Abstract
Recent reports have suggested critical roles of myeloid cells in tumor invasion and metastasis, although these findings have not led to therapeutics. Using a mouse model for liver dissemination, we show that mouse and human colon cancer cells secrete CC-chemokine ligands CCL9 and CCL15, respectively, and recruit CD34+ Gr-1− immature myeloid cells (iMCs). They express CCL9/15 receptor CCR1 and produce matrix metalloproteinases MMP2 and MMP9. Lack of the Ccr1, Mmp2, or Mmp9 gene in the host dramatically suppresses outgrowths of disseminated tumors in the liver. Importantly, CCR1 antagonist BL5923 blocks the iMC accumulation and metastatic colonization and significantly prolongs the survival of tumor-bearing mice. These results suggest that CCR1 antagonists can provide antimetastatic therapies for patients with disseminated colon cancer in the liver.
Keywords: chemokine, metalloproteinase, stromal cell
Colon cancer is one of the leading causes of cancer-related deaths (1). Although most primary tumors can be resected surgically, colorectal cancer frequently spreads to the liver, which is responsible for the high mortality of the disease (2). For successful metastasis, cancer cells need to invade surrounding tissues, penetrate microvessels, survive in circulation, disseminate to distant organs, form micrometastases, and expand into macrometastases. To progress through these steps, tumor cells often acquire the capability of survival and invasion by activating metastatic signaling pathways or inactivating metastasis suppressor genes (2, 3). In addition to these cell autonomous changes, tumor stromal cells, especially bone marrow-derived myeloid cells, actively participate in early steps of the metastatic cascade in some mouse models (4). For example, tumor-associated macrophages (TAMs) promote migration and intravasation of mammary tumor cells (5, 6). Bone marrow-derived cells that express myeloid cell marker CD11b and granulocyte marker Gr-1 (CD11b+ Gr-1+) also promote metastasis of breast cancer cells, likely through promotion of intravasation and suppression of immune responses (7). Furthermore, CD11b+ myeloid cells that express vascular endothelial growth factor receptor 1 (VEGFR1) accumulate at the metastatic sites before the arrival of lung cancer and melanoma cells and foster the dissemination of the cancer cells (8). These reports suggest that bone marrow-derived myeloid cells can help cancer epithelium in early steps of metastasis. It remains to be determined whether therapeutics targeting such myeloid cells can prevent cancer metastasis (9).
As a model for invasive colon cancer, we previously constructed cis-Apc+/Δ716 Smad4+/− (Apc/Smad4) mice that develop intestinal adenocarcinomas with marked invasions by loss of Apc and Smad4 tumor suppressor genes in the intestinal epithelium (10, 11). In the Apc/Smad4 tumors, we reported that the invading cancer epithelium is associated with immature myeloid cells (iMCs) that express myeloid progenitor cell marker CD34 and CD11b (12). Because these iMCs do not express Gr-1 or VEGFR1, they belong to a different subclass from the Gr-1+ iMCs in breast cancer (7) or VEGFR1+ myeloid cells in premetastatic niches (8). Using the Apc/Smad4 mice, we further showed that the CD34+ Gr-1− iMCs promote colon cancer invasion into the adjacent tissues (12).
However, whether colon cancer cells in the metastasizing sites can recruit the iMCs like those in the primary sites has not been investigated. Because the intestinal tumors in Apc/Smad4 mice do not metastasize to the liver or lung during their short lifespan (11), and because no practical models are available whose endogenous (i.e., nontransplant) tumors progress and metastasize to the liver (or lung), here we have resorted to a transplantation model to determine whether colon cancer cells can recruit the iMCs in the metastasizing sites.
Results
Mouse Colon Cancer Cells Disseminated to the Liver Are Associated with iMCs.
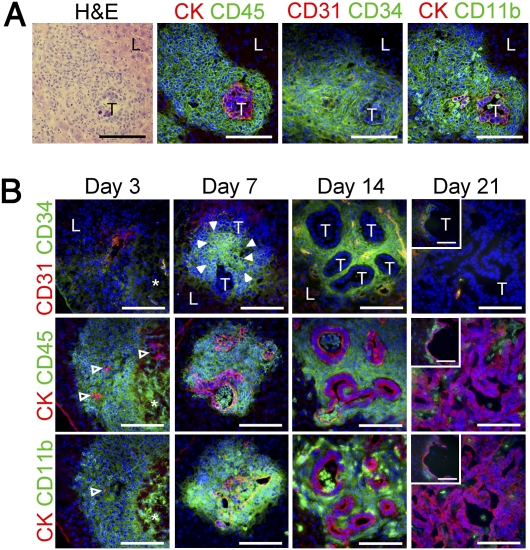
To investigate possible roles of the CD34+ Gr-1− iMC subclass in colon cancer metastasis, we injected CMT93 mouse colon cancer cells (Table S1) into the spleen of syngeneic C57BL/6 mice, which allowed efficient dissemination of tumor cells to the liver. We found massive accumulations of stromal cells that expressed CD34, CD11b, and CD45, but not Gr-1 or VEGFR1 (Fig. 1A and Fig. S1A). We further confirmed that they did not express CD31 (a marker for endothelial cells), CD14 (monocytes), B2.20 (B-cells), CD3ε (T cells), or αSMA (myofibroblasts). These characteristics fit those of the iMC subclass in the primary colon cancer of Apc/Smad4 mice (12). Of note, some of these stromal cells in the liver expressed macrophage marker F4/80 and dendritic cell marker CD11c that were absent in the Apc/Smad4 primary tumors (Fig. S1A). We also verified their bone marrow-origin by transplanting bone marrow cells containing GFP into irradiated recipients (Fig. S1 B and C). Based on these results, we concluded that the cancer-associated stromal myeloid cells in the liver belong to the CD34+ Gr-1− iMC subclass. Further analyses showed that the iMCs started to accumulate at day 7 postinjection when disseminated cancer cells began to form tumor glands. The iMCs accumulated further by day 14 with expansion of the tumor glands in the liver (Fig. 1B). By day 21, the iMCs disappeared from the metastatic lesions where the cancer cells formed massive glands or carcinoma cysts, suggesting the possibility that the iMCs contributed to an early phase of metastatic expansion. As mentioned above, we could not find VEGFR1+ cells in the liver lesions during 2 wk after injection (Fig. S1A), although such cells were reported in lung metastatic sites at day 12 after s.c. injection of melanoma cells (8).
Fig. 1.
Accumulation of iMCs around disseminated cancer cells in the liver. (A) A metastatic focus in the liver stained with H&E (Far Left). Serial sections were immunostained for pan-cytokeratin (CK), CD45, CD31, CD34, and CD11b. T, metastasizing tumor; L, adjacent normal liver. (Scale bars, 100 μm.) (B) Liver metastasis foci dissected at days 3, 7, 14, and 21 postinjection. Open arrowheads, disseminated tumor cells; filled arrowheads, a cluster of the iMCs; asterisks, a necrotic area. T, tumor glands; L, adjacent normal liver. (Insets) A representative carcinoma cyst. (Scale bars, 100 μm.)
Mouse Colon Cancer Cells Secrete CCL9 and Recruit CCR1-Expressing iMCs to the Liver.
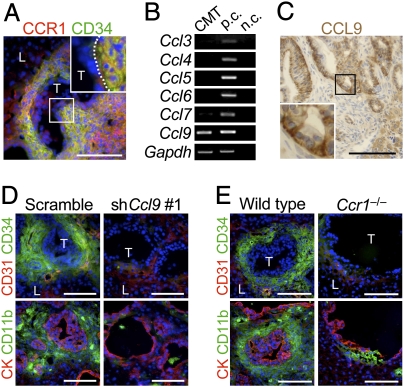
We next investigated CCR1 expression, another important characteristic of the iMCs. Most iMCs at the metastatic foci expressed CCR1, whereas the cancer epithelium did not (Fig. 2A). Because specific ligands activate CCR1 (13) and recruit the iMCs (12), we looked for such ligands that were expressed by the cancer cells. Among them, CMT93 cells expressed only Ccl9 mRNA but others did not (Fig. 2B). Consistently, cultured CMT93 cells secreted CCL9 protein (25 ± 3 pg/105 cells) at a similar level to that by intestinal cancer cells from Apc/Smad4 polyps (37 ± 16 pg/105 cells). Immunostaining data also confirmed that metastasized CMT93 cells expressed CCL9 but the surrounding stromal cells did not (Fig. 2C).
Fig. 2.
Inactivation of CCL9-CCR1 signaling blocks accumulation of the iMCs in the liver. (A) A metastatic focus in the liver immunostained for CCR1 and CD34. (Inset) A higher magnification of the boxed area. T, tumor; L, liver. (Scale bar, 100 μm.) (B) Expression of mRNAs for CCR1 ligands determined by RT-PCR. Total RNA was isolated from cultured CMT93 cells. Total RNA from intestinal polyps of Apc/Smad4 mice was used as a control (p.c.). Negative controls (n.c.) had distilled water instead of RNA. (C) A metastatic focus in the liver immunostained for CCL9 (brown DAB staining with hematoxylin counterstaining). (Inset) A higher magnification of the boxed area. (Scale bar, 100 μm.) (D) Liver metastasis foci in mice injected with CMT93 cells expressing scramble RNA (Scramble) and shRNA against Ccl9 (shCcl9#1). Serial sections were immunostained for CK, CD31 and the iMC markers (CD34 and CD11b). T, tumor; L, liver. (Scale bars, 100 μm.) (E) Liver metastasis foci in wild-type and Ccr1−/− mice injected with CMT93 cells. (Scale bars, 100 μm.)
To assess the importance of CCL9 in iMC accumulation in the liver, we prepared CMT93 derivatives that contained shRNA against Ccl9 or control scramble RNA. Two constructs of the shRNA, shCcl9#1 and shCcl9#2, reduced the CCL9 levels to 25% and 47% of control CMT93-scramble cells, respectively (i.e., 7 ± 1, 14 ± 2, and 29 ± 7 pg/105 cells for CMT-shCcl9#1, CMT-shCcl9#2, and CMT93-scramble, respectively). As expected, almost all metastatic foci formed by control cells accumulated iMCs markedly. In contrast, such accumulations were not observed in the majority of foci by the CCL9-reduced CMT93 cells (CMT93-shCcl9#1; Fig. 2D). We further injected CMT93 cells into Ccr1−/− mice and found that 7 of 10 mice formed no visible metastatic foci in the liver, although the remaining three developed some foci (see below). We therefore examined the minor population of mice for liver metastases, but iMCs were missing around most such lesions (Fig. 2E). Collectively, these results suggest that activation of CCR1 by cancer-secreted CCL9 is critical for the iMCs to accumulate at the metastatic foci. As an exception, we found several small metastatic lesions where numerous iMCs accumulated despite the lack of CCR1 (Fig. S2), suggesting a rare alternative mechanism that can recruit iMCs independent of CCR1.
Inactivation of the Mouse CCL9-CCR1 Signaling Blocks Metastatic Expansion of Cancer in the Liver, and Prolongs Host Survival.
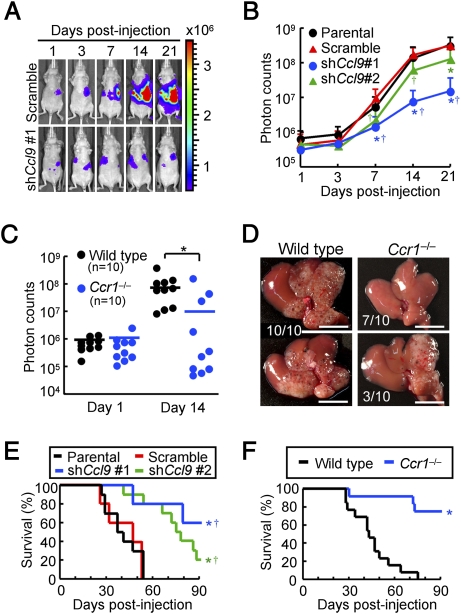
Because the iMCs helped invasion of primary tumors in the intestines (12), we hypothesized that accumulation of the iMCs could also promote the metastatic expansion of disseminated colon cancer in the liver. We therefore injected luciferase-expressing CMT93 cells into nu/nu mice (nude mice), which enabled monitoring of the liver tumors by bioluminescence over time. In mice injected with the parental CMT93 or CMT93-scramble cells, the intensity of bioluminescence began to increase at day 7 postinjection and increased exponentially thereafter (Fig. 3 A and B). In contrast, such an increase in luminescence was markedly suppressed in mice injected with CMT93-shCcl9#1 cells, although they showed essentially the same levels of luminescence as control groups up to day 3. Another shRNA construct (shCcl9#2) showed a similar effect, although at a weaker level than that of shCcl9#1 (Fig. 3B). Of note, all CMT93 derivatives showed essentially the same luciferase activity and proliferation rate in culture. We then injected luciferase-expressing CMT93 cells into the wild-type and Ccr1−/− mice. Although all wild-type mice showed marked increases in the luminescence level at day 14 postinjection, such an increase was not observed in 70% (7/10) of Ccr1−/− mice (Fig. 3C). In these hosts, we did not find any macroscopic foci on liver surfaces (Fig. 3D). These results indicate that accumulation of the iMCs via CCL9-CCR1 axis plays a major role in the expansion of liver metastasis foci. Although all mice injected with the CMT93-scramble or parental cells became moribund within 54 d, 60% (3/5) and 20% (2/10) of mice injected with CMT93-shCcl9 #1 and CMT93-shCcl9 #2 cells, respectively, remained alive till the end of analysis (day 90; Fig. 3E). Likewise, 75% (9/12) of the tumor-transplanted Ccr1−/− mice survived to day 90, in contrast to the wild type that became moribund before day 75 (Fig. 3F). These results indicate that inactivation of the CCL9-CCR1 signaling prolongs the survival by blocking early metastatic expansion.
Fig. 3.
Blockade of CCL9-CCR1 signaling suppresses expansion of metastatic lesions and prolongs host survival. (A) Representative in vivo bioluminescence images of mice injected with luciferase-expressing CMT93 cells that contained scramble RNA (Upper) or shRNA against Ccl9 (Lower). Colored scale bar represents intensity of bioluminescence in photons/sec. (B) Quantification of metastatic lesions by bioluminescence (photon counts) from luciferase-expressing CMT93 cells (Parental), or their derivatives that contained scramble RNA (Scramble) or shRNA against Ccl9 (shCcl9#1 or shCcl9#2). Results are given as the means ± SD *P < 0.02 and †P < 0.02 compared with Parental and Scramble, respectively (n = 8–11 mice in each group). (C) Quantification of metastatic lesions by bioluminescence from luciferase-expressing CMT93 cells injected into wild-type and Ccr1−/− mice. Each circle represents an individual mouse. Horizontal lines show the means of the respective groups. *P < 0.01. (D) Representative macroscopic views of the liver dissected from wild-type and Ccr1−/− mice injected with CMT93 cells. (Scale bars, 10 mm.) (E) Kaplan-Meier plot showing survival of wild-type hosts injected with control CMT93 cells (Prnt and Scr) or those containing shRNA constructs against Ccl9 (shCcl9#1 or shCcl9#2). *P < 0.01 and †P < 0.02 compared with Prnt and Scr, respectively (n = 5–10 mice in each group). (F) Survival rates of wild-type and Ccr1−/− mice injected with parental CMT93 cells. *P = 0.0001(n = 12–13 mice in each group).
Lack of MMP2 or MMP9 in the Host Mouse Suppresses Expansion of Metastatic Lesions in the Liver.
