Abstract
Increasing the expression of Hsp70 (heat-shock protein 70) can inhibit sensory neuron degeneration after axotomy. Since the onset of DPN (diabetic peripheral neuropathy) is associated with the gradual decline of sensory neuron function, we evaluated whether increasing Hsp70 was sufficient to improve several indices of neuronal function. Hsp90 is the master regulator of the heat-shock response and its inhibition can up-regulate Hsp70. KU-32 (N-{7-[(2R,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyl-tetrahydro-2H-pyran-2-yloxy]-8-methyl-2-oxo-2H-chromen-3-yl}acetamide) was developed as a novel, novobiocin-based, C-terminal inhibitor of Hsp90 whose ability to increase Hsp70 expression is linked to the presence of an acetamide substitution of the prenylated benzamide moiety of novobiocin. KU-32 protected against glucose-induced death of embryonic DRG (dorsal root ganglia) neurons cultured for 3 days in vitro. Similarly, KU-32 significantly decreased neuregulin 1-induced degeneration of myelinated Schwann cell DRG neuron co-cultures prepared from WT (wild-type) mice. This protection was lost if the co-cultures were prepared from Hsp70.1 and Hsp70.3 KO (knockout) mice. KU-32 is readily bioavailable and was administered once a week for 6 weeks at a dose of 20 mg/kg to WT and Hsp70 KO mice that had been rendered diabetic with streptozotocin for 12 weeks. After 12 weeks of diabetes, both WT and Hsp70 KO mice developed deficits in NCV (nerve conduction velocity) and a sensory hypoalgesia. Although KU-32 did not improve glucose levels, HbA1c (glycated haemoglobin) or insulin levels, it reversed the NCV and sensory deficits in WT but not Hsp70 KO mice. These studies provide the first evidence that targeting molecular chaperones reverses the sensory hypoalgesia associated with DPN.
Keywords: diabetic neuropathy, dorsal root ganglia neuron, heat-shock protein 70, molecular chaperone, nerve conduction velocity, neurodegeneration
Abbreviations: AM, acetoxymethyl ester; DAPI, 4′,6-diamidino-2-phenylindole; DMEM, Dulbecco's modified Eagle's medium; DPN, diabetic peripheral neuropathy; DRG, dorsal root ganglion; Drp1, dynamin-related protein 1; FBG, fasting blood glucose; FCS, fetal calf serum; Hsc70, heat-shock cognate 70 stress protein; HSF1, heat-shock factor 1; Hsp90, heat-shock protein 90; HSR, heat-shock response; JNK, c-Jun N-terminal kinase; KO, knockout; KU-32, N-{7-[(2R,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyl-tetrahydro-2H-pyran-2-yloxy]-8-methyl-2-oxo-2H-chromen-3-yl}acetamide; LC-MS, liquid chromatography MS; MBP, myelin basic protein; MNCV, motor NCV; NCV, nerve conduction velocity; NGF, nerve growth factor; NRG1, human recombinant neuregulin-1-β1 epidermal growth factor domain; SC-DRG, Schwann cell DRG; SNCV, sensory NCV; STZ, streptozotocin; WT, wild-type
INTRODUCTION
DPN (diabetic peripheral neuropathy) is a neurodegenerative complication of diabetes that has proved difficult to manage pharmacologically since it does not result from a single biochemical aetiology that is uniformly manifested for the disease's duration (10–30 years). Indeed, small molecule inhibitors against targets that are relatively diabetes-specific, i.e. aldose reductase and advanced glycation end products, have not effectively halted the progressive degeneration of sensory fibres in human DPN (Tomlinson and Gardiner, 2008). On the other hand, targeting pathways that contribute to disease progression, but that are not necessarily diