, in particular an important interaction between murine D314A and H3R2, with functional consequences in both chromatin binding and PTA expression levels (5, 6).
Cultured U2OS cells overexpressing Aire and the H3K4-specific demethylase, PLU-1, yielded an unexpected result: Aire's transcriptional footprint remained largely unchanged, with only a partial derepression of a subset of genes already accessed by Aire. These results argue that the Aire-PHD:H3K4 interaction is not sufficient for Aire to productively target a given gene. This behavior may reflect local effects. For example, methylation of H3R2 and phosphorylation of H3T3, modifications that are not PLU-1-sensitive, are known to strongly inhibit Aire binding to the H3 tail (3, 4). These modifications also had a substantial impact on Aire's chromatin binding as well as its induction of PTA transcripts (5). Indeed, global hypermethylation via overexpression of the H3R2 methyltransferase, PRMT6, in HEK293 cells blocked Aire-dependent PTA induction (5). Although it has been shown that methylation at H3R2 and H3K4 are mutually exclusive (22, 23), implying that H3R2 residues near PLU-1–demethylated H3K4 residues probably would not be methylated, this situation could rapidly change, given the dynamic nature of histone methylation.
More distal effects might also play a role in Aire's targeting of weakly transcribed genes. For instance, a recent report suggested that Aire might function, at least in part, in a manner analogous to the manner in which the topoisomerase-2 (TOP2) poison, etoposide, operates (24). According to the proposed scenario, Aire would localize to weakly transcribed chromatin regions via its recognition of lightly methylated H3K4 marks. Within these locales, TOP2 would reside along genes undergoing transcription or replication, and Aire would stabilize TOP2-initiated DNA double-stranded breaks, inhibiting their religation and thereby activating the DNA-damage response and promoting transcription (25). In the absence of TOP2, Aire specifically fails to induce PTA expression in cultured cell lines (7), providing for a supplementary layer of gene-targeting specificity. It is also possible that Aire's interaction with TOP2 [or some other element(s)] is the primary targeting event, and that its binding to H3K4 provides additional stability needed to launch downstream events. This view is in line with recent thoughts on the residence of inhibitor of growth 2 (ING2) repressor complex or nucleosome remodeling factor (NURF) activating complex on active loci (15). Although effector modules from both of these complexes are specific for the same histone modification (H3K4me3), their recruitment to target genes is dictated by distinct site-specific factors: ING2 requires binding to the lipid signaling molecule phophatidylinositol-5-phosphate (26) and NURF requires interaction with GAGA factor (27) or ecdysone receptor (28). For a more profound mechanistic understanding of Aire targeting and function, it will be important to biochemically confirm the spatiotemporal localization of Aire, TOP2, and other interacting factors, along with histone modifications at specific loci as well as genome-wide.
Materials and Methods
Mice.
The D299A mutation was introduced into the TOA construct using QuikChange (Stratagene). iA mice were generated, as previously described (19) and as detailed in SI Materials and Methods.
Thymic Epithelial Cell Isolation and Intracellular Aire Staining.
Suspensions of thymic stroma were prepared and stained for intracellular Aire as previously described (29). Details are given in SI Materials and Methods.
Antibodies and Flow Cytometry.
Flow cytometric analysis was performed as previously described (29) and is discussed in SI Materials and Methods.
RNA Amplification, Microarray Hybridization, and Data Analysis.
RNA amplification, microarray hybridization and data analysis was performed as previously described (24, 30). Details are provided in SI Materials and Methods.
Histopathology.
Histopathology was performed as previously described (31) and as detailed in SI Materials and Methods.
Transfection.
U2OS cells were harvested 40 h after transfection in six-well plates with Lipofectamine according to the manufacturer's protocol (Invitrogen). Cells were harvested with 0.05% trypsin, sorted for GFP or mCherry on the FACSAria, and total RNA was extracted with TRIzol (Invitrogen).
Immunostaining.
Immunostaining was performed as previously described (32); details are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Yang Shi and Shigeki Iwase (Department of Pathology, Harvard Medical School, Boston, MA) for reagents, and Matthew Simon and Adam Matthews for insightful discussions. We also thank Kristen Leatherbee, Scott Davis, Kimie Hattori, and John Stockton for technical assistance. This work was supported by the Manipulated NOD Mouse Core of the Juvenille Diabetes Research Foundation Center at Harvard Medical School, and by National Institutes of Health Grants RO1 DK60027 (to D.M. and C.B.), RO1 GM079641 (to R.E.K.), and a Ruth L. Kirschstein predoctoral fellowship, National Research Service Award T32 DK07260 (to A.S.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004436107/-/DCSupplemental.