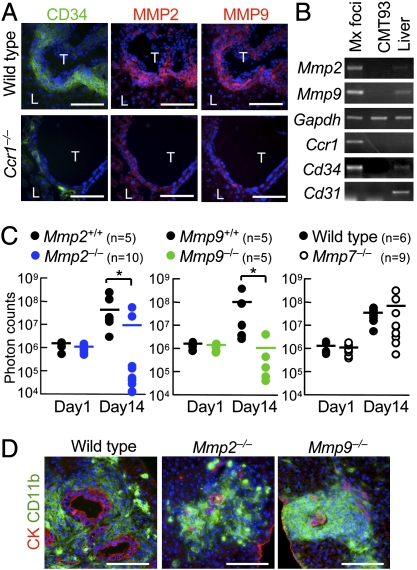
For metastatic expansion in the liver, disseminated cancer cells need to invade the liver parenchyma. We therefore hypothesized that the iMCs promote intrahepatic invasion of disseminated cancer through secretion of proteases. To identify such enzymes, we compared mRNA levels in the metastatic foci between the wild-type and Ccr1−/− mice by microarray and RT-PCR (Fig. S3A). The results showed more than twofold increases in the levels of Mmp2, Mmp7, Mmp9, and Mmp13 in the iMC-associated foci in wild-type mice (Fig. S3B). In such lesions, MMP2 and MMP9 were expressed in the stromal iMCs, but not in the cancer epithelium (Fig. 4 A and B), whereas MMP7 and MMP13 were found primarily in the epithelium (Fig. S3C). Consistently, MMP2 and MMP9 were absent around the liver foci in Ccr1−/− mice where the iMCs were missing, although MMP7 and MMP13 were present (Fig. 4A and Fig. S3C). We then injected luciferase-expressing CMT93 cells into Mmp2−/− mice and found significant reduction in the liver luminescence at day 14, compared with those in Mmp2+/+ littermates (Fig. 4C). Likewise, the luciferase luminescence was much lower in Mmp9−/− mice than Mmp9+/+ littermates, whereas such a reduction was not observed in Mmp7−/− mice (Fig. 4C). Notably, almost all metastatic foci in the Mmp2−/− and Mmp9−/− mice consisted of smaller cancer glands than those in wild-type mice, although they were associated with the iMCs (Fig. 4D). These results suggest that MMP2 and MMP9 are critical for the iMCs to promote metastatic expansion of colon cancer cells, but not to accumulate in the liver.
Fig. 4.
Lack of MMP2 or MMP9 inhibits expansion of metastatic liver lesions. (A) Liver metastasis foci from wild-type and Ccr1−/− mice injected with CMT93 cells. Serial sections were stained for CD34, MMP2, and MMP9. T, tumor; L, liver. (Scale bars, 100 μm.) (B) Expression of MMP mRNAs determined by RT-PCR. Total RNA was isolated from metastatic foci in wild-type mice (Mx foci), cultured CMT93 cells, and normal mouse liver. Expression of mRNAs for CCR1, CD34, and CD31 were also determined. (C) Quantification of metastatic lesions by bioluminescence from luciferase-expressing CMT93 cells. Each circle represents an individual mouse. Horizontal lines show the means of the respective groups. *P < 0.01 (n = 9–11 mice in each group). (D) Liver metastasis foci in wild-type, Mmp2−/−, and Mmp9−/− mice injected with CMT93 cells. (Scale bars, 100 μm.)
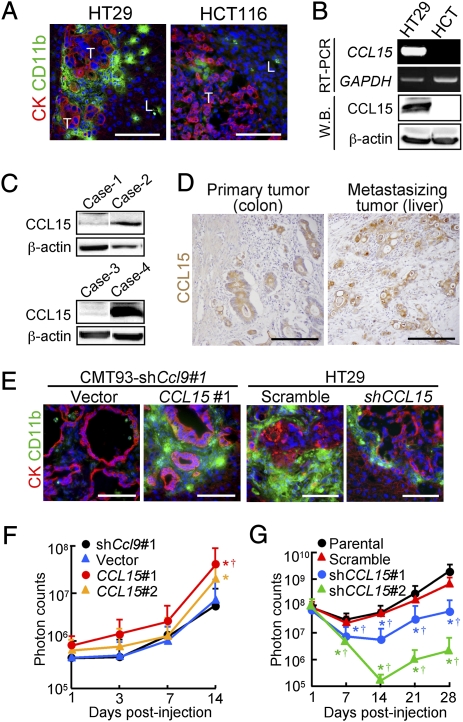
Some Human Colon Cancer Cells Express CCR1 Ligand CCL15.
To test whether human colon cancer cells could recruit iMCs, we injected four cell lines into the spleen of nu/nu mice and found that HT29 cells were associated with the iMCs in the liver, whereas HCT116, DLD-1, or SW620 cells were not (Fig. 5A and Fig. S4A). Based on the structural similarity, human orthologs of mouse CCL9 have been suspected to be CCL15 and/or CCL23 (14–16). Thus, we determined the levels of these chemokines in 11 human colon cancer cell lines, and found that six of them including HT29 expressed CCL15 mRNA and protein at high levels, but not CCL23 (Fig. 5B, Fig. S4 B and C, and Table S1). We could not detect mRNAs for any other CCR1 ligands; CCL3, 4, 5, 7, 14, or 16 (Fig. S4C). We further verified by Western blotting that 28% (13/47) of human colon cancer specimens expressed CCL15 protein (Fig. 5C). An immunostaining analysis also showed that 29% (12/41) of primary tumors and 29% (12/41; a separate combination) of liver metastases expressed CCL15 in the cancer epithelium (Fig. 5D). These results demonstrate that a subset of human colon cancer cases secretes CCL15 and suggest that it may recruit CCR1+ human iMCs.
Fig. 5.
