Abstract
Plant heat shock protein Hsp70 is the major target of HopI1, a virulence effector of pathogenic Pseudomonas syringae. Hsp70 is essential for the virulence function of HopI1. HopI1 directly binds Hsp70 through its C-terminal J domain and stimulates Hsp70 ATP hydrolysis activity in vitro. In plants, HopI1 forms large complexes in association with Hsp70 and induces and recruits cytosolic Hsp70 to chloroplasts, the site of HopI1 localization. Deletion of a central P/Q-rich repeat region disrupts HopI1 virulence but not Hsp70 interactions or association with chloroplasts. Thus, HopI1 must not only bind Hsp70 through its J domain, but likely actively affects Hsp70 activity and/or specificity. At high temperature, HopI1 is dispensable for P. syringae pathogenicity, unless excess Hsp70 is provided. A working hypothesis is that Hsp70 has a defense-promoting activity(s) that HopI1 or high temperature can subvert. Enhanced susceptibility of Hsp70-depleted plants to nonpathogenic strains of P. syringae supports a defense-promoting role for Hsp70.
Keywords: Hsp70, plant defense, type III effector, HopI1, chloroplast
To cause a successful infection, many plant pathogenic bacteria use a type III secretion system through which dozens of different effector proteins are injected into plant cells (1). Pseudomonas syringae, a type III-requiring pathogen, causes disease on foliage and fruits of diverse plants. Some P. syringae effectors can restrict host range and/or disease potential, rendering the pathogen “avirulent,” when they are recognized by a plant's defense machinery (2). However, in many cases, effectors suppress plant immune responses (1, 3). These virulence effectors are of intense interest, because their study not only gives insight into pathogenic mechanisms, but they can be used to identify previously unknown defense components (1, 4, 5) whose engineering may lead to novel approaches for creating disease-resistant plants.
The P. syringae pv. maculicola ES4326 (Pma) HopI1 effector, a J protein (i.e., one that contains a J domain) suppresses accumulation of the defense regulator salicylic acid (SA) and related plant defenses (6). HopI1 localizes to chloroplasts where SA is synthesized (7) and also affects thylakoid stack structure within chloroplasts (6). HopI1-expressing plants can rescue the virulence defect of PmaΔhopI1 bacteria, indicating that HopI1 exerts its effects from within plant cells. All pathogenic P. syringae examined have a HopI1 allele with a conserved 190-amino acid N-terminal region of unknown function, a middle region with variable numbers of P/Q-rich 37/38 amino acid repeats (1–6) and a conserved 70-amino acid J domain. Several alleles with different repeat numbers can complement the virulence defect of PmaΔhopI1, indicating they all function similarly (6).
The J domain of HopI1 provides a clue to HopI1’s possible mechanism of action. J proteins bind Hsp70 through the J domain and stimulate Hsp70’s ATP hydrolysis activity as well as other activities such as de novo folding of client proteins, intervening when proteins are improperly folded—often during stress conditions, protein degradation, and the disassembly of complexes, protein translocation, and trafficking (8–10). A conserved HPD loop of J domains is essential for interaction with Hsp70 and modulating Hsp70 activities (8). Arabidopsis has 16 Hsp70s, at least two in each cellular compartment (11, 12) and >100 J proteins (13, 14). J proteins are divided to three classes depending on the presence of other conserved domains. Classic cochaperones of Hsp70 with similar organization as Hsp40 form class I (15). HopI1 belongs to class III, because it has no other domains found in Hsp40 homologs. Known class III J proteins in plants play roles in chloroplast movements and uncoating of clathrin vesicles (14).
HopI1’s J domain can functionally substitute for the J domain of Ydj1 in yeast, because chimeric HopI1(J domain)-Ydj1ΔJ rescues Δydj1 yeast growth at high temperature (6). An HPD loop mutant (HPD/QAA) disrupts the ability of the J domain of HopI1 to function in yeast and the ability of HopI1 to complement the virulence defect of PmaΔhopI1 (6), proving that the J domain of HopI1 is functional.
Here, we provide biochemical evidence for the basis of HopI1’s virulence activity, identify a region of HopI1 that is essential for its function in promoting pathogen growth, and define the environmental conditions under which HopI1’s role during infection is important. We show that Hsp70 is essential for mediating HopI1’s virulence effect and plays a role in basal resistance to a nonpathogenic strain of P. syringae.
Results
HopI1 Is a Virulence Factor on Many Crops.
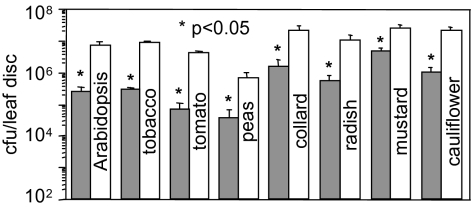
During Pma infections of the model plants Arabidopsis thaliana, Nicotiana benthamiana, and N. tabacum, the HopI1 effector has a strong virulence role (6). Because P. syringae causes disease on diverse plants of agricultural importance, we tested the virulence role of HopI1 during infection of various crops. The PmaΔhopI1 strain grew less (Fig. 1) and caused less disease symptoms than Pma (Fig. S1) on tomato (several cultivars), peas, and many mustard family plants related to radish from which Pma was isolated (16). Thus, HopI1 is an effective virulence factor on all tested crop plants.
Fig. 1.
HopI1Pma is a virulence factor on many crops. Arabidopsis, tobacco, and peas were infiltrated with bacteria (OD600 = 0.0003), and bacterial growth was quantified 3 days after inoculation (dpi). Tomato and mustard family plants were sprayed with bacteria (OD600 = 0.005), and bacterial growth was quantified 7 dpi. Deletion of hopI1 resulted in reduced bacterial growth (*P < 0.05). Gray bars, PmaΔhopI1 strain; white bars, PmaES4326 strain. Growth experiments were repeated two or more times with similar results.
HopI1 Interacts with Hsp70 and Stimulates ATP Hydrolysis via the J Domain in Vitro.
