Abstract
Vertebrate embryo somite formation is temporally controlled by the cyclic expression of somitogenesis clock genes in the presomitic mesoderm (PSM). The somitogenesis clock is believed to be an intrinsic property of this tissue, operating independently of embryonic midline structures and the signaling molecules produced therein, namely Sonic hedgehog (Shh). This work revisits the notochord signaling contribution to temporal control of PSM segmentation by assessing the rate and number of somites formed and somitogenesis molecular clock gene expression oscillations upon notochord ablation. The absence of the notochord causes a delay in somite formation, accompanied by an increase in the period of molecular clock oscillations. Shh is the notochord-derived signal responsible for this effect, as these alterations are recapitulated by Shh signaling inhibitors and rescued by an external Shh supply. We have characterized chick smoothened expression pattern and have found that the PSM expresses both patched1 and smoothened Shh signal transducers. Upon notochord ablation, patched1, gli1, and fgf8 are down-regulated, whereas gli2 and gli3 are overexpressed. Strikingly, notochord-deprived PSM segmentation rate recovers over time, concomitant with raldh2 overexpression. Accordingly, exogenous RA supplement rescues notochord ablation effects on somite formation. A model is presented in which Shh and RA pathways converge to inhibit PSM Gli activity, ensuring timely somite formation. Altogether, our data provide evidence that a balance between different pathways ensures the robustness of timely somite formation and that notochord-derived Shh is a component of the molecular network regulating the pace of the somitogenesis clock.
Keywords: somitogenesis, molecular clock, notochord
Vertebrate body segmented organization relies on the progressive formation of early embryonic vertebrae precursor structures—somites—flanking bilaterally the axial organs, notochord, and neural tube. Somitogenesis is a reiterated process occurring over time with strict periodicity. In the trunk of the chicken embryo, a pair of somites is formed every 90 min from the anterior tip of presomitic mesoderm (PSM). As development proceeds, ventral somitic cells migrate around the axial organs, giving rise to segmented structures such as vertebrae, intervertebral disks, and ribs, whereas more dorsal somitic cells give rise to the dermis of the back and all of the striated muscles of the adult body (1). Underlying somitogenesis periodicity is a molecular clock first evidenced by the cyclic expression of hairy1 in chick PSM cells with 90-min periodicity, corresponding to the time of somite formation (2). Currently, multiple genes belonging to Notch, Wnt, and Fgf signaling pathways are known to have similar oscillatory behavior and to participate in the embryo segmentation clock (1, 3). Posterior PSM cells in an undetermined state are influenced by high FGF/Wnt levels, which fade away anteriorly, counteracted by an opposing gradient of RA (4 –6). A determination front is established in the intersection of these opposite gradients at the level of somites –III to –IV, and cells located anteriorly to this front are determined to form somites (6).
Packard et al. described somite formation to be independent of the neural tube and notochord (7, 8) and today this is widely accepted. The somitogenesis clock is also believed to operate independently of these axial structures (2). However, there is an intriguing report showing that when a quail PSM is grafted into a chicken embryo, the grafted tissue progressively adjusts somite formation to that of the host contralateral part (9). This is particularly evident in the somites formed by cells that were located posterior to the donor determination front when the graft was performed, strongly suggesting that undetermined PSM requires a signal coming from the embryo midline structures for subsequent somite formation. Notochord and neural tube floor plate produce Sonic hedgehog (Shh), a morphogen central to growth, patterning, and morphogenesis (10), implicated in somite sclerotome induction (11) and in survival of medial somitic cells (12, 13). These functions have been described to account for the lack of spinal column observed in Shh KO mice (14). However, it is commonly believed that Shh signaling does not play a role in PSM segmentation or in the somitogenesis clock.
In the present work, we evaluated the notochord and Shh signaling contribution to PSM segmentation preceding somite formation. We show that notochord ablation delays somite formation and molecular clock oscillations, and that Shh is the notochord-derived signal regulating these events. Furthermore, our results show that the delay in somite formation in notochord-deprived PSMs is overcome in time, mediated by RA signaling. Altogether, our data identify embryo midline-derived Shh as a component of the molecular network regulating the pace of the somitogenesis clock and reveal a cross-talk with the RA pathway to ensure the robustness of somite formation.
Results
Timely Somite Segmentation of Undetermined PSM Tissue Requires Presence of Notochord.
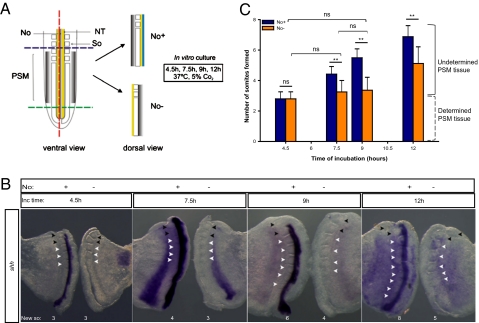
To evaluate the role of the notochord in PSM segmentation, the posterior part of chicken embryos was cut along the embryo midline, generating two embryo explants (Fig. 1A): a control explant containing half of the neural tube, the floor plate, and the notochord (No+), and an experimental explant with half of the neural tube but without the floor plate and notochord (No−). Explant pairs were cultured separately for different time periods (4.5, 7.5, 9, and 12 h), and the number of somites formed in culture was assessed (Fig. 1 B and C). The presence or absence of notochord on the control and experimental explants was randomly confirmed by in situ hybridization using a sonic hedgehog (shh) antisense RNA probe (Fig. 1B).