Human CCL15 recruits the iMCs and promotes expansion of metastatic lesions in mouse liver. (A) Liver metastasis foci formed by human colon cancer cells HT29 and HCT116. T, tumor; L, liver. (Scale bars, 100 μm.) (B) Levels of CCL15 mRNA and protein determined by RT-PCR and Western blotting, respectively. Total RNA and lysates were prepared from metastatic foci formed by the human colon cancer cell lines in mouse liver. (C) Determination of CCL15 protein levels by Western blotting. Lysates were prepared from human colon cancer specimens. Cases-2 and 4 expressed CCL15. β-actin was used as a loading control. (D) Human colon cancer specimens immunostained for CCL15 (hematoxylin counterstaining). Tumors were dissected from the primary (colon) and metastatic (liver) sites of the same patients. (Scale bars, 100 μm.) (E) Liver metastasis foci from mice with CMT93-shCcl9#1 cells containing empty vector (Vector) or the CCL15 gene (CCL15#1) (Left), and with HT29 cells expressing scramble RNA (HT29-scramble) or shRNA against CCL15 (HT29-shCCL15) (Right). (Scale bars, 100 μm.) (F) Quantification of the metastatic lesions in mice injected with CMT93-shCcl9 cells (shCcl9#1), or their derivatives with empty (Vector) or CCL15-expressing vector (CCL15#1 or CCL15#2). Results are given as the means ± SD *P < 0.04 and †P < 0.03 compared with shCcl9#1 and Vector, respectively (n = 5–19 mice in each group). Note that the ordinate is in a logarithmic scale. (G) Quantification of the metastatic lesions in mice injected with luciferase-expressing HT29 cells (Parental), or their derivatives expressing scramble RNA (Scramble) or shRNA constructs against CCL15 (shCCL15#1 or shCCL15#2). Results are given as the means ± SD *P < 0.04 and †P < 0.04 compared with Parental and Scramble, respectively (n = 6–12 mice in each group).
Human CCL15 Promotes Accumulation of the iMCs and Expansion of Metastatic Foci in Mouse Liver.
To assess the role of human CCL15 in iMC accumulation and following metastatic expansion of cancer in the liver, we constructed derivatives of the CMT93-shCcl9#1 cells where not only mouse CCL9 expression was suppressed by an shRNA, but also human CCL15 was introduced. These cells secreted essentially the same level of CCL15 as those by HT29 cells (321 ± 16, 315 ± 34, and 302 ± 18 pg/105 cells for CMT-shCcl9:CCL15#1, CMT-shCcl9:CCL15#2, and HT29, respectively), whereas CMT93-shCcl9#1 cells with the empty vector (CMT-shCcl9:Vector) did not at any detectable levels. As expected, iMC accumulation was blocked in the majority of metastatic foci formed by CMT93-shCcl9:Vector cells. In contrast, numerous iMCs accumulated in most tumors of CMT93-shCcl9:CCL15#1 cells (Fig. 5E). We further found that mice injected with CMT93-shCcl9:CCL15#1 and CMT-shCcl9:CCL15#2 cells showed higher luminescence levels in the liver than those with control cells at day 14 (Fig. 5F). Of note, forced expression of CCL15 did not affect proliferation of CMT93 cells in culture. We next prepared HT29 cells containing shRNA against human CCL15 (HT29-shCCL15#1 and HT29-shCCL15#2) where expression of CCL15 was reduced to a negligible level (0.1 ± 0.2 and 4 ± 3 pg/105 cells for HT29-shCCL15#1 and HT29-shCCL15#2, respectively). In metastatic foci formed by HT29-shCCL15#1 cells, accumulation of the iMCs was markedly reduced compared with those by HT29-scramble cells (Fig. 5E). Furthermore, shRNA against CCL15 (shCCL15#1 and shCCL15#2) significantly reduced the luminescence levels in mice injected with HT29 cells (Fig. 5G). These results indicate that CCL15 secreted from human colon cancer cells can recruit mouse CCR1+ iMCs, and promote their metastatic expansion.
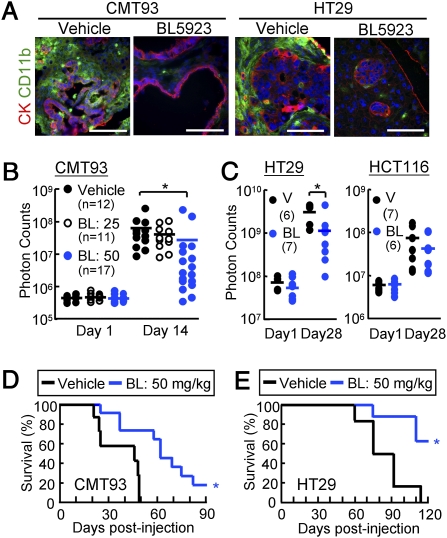
CCR1 Antagonist BL5923 Blocks Metastatic Expansion of Cancer in the Mouse Liver and Prolongs Host Survival.
Finally, we evaluated a CCR1 antagonist for its suppressive effects on colon cancer metastasis in the mouse liver dissemination model. We administered BL5923 (ref. 17, and SI Methods) or the vehicle to ν/ν mice that were injected with mouse (CMT93) or human (HT29) colon cancer cells as described above. Treatment of the host mice with 50 mg/kg of BL5923, but not the vehicle, markedly reduced the accumulation of iMCs around the metastatic foci of CMT93 as well as HT29 (Fig. 6A). In mice injected with the luciferase-expressing CMT93 cells, BL5923 (at 50 mg/kg) significantly reduced the luminescence levels at day 14, compared with the markedly increased levels in the vehicle-treated mice (Fig. 6B and Fig. S5A). A lower dose of the compound (25 mg/kg) was ineffective to block the metastatic expansion. As anticipated, BL5923 did not affect the proliferation rate of CMT93 cells in culture (Fig. S5B). Likewise, expansion of HT29 cells in the liver was significantly blocked by treatment with 50 mg/kg of BL5923 (Fig. 6C and Fig. S5C). In contrast, it did not suppress the metastatic expansion of HCT116 cells that could form metastases without iMC accumulation (Figs. 5A and 6C), consistent with the mechanism that BL5923 blocks tumor metastasis through CCR1 inhibition in the iMCs. We further found that the BL5923 treatment prolonged the mean survival of the hosts from 37 d postinjection to 62 d and from 84 d to 113 d when CMT93 and HT29 cells were injected, respectively (Fig. 6 D and E). These results strongly suggest that CCR1 antagonist BL5923 suppresses accumulation of the iMCs and early metastatic expansion of colon cancer, allowing the prolonged host survival.