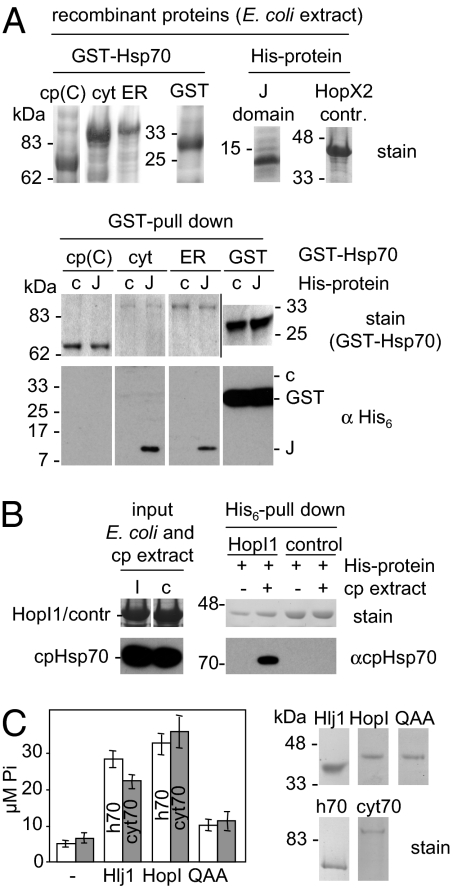
Because many J domains bind directly to Hsp70 proteins (8), we tested whether HopI1’s J domain can directly interact with plant Hsp70s. Recombinant His-tagged HopI1Pma J domain, but not the control protein His6-HopX2, specifically bound in vitro full-length plant cytosolic and ER Hsp70 isoforms fused to GST (Fig. 2A). It did not interact with a truncated version of chloroplast Hsp70-GST that lacked the N-terminal region of Hsp70 required for interaction with J domains (15). Because we did not have soluble full-length recombinant chloroplast Hsp70, we tested whether HopI1 can bind cpHsp70 from isolated chloroplasts. Recombinant full-length His6-HopI1 from P. syringae pv. syringae strain B728a (HopI1Psy; HopI1Pma was not tested because of its low solubility in Escherichia coli) immobilized on Ni2+-beads pulled down cpHsp70 from pea chloroplast extracts, whereas His6-HopX2 did not (Fig. 2B). Thus, HopI1 can interact with different isoforms of plant Hsp70s in vitro, and J domain alone is sufficient for binding.
Fig. 2.
HopI1 specifically interacts with Hsp70. (A) The J domain of HopI1 specifically interacts with full-length Arabidopsis Hsp70s in vitro. (Upper) Arabidopsis Hsp70 fused to GST and J domain of HopI1 fused to His6 were expressed in E. coli (SDS/PAGE gel stained with Coomassie blue is shown; GST proteins are from one gel and His-proteins from a different gel). cp(C), C-terminal part of cpHsp70-2 (Hsp70-7, amino acids 413–718); cyt, cytosolic Hsp70-1; ER, ER Bip2 (Hsp70-11); GST, GST control; J, J domain of HopI1Pma (amino acids 334–432, 12 kDa with a His tag); c, HopX2 control (40 kDa). (Lower) GST-pull down. Recombinant GST-Hsp70s and GST control were immobilized on glutathione-agarose and incubated with an extract from E. coli expressing the J domain of HopI1 with a His tag (J) or control His6-HopX2 (c). Eluted proteins were separated by SDS/PAGE and detected with Coomassie stain (GST proteins) and with His6 antibody. HopI1 J domain interacted with different full length Hsp70s but not with the C-terminal half (C) of cpHsp70-2 (the N-terminal part of Hsp70s is necessary for the interaction with J proteins; ref. 15). Strong signal in GST control is cross-reaction with GST (27 kDa), not visible in GST-Hsp70s, because GST alone was purified in a higher amount because of differences in solubility. Pulled-down J protein was not detected by Coomassie stain. Signals for all samples are from one exposure of one continuous membrane/gel. (B) HopI1 specifically interacts with Hsp70 from pea chloroplasts. Recombinant His6-HopI1Psy was immobilized on Ni2+-NTA and incubated with pea chloroplast extract (cp). Eluted proteins were separated by SDS/PAGE and detected with Coomassie stain (His6 proteins) and antibody that specifically recognizes chloroplast cpHsp70. Control is His6-HopX2. Signals for all samples are from one exposure of one continuous membrane except recombinant protein input (E. coli extracts), which are from different gel than pull-down samples. (C) HopI1 stimulates ATPase activity of Hsp70 in vitro. (Left) White bars, human Hsp70 (h70); gray bars, GST-AtHsp70-1 (cyt70); -, no J protein; Hlj1, yeast J domain-GST (positive control); HopI, His6-HopI1Psy; QAA, His6-HPD/QAA HopI1Psy mutant. ATP hydrolysis was measured by using 0.3 μM Hsp70 and 0.5 μM J protein, in duplicates. Average of Hsp70 ATPase activity after 2.5 h assayed in three experiments (using two different recombinant protein preparations) is shown with SEs. Recombinant proteins expressed and purified from E. coli are shown on Right [HopI1, QAA, and AtHsp70-1 (cyt70) are from one gel and Hlj1 and human Hsp70 (h70) from another gel].
Like other J proteins, HopI1Psy acted as a typical cochaperone of Arabidopsis or human Hsp70 by increasing their ATP hydrolysis activities (Fig. 2C). This stimulatory activity largely depended on an intact J domain, because an HPD/QAA J domain loop variant of HopI1Psy stimulated Hsp70 ATPase much less (Fig. 2C). Thus, HopI1 has features of a typical J protein, because it can bind Hsp70 and stimulate its activity.
HopI1 Interacts Mainly with Hsp70 in Vivo and Forms Large Complexes.