Fig. 1.
Notochord removal impairs timely somite segmentation of the undetermined PSM in vitro. (A) Schematic representation of explant delimitation and excision. No, notochord; NT, neural tube; So, somite; PSM, presomitic mesoderm. (B) Evidence for the presence (No+)/absence (No−) of the notochord in control and experimental explants, by in situ hybridization for shh. Black arrowheads indicate somites formed before culture; white arrowheads indicate somites formed during incubation (New so). (C) Graphic representation of mean number of somites formed de novo upon 4.5, 7.5, 9, or 12 h of incubation. Data are mean ± SD. **P < 0.05. ns, not statistically significant.
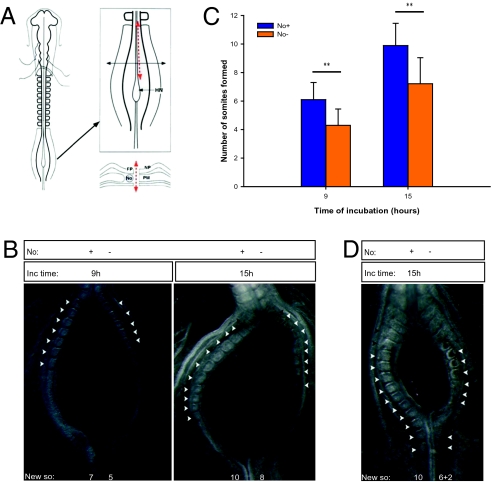
Explants incubated for 4.5 h formed three somites at the expected rate of one somite/90 min, even in the absence of the notochord (n = 68) (Fig. 1 B and C). Surprisingly, for greater incubation periods of 7.5 h (n = 33) and 9 h (n = 100), the PSM tissue did not segment to the same extent in the absence of notochord as in control explants (Fig. 1 B and C). In fact, somite formation considerably slowed down from 4.5 h to 9 h of incubation. Both PSM cell apoptosis and proliferation levels were assessed, and showed that the results obtained were not caused by alterations in either of these cellular processes (Fig. S1). Strikingly, from 9 h to 12 h (n = 25), an average of two new somites was formed even in the absence of the notochord, suggesting a recovery of the system (Fig. 1 B and C). Our experimental approach was reproduced in ovo; upon 9 h (n = 28) or 15 h (n = 24) of reincubation, the results obtained recapitulate those observed in explant cultures: the side without notochord consistently formed less somites than the control part (Fig. 2 and Movie S1). Moreover, somite formation rate is recovered over time and, accordingly, a difference of two somites in control and experimental sides is maintained after 9, 12, or 15 h of incubation (Figs. 1C and 2C). In some embryos the size of the slit allowed to observe a delay in somite segmentation in the PSM facing the incision, whereas timely bilateral somite formation occurred caudal to the slit, leaving a rostral gap of unsegmented tissue with approximately two somites length (Fig. 2D). Because somite formation periodicity in the absence of the notochord is already reestablished at 15 h (Figs. 1C and 2C), No− tissue will undergo further segmentation in a timely fashion, concomitantly with the generation of new somites caudal to the slit. Accordingly, after a 24 h incubation, both control and experimental PSMs are completely segmented indicating that notochord deprivation does not impair, but transiently delays somite formation (Fig. S2 A and B).
Fig. 2.
Notochord ablation in ovo delays somite segmentation of the undetermined PSM. (A) Schematic representation of embryo microsurgical operation. Only one of the embryo's PSMs (PM) was left in contact with the notochord (No). HN, Hensen's node; FP, floor plate; NP, neural plate. (B) Representative results obtained in operated embryos after a reincubation period of 9 or 15 h. (C) Graphic representation of the mean number of somites formed after 9 or 15 h. Data are mean ± SD. **P < 0.001. (D) Operated embryo reincubated for 15 h where a gap in somite formation is observed, resulting from the absence of notochord (No−) in the PSM tissue juxtaposed to the surgical slit, whereas reestablished contact of the posterior PSM with the notochord reinstates timely somite formation. Presence (No+) or absence (No−) of notochord is indicated. Black arrowheads indicate somites formed before the surgical intervention; white arrowheads indicate somites formed during reincubation (New so).
Altogether, we show that the undetermined PSM tissue depends on the presence of the notochord for timely somite segmentation. Moreover, as long as the PSM cells retain contact with the notochord, they preserve autonomous correct temporal information. PSM rostral to the determination front no longer requires the notochord to segment at normal rate.
Notochord Ablation Alters Clock and Determination Front Gene Expression.
