Abstract
Ecologists have long recognized the roles of competition and disturbance in shaping ecological communities, and the combinatorial effects of these two factors have been the subject of substantial ecological research. Nevertheless, it is still unclear whether competition remains as an important structuring force in habitats strongly influenced by disturbance. The conventional belief remains that the importance of competition decreases with increasing disturbance, but limited theory suggests otherwise. Using protist communities established in laboratory microcosms, we demonstrate that disturbance does not diminish the importance of competition. Interspecific competition significantly increased rates of species extinction over a broad disturbance gradient, and increasing disturbance intensities increased, rather than decreased, the tempo of competitive exclusion. This community-level pattern is linked to the species-level pattern that interspecific competition led to most frequent extinctions of each species at the highest level of disturbance that the species can tolerate. Consequently, despite a strong tradeoff between competitive ability and disturbance tolerance across the competing species, species diversity generally declined with disturbance. The consistent structuring role of competition throughout the disturbance gradient underscores the need to understand competitive interactions and their consequences even in highly disturbed habitats.
Keywords: competitive interactions, species coexistence, species extinction, species richness
One of the key aims of community ecology is to understand the role of various factors, including competition as a major type of biotic interactions, in regulating the structure of ecological communities along environmental gradients (1–3). It has often been suggested that the role of interspecific competition changes along environmental gradients such that it is most important in low-stress/disturbance conditions and least important in high-stress/disturbance conditions (1, 4, 5). This intuitively appealing idea is deeply entrenched in ecology and has become a cornerstone of some of the most influential ecological theories (4–8). However, there is some, albeit limited, theoretical evidence suggesting that disturbance, as an agent of mortality and biomass reduction, may not necessarily reduce the role of competition in structuring ecological communities (9). This prediction arises from the fact that species enduring greater disturbances often attain smaller population sizes due to their higher average mortality rates, which could make them less tolerant to competition (9).
Although the effects of disturbance on competition have been investigated empirically (10, 11), little is known about the actual importance of competition in regulating community structure along disturbance gradients. The importance of competition in structuring communities (that is, its effects on species abundances and community-level attributes), which differs from its intensity (9, 12), is best assessed by analyzing competitive outcomes with long-term data (13). Existing studies on disturbance and competition, typically conducted in terrestrial plant communities within a single growing season, generally cannot observe competitive exclusion or stable coexistence of community members. As a result, none of these disturbance-competition studies have established the linkage between disturbance and the importance of competition.
To examine the effects of disturbance on competition and species diversity, we conducted a set of laboratory microcosm experiments that subjected communities of freshwater bacterivorous protists to a broad range of disturbance intensity. Mortality-causing disturbance was imposed via sonication, an effective means of introducing mortality without bringing other adverse effects in microcosms (14, 15). The disturbance gradient consisted of 11 different levels, ranging from weak disturbances that had little effect on most species to strong disturbances that caused the direct extinction of most species (Results). A key advantage of this system is that the rapid reproduction of protists allowed the examination of multigenerational community dynamics, including competitive exclusion and stable coexistence, in a period of a few weeks. Protist microcosm experiments thus have made valuable contributions to our understanding of the ecological consequences of disturbance (14–19). A pool of 11 ciliated protist species (Fig. 1), occupying the same trophic level as bacterivorous consumers, was used to set up a total of 583 single-, bi-, and multispecies aquatic microcosms (Materials and Methods), so as to assess the ability of each species to cope with disturbance in the absence of interspecific competition, the competitive ability of species in the absence of disturbance, and the structuring role of interspecific competition under disturbance, respectively.
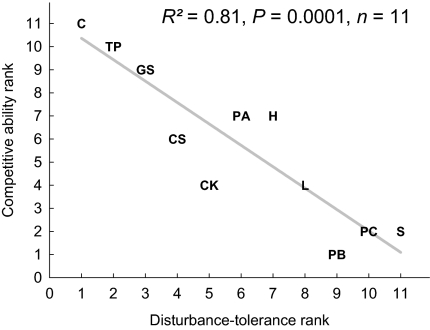
Fig. 1.