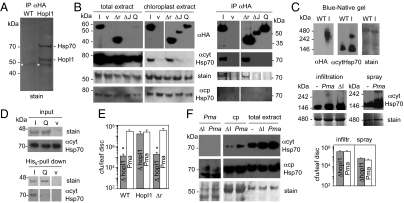
To discover the main HopI1-interacting proteins in vivo, we immunoprecipitated (IP) complexes with an anti-HA matrix from total or chloroplast-enriched extracts of HopI1Pma-HA-expressing Arabidopsis (both uninfected and PmaΔhopI1-infected) (Fig. 3A). Proteins in the two major specific bands found in the HopI1 complexes, but absent from control plants, were identified by LC-MS/MS as HopI1 and several forms of Hsp70, most predominantly Hsp70-1 and -3, and one peptide specific for chloroplast cpHsp70-1 (Hsp70-6) (Table S1). Hsp70-1 is a cytosolic isoform but was also found in chloroplast stroma in proteomic studies (17, 18). Many Hsp70 peptides identified by LC-MS/MS matched more than one Hsp70 isoform. Consistent with the LC-MS/MS analysis, an antibody specific for cytosolic Hsp70s (cytHsp70) (19) showed strong immunoreactivity with a 70-kDa band from HopI1-containing complexes (Fig. 3B and Fig. S2), which was absent in control IPs from vector-transformed Arabidopsis and N. benthamiana. Hsp70 isoforms were the main interactors of HopI1 visible on Coomassie-stained gels of proteins precipitated with HopI1-HA from total and chloroplast-enriched plant extracts (Fig. 3 A and B and Fig. S2). Blue native and 2D gels showed that HopI1 formed large complexes (240–480 kDa) in vivo of similar sizes to complexes formed by cytosolic Hsp70 in HopI1-expressing plants (Fig. 3C and Fig. S3). Hsp70 was not detectable in such large complexes in WT Arabidopsis (Fig. 3C and Fig. S3); however, such complexes were formed during infection of WT plants with PmaES4326, albeit at lower levels than in transgenic plants (Fig. 3C).
Fig. 3.
HopI1 forms complexes with Hsp70 in planta and induces Hsp70 levels. (A) Hsp70 is a major interactor of HopI1 in planta. Proteins were immunoprecipitated with anti-HA matrix from control (WT) and HopI1Pma-HA-expressing Arabidopsis, separated by SDS/PAGE and stained with Coomassie blue. Experiment was repeated with plants infected with PmaΔhopI1 with the same results. Strong bands were identified by LC-MS/MS as Hsp70 isoforms and HopI1. *, not specific (antibody). (B) J domain and HPD loop are necessary for HopI1 interaction with Hsp70. P/Q repeats of HopI1 are dispensable for this interaction. (Left) Western blots with HA antibody show that chloroplast-enriched fractions from transgenic Arabidopsis were also enriched in HopI1Pma-HA variants (I, HopI1; Δr, Δrepeats; ΔJ, ΔJ domain; Q, HPD/QAA mutant; v, vector control plants) comparing with total extracts. Plants expressing HopI1 and Δrepeats variants had elevated levels of cytHsp70, especially associated with chloroplasts (quantification is shown in Fig. S4B), whereas levels of chloroplast Hsp70 isoforms were not changed. Coomassie-stained membrane (Rubisco) shows similar loading. The same membrane was incubated with cytosolic Hsp70 monoclonal antibody and later with cpHsp70 polyclonal antibody and stained with Coomassie blue; HA signals are from another membrane with the same samples. Signals for all extract samples are from one exposure of one continuous membrane. (Right) Proteins from chloroplast-enriched extracts were immunoprecipitated with anti-HA matrix (due to uneven accumulation of HopI1-HA variants, twice more plant extract was used for IP of QAA and ΔJ than for HopI1 and Δr), separated by SDS/PAGE and stained with Coomassie blue or detected by HA antibody or cytosolic Hsp70 antibody (in separate gels/membranes). cytHsp70 precipitated with HopI1 and Δrepeats. cpHsp70 was not detected with cpHsp70 antibody in IP. Signals for all IP samples are from one exposure of one continuous membrane. IPs and immunoanalyses were repeated at least twice each with transgenic Arabidopsis plants and different HopI1 variants transiently expressed in N. benthamiana (Fig. S2), with the same results. LC-MS/MS analysis was done for two independent IPs from Arabidopsis total extracts and one from chloroplasts. (C) HopI1 and Hsp70 form high molecular mass complexes in planta. Blue-native gel of protein leaf extracts from WT and HopI1-HA (I)-expressing Arabidopsis shows HopI1 in 240–480 kDa complexes (Top). In plants expressing HopI1, Hsp70 is recruited to such high molecular mass complexes (300–350 kDa), larger than Hsp70 complexes in WT plants. The same membrane was incubated with HA antibody, and later with cytosolic Hsp70 antibody and stained with Coomassie blue. Two-dimensional gels of the same samples in Fig. S3 show that signals are from proteins of correct sizes. Large cytHsp70 complexes of similar size as in HopI1-expressing plants also formed in plants infected with Pma 1 d after infiltration at OD600 = 0.01 or spraying at OD600 = 0.1 (Middle). Levels of Pma and PmaΔhopI1 (ΔI) bacteria were similar 1 dpi (P > 0.3; Bottom). –, uninfected plants. Signals for infiltrated plants are from one exposure of one membrane and for sprayed plants from another membrane. (D) Interaction with Hsp70 depends on HopI1’s HPD loop. Recombinant His6-HopI1Psy (I) and HPD/QAA mutant of HopI1Psy (Q) were immobilized on Ni2+-NTA and incubated with Arabidopsis protein extract. Extract from E. coli transformed with empty vector was a control (v). Eluted proteins were separated by SDS/PAGE and detected with Coomassie stain (His6 proteins) and cytHsp70 antibody. Inputs are E. coli and plant extracts. Signals for all pull-down samples are from one exposure of one continuous membrane and input samples are from another membrane. Pull-down experiments were done twice with similar results. (E) P/Q repeats are necessary for virulence function of HopI1. Growth of PmaΔhopI1 strain in planta (infiltrated at OD600 = 0.0003; 3 dpi) was rescued in HopI1-expressing, but not HopI1Δrepeats-expressing Arabidopsis (protein accumulation in transgenic plants is shown in B). *P < 0.05. Growth experiments were repeated at least twice with each of two independent HopI1Δrepeats transgenic lines, giving similar results. (F) HopI1 induces and recruits Hsp70 to chloroplasts during infection. Cytosolic Hsp70 was induced by infection and recruited to chloroplasts to a greater extent when Pma harbored the HopI1 effector. Chloroplast-resident cpHsp70 levels were unaffected by infection. The same membrane was incubated with cytosolic Hsp70 monoclonal antibody and later with cpHsp70 polyclonal antibody and stained with Coomassie blue. Pma, PmaES4326 extracts showing that the cytHsp70 antibody does not recognize bacterial proteins; cp, chloroplast extracts; ΔI, PmaΔhopI1; Pma, PmaES4326; –, uninfected plants. Arabidopsis was sprayed with bacteria at OD600 = 0.1 or infiltrated at OD600 = 0.01 (shown) and Hsp70 levels were examined by Western blot analysis 1 dpi, when levels of both bacteria strains were similar (C). The average amount of cytosolic Hsp70 associated with chloroplasts was 2.2 times higher and total cytosolic Hsp70 1.5 times higher in plants infected with WT Pma than PmaΔhopI1 (Fig. S4B). At least six independent samples in two or more experiments were evaluated. Signals for all samples are from one exposure of one continuous membrane.