References
- 1.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 2.Mathis D, Benoist C. AIRE. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 3.Org T, et al. The autoimmune regulator PHD finger binds to non-methylated histone H3K4 to activate gene expression. EMBO Rep. 2008;9:370–376. doi: 10.1038/embor.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koh AS, et al. Aire employs a histone-binding module to mediate immunological tolerance, linking chromatin regulation with organ-specific autoimmunity. Proc Natl Acad Sci USA. 2008;105:15878–15883. doi: 10.1073/pnas.0808470105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chignola F, et al. The solution structure of the first PHD finger of autoimmune regulator in complex with non-modified histone H3 tail reveals the antagonistic role of H3R2 methylation. Nucleic Acids Res. 2009;37:2951–2961. doi: 10.1093/nar/gkp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakravarty S, Zeng L, Zhou MM. Structure and site-specific recognition of histone H3 by the PHD finger of human autoimmune regulator. Structure. 2009;17:670–679. doi: 10.1016/j.str.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Org T, et al. AIRE activated tissue specific genes have histone modifications associated with inactive chromatin. Hum Mol Genet. 2009;18:4699–4710. doi: 10.1093/hmg/ddp433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan F, et al. Recognition of unmethylated histone H3 lysine 4 links BHC80 to LSD1-mediated gene repression. Nature. 2007;448:718–722. doi: 10.1038/nature06034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ooi SK, et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature. 2007;448:714–717. doi: 10.1038/nature05987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourc'his D, Bestor TH. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature. 2004;431:96–99. doi: 10.1038/nature02886. [DOI] [PubMed] [Google Scholar]
- 11.Bourc'his D, et al. Dnmt3L and the establishment of maternal genomic imprints. Science. 2001;294:2536–2539. doi: 10.1126/science.1065848. [DOI] [PubMed] [Google Scholar]
- 12.Hata K, Okano M, Lei H, Li E. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development. 2002;129:1983–1993. doi: 10.1242/dev.129.8.1983. [DOI] [PubMed] [Google Scholar]
- 13.Taverna SD, et al. Yng1 PHD finger binding to H3 trimethylated at K4 promotes NuA3 HAT activity at K14 of H3 and transcription at a subset of targeted ORFs. Mol Cell. 2006;24:785–796. doi: 10.1016/j.molcel.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sims RJ, III, et al. Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription postinitiation factors and pre-mRNA splicing. Mol Cell. 2007;28:665–676. doi: 10.1016/j.molcel.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruthenburg AJ, Allis CD, Wysocka J. Methylation of lysine 4 on histone H3: Intricacy of writing and reading a single epigenetic mark. Mol Cell. 2007;25:15–30. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Musco G, Peterson P. PHD finger of autoimmune regulator: An epigenetic link between the histone modifications and tissue-specific antigen expression in thymus. Epigenetics. 2008;3:310–314. doi: 10.4161/epi.3.6.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venanzi ES, Melamed R, Mathis D, Benoist C. The variable immunological self: Genetic variation and nongenetic noise in Aire-regulated transcription. Proc Natl Acad Sci USA. 2008;105:15860–15865. doi: 10.1073/pnas.0808070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerau-de-Arellano M, Mathis D, Benoist C. Transcriptional impact of Aire varies with cell type. Proc Natl Acad Sci USA. 2008;105:14011–14016. doi: 10.1073/pnas.0806616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D. Neonatal tolerance revisited: A perinatal window for Aire control of autoimmunity. J Exp Med. 2009;206:1245–1252. doi: 10.1084/jem.20090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen J, et al. RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell. 2007;128:1063–1076. doi: 10.1016/j.cell.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Yamane K, et al. PLU-1 is an H3K4 demethylase involved in transcriptional repression and breast cancer cell proliferation. Mol Cell. 2007;25:801–812. doi: 10.1016/j.molcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Kirmizis A, et al. Arginine methylation at histone H3R2 controls deposition of H3K4 trimethylation. Nature. 2007;449:928–932. doi: 10.1038/nature06160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guccione E, et al. Methylation of histone H3R2 by PRMT6 and H3K4 by an MLL complex are mutually exclusive. Nature. 2007;449:933–937. doi: 10.1038/nature06166. [DOI] [PubMed] [Google Scholar]
- 24.Abramson J, Giraud M, Benoist C, Mathis D. Aire's partners in the molecular control of immunological tolerance. Cell. 2010;140:123–135. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 25.Muslimovic A, Nystrom S, Gao Y, Hammarsten O. Numerical analysis of etoposide induced DNA breaks. PLoS ONE. 2009;4:e5859. doi: 10.1371/journal.pone.0005859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gozani O, et al. The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell. 2003;114:99–111. doi: 10.1016/s0092-8674(03)00480-x. [DOI] [PubMed] [Google Scholar]
- 27.Xiao H, et al. Dual functions of largest NURF subunit NURF301 in nucleosome sliding and transcription factor interactions. Mol Cell. 2001;8:531–543. doi: 10.1016/s1097-2765(01)00345-8. [DOI] [PubMed] [Google Scholar]
- 28.Badenhorst P, et al. The Drosophila nucleosome remodeling factor NURF is required for Ecdysteroid signaling and metamorphosis. Genes Dev. 2005;19:2540–2545. doi: 10.1101/gad.1342605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray DH, et al. Unbiased analysis, enrichment and purification of thymic stromal cells. J Immunol Methods. 2008;329:56–66. doi: 10.1016/j.jim.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Hill JA, et al. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Jiang W, et al. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202:805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray DH, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007;204:2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.