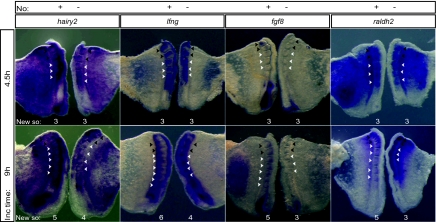
Because notochord ablation affected temporal control of morphological somite formation, we evaluated the expression of genes involved in the somitogenesis clock and determination front establishment. Explants such as those previously described (No+ and No−) were incubated for 4.5 or 9 h and hybridized with probes for the clock genes hairy2 and lunatic fringe (lfng), and determination front genes fgf8 and raldh2 (Fig. 3). Molecular clock gene expression was significantly altered in the absence of the notochord both after 4.5 h (lfng, n = 4/4; hairy2, n = 8/11) and 9 h (lfng, n = 19/22; hairy2, n = 15/18) (Fig. 3). The same could be observed upon in ovo microsurgery (Fig. S2C). Furthermore, in notochord-deprived explants the expression domains of both fgf8 (Fig. 3) and its downstream target sprouty1 (Fig. S3) were shifted caudally. However, this was not the primary cause for somite formation delay, because FGF8 supplementation did not rescue somite formation in No− explants and inhibition of FGF signaling in No+ explants did not mimic Shh deprivation (Fig. S4). Raldh2 expression was unaltered by notochord ablation upon 4.5 h of incubation (n = 9/10) but was highly overexpressed after 9 h (n = 4/5) (Fig. 3). The expression of meis2, a RA downstream target, further confirms these results and was already slightly up-regulated after 4.5 h (Fig. S3). As a control, two equivalent No+ explants were generated by splitting the notochord in half, and no alterations in segmentation rate or gene expression were observed (Fig. S5). In conclusion, somite formation delay in the absence of the notochord is accompanied by alterations in both molecular clock and determination front gene expression.
Fig. 3.
Notochord ablation alters somitogenesis clock and determination front gene expression. Expression patterns of hairy2, lfng, fgf8, and raldh2, evaluated by in situ hybridization in explants cultured for 4.5 or 9 h. Presence (No+) or absence (No−) of the notochord is indicated. Black arrowheads indicate somites formed before culture; white arrowheads indicate somites formed during incubation (New so).
Ectopic Shh Replaces Notochord in Temporal Control of Gene Expression and Somite Formation.
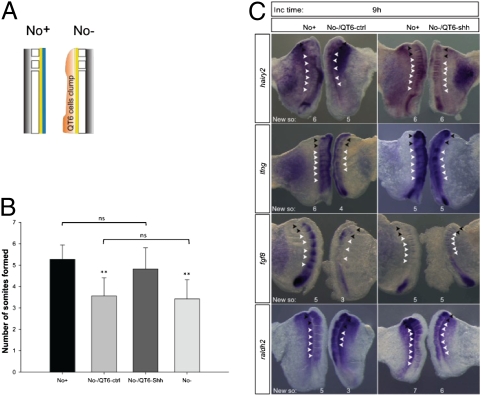
Shh is one of the most well-studied signaling molecules produced by the notochord. Shh-producing (QT6-Shh) or control (QT6-ctrl) QT6 cells were juxtaposed to No− explants which were cultured for 9 h in parallel to their No+ counterpart (Fig. 4A). No−/QT6-ctrl (n = 43) failed to form the expected number of somites and presented gene expression perturbations, similarly to what had been previously observed for the No− explants (Fig. 4 B and C). Notably, No−/QT6-Shh explants and No+ controls presented the same number of somites and comparable expression patterns for the molecular clock genes hairy2 (n = 9/14) and lfng (n = 9/12) and for the determination front genes fgf8 (n = 4/6) and raldh2 (n = 3/3) (Fig. 4 B and C). Altogether, the results indicate that Shh is able to replace the notochord in control of timely somite segmentation and underlying gene expression dynamics.
Fig. 4.
Exogenous Shh supply replaces the notochord in temporal control of somite formation and gene expression. (A) Schematic representation of a clump (pink) of control QT6 fibroblasts (QT6-ctrl) or QT6-producing Shh cells (QT6-Shh) juxtaposed to the neural tube (yellow) of explants without notochord (No−). (B) Graphic representation of mean number of somites formed by explants with notochord (No+), without notochord (No−), and without notochord but with control (No−/QT6-ctrl) or Shh-producing (No−/QT6-Shh) cells. **P < 0.05 vs. No+ or No−/QT6-Shh. ns, not statistically significant. Data are mean ± SD. (C) Explants with juxtaposed cells were hybridized for hairy2, lfng, fgf8, and raldh2. Black arrowheads indicate somites formed before culture; white arrowheads indicate somites formed during incubation (New so).
Shh Signaling Controls Clock Pace and Somite Formation.
Shh signaling is transduced by a receptor complex that includes two transmembrane proteins, Patched (Ptch) and Smoothened (Smo). Shh association with Ptch allows Smo activity, whereas in the absence of Shh, Ptch binds and inhibits Smo. Among the many target genes regulated by Shh signaling are ptch and gli signal transducers (15). To investigate the Shh pathway components operating in somite segmentation, we characterized the expression patterns of smo and ptch1/2 (Fig. S6). We found that notochord-adjacent PSM cells express both smo and ptch1/2, enabling them to respond to notochord-derived Shh.