J Domain and Its HPD Loop Are Critical for Hsp70 Binding.
Hsp70 did not co-IP with HopI1-HA protein variants lacking the entire J domain or harboring the J domain HPD/QAA loop mutation stably expressed in Arabidopsis or transiently expressed in N. benthamiana (Fig. 3B and Fig. S2). Because QAA and ΔJ variants accumulate in plants to lower levels than WT HopI1 (probably due to the lack of stabilization by Hsp70), we confirmed the requirement for HPD loop in the J domain for interaction with Hsp70 by pull down with equal amounts of recombinant HopI1Psy and QAAPsy mutant (Fig. 3D). The loss of the interaction of HopI1 J domain mutants with Hsp70 likely explains why they lack virulence function (6). J domain loss of function phenotypes cannot be explained by mislocalization, because HopI1 variants were targeted to chloroplasts, similarly to full-length HopI1 (Fig. 3B).
HopI1’s P/Q-Rich Region Is Dispensable for Interaction with Hsp70 but Is Essential for Virulence.
Deletion of the P/Q repeats of HopI1 did not influence its ability to interact with Hsp70 in planta (Fig. 3B and Fig. S2). A P/Q repeats deletion (Δr) variant of HopI1 was unstable in Pma (6); therefore, we tested its virulence function by attempting to rescue the attenuated growth of PmaΔhopI1 in Arabidopsis expressing HopI1PmaΔr-HA. Although HopI1Pma-HA expressed in Arabidopsis rescued the virulence defect of PmaΔhopI1, HopIPmaΔr-HA did not (Fig. 3E), even though HopI1PmaΔr-HA accumulated in plants to high levels and was present in chloroplasts (Fig. 3B). Thus, the P/Q repeat region is important for HopI1’s function in virulence.
HopI1 Affects the Abundance and Location of Cytosolic Hsp70.
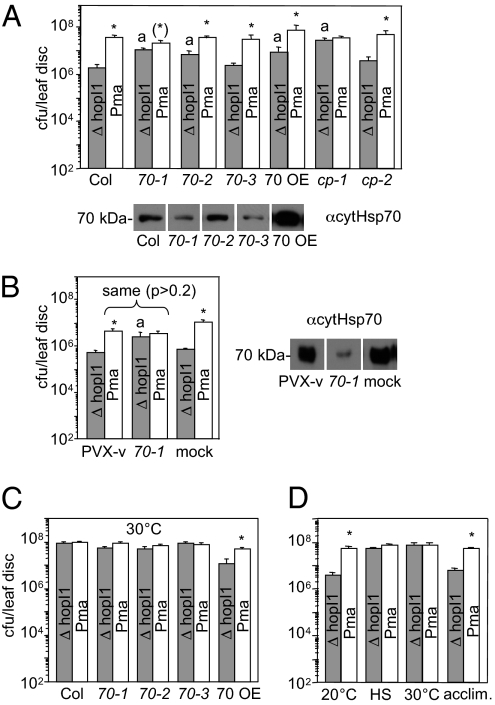
Infection with pathogens increases the level of Hsp70 transcripts (20, 21) and protein (21). To test whether HopI1 might specifically affect Hsp70 amount and/or localization, we monitored Hsp70 levels in HopI1-expressing and control plants with two antibodies, α-cytHsp70 (19) and α-cpHsp70, which is specific for the chloroplast Hsp70s (22). HopI1-expressing Arabidopsis had higher cytHsp70 levels and showed an increase of cytHsp70 that was recruited to chloroplasts (Fig. 3B and Fig. S4 A and B). Although cytHsp70 is present mainly in cytosol, it has been reported in chloroplasts, similarly as numerous other proteins without apparent transit peptides (17, 18). We also observed HopI1-dependent increased levels and chloroplast association of cytHsp70 in plants infected with Pma (Fig. 3F and Fig. S4B). The level of chloroplast-specific isoforms (cpHsp70) of Hsp70 was not altered in HopI1-expressing plants or after infection (Fig. 3 B and F). Quantitation of total and chloroplast-associated cytHsp70 in plants with modulated Hsp70 levels relative to cytHsp70 in WT plants and controls for contamination are shown in Fig. S4. Plants that overexpressed Hsp70-1 (Hsp70-1-OE) or were treated with high temperature showed proportionally increased cytHsp70 in chloroplast-enriched and total fractions (Fig. S4). However, the presence of HopI1 (expressed in plants or during Pma infection) caused significantly higher fold enrichments of cytHsp70 associated with chloroplasts. Elevated cytHsp70 levels and/or its altered localization could not fully mimic the effects of WT HopI1. Indeed, cytHsp70 levels and localization were also altered in HopI1Δr-expressing plants (Fig. 3B and Fig. S4B), even though these plants could not rescue the attenuated growth of PmaΔhopI1 (Fig. 3E). Moreover, Hsp70-1-OE plants did not rescue the virulence defect of PmaΔhopI1 (Fig. 4A). These observations suggest that HopI1 has a specific function in addition to causing an increase in the Hsp70 level.