Tradeoff between competitive ability and disturbance tolerance exhibited by the studied species. Rankings of species disturbance-tolerance ability (best tolerator ranked as the first) and competitive ability (best competitor ranked as the first) were obtained based on the results of the single-species and bispecies experiments, respectively (Materials and Methods). Capital letters correspond to the names of the ciliated protist species used in the experiments. C, Colpoda sp.; CK, Colpidium kleini; CS, Colpidium striatum; GS, Glaucoma scintillans; H, Halteria grandinella, L, Loxocephalus sp.; PA, Paramecium aurelia; PB, Paramecium bursaria; PC, Paramecium caudatum; S, Spirostomum teres; TP, Tetrahymena pyriformis. Linear regression line is shown along with the data.
Results and Discussion
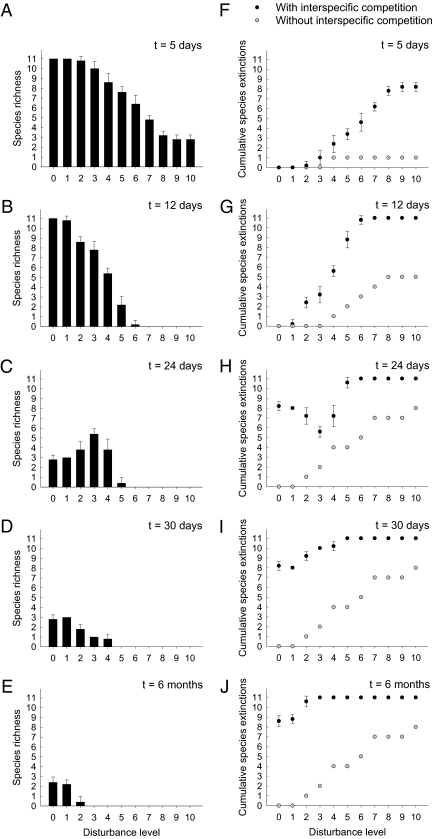
The 11 protist species differed markedly in their competitive ability (Fig. 1) and in their ability to tolerate disturbance (Fig. S1). When considered together, there was a strong tradeoff between the two traits (Fig. 1). This tradeoff appeared to be mediated by body size, as smaller species, which tended to be weaker competitors, were better at tolerating disturbance (Fig. S2). The better tolerance of smaller species largely reflected their greater resilience afforded by their higher growth rates and larger carrying capacities, as they showed similar vulnerability to the imposed disturbance as larger species (Fig. S3). Note that the tradeoff between species competitive ability and the ability to cope with disturbance, together with the assumption of competition being less important with increasing disturbance, formed the base of the intermediate disturbance hypothesis (IDH) (4, 6, 20), also known as the hump-back model (1). This hypothesis predicts that species richness peaks at intermediate levels of disturbance, with diversity limited by competitive exclusion and disturbance-induced extinction at the low and high ends of the disturbance gradient, respectively. However, we found that this unimodal relationship between disturbance and species diversity was short-lived, emerging only on day 24 of our multispecies experiment (Fig. 2C). Before day 22, species extinction occurred mostly at the high end of the disturbance gradient, resulting from the joint effects of competition and disturbance (discussed below). Between day 22 and day 24, however, competitive exclusion occurred rather rapidly at low disturbance levels, presumably because of strong competition associated with high population densities. As a result, diversity was temporarily high at intermediate disturbance levels on day 24. On all other sampling days, however, species richness always decreased monotonically with disturbance (Fig. 2 A, B, D, and E).
Fig. 2.
Temporal patterns of species richness and extinction along the disturbance gradient. (A–E) Observed species richness in the multispecies experiment was plotted against disturbance intensity at different sampling dates; data are means ± SE. (F–J) Number of cumulative species extinctions, with and without interspecific competition, was plotted against disturbance intensity at each sampling date. As all populations reached equilibrium states in the single-species experiment by day 30, the expected cumulative species extinctions at t = 6 mo were the same as those on day 30.