Fig. 6.
CCR1 antagonist BL5923 blocks expansion of metastatic lesions and prolongs host survival. (A) Liver metastasis foci from mice treated with vehicle or BL5923 (50 mg/kg). Mice were injected with CMT93 cells (Left) or HT29 cells (Right). (Scale bars, 100 μm.) (B) Quantification of metastatic lesions in mice injected with CMT93 cells, and treated with the vehicle or BL5923 at 20 or 50 mg/kg (BL: 25 or BL: 50, respectively). Each circle represents an individual mouse. Horizontal lines show the means of the respective groups. *P < 0.01 compared with Vehicle (n = 11–17 mice in each group). (C) Quantification of the metastatic lesions in mice treated with vehicle (V) or 50 mg/kg of BL5923 (BL). Mice were injected with HT29 (Left) or HCT116 (Right) cells. Each circle represents an individual mouse. Horizontal lines show the means of the respective groups. *P < 0.04 compared with vehicle (n = 6–7 mice in each group). (D and E) Kaplan-Meier plots showing survivals of the host mice treated with the vehicle or BL5923 (BL: 50 mg/kg). Mice were injected with CMT93 (D) or HT29 (E). *P = 0.004 compared with vehicle (n = 7–11 mice in each group).
Discussion
In the mouse model, we have found that colon cancer cells disseminated to the liver begin to form micrometastases by day 7 postinjection and rapidly expand thereafter mirroring the extent of iMC accumulation (Figs. 1B and 3B). Because the disseminated cancer cells cannot expand when the iMC accumulation is blocked, these results suggest that the CD34+ Gr-1− iMCs promote a late step of the metastatic cascade: expansion of disseminated cancer and colonization. Because the iMCs have disappeared by day 21 postinjection when cancer cells colonize in the liver, they may support an early phase of metastatic expansion that is rate limiting in the metastatic cascade (18). Consistently, suppression of such early metastatic expansion prolongs survival of mice with disseminated colon cancer in the liver. On the other hand, different subclasses of myeloid cells such as TAMs and Gr-1+ iMCs help breast cancer cells to invade microvessels, an early step of the metastatic cascade (5–7). However, the CD34+ Gr-1− iMCs appear to have only a minor role, if any, in the intravasation and dissemination because the primary colon cancer in Apc/Smad4 mice do not metastasize despite marked accumulation of the iMCs at the invasion fronts and strong local invasion (10–12).
Interestingly, we have found that CMT93 mouse colon cancer cells recruit the iMCs from the bone marrow to metastatic lesions through the same mechanism as that underlying recruitment of the cells to the primary tumors, activation of CCR1 on the iMCs. Like intestinal tumor epithelium of Apc/Smad4 mice (12), CMT93 colon cancer cells secrete CCL9, a CCR1 ligand and strong chemoattractant for bone marrow cells (19). Because CMT93 cells do not express other CCR1 ligands (CCL3-CCL7), CCL9 appears to be the only ligand that plays a critical role in recruiting the iMCs. Although human ortholog of mouse CCL9 has not been identified, we have shown here that one-third of human colon cancer metastases express CCL15 that has a structural similarity with CCL9 (14–16). We have further demonstrated that HT29 human colon cancer cells secrete CCL15, recruit mouse iMCs, and expand in the liver, whereas the CCL15-reduced HT29 cells fail to do so. These results suggest that CCL15 secreted from human colon cancer plays a critical role in the recruitment of bone marrow-derived iMCs as a functional homolog of mouse CCL9. Consistently, human CCL15 can promote in vitro migration of mouse mononuclear cells isolated from the bone marrow (20). Although we have been unable to detect CCR1-expressing bone marrow-derived cells in clinical specimens of colon cancer metastasis, it is likely because access is limited only to those in late stages. The transient nature of iMC accumulation in metastasis remains to be investigated further.
In our liver metastasis model, we have found that the iMCs express MMP2 and MMP9, but the tumor epithelium does not. These results are consistent with previous reports that show stroma-restricted expression of the MMPs in human liver metastases of colorectal cancer (21, 22). Abundant expression of MMP2 or MMP9 is associated with poor prognosis and high mortality of colon cancer patients (23–27). Although selective inhibitors of MMP2/9 significantly block mouse liver metastasis of colon cancer and prolong the survival of tumor-bearing mice (28), clinical trials for MMP inhibitors have failed because of severe side effects such as musculoskeletal pain and inflammation (29–31). Alternatively, we have shown here in a mouse model that reduced iMC accumulation by inactivation of CCR1 can suppress metastatic expansion of colon cancer. These results provide the rationale for application of CCR1 antagonists to colon cancer treatment that targets the MMP-expressing myeloid cells, rather than direct and systemic inhibition of MMPs (32). Supporting this novel strategy of “cellular target therapy” (12), we have demonstrated here that CCR1 antagonist BL5923 significantly blocks liver metastasis of mouse (CMT93) and human (HT29) colon cancer cells and prolongs the host survival. Because Ccr1−/− mice are healthy unless challenged with specific pathogens (33), therapeutic inactivation of CCR1 may exhibit few side effects. Consistently, several CCR1 antagonists were well tolerated in phase II trials for rheumatoid arthritis and multiple sclerosis (34). It is therefore possible that administration of CCR1 antagonists as an adjuvant therapy after surgical resection of the primary tumors can improve the survival of patients with colorectal cancer that expresses CCL15. Our present results await clinical trials for such therapies.
Methods
Mice.
C57BL/6, Ccr1−/− (33), Mmp2−/− (35), Mmp7−/− (36), Mmp9−/− (37), and nu/nu mice at 7 wk of age were used as hosts of tumor injections (see below). All animals were bred and maintained according to the protocol approved by the Animal Care and Use Committee of Kyoto University. Genetic background and origin of the mice are detailed in SI Methods.
Cell Lines.
CMT93 mouse colon cancer cells (derived from C57BL/6 strain), HT29, DLD-1, SW620, and HCT116 human colon cancer cells were cultured at 37 °C under 5% CO2 in DMEM with 10% FCS. These cells were transfected with expression vectors encoding luciferase or CCL15, or shRNA expression vectors targeting Ccl9 and CCL15 (SI Methods).
Clinical Samples.
Colon cancer samples for Western blotting were prepared from primary tumors of 47 patients who underwent operations with informed consents. For immunostaining, we prepared an additional 41 sets of the primary colon cancers and liver metastases derived from the same patients.
Experimental Metastasis Model.
We injected 1.5 × 106 of CMT93 cells into the spleens of C57BL/6 or knockout mice (C57BL/6 backgrounds) under anesthesia. We also injected 1 × 106 of CMT93 or 3 × 106 of human cancer cells into ν/ν mice (SI Methods). The spleen was removed 1 min after tumor injection to prevent splenic tumor formation, so that metastatic lesions developed only in the liver.
In Vivo Bioluminescence Imaging.
We injected 100 μL of D-luciferin solution (10 mg/mL in PBS, i.p.; Promega) into anesthetized tumor-bearing mice 15 min before imaging. Bioluminescence from the luciferase-expressing tumor cells was determined at days 1, 3, 7, 14, 21, and 28 postinjection, using IVIS-SPECTRUM in vivo photon-counting device (Caliper Life Sciences). Images were quantified as photon counts/second using the Living Image software (Caliper Life Sciences).
Histological Analyses.
The methods used for immunostaining were based on those previously described (12). Details are given in SI Methods.
Western Blotting.
Lysates (50 μg protein each) were separated in 10–20% gradient SDS-gels (WAKO), and Western blotting was performed as described previously (38). CCL15 was detected with a goat polyclonal antibody for human CCL15 (R&D) and Qentix Western Blot Signal Enhancer (Pierce).
RT-PCR.
Expression of mRNAs was determined by RT-PCR as described previously (12). Primer sequences are shown in SI Methods.
Quantifications of CCL9 and CCL15 Proteins.
Conditioned media were collected from cultured CMT93 and HT29 cells, and their derivatives. The levels of CCL9 (MIP-1γ), and CCL15 (MIP-1δ) were determined using the Mouse MIP-1γ Immunoassay Kit, and Human CCL15/MIP-1δ DuoSet ELISA Development Kit (R&D), respectively.
Drug Treatment.
BL5923 (Novartis) was dissolved in 0.5% (wt/vol) hydroxyethyl cellulose (17), and administered to mice twice daily by oral gavages at doses of 25 or 50 mg/kg. The relevant solvent was administered to control animals. The treatments were started at 3 d before tumor injection, and continued to the end of analyses.
Statistical Analyses.
Statistical significance was evaluated with the Student's t test or nonparametric Wilcoxon u test. The log-rank test was used for analysis of Kaplan-Meier survival curves. All statistical analyses were performed with JMP 7 (SAS Institute). P < 0.05 was considered as statistically significant. Data represent the means ± SD.
Supplementary Material
Acknowledgments
We thank M. Okabe (Osaka University) for EGFP transgenic mice, P. M. Murphy (National Institutes of Health) for Ccr1 knockout mice, and Z. Werb (University of California, San Francisco), S. Itohara [The Institute of Physical and Chemical Research (Japan) Brain Science Institute], and C. Takahashi (Kyoto University) for Mmp2 and Mmp9 knockout mice. We also thank T. Inai and E. Hirose (Kyushu University) for CMT93 subtypes, T. Sudo (TORAY Industries Inc., Tokyo, Japan) for performing DNA microarray analysis, and Dr. Kawada (Kyoto University) for preparing human specimens. This work was supported by the Jeannik M. Littlefield-American Association for Cancer Research (AACR) Grants in Metastatic Colon Cancer and Grant-in Aid for Scientific Research from the Ministry of Education, Culture, Sports and Technology of Japan (to M.M.T).
Footnotes
Conflict of interest statement: P.L. and L.R. are employees of Novartis Pharma, AG that holds patents for BL5923.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002372107/-/DCSupplemental.