Fig. 4.
Hsp70 is necessary for HopI1 virulence function. (A) Compared with WT Col, the difference in growth of ΔhopI1 and WT Pma strains was significantly smaller on Arabidopsis with decreased amounts of Hsp70-1 (on average ≈10% of the difference on WT Col in five experiments), cpHsp70-1 (≈2%) and (to lower degree) Hsp70-2 (≈20%). a, growth of ΔhopI1 strain was higher on hsp70-1, hsp70-2, and cphsp70-1 mutants than on WT Arabidopsis (P < 0.005); *, growth of Pma strain was higher than ΔhopI1 on Col, hsp70-2, hsp70-3, Hsp70 OE, and cphsp70-2 plants (P < 0.05). In some experiments (3 of 5), growth of the Pma strain was slightly lower on hsp70-1 mutant than on WT Arabidopsis (P < 0.05) and slightly higher (*) than the growth of ΔhopI1 (P < 0.05). 70-1, 70-2, 70-3, 70 OE,hsp70 mutants, and overexpressing plants; cp-1 and cp-2, chloroplast cphsp70 mutants. Plants were spray inoculated at OD600 = 0.01, and bacterial growth was assayed 3 and 5 dpi (shown). (B) Silencing Hsp70-1 in N. benthamiana complemented growth defect of ΔhopI1 strain. Plants were infected with PVX-vector (PVX-v), PVX-NbHsp70-1 silencing construct (70-1), or mock treated with buffer (mock). Eighteen days later, upper leaves were infiltrated with P. syringae at OD600 = 0.0003 and bacterial growth was assayed 3 dpi. a, growth of ΔhopI1 strain was higher on hsp70-1 silenced N. benthamiana than on control (P < 0.05); *P < 0.002. Western blots in A and B show Hsp70 proteins detected with cytosolic Hsp70 antibody in mutant and silenced plants. Samples shown are from the same membrane exposure. Expression of chloroplast Hsp70 in cphsp70 mutants was reported (23). (C) At 30 °C, ΔhopI1 strain grew as well as WT Pma on Col and hsp70 mutants (P > 0.05). At this temperature, HopI1 was needed for virulence only on plants overexpressing Hsp70 (*P < 0.05). Plants initially grown at 20 °C were transferred and kept at 30 °C after infection. (D) Acute heat shock (35 min at 50 °C; HS) before infection at 20 °C also abolished the growth defect of PmaΔhopI1, as in plants kept at 30 °C after infection (P > 0.07). On Arabidopsis acclimated to 30 °C 1 d before infection (acclim.), HopI1 was needed for full Pma virulence (*P < 0.01). The growth experiments were repeated two (HS, acclimation) or more times (all other experiments) with similar results.
Hsp70-1 Is Necessary for HopI1’s Virulence Role.
To test the hypothesis that HopI1’s virulence function depends on Hsp70, we analyzed the growth of Pma and PmaΔhopI1 in Arabidopsis-harboring mutations in various Hsp70-encoding genes. Cytosolic and chloroplast hsp70 mutant lines were characterized and shown to have reduced expression of specific Hsp70 genes (21, 23). As described, the cphsp70-1 line was small with abnormally shaped leaves (23) and Hsp70-1-OE plants were small (21, 24), whereas other mutants were morphologically normal (21, 24). Hsp70-1, and to a lesser degree Hsp70-2, were important for HopI1’s virulence role. In hsp70-1 and hsp70-2 mutants, the difference in the growth of Pma and PmaΔhopI1 strains was reduced or absent, whereas infection of WT Arabidopsis resulted in a large growth difference (Fig. 4A). The hsp70-1 and hsp70-2 mutants were more susceptible than WT plants to PmaΔhopI1, but not to Pma.
We confirmed the importance of Hsp70 for HopI1’s virulence role in N. benthamiana by using virus-induced gene silencing. PVX-hsp70-1 specifically silenced only the hsp70-1 allele (25). Hsp70-1-silenced N. benthamiana were stunted, as described (25) and had reduced cytosolic Hsp70 protein level compared with PVX-vector infected plants (Fig. 4B). In hsp70-1-silenced N. benthamiana, PmaΔhopI1 grew to similar level as Pma (Fig. 4B). The growth difference between Pma and PmaΔhopI1 in the Arabidopsis chloroplast cphsp70-1 mutant was also highly reduced (Fig. 4A). Together with the observed HopI1-dependent increase of cytosolic Hsp70 associated with chloroplasts, these results suggest that Hsp70 in chloroplasts is critical for HopI1 function.
High Temperature and Hsp70 Levels Affect the Requirement of HopI1 for Pma Virulence.
Our working hypothesis is that HopI1 reduces defenses by suppressing (or reversing) a Hsp70 defense-promoting function. Hsp70 also helps plants cope with temperature-induced stress (26), a role that might supercede its defense role. Consistent with these ideas, HopI1 is dispensable for virulence at high temperature. Plants grown at 20 °C and then shifted to 30 °C at the time of infection supported the same amount of high growth of both PmaΔhopI1 and Pma (Fig. 4C). An acute, transient temperature shock (35 min at 50 °C) followed by infection at 20 °C also resulted in plants on which PmaΔhopI1 and Pma grew to the same high level (Fig. 4D). Overexpression of Hsp70 restored the virulence role for HopI1; in Hsp70-1-OE plants, PmaΔhopI1 grew less than Pma during high temperature infections (Fig. 4C). In WT plants infected at 30 °C, the PmaΔhopI1 growth defect was detected when plants were allowed to acclimate to 30 °C for 1 d before infection (Fig. 4D). Thus, HopI1’s virulence effect can occur at high temperature, either when excess Hsp70 is provided or there is an adaptation period that allows Hsp70 to be available for defense.