Given the absence of all species across much of the disturbance gradient at the end of the multispecies experiment (Fig. 2E), one likely explanation for the observed decline of species diversity with disturbance is that high levels of disturbances directly eliminated most or all species. This idea, however, was not supported by our single-species experiment, which showed that several species, when alone, were able to sustain viable populations even at the highest disturbance intensities (Fig. S1). It should be noted that, under conventional thinking, these disturbance-tolerant species are expected to dominate under high disturbances; instead, all of these species became extinct under interspecific competition in our multispecies experiment (Fig. 2). These results support the hypothesis that competition can still play a significant structuring role in highly disturbed habitats (9).
If competition consistently regulates community structure throughout the disturbance gradient, the combined effect of competition and disturbance may potentially explain the negative disturbance–diversity relationships observed in our multispecies experiment. To explore this possibility, we assessed the importance of interspecific competition more quantitatively using a null community model approach (21). This was done by comparing the actual number of species extinctions in the presence of interspecific competition (that is, extinctions observed in the multispecies experiment) with the expected number of species extinctions in the absence of interspecific competition (that is, predicted extinctions based on the results of the single-species experiment) (Materials and Methods). We found that species extinctions that were attributable to interspecific competition occurred throughout the entire disturbance gradient (Fig. 2 F–J). Most interestingly, the tempo of competitive exclusion was greater at higher, not lower, disturbance intensities (Fig. 2 F–J). At the early stage (week 1) of the multispecies experiment, interspecific competition already resulted in the extinction of seven species at the two highest disturbance levels, contrasting with no species extinction observed at the two lowest disturbance levels (Fig. 2F). Most competitive extinctions at the lowest disturbance levels did not occur until after week 3 of the experiment (Fig. 2 G–J). Therefore, contrary to the common perception that disturbance tends to reduce the importance of competition and mitigate competitive exclusion (5, 6, 17), increasing disturbance intensities actually increased the importance of competition in controlling species extinction and community richness.
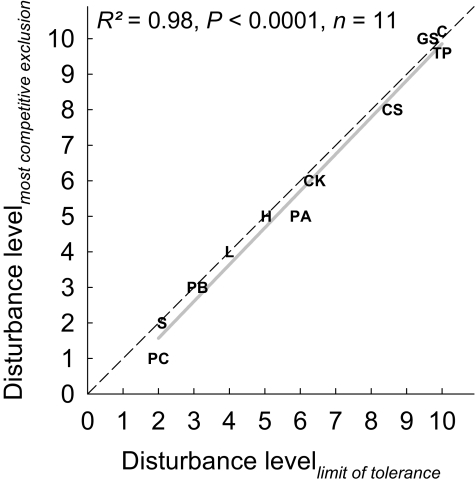
The above results may possibly be explained by increasing disturbance resulting in incrementally lower population densities of all species (Fig. S1), making these species (including disturbance-tolerant species) less tolerant of competition. To confirm this, we identified for each species the level of disturbance at which interspecific competition led to its most frequent extinctions, and compared this level to the highest disturbance level that the species can tolerate in the absence of interspecific competition (Materials and Methods). Strikingly, when all species were considered together, there was a strong positive relationship between the two levels, with the slope of the regression close to 1 (Fig. 3). This indicates that each species was most vulnerable to interspecific competition at its upper disturbance limit at which its density was most severely reduced, a finding supportive of the above hypothesis.
Fig. 3.
The relationship between the highest disturbance level that the species can tolerate in the absence of interspecific competition as recorded in the single species experiment (Disturbance levellimit of tolerance) and the level of disturbance at which interspecific competition led to the most frequent extinctions of each species in the multispecies experiment (Disturbance levelmost competitive exclusion). Solid and broken lines are the linear regression line and 1:1 line, respectively.