References
- 1.Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 3.Smith SC, Theodorescu D. Learning therapeutic lessons from metastasis suppressor proteins. Nat Rev Cancer. 2009;9:253–264. doi: 10.1038/nrc2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goswami S, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 6.Wyckoff JB, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, et al. Abrogation of TGF β signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadea BB, Joyce JA. Tumour-host interactions: Implications for developing anti-cancer therapies. Expert Rev Mol Med. 2006;8:1–32. doi: 10.1017/S1462399406000172. [DOI] [PubMed] [Google Scholar]
- 10.Takaku K, et al. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998;92:645–656. doi: 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- 11.Taketo MM, Edelmann W. Mouse models of colon cancer. Gastroenterology. 2009;136:780–798. doi: 10.1053/j.gastro.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura T, et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet. 2007;39:467–475. doi: 10.1038/ng1997. [DOI] [PubMed] [Google Scholar]
- 13.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Bacon KB, Oldham ER, Schall TJ. Molecular cloning and functional characterization of human MIP-1 δ, a new C-C chemokine related to mouse CCF-18 and C10. J Clin Immunol. 1998;18:214–222. doi: 10.1023/a:1020535106684. [DOI] [PubMed] [Google Scholar]
- 15.Zlotnik A, Morales J, Hedrick JA. Recent advances in chemokines and chemokine receptors. Crit Rev Immunol. 1999;19:1–47. [PubMed] [Google Scholar]
- 16.Forssmann U, Mägert H-J, Adermann K, Escher SE, Forssmann W-G. Hemofiltrate CC chemokines with unique biochemical properties: HCC-1/CCL14a and HCC-2/CCL15. J Leukoc Biol. 2001;70:357–366. [PubMed] [Google Scholar]
- 17.Revesz L, et al. Bridged piperazines and piperidines as CCR1 antagonists with oral activity in models of arthritis and multiple sclerosis. Lett Drug Des Discov. 2006;3:689–694. [Google Scholar]
- 18.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 19.Yang M, Odgren PR. Molecular cloning and characterization of rat CCL9 (MIP-1γ), the ortholog of mouse CCL9. Cytokine. 2005;31:94–102. doi: 10.1016/j.cyto.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Kominsky SL, Abdelmagid SM, Doucet M, Brady K, Weber KL. Macrophage inflammatory protein-1 δ: A novel osteoclast stimulating factor secreted by renal cell carcinoma bone metastasis. Cancer Res. 2008;68:1261–1266. doi: 10.1158/0008-5472.CAN-07-6122. [DOI] [PubMed] [Google Scholar]
- 21.Théret N, et al. Overexpression of matrix metalloproteinase-2 and tissue inhibitor of matrix metalloproteinase-2 in liver from patients with gastrointestinal adenocarcinoma and no detectable metastasis. Int J Cancer. 1997;74:426–432. doi: 10.1002/(sici)1097-0215(19970822)74:4<426::aid-ijc11>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Zeng ZS, Guillem JG. Distinct pattern of matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 mRNA expression in human colorectal cancer and liver metastases. Br J Cancer. 1995;72:575–582. doi: 10.1038/bjc.1995.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho YB, et al. Matrix metalloproteinase-9 activity is associated with poor prognosis in T3-T4 node-negative colorectal cancer. Hum Pathol. 2007;38:1603–1610. doi: 10.1016/j.humpath.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Hilska M, et al. Prognostic significance of matrix metalloproteinases-1, -2, -7 and -13 and tissue inhibitors of metalloproteinases-1, -2, -3 and -4 in colorectal cancer. Int J Cancer. 2007;121:714–723. doi: 10.1002/ijc.22747. [DOI] [PubMed] [Google Scholar]
- 25.Sutnar A, et al. Clinical relevance of the expression of mRNA of MMP-7, MMP-9, TIMP-1, TIMP-2 and CEA tissue samples from colorectal liver metastases. Tumour Biol. 2007;28:247–252. doi: 10.1159/000110897. [DOI] [PubMed] [Google Scholar]
- 26.Langers AMJ, et al. MMP-2 geno-phenotype is prognostic for colorectal cancer survival, whereas MMP-9 is not. Br J Cancer. 2008;98:1820–1823. doi: 10.1038/sj.bjc.6604380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inafuku Y, et al. Matrix metalloproteinase-2 expression in stromal tissues is a consistent prognostic factor in stage II colon cancer. Cancer Sci. 2009;100:852–858. doi: 10.1111/j.1349-7006.2009.01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagenaar-Miller RA, Gorden L, Matrisian LM. Matrix metalloproteinases in colorectal cancer: Is it worth talking about? Cancer Metastasis Rev. 2004;23:119–135. doi: 10.1023/a:1025819214508. [DOI] [PubMed] [Google Scholar]
- 29.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 30.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 31.Overall CM, Kleifeld O. Tumour microenvironment—opinion: Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 32.Kitamura T, Taketo MM. Keeping out the bad guys: Gateway to cellular target therapy. Cancer Res. 2007;67:10099–10102. doi: 10.1158/0008-5472.CAN-07-2100. [DOI] [PubMed] [Google Scholar]
- 33.Gao J-L, et al. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959–1968. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribeiro S, Horuk R. The clinical potential of chemokine receptor antagonists. Pharmacol Ther. 2005;107:44–58. doi: 10.1016/j.pharmthera.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Itoh T, et al. Unaltered secretion of β-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem. 1997;272:22389–22392. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- 36.Wilson CL, Heppner KJ, Labosky PA, Hogan BLM, Matrisian LM. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA. 1997;94:1402–1407. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vu TH, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujishita T, Aoki K, Lane HA, Aoki M, Taketo MM. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice. Proc Natl Acad Sci USA. 2008;105:13544–13549. doi: 10.1073/pnas.0800041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.