In support of the idea that defenses are limited at high temperature, the Arabidopsis Nossen accession (on which Pma is not an aggressive pathogen) showed highly increased growth and symptoms of both Pma and PmaΔhopI1 at 30 °C relative to infection at 20 °C (Fig. S5). HopI1 was dispensable on Nossen at 30 °C as well (Fig. S5).
A Role for Hsp70 in Basal Disease Resistance.
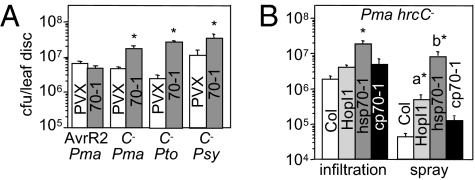
The host plant proteins targeted by effectors often function in defense. Indeed, N. benthamiana with reduced Hsp70-1 support increased growth of the nonhost pathogen P. chicorii (25). To test whether Hsp70 might have a defense role during Pma infections, we measured the growth of avirulent, virulent, and type III secretion-deficient P. syringae strains on plants with reduced Hsp70-1 levels. The growth of avirulent Pma carrying avrRpt2 (Fig. 5A) and virulent Pma (Fig. 4B) was not affected in hsp70-1-silenced N. benthamiana and not affected or slightly reduced in hsp70-1 Arabidopsis (Fig. 4A; ref. 21). However, three type III secretion-deficient P. syringae strains (hrcC−) grew to much higher levels in N. benthamiana with reduced Hsp70-1 (Fig. 5A). Thus, Hsp70-1 has a large role in basal disease resistance. HopI1-expressing Arabidopsis also supported higher growth of the Pma hrcC− strain when bacteria where sprayed, but not when they were infiltrated (Fig. 5B; ref. 6). However, growth of Pma hrcC− was higher in hsp70-1 plants than in HopI1-expressing Arabidopsis. Thus, HopI1 may partially suppress Hsp70’s function in basal defense.
Fig. 5.
Hsp70-1 has a role in basal resistance. (A) Type III secretion-deficient strains (C−, hrcC−) of Pma, PtoDC3000 (Pto), and PsyB728a (Psy) grew to higher levels on hsp70-1-silenced N. benthamiana than control PVX-treated plants; the growth of Pma with AvrRpt2 (AvrR2) was not affected. Bacteria were infiltrated at OD600 = 0.01 and growth was measured 3 dpi. (B) Pma hrcC−grew more on Arabidopsis hsp70-1 mutant than on WT plants and cphsp70-1 mutant when bacteria where infiltrated (at OD600 = 0.01) or sprayed (at OD600 = 0.1) and on HopI1-expressing Arabidopsis infected by spraying. *P < 0.05. The growth was measured 5 dpi. a and b indicate that the growth of bacteria was higher on hsp70-1 than HopI1 plants (P < 0.05). These experiments were repeated twice with similar results.
Discussion
The ubiquitous effector HopI1 of pathogenic P. syringae has a critical role in virulence on many crop plants. A key feature of HopI1’s biochemical mechanism of action is its interaction with plant Hsp70 proteins, which HopI1 binds through its J domain. HopI1 has bona fide J protein activity, because it can stimulate the ATP hydrolysis by Hsp70. This activity is an essential part of Hsp70’s biochemical mechanism (8). Hsp70 is not only the major interactor of HopI1 in planta, but it is necessary for the virulence function of HopI1. We have strong biochemical and genetic evidence that HopI1 targets plant Hsp70 and recruits it to plant chloroplasts where the large complex likely actively suppresses plant defenses. HopI1 induces similar changes in Hsp70 complexes as happen during infection.
In addition to the importance of the J domain, the P/Q-rich region is essential for HopI1’s virulence role, but is not necessary for binding Hsp70 or chloroplast localization. Therefore, HopI1 does not act by simply binding Hsp70 to compete with other Hsp70-binding proteins. Because the P/Q-rich region is predicted to be unstructured, it may form a flexible linker between the N terminus and the J domain. The G/F region in class I J proteins provides such an unstructured linker between Hsp70 binding J domain and client binding domain (14), bringing a client to Hsp70. The role of P/Q repeats in HopI1 may be similar, or they may directly bind client proteins.
HopI1 joins a growing list of pathogen effectors that induce and/or recruit host target proteins to a specific subcellular compartment (4, 27, 28). We don't know the specific importance of the induction of cytHsp70 accumulation and recruitment to chloroplasts in response to HopI1. However, given the known localization to and role of HopI1 in chloroplasts and its role in suppressing accumulation of chloroplast-synthesized SA, it is plausible that the basal level of Hsp70 in chloroplasts is too low for HopI1 to act without the additional recruitment of cytHsp70. HopI1 function may also require a specific isoform(s) of Hsp70. Plants may have to compensate for the amount of Hsp70 bound to HopI1 (to provide enough Hsp70 for normal cell functions) and, therefore, accumulate more cytHsp70.
Why does HopI1 target Hsp70? One possibility is that Hsp70 affects the folding/complex assembly of a chloroplast-resident defense factor (possibly SA-biosynthesis or transport components). When HopI1 is present, it might interfere with defense by actively switching Hsp70 to a mode where it facilitates degradation or disassembly the defense-promoting complex. The class III J protein auxilin has a role in complex disassembly, so there is precedent for this type of J protein-Hsp70 activity (14). This scenario could explain why reducing Hsp70 levels phenocopies plants in which HopI1 is present: Plants with reduced Hsp70 might promote the growth of PmaΔhopI1 because of the reduced folding/assembly of defense complexes. Other mechanisms are also possible, e.g., reduced turnover of a negative defense regulator. Ultimately, when the clients of the HopI1-Hsp70 complex are known, the exact mechanism can be clarified.