Current empirical evidence indicates that although the unimodal disturbance–diversity pattern predicted by the IDH has been frequently documented (4, 6, 22–27), other forms of disturbance–diversity relationships in fact tend to be more common (28). The persistent role of competition along the disturbance gradient found in our experiment, which challenges a major IDH assumption, provides a possible explanation for this mismatch between theory and empirical data. However, the extent to which this explanation can account for the abundant nonunimodal disturbance–diversity patterns observed in various systems remains unclear. In particular, we note three characteristics of our experimental system that may influence the applicability of our results to some other systems. First, protist species in our experiments competed exploitatively for shared bacterial resources. However, theory suggests that the mechanism of competition may influence how disturbance alters competitive outcomes (29). It thus may not be straightforward to extrapolate our findings based on exploitive competition to communities characterized by interference competition (e.g., competition for space). Second, there was no outside immigration into our experimental microcosms, contrasting with natural communities subject to various degrees of immigration. We chose to control for immigration because it would have effectively prevented species extinction, which may compromise our ability to estimate the importance of competition. This decision, however, means the loss of the opportunity of evaluating whether the competition-colonization tradeoff, known to exist among our study organisms (30), can result in IDH patterns, as often predicted (31, 32). More generally, we note that few empirical studies have established the linkage between the competition–colonization tradeoff and the unimodal disturbance–diversity pattern (33), an issue that clearly warrants further investigation. Third, given the small microcosm size, disturbance in our experiments can be considered as having a “global” effect throughout each microcosm. This contrasts with the more common situation in nature whereby localized disturbance generates spatial heterogeneity among different habitat patches, which characterizes many empirical studies supportive of the IDH (31). Direct extrapolation of our results to cases influenced by local disturbance is hampered by the fact that spatial heterogeneity in the latter case may additionally influence species coexistence and diversity (34, 35). Nevertheless, there is theoretical evidence that similar mechanisms could operate in both cases to generate IDH patterns (31), as well as experimental evidence that IDH patterns can emerge in the presence of global disturbance (14, 36). Comparable work in other systems is needed to determine the generality of our results. In addition to the above limitations, our explanation of persistent competition underlying the lack of IDH patterns is also complicated by the fact that a host of other factors, such as productivity (5), trophic interactions (29), and history of community assembly (14), may alter disturbance–diversity relationships.
Despite the above caveats regarding our results in relation to the IDH, our study provides unique experimental evidence that competition can consistently regulate species extinction and community richness over broad disturbance gradients. This result challenges conventional thinking (4–6) but is consistent with the prediction of the few theoretical investigations (9), with important implications for understanding relevant ecological issues, such as those related to biodiversity, community assembly, and conservation. For example, our study suggests that the reduced importance of competition with increasing disturbance is unlikely to be behind empirical patterns consistent with the IDH. More attention should thus be focused on examining other candidate mechanisms that may potentially contribute to IDH patterns (e.g., disturbance-induced spatial structure). Another corollary of our result is that priority effects associated with varying histories of community assembly may not necessarily be weak in highly disturbed habitats as previously thought (7, 14, 37), a hypothesis that needs to be evaluated experimentally. Our results also suggest that when evaluating species extinction risk, one needs to take into account the effect of interspecific competition, even in habitats strongly influenced by disturbance. Given that predation can reduce population sizes and make species more vulnerable to disturbance in a similar way (38), conservation biology can benefit from considering the roles of both abiotic and biotic interactions.
Materials and Methods
Single-, Bi-, and Multispecies Experiments.
The single-species experiment, which consisted of 363 microcosms, assessed the ability of each species to cope with disturbance in the absence of interspecific competition. Daily disturbance started 3 d after the introduction of species into microcosms, and population density of each species was estimated every 2–3 d until carrying capacity or extinction was reached (∼1 mo). Species were ranked by their ability to tolerate disturbance (discussed below). The bispecies experiment, which consisted of all possible species pairwise interactions established in 165 microcosms, assessed species’ competitive ability in the absence of disturbance. Weekly samples were taken from each microcosm to record species presence/absence until week 10, when competitive exclusion and stable coexistence (i.e., no trend of population decline) were observed. Species were ranked by their competitive ability, assessed by the number of pairwise combinations in which they persisted at the end of the experiment (30). The multispecies experiment, consisting of 55 microcosms, subjected communities of the 11 protist species to both disturbance and interspecific competition. Daily disturbance again started 3 d after the introduction of species into microcosms, and was suspended after 30 d. We continued running the experiment without disturbance for another 5 mo to confirm that our results on day 30 were not transient. The population density of each species was recorded every 2–3 d during the first 30 d and every 2 wk during the following 5 mo.
Microcosms.
Microcosms were 250-mL glass bottles each filled with 100 mL aqueous bacterized medium. The medium was prepared using a formula of 0.55 g of protozoan pellet (Carolina Biological Supply Co.) per 1 L deionized water. Experimental containers and medium were autoclaved, after which the medium was inoculated with three bacterial species (Bacillus cereus, Bacillus subtilis, and Serratia marcescens). The laboratory stock cultures of the studied protist species were also raised on the same three bacterial species. The bacterized medium was distributed into individual microcosms 48 h after bacterial inoculation. In all experiments, ∼100 individuals of each protist species were used as a starting density. All microcosms were maintained at 22 °C in a 12:12-h light:dark cycle. We replenished 10% medium of each microcosm at a weekly interval for the duration of the experiments.
Disturbance Manipulation.
We simulated mortality-causing disturbances using sonication, as in previous microcosm studies (14, 15). The intensity of disturbance was manipulated by sonicating each microcosm with a Sonic Dismembrator Model 100 (Fisher Scientific) at 10 different power levels (from 1 to 10) for 40 s every 24 h (±30 min). A control treatment without disturbance (level 0) was also included. Altogether, each microbial community type was subjected to a gradient of disturbance intensity with 11 levels. Although our experiments showed strong effects of disturbance, in the form of sonication, on protist populations, we did not monitor its effects on bacteria. We focused our attention on protists, as bacteria can presumably recover from disturbance faster than protists, because of their shorter generation times. This is supported by two protist microcosm experiments that reported little effect of disturbance (in the form of sonication and temperature shock, respectively) on bacteria (15, 19).
Assessing Species’ Disturbance-Tolerance Ability from the Single-Species Experiment.
To quantify species’ ability to tolerate disturbance, we applied generalized linear models to species presence/absence data at the end of the single-species experiment. In the models, the logit of the probability of occurrence of species i was a linear function of disturbance described as:
where pi,x is the conditional probability of species i being present at disturbance level x, and bi,j is the jth regression coefficient for species i. Likelihood ratio tests were used to assess the significance of the logit models as compared with null models. All logit regressions were highly significant (P < 0.0005 for all species). We estimated species’ distribution over the disturbance gradient using 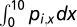 . This number was used to represent species’ ability to tolerate disturbance, as species more tolerant to disturbance tend to be more widely distributed along the disturbance gradient. This number was strongly correlated with the maximum disturbance that a species can tolerate (R2 = 0.98, P < 0.0001, n = 11).
. This number was used to represent species’ ability to tolerate disturbance, as species more tolerant to disturbance tend to be more widely distributed along the disturbance gradient. This number was strongly correlated with the maximum disturbance that a species can tolerate (R2 = 0.98, P < 0.0001, n = 11).
For each species, the maximum disturbance level that it can tolerate in the absence of interspecific competition (Distubance levellimit of tolerance) was identified as the disturbance level at which its extinction probability (1 − pi) reaches 1.
Measuring the Importance of Competition.
At the community level, we assessed the importance of competition by comparing the number of species extinctions in the multispecies experiment with the number of expected species extinctions without interspecific competition, calculated as the total number of extinct species in the single-species experiment at each disturbance level (21).