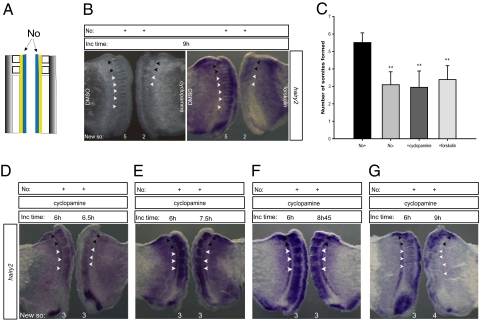
We performed chemical inhibition of Shh pathway in No+ explants and the results obtained mimicked the absence of notochord, leading to a similar delay in somite formation (Fig. 5, A–C). Furthermore, the expression patterns of molecular clock genes are different in control and Shh-inhibited explants (Fig. 5B). Shh inhibition, however, does not halt molecular clock oscillations: cyclopamine-treated explants incubated for time periods differing by 30 min (6 h and 6.5 h) presented dissimilar phases of molecular clock gene expression (Fig. 5D). In accordance to the observed delay in somite formation, Shh-inhibited explants incubated for time periods differing by 90 min (6 h and 7.5 h) differed in the phases of molecular clock gene expression (Fig. 5E). Taken together, these results indicate that, in the absence of Shh signaling, the period of the clock is no longer 90 min.
Fig. 5.
Inhibition of Shh signaling delays the pace of the clock. (A) Schematic representation of explant delimitation and excision. (B) Bisected-notochord explants were incubated for 9 h with DMSO (control explant) and with either 10 μg/mL cyclopamine or 25 μg/mL forskolin (experimental explant). Forskolin-treated explant and corresponding control were hybridized for hairy2. (C) Graphic representation of mean number of somites formed by explants with notochord (No+), without notochord (No−), and with notochord but incubated in the presence of cyclopamine or forskolin after 9 h of incubation. **P < 0.05 vs. No+. ns, Not statistically significant. Data are mean ± SD. (D–G) Explants with bisected notochord treated with cyclopamine were cultured for the indicated time periods and hybridized with hairy2 probe. Black arrowheads indicate somites formed before culture; white arrowheads indicate somites formed during incubation (New so).
To evaluate the delay in the molecular clock, we cultured pairs of Shh-inhibited explants for time periods differing by 1 h 10 min, 2 h, 2 h 15 min, or 2.5 h. All explant pairs presented different phases of expression of the clock and the same number of somites. When explants were incubated with a time difference of 2 h 45 min (Fig. 5F), 27% of the cases presented similar clock gene expression, but no extra somite formed. Finally, when differing by 3 h, 85% of the explants had formed one extra somite but already presented different molecular clock patterns. These results indicate that clock oscillations are severely delayed in the absence of Shh, with a new time period between 2 h 45 min and 3 h. The imprecision in the determination of the new oscillation period could be due to differing levels of perturbation of the system imposed by Shh deprivation, which can be dependent on the phase of the molecular clock at the moment of notochord ablation. In conclusion, Shh ensures timely somite segmentation by setting the pace of the somitogenesis molecular clock.
Retinoic Acid Is Able to Recover Somite Formation Rate in the Absence of Notochord.
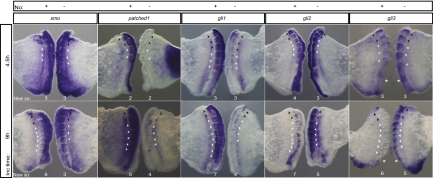
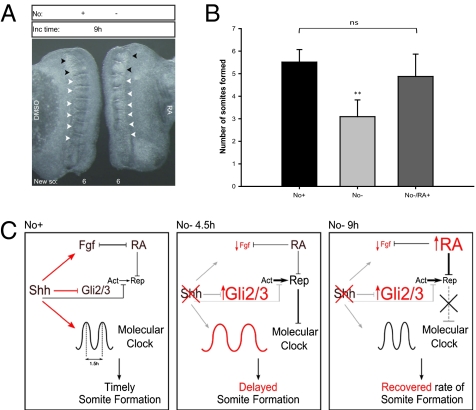
Notochord-deprived PSMs present delayed somite formation from 4.5 h of incubation onward, accompanied by an overexpression of gli2 (n = 4/4) and gli3 (n = 11/11), a slight up-regulation of smo (n = 5/5) and down-regulation of both ptch1 (n = 5/5) and gli1 (n = 3/3) (Fig. 6). Timely somite formation is resumed after 9 h and this is not due to a re-establishment of Shh pathway components throughout this time period, as their levels of expression do not revert to basal state (Fig. 6). At 9 h, an intriguing overexpression of raldh2 is observed that is not present at 4.5 h (Fig. 3). To test whether increased amounts of retinoic acid (RA) could mediate the recovery of somite formation rate, No− explants were incubated in RA-conditioned medium, and the number of formed somites after 9 h was assessed (Fig. 7 A and B). Noticeably, a greater number of somites is formed in No− explants when the medium is supplemented with RA. Moreover, the total number of somites formed in these conditions is not significantly different from that observed in the No+ explant (Fig. 7B). Our results strongly suggest that an increase in RA signaling can mediate the recovery of somite formation in the absence of Shh. However, greatly increasing the amounts of RA, Shh, or both could not accelerate somite formation rate (Fig. S7).