Our experiments suggest that the amount of Hsp70 available for defense functions that can be suppressed by HopI1 is limiting during the high temperature infections. The observation that heat shock or high temperature disrupts SA accumulation and/or resistance responses to different pathogens (29, 30), and in the defense mutant bon-1 (31), could also be due to pool of Hsp70 being diverted to stress functions at the expense of the defense response.
Hsp70 has a role in basal resistance to P. syringae that likely goes beyond the function that HopI1 targets. This role is evidenced by the hypersusceptibility of hsp70-1 mutants/down-regulated plants to type III-deficient Pma; HopI1-expressing plants are also more susceptible to these nonpathogenic bacteria, but to a lower level than hsp70-1 plants and only when inoculated by spraying. The large requirement of Hsp70 for basal resistance could reflect a role for the cytosolic pool of Hsp70 in basal defense. Hsp70 is important for nonhost resistance to P. chicorii in N. benthamiana (25), and it is a part of immune complex with SGT1 and Hsp90 (21). If Hsp70 is involved in defense, its not clear why Hsp70-1-OE Arabidopsis are hypersusceptible to P. syringae pv. tomato strain DC3000 and two avirulent derivatives (21). However, these strains also harbor hopI1; it is possible that the increased susceptibility occurs through a HopI1-dependent mechanism. Hsp70 is a common target of plant and animal pathogens, which either exploit Hsp70 activity or suppress it (20, 21, 32, 33). A central goal will be to discern the mechanism by which Hsp70 participates in interactions with different pathogens (identify client proteins and cellular processes that involve Hsp70) that influence the outcome of an infection. In this regard, HopI1 will be a useful tool for determining the specificity of different Hsp70-dependent events.
Materials and Methods
Bacteria and plant genotypes, antibodies, and detailed methods are provided in SI Materials and Methods.
Infections.
HA-tagged HopI1Pma (JJ30) full length and mutant versions and Agrobacterium-mediated plant transformation were described in refs. 6 and 34. Two Col and Nossen lines expressing Δrepeats (JJ196), QAA (JJ202), and ΔJ (JJ197) were used. Bacterial infections and growth were as in ref. 35 and SI Materials and Methods.
Pull Down Assays, Immunoprecipitation, and Protein Analysis.
For pull downs, recombinant proteins (SI Materials and Methods) from E. coli lysates were immobilized on on Ni-NTA or gluthatione resins, incubated for 1h at 4°C with E. coli lysate containg interacting partner or with plant extract, and eluted and analyzed by immunoblotting. IP with anti-HA matrix (Roche) followed manufacturer IP protocol (details in SI Materials and Methods). LC-MS/MS protein identification was performed at Chicago Biomedical Consortium and Stanford University and data analyzed with Sequest and Mascot software.
ATPase Activity of Hsp70.
Phosphate released by ATP hydrolysis was measured in colorimetric assay with molybdate and malachite green reagent (see SI Materials and Methods).
Protein Complexes.
Protein complexes in total and chloroplast enriched extracts (see SI Materials and Methods) were analyzed by blue native (BN) and 2-dimensional (2D) PAGE using Invitrogen gels according to manufacturer protocol and visualized by immunoblotting.
Supplementary Material
Acknowledgments
We thank D. Duncan and D. Blumenthal (University of Chicago) for help with cloning, J. Brodsky (University of Pittsburgh, Pittsburgh) for proteins and useful discussions, K. Keegstra (Michigan State University, East Lansing, MI) and T. Leustek (Rutgers University, New Brunswick, NJ) for antibodies, and J. Parker (Max-Planck Institute, Cologne, Germany), R. Terauchi (Iwate Biotechnology Research Center, Iwate, Japan), H. M. Li (Institute of Molecular Biology, Academia Sinica, Taipei, Taiwan), C. Guy (University of Florida, Gainesville, FL), L. Noël (Centre National de la Recherche Scientifique-Commissariat à l'Énergie Atomique, St. Paul-lez-Durance, France), and A. Joachimiak (Argonne National Laboratory, Argonne, IL) for seeds and/or plasmids. This work was supported by National Science Foundation Grant IOS0822393, US Department of Agriculture Grant NRI-2005-35319-16136 USDA, and National Institutes of Health Grant GM054292 (to J.T.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0910943107/-/DCSupplemental.