At the species level, we measured the importance of competition at a given disturbance level as the absolute difference between population extinction rates with and without interspecific competition, recorded at the end of the multispecies and single-species experiments, respectively. Based on this measure, we identified the disturbance level at which the importance of competition was maximum for each species (Disturbance levelmost competitive exclusion). We obtained similar results when related measures, such as relative differences in population extinction rates, differences in extinction times, and differences in population growth rates, were used.
Supplementary Material
Acknowledgments
We thank Peter Chesson, Philip Grime, and Peter Morin for comments and discussion. This project was supported by National Science Foundation Grant DEB-0640416 (to L.J.) and by Georgia Institute of Technology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000699107/-/DCSupplemental.
References
- 1.Grime JP. Plant Strategies and Vegetation Processes. Chichester: Wiley; 1979. [Google Scholar]
- 2.Tilman D. Dynamics and Structure of Plant Communities. Princeton: Princeton University Press; 1988. [Google Scholar]
- 3.Whittaker RH. Dominance and diversity in land plant communities: Numerical relations of species express the importance of competition in community function and evolution. Science. 1965;147:250–260. doi: 10.1126/science.147.3655.250. [DOI] [PubMed] [Google Scholar]
- 4.Grime JP. Competition exclusion in herbaceous vegetation. Nature. 1973;242:344–347. [Google Scholar]
- 5.Huston M. A general hypothesis of species diversity. Am Nat. 1979;113:81–101. [Google Scholar]
- 6.Connell JH. Diversity in tropical rain forests and coral reefs. Science. 1978;199:1302–1310. doi: 10.1126/science.199.4335.1302. [DOI] [PubMed] [Google Scholar]
- 7.Weiher E, Keddy PA. Assembly rules, null models, and trait dispersion: New questions from old patterns. Oikos. 1995;74:159–164. [Google Scholar]
- 8.Pianka E. Latitudinal gradients in species diversity: A review of concepts. Am Nat. 1966;100:33–46. [Google Scholar]
- 9.Chesson P, Huntly N. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. Am Nat. 1997;150:519–553. doi: 10.1086/286080. [DOI] [PubMed] [Google Scholar]
- 10.Campbell BD, Grime JP. An experimental test of plant strategy theory. Ecology. 1992;73:15–29. [Google Scholar]
- 11.Turkington R, Klein E, Chanway C. Interactive effects of nutrients and disturbance: An experimental test of plant strategy theory. Ecology. 1993;74:863–878. [Google Scholar]
- 12.Welden CW, Slauson WL. The intensity of competition versus its importance: An overlooked distinction and some implications. Q Rev Biol. 1986;61:23–44. doi: 10.1086/414724. [DOI] [PubMed] [Google Scholar]
- 13.Freckleton R, Watkinson A, Rees M. Measuring the importance of competition in plant communities. J Ecol. 2009;97:379–384. [Google Scholar]
- 14.Jiang L, Patel SN. Community assembly in the presence of disturbance: A microcosm experiment. Ecology. 2008;89:1931–1940. doi: 10.1890/07-1263.1. [DOI] [PubMed] [Google Scholar]
- 15.Steiner CF. Impacts of density-independent mortality and productivity on the strength and outcome of competition. Ecology. 2005;86:727–739. [Google Scholar]
- 16.Gallet R, et al. Predation and disturbance interact to shape prey species diversity. Am Nat. 2007;170:143–154. doi: 10.1086/518567. [DOI] [PubMed] [Google Scholar]
- 17.McGrady-Steed J, Morin P. Disturbance and the species composition of rain pool microbial communities. Oikos. 1996;76:93–102. [Google Scholar]