Fig. 6.
Expression of Shh pathway components in the PSM is altered by notochord ablation. Expression patterns of smo, ptch1, gli1, gli2, and gli3 genes evaluated by in situ hybridization in explants cultured for 4.5 or 9 h. *Neural tube removal from explants for better visualization of PSM gli3 expression. Presence (No+) or absence (No−) of the notochord is indicated. Black arrowheads indicate somites formed before culture: white arrowheads indicate somites formed during incubation (New so).
Fig. 7.
Shh and RA signaling pathways interplay in the PSM to ensure timely somite formation. (A and B) Retinoic acid mediates the recovery of timely somite formation in absence of notochord. (A) Explants with (No+) or without (No−) notochord were cultured for 9 h with DMSO (control explant) or with 15 μM of retinoic acid (RA) (experimental explant). Black arrowheads indicate somites formed before culture; white arrowheads indicate somites formed during incubation period (New so). (B) Graphic representation of mean number of somites formed by explants with notochord (No+), without notochord (No−) or without notochord but with RA (No−/RA+). **P < 0.05 vs. No+ or No−/RA. ns, Not statistically significant. Data are mean ± SD. (C) Proposed model for molecular events underlying temporal control of somite formation in the presence (No+) or absence (No−) of notochord over time. In the presence of notochord-derived Shh, gli2/3 expression in the PSM is repressed, and timely molecular clock oscillations and somite formation occur. When Shh signaling is disrupted, gli2/3 are overexpressed, and there is a delay in both the clock period and PSM morphological segmentation. The normal rate of these events is reestablished over time due to an up-regulation of RA that counteracts Gli activity (detailed in text).
Discussion
Somite Segmentation Relies on Axial Structures.
Segmentation of paraxial mesoderm has been classically described as an intrinsic mechanism independent of axial structures (16). PSM tissue incubated for 14 h, with or without the axial structures, formed the same number of somites (7, 8), suggesting that these structures do not contribute to somite formation. However, the rate of PSM segmentation during the incubation period was not evaluated. When the molecular clock genes were described, their expression oscillations were also shown to occur independently of axial structures (2, 17, 18). Nevertheless, these studies were performed only up to 4.5 h of incubation, and we now know that this accounts only for determined PSM segmentation (6). Only one report has suggested that contact with tissues near the midline influences somite formation (9).
We have revisited this issue by evaluating somite formation rate over time in the presence or absence of the notochord. The temporal framework used allowed us to assess segmentation of both determined and undetermined PSM. Our results clearly show that the anterior third of the PSM does not require the notochord for timely segmentation, indicating that this tissue is determined not only spatially (6) but also temporally (this work). Segmentation of the undetermined PSM, however, is significantly delayed in the absence of the notochord. This is particularly evident for the first three undetermined somites, which resume proper segmentation only after 9–12 h of incubation. Hence, although the absence of the notochord does not impair segmentation (this work; 7, 8), we show that the strict, characteristic rate of embryo somite formation is dependent upon a signal from the notochord.
Shh Produced by Embryo Midline Structures Ensures Somite Formation Rate.
Shh signaling pathway is not classically associated with somite segmentation. In fact, neither the Shh downstream Gli effectors nor the pathway's membrane receptors have been reported to be expressed throughout the PSM (19, 20). We show that ptch1 is expressed in the medial PSM tissue and that smo is ubiquitously present in the PSM, creating the conditions for Shh signaling readout in this tissue.
Embryo midline structures produce diffusible Shh. Our results indicate that the notochord role on somite formation rate may be the production of a Shh signal delivered to the adjacent PSM. In fact, exogenous supply of this protein rescued timely somite formation in the absence of the notochord. Moreover, in the presence of canonical Shh inhibitors somite formation rate was perturbed to the same extent as when the notochord was removed. These experiments strongly suggest that embryo midline structures ensure strict control of somite formation rate through the supply of canonical Shh signal to the PSM.
Somitogenesis Clock Periodicity Is Modulated by Shh Signaling.