References
- 1.Block A, Li G, Fu ZQ, Alfano JR. Phytopathogen type III effector weaponry and their plant targets. Curr Opin Plant Biol. 2008;11:396–403. doi: 10.1016/j.jbi.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dangl JL, Jones JD. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 3.Zhou JM, Chai J. Plant pathogenic bacterial type III effectors subdue host responses. Curr Opin Microbiol. 2008;11:179–185. doi: 10.1016/j.mib.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Lee MW, Jelenska J, Greenberg JT. Arabidopsis proteins important for modulating defense responses to Pseudomonas syringae that secrete HopW1-1. Plant J. 2008;54:452–465. doi: 10.1111/j.1365-313X.2008.03439.x. [DOI] [PubMed] [Google Scholar]
- 5.Nomura K, et al. A bacterial virulence protein suppresses host innate immunity to cause plant disease. Science. 2006;313:220–223. doi: 10.1126/science.1129523. [DOI] [PubMed] [Google Scholar]
- 6.Jelenska J, et al. A J domain virulence effector of Pseudomonas syringae remodels host chloroplasts and suppresses defenses. Curr Biol. 2007;17:499–508. doi: 10.1016/j.cub.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strawn MA, et al. Arabidopsis isochorismate synthase functional in pathogen-induced salicylate biosynthesis exhibits properties consistent with a role in diverse stress responses. J Biol Chem. 2007;282:5919–5933. doi: 10.1074/jbc.M605193200. [DOI] [PubMed] [Google Scholar]
- 8.Kelley WL. The J-domain family and the recruitment of chaperone power. Trends Biochem Sci. 1998;23:222–227. doi: 10.1016/s0968-0004(98)01215-8. [DOI] [PubMed] [Google Scholar]
- 9.Höhfeld J, Cyr DM, Patterson C. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep. 2001;2:885–890. doi: 10.1093/embo-reports/kve206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riordan M, et al. HSP70 binding modulates detachment of Na-K-ATPase following energy deprivation in renal epithelial cells. Am J Physiol Renal Physiol. 2005;288:F1236–F1242. doi: 10.1152/ajprenal.00438.2004. [DOI] [PubMed] [Google Scholar]
- 11.Lin BL, et al. Genomic analysis of the Hsp70 superfamily in Arabidopsis thaliana. Cell Stress Chaperones. 2001;6:201–208. doi: 10.1379/1466-1268(2001)006<0201:gaoths>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sung DY, Vierling E, Guy CL. Comprehensive expression profile analysis of the Arabidopsis Hsp70 gene family. Plant Physiol. 2001;126:789–800. doi: 10.1104/pp.126.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miernyk JA. The J-domain proteins of Arabidopsis thaliana: An unexpectedly large and diverse family of chaperones. Cell Stress Chaperones. 2001;6:209–218. doi: 10.1379/1466-1268(2001)006<0209:tjdpoa>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajan VBV, D'Silva P. Arabidopsis thaliana J-class heat shock proteins: Cellular stress sensors. Funct Integr Genomics. 2009;9:433–446. doi: 10.1007/s10142-009-0132-0. 10.1007/s10142-009-0132-0. [DOI] [PubMed] [Google Scholar]
- 15.Qiu XB, Shao YM, Miao S, Wang L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci. 2006;63:2560–2570. doi: 10.1007/s00018-006-6192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis KR, Schott E, Ausubel FM. Virulence of selected phytopathogenic pseudomonads in Arabidopsis thaliana. Mol Plant Microbe Interact. 1991;4:477–488. [Google Scholar]
- 17.Kleffmann T, et al. The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Curr Biol. 2004;14:354–362. doi: 10.1016/j.cub.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 18.Zybailov B, et al. Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS One. 2008;3:e1994. doi: 10.1371/journal.pone.0001994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson JV, Li QB, Haskell DW, Guy CL. Structural organization of the spinach endoplasmic reticulum-luminal 70-kilodalton heat-shock cognate gene and expression of 70-kilodalton heat-shock genes during cold acclimation. Plant Physiol. 1994;104:1359–1370. doi: 10.1104/pp.104.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen ZR, et al. Influence of cytoplasmic heat shock protein 70 on viral infection of Nicotiana benthamiana. Mol Plant Pathol. 2008;9:809–817. doi: 10.1111/j.1364-3703.2008.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noël LD, et al. Interaction between SGT1 and cytosolic/nuclear HSC70 chaperones regulates Arabidopsis immune responses. Plant Cell. 2007;19:4061–4076. doi: 10.1105/tpc.107.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akita M, Nielsen E, Keegstra K. Identification of protein transport complexes in the chloroplastic envelope membranes via chemical cross-linking. J Cell Biol. 1997;136:983–994. doi: 10.1083/jcb.136.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su PH, Li HM. Arabidopsis stromal 70-kD heat shock proteins are essential for plant development and important for thermotolerance of germinating seeds. Plant Physiol. 2008;146:1231–1241. doi: 10.1104/pp.107.114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sung DY, Guy CL. Physiological and molecular assessment of altered expression of Hsc70-1 in Arabidopsis. Evidence for pleiotropic consequences. Plant Physiol. 2003;132:979–987. doi: 10.1104/pp.102.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanzaki H, et al. Cytosolic HSP90 and HSP70 are essential components of INF1-mediated hypersensitive response and non-host resistance to Pseudomonas cichorii in Nicotiana benthamiana. Mol Plant Pathol. 2003;4:383–391. doi: 10.1046/j.1364-3703.2003.00186.x. [DOI] [PubMed] [Google Scholar]
- 26.Kotak S, et al. Complexity of the heat stress response in plants. Curr Opin Plant Biol. 2007;10:310–316. doi: 10.1016/j.pbi.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Caplan JL, Mamillapalli P, Burch-Smith TM, Czymmek K, Dinesh-Kumar SP. Chloroplastic protein NRIP1 mediates innate immune receptor recognition of a viral effector. Cell. 2008;132:449–462. doi: 10.1016/j.cell.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernoux M, et al. RD19, an Arabidopsis cysteine protease required for RRS1-R-mediated resistance, is relocalized to the nucleus by the Ralstonia solanacearum PopP2 effector. Plant Cell. 2008;20:2252–2264. doi: 10.1105/tpc.108.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: A likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Bao Z, Zhu Y, Hua J. Analysis of temperature modulation of plant defense against biotrophic microbes. Mol Plant Microbe Interact. 2009;22:498–506. doi: 10.1094/MPMI-22-5-0498. [DOI] [PubMed] [Google Scholar]
- 31.Yang S, Hua J. A haplotype-specific Resistance gene regulated by BONZAI1 mediates temperature-dependent growth control in Arabidopsis. Plant Cell. 2004;16:1060–1071. doi: 10.1105/tpc.020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Multhoff G. Heat shock proteins in immunity. Handb Exper Pharmacol. 2006;172:279–304. doi: 10.1007/3-540-29717-0_12. [DOI] [PubMed] [Google Scholar]
- 33.Axsen WS, Styer CM, Solnick JV. Inhibition of heat shock protein expression by Helicobacter pylori. Microb Pathog. 2009;47:231–236. doi: 10.1016/j.micpath.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vinatzer BA, et al. The type III effector repertoire of Pseudomonas syringae pv. syringae B728a and its role in survival and disease on host and non-host plants. Mol Microbiol. 2006;62:26–44. doi: 10.1111/j.1365-2958.2006.05350.x. [DOI] [PubMed] [Google Scholar]
- 35.Mohr TJ, et al. Naturally occurring nonpathogenic isolates of the plant pathogen Pseudomonas syringae lack a type III secretion system and effector gene orthologues. J Bacteriol. 2008;190:2858–2870. doi: 10.1128/JB.01757-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.