- 18.Haddad NM, et al. Species’ traits predict the effects of disturbance and productivity on diversity. Ecol Lett. 2008;11:348–356. doi: 10.1111/j.1461-0248.2007.01149.x. [DOI] [PubMed] [Google Scholar]
- 19.Scholes L, Warren P, Beckerman A. The combined effects of energy and disturbance on species richness in protist microcosms. Ecol Lett. 2005;8:730–738. [Google Scholar]
- 20.Horn H. In: Ecology and Evolution of Communities. Cody M, Diamond J, editors. Cambridge, MA: Belknap; 1975. pp. 196–211. [Google Scholar]
- 21.Goldberg D. Influence of competition at the community level: An experimental version of the null models approach. Ecology. 1994;75:1503–1506. [Google Scholar]
- 22.Sousa W. Disturbance in marine intertidal boulder fields: The non-equilibrium maintenance of species diversity. Ecology. 1979;60:1225–1239. [Google Scholar]
- 23.Paine R, Levin S. Intertidal landscapes: Disturbance and the dynamics of pattern. Ecol Monogr. 1981;5:145–178. [Google Scholar]
- 24.Molino J-F, Sabatier D. Tree diversity in tropical rain forests: A validation of the intermediate disturbance hypothesis. Science. 2001;294:1702–1704. doi: 10.1126/science.1060284. [DOI] [PubMed] [Google Scholar]
- 25.Platt W, Beckage B, Doren R, Slater H. Interactions of large-scale disturbances: Prior fire regimes and hurricane mortality of savanna pines. Ecology. 2002;83:1566–1572. [Google Scholar]
- 26.Svensson JR, et al. Maximum species richness at intermediate frequencies of disturbance: Consistency among levels of productivity. Ecology. 2007;88:830–838. doi: 10.1890/06-0976. [DOI] [PubMed] [Google Scholar]
- 27.Bongers F, Poorter L, Hawthorne WD, Sheil D. The intermediate disturbance hypothesis applies to tropical forests, but disturbance contributes little to tree diversity. Ecol Lett. 2009;12:798–805. doi: 10.1111/j.1461-0248.2009.01329.x. [DOI] [PubMed] [Google Scholar]
- 28.Mackey R, Currie D. The diversity-disturbance relationship: Is it generally strong and peaked? Ecology. 2001;82:3479–3492. [Google Scholar]
- 29.Wootton JT. Effects of disturbance on species diversity: A multitrophic perspective. Am Nat. 1998;152:803–825. doi: 10.1086/286210. [DOI] [PubMed] [Google Scholar]
- 30.Cadotte MW, et al. On testing the competition-colonization trade-off in a multispecies assemblage. Am Nat. 2006;168:704–709. doi: 10.1086/508296. [DOI] [PubMed] [Google Scholar]
- 31.Roxburgh SH, Shea K, Wilson JB. The intermediate disturbance hypothesis: Patch dynamics and mechanisms of species coexistence. Ecology. 2004;85:359–371. [Google Scholar]
- 32.Petraitis P, Latham R, Niesanbaum R. The maintenance of species diversity by disturbance. Q Rev Biol. 1989;64:393–418. [Google Scholar]
- 33.Cadotte MW. Competition-colonization trade-offs and disturbance effects at multiple scales. Ecology. 2007;88:823–829. doi: 10.1890/06-1117. [DOI] [PubMed] [Google Scholar]
- 34.Huffaker C. Experimental studies on predation: Dispersion factors and predator-key oscillations. Hilgardia. 1958;27:343–383. [Google Scholar]
- 35.Amarasekare P. Competitive coexistence in spatially structure environments: A synthesis. Ecol Lett. 2003;6:1109–1122. [Google Scholar]
- 36.Buckling A, Kassen R, Bell G, Rainey PB. Disturbance and diversity in experimental microcosms. Nature. 2000;408:961–964. doi: 10.1038/35050080. [DOI] [PubMed] [Google Scholar]
- 37.Chase JM. Community assembly: When should history matter? Oecologia. 2003;136:489–498. doi: 10.1007/s00442-003-1311-7. [DOI] [PubMed] [Google Scholar]
- 38.Schoener TW, Spiller DA, Losos JB. Predators increase the risk of catastrophic extinction of prey populations. Nature. 2001;412:183–186. doi: 10.1038/35084071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.