Could a perturbation in the clock period underlie the delay in somite formation observed in the absence of Shh? When PSM tissue is deprived of Shh signaling, either by notochord ablation or chemical inhibition, hairy2/lfng expression patterns are significantly different from those obtained in control conditions. Moreover, an exogenous Shh supply to notochord-ablated explants recovered molecular clock expression patterns. Hence, we could detect a clear correlation between the presence of Shh and both timely somite formation and molecular clock gene oscillations. Our group has previously shown that only the medial-most PSM (M-PSM) cells possess intrinsic information for both somite formation and molecular clock gene expression (21). The study suggested that a signal supplied by M-PSM travels along the medial-lateral PSM axis, ensuring both timely segmentation and cyclic clock expression. However, the nature of this signal was not identified (21). The results obtained throughout the present work suggest that M-PSM cells are exposed to notochord-derived Shh signaling, as evidenced by ptch1/2 restricted expression, providing them crucial information for timely clock oscillations and somite formation. M-PSM cells are then capable of instructing and recruiting their lateral neighbors for segmentation. This hypothesis is supported by the observation that somites formed in the absence of Shh are smaller along the medial-lateral (M-L) axis (Fig. S2B). How this is achieved in vivo is subject of ongoing studies in the laboratory.
Inhibition of Wnt signaling has also been described to perturb the molecular clock (22). We found that axin2 expression is unaltered in 60% of the notochord-ablated explants (Fig. S8) and the other 40% are only slightly down-regulated after 4.5 h. Because clock expression is perturbed in a much higher percentage of cases (80%), somite formation delay in the absence of Shh should not be due primarily to an impairment of Wnt signaling. Moreover, Wnt does not seem to be involved in the recovery of timely segmentation, as axin2 is clearly down-regulated after 9 h (Fig. S8).
Somitogenesis clock gene expression perturbation in the absence of Shh signaling was not due to a halt in gene oscillations, but to an alteration of the clock period. In fact, the period of hairy2 expression cycles is considerably enlarged in the absence of Shh signaling, from 1.5 h to a time point between 2 h 45 min and 3 h. Significantly, somite formation in these conditions is also greatly delayed, reinforcing the dependence of timely somite formation on the pace of clock oscillations. Interestingly, somite formation delay observed upon Shh inhibition was dose dependent, as fewer somites were formed with increased inhibitor concentrations (Fig. S9). In situ hybridization on sections suggests the existence of a ptch1 expression gradient, with higher levels in the determined PSM (Fig. S6D). It is tempting to postulate that an anterior–posterior gradient of Shh activity could account for the different segmentation rates observed for rostral (early) and caudal (late) somites. Shh knock-out mice present a striking absence of the entire spinal column and most of the ribs (14). Our data suggest that a perturbation in somite segmentation could be contributing to this phenotype.
Shh and RA Pathways Interplay with the Molecular Clock to Ensure Timely Somite Formation.
This work shows that Shh produced by the notochord/floor plate is required to maintain timely operation of the molecular clock and the correct pace of somite formation (Fig. 7C , Left). An important consequence of PSM isolation from its source of Shh is the up-regulation of gli2 and gli3 (Fig. 7C , Middle), which corroborates previous reports (19). This strongly suggests that low gli levels in the PSM are not due to the lack of Shh signaling readout, as is commonly believed, but rather are actively restricted by notochord-derived Shh. Another striking molecular consequence of notochord ablation is the down-regulation of fgf8 expression in the PSM, showing that Shh signaling is required to preserve the fgf8 gradient underlying the determination front. These molecular changes, observed in the PSM after 4.5 h of incubation in the absence of Shh, most probably account for the subsequent delay in somite formation rate and molecular clock oscillations.
The PSM fgf8 gradient counteracts an opposing RA gradient (5). Hence, the reduction of fgf8 levels in the absence of Shh could allow the observed up-regulation of Raldh2 after 9 h of incubation (Fig. 7C , Right). Under these molecular conditions, there is a recovery of somite formation rate. Accordingly, we show that an external RA supply is able to rescue timely somite formation in the absence of Shh (Fig. 7 A and B). Although RA has been shown to inhibit gli2 expression (23), this does not occur within our experimental time frame. Because RA also modulates Gli activity (24), our results suggest that raldh2 overexpression after 9 h of notochord ablation rescues somite formation through an inhibition of Gli2/3 activity in the PSM (Fig. 7C , Right). It is plausible that somite formation recovery may further involve the inhibition of gli2 expression levels later in time. Our work strongly suggests that Shh and RA pathways interplay in the PSM to ensure timely somite formation and positions Gli2/3 at the intersection of these two pathways. Gli2 was recently reported to exert direct transcriptional regulation on the murine hes gene in retinal explants (25). In our experimental setup, gli2 overexpression was concomitant with a deregulation in the tempo of hairy2 transcriptional bursts, suggesting that in the PSM the same transcriptional regulation could take place. Hence, Shh-mediated restriction of Gli activity in the PSM seems to be essential to ensure the pace of both molecular clock oscillations and somite formation. Interestingly, Shh has been recently described to be essential for proper timing of neural progenitor differentiation, and RA was proposed to compensate for Shh deficiency in this developmental system (26).
Somitogenesis is a robust developmental mechanism. This can be achieved only by the integration of multiple signaling pathways. In fact, Notch, Wnt, Fgf, and RA signaling pathways operate in the PSM molecular clock and/or determination front (3, 4, 6). In the present work, we provide conclusive evidence that Shh signaling is a component of the intricate molecular machinery responsible for temporal control of somite formation.
Multiple congenital vertebral malformations have been associated with mutations in somitogenesis clock genes (1, 3). Furthermore, there are reports associating the Shh signaling pathway with congenital vertebral anomalies (27, 28). We report a previously uncharacterized association between these two molecular systems operating in the formation of early embryonic vertebrae precursor structures. A deeper understanding of the multiple molecular interactions occurring during the somitogenesis process is crucial for an adequate scientific contribution to the medical community, striving to better comprehend and treat such human malformations.
Materials and Methods
Detailed materials and methods can be found in SI Materials and Methods. Chick embryos were staged according to Hamburger and Hamilton (HH) (29), and somites were considered to be formed when a definite cleft was observed (30). Antisense digoxigenin-labeled RNA probes were produced as described: shh (31), hairy2 (17), lunatic fringe (32), fgf8 (33), raldh2 (34), patched-1 (20), gli1 and gli2 (35), and gli3 (19). A smoothened-specific probe was generated. For explant cultures, ventrally positioned embryos were divided into two halves by cutting across the three germ layers at the midline level, either parallel to the embryonic axis or bisecting the axial structures. In Shh-graft experiments, clumps of either QT6-ctrl or QT6-Shh cells (36) were juxtaposed to the No− experimental explants. For chemical treatments, 15 μM of RA (Sigma), 10 μg/mL cyclopamine (Calbiochem), 25 μg/mL forskolin (Sigma), or DMSO was added to the culture medium. In situ hybridization was performed as previously described (37).
Supplementary Material
Acknowledgments
We thank R. Moura, C.J. Sheeba, F. Bajanca, and O. Martinho for helpful input regarding this work, and N. Le Dourain and L. Saúde for critical reading of the manuscript. We also wish to acknowledge stimulating insights provided by the Reviewers. T.P.R was supported by Fundação para a Ciência e a Tecnologia (FCT), Portugal (SFRH/BD/27796/2006); R.P.A. is funded by a Ciencia2007 Program Contract (Portuguese Government). This work was supported by FCT, Portugal (Projects PTDC/SAU-OBD/099758/2008 and PTDC/SAU-OBD/105111/2008), the EU/FP6—Network of Excellence—“Cells into Organs” and IBB/CBME, LA, FEDER/POCI 2010.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000979107/-/DCSupplemental.
References
- 1.Andrade RP, Palmeirim I, Bajanca F. Molecular clocks underlying vertebrate embryo segmentation: A 10-year-old hairy-go-round. Birth Defects Res C Embryo Today. 2007;81:65–83. doi: 10.1002/bdrc.20094. [DOI] [PubMed] [Google Scholar]
- 2.Palmeirim I, Henrique D, Ish-Horowicz D, Pourquié O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell. 1997;91:639–648. doi: 10.1016/s0092-8674(00)80451-1. [DOI] [PubMed] [Google Scholar]
- 3.Dequéant ML, et al. A complex oscillating network of signaling genes underlies the mouse segmentation clock. Science. 2006;314:1595–1598. doi: 10.1126/science.1133141. [DOI] [PubMed] [Google Scholar]
- 4.Aulehla A, et al. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell. 2003;4:395–406. doi: 10.1016/s1534-5807(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 5.Diez del Corral R, et al. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- 6.Dubrulle J, McGrew MJ, Pourquié O. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell. 2001;106:219–232. doi: 10.1016/s0092-8674(01)00437-8. [DOI] [PubMed] [Google Scholar]
- 7.Packard DS., Jr Somitogenesis in cultured embryos of the Japanese quail, Coturnix coturnix japonica. Am J Anat. 1980;158:83–91. doi: 10.1002/aja.1001580108. [DOI] [PubMed] [Google Scholar]
- 8.Packard DS, Jr, Jacobson AG. The influence of axial structures on chick somite formation. Dev Biol. 1976;53:36–48. doi: 10.1016/0012-1606(76)90207-4. [DOI] [PubMed] [Google Scholar]
- 9.Packard DS, Jr, Zheng R-Z, Turner DC. Somite pattern regulation in the avian segmental plate mesoderm. Development. 1993;117:779–791. doi: 10.1242/dev.117.2.779. [DOI] [PubMed] [Google Scholar]
- 10.Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 11.Fan C-M, Tessier-Lavigne M. Patterning of mammalian somites by surface ectoderm and notochord: Evidence for sclerotome induction by a hedgehog homolog. Cell. 1994;79:1175–1186. doi: 10.1016/0092-8674(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 12.Rong PM, Teillet M-A, Ziller C, Le Douarin NM. The neural tube/notochord complex is necessary for vertebral but not limb and body wall striated muscle differentiation. Development. 1992;115:657–672. doi: 10.1242/dev.115.3.657. [DOI] [PubMed] [Google Scholar]
- 13.Teillet MA, LaPointe F, Le Douarin NM. The relationships between notochord and floor plate in vertebrate development revisited. Proc Natl Acad Sci USA. 1998;95:11733–11738. doi: 10.1073/pnas.95.20.11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang C, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 15.Varjosalo M, Taipale J. Hedgehog: Functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 16.Palmeirim I, Rodrigues S, Dale JK, Maroto M. Development on time. Adv Exp Med Biol. 2008;641:62–71. doi: 10.1007/978-0-387-09794-7_5. [DOI] [PubMed] [Google Scholar]
- 17.Jouve C, et al. Notch signalling is required for cyclic expression of the hairy-like gene HES1 in the presomitic mesoderm. Development. 2000;127:1421–1429. doi: 10.1242/dev.127.7.1421. [DOI] [PubMed] [Google Scholar]
- 18.McGrew MJ, Dale JK, Fraboulet S, Pourquié O. The lunatic fringe gene is a target of the molecular clock linked to somite segmentation in avian embryos. Curr Biol. 1998;8:979–982. doi: 10.1016/s0960-9822(98)70401-4. [DOI] [PubMed] [Google Scholar]
- 19.Borycki A-G, Brown AMC, Emerson CP., Jr Shh and Wnt signaling pathways converge to control Gli gene activation in avian somites. Development. 2000;127:2075–2087. doi: 10.1242/dev.127.10.2075. [DOI] [PubMed] [Google Scholar]
- 20.Marigo V, Tabin CJ. Regulation of patched by sonic hedgehog in the developing neural tube. Proc Natl Acad Sci USA. 1996;93:9346–9351. doi: 10.1073/pnas.93.18.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freitas C, Rodrigues S, Charrier JB, Teillet MA, Palmeirim I. Evidence for medial/lateral specification and positional information within the presomitic mesoderm. Development. 2001;128:5139–5147. doi: 10.1242/dev.128.24.5139. [DOI] [PubMed] [Google Scholar]
- 22.Gibb S, et al. Interfering with Wnt signalling alters the periodicity of the segmentation clock. Dev Biol. 2009;330:21–31. doi: 10.1016/j.ydbio.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribes V, Le Roux I, Rhinn M, Schuhbaur B, Dollé P. Early mouse caudal development relies on crosstalk between retinoic acid, Shh and Fgf signalling pathways. Development. 2009;136:665–676. doi: 10.1242/dev.016204. [DOI] [PubMed] [Google Scholar]
- 24.Goyette P, et al. Regulation of gli activity by all-trans retinoic acid in mouse keratinocytes. Cancer Res. 2000;60:5386–5389. [PubMed] [Google Scholar]
- 25.Wall DS, et al. Progenitor cell proliferation in the retina is dependent on Notch-independent Sonic hedgehog/Hes1 activity. J Cell Biol. 2009;184:101–112. doi: 10.1083/jcb.200805155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh S, Huang X, Liu J, Litingtung Y, Chiang C. Shh and Gli3 activities are required for timely generation of motor neuron progenitors. Dev Biol. 2009;331:261–269. doi: 10.1016/j.ydbio.2009.05.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celli J, van Bokhoven H, Brunner HG. Feingold syndrome: Clinical review and genetic mapping. Am J Med Genet A. 2003;122A:294–300. doi: 10.1002/ajmg.a.20471. [DOI] [PubMed] [Google Scholar]
- 28.Kim PC, Mo R, Hui Cc C. Murine models of VACTERL syndrome: Role of sonic hedgehog signaling pathway. J Pediatr Surg. 2001;36:381–384. doi: 10.1053/jpsu.2001.20722. [DOI] [PubMed] [Google Scholar]
- 29.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- 30.Pourquié O, Tam PPL. A nomenclature for prospective somites and phases of cyclic gene expression in the presomitic mesoderm. Dev Cell. 2001;1:619–620. doi: 10.1016/s1534-5807(01)00082-x. [DOI] [PubMed] [Google Scholar]
- 31.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 32.Sakamoto K, et al. Identification of a chick homologue of Fringe and C-Fringe 1: Involvement in the neurogenesis and the somitogenesis. Biochem Biophys Res Commun. 1997;234:754–759. doi: 10.1006/bbrc.1997.6652. [DOI] [PubMed] [Google Scholar]
- 33.Crossley PH, Minowada G, MacArthur CA, Martin GR. Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell. 1996;84:127–136. doi: 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- 34.Tsukui T, et al. Multiple left-right asymmetry defects in Shh(-/-) mutant mice unveil a convergence of the shh and retinoic acid pathways in the control of Lefty-1. Proc Natl Acad Sci USA. 1999;96:11376–11381. doi: 10.1073/pnas.96.20.11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marigo V, Scott MP, Johnson RL, Goodrich LV, Tabin CJ. Conservation in hedgehog signaling: Induction of a chicken patched homolog by Sonic hedgehog in the developing limb. Development. 1996;122:1225–1233. doi: 10.1242/dev.122.4.1225. [DOI] [PubMed] [Google Scholar]
- 36.Duprez D, Fournier-Thibault C, Le Douarin N. Sonic Hedgehog induces proliferation of committed skeletal muscle cells in the chick limb. Development. 1998;125:495–505. doi: 10.1242/dev.125.3.495. [DOI] [PubMed] [Google Scholar]
- 37.Henrique D, et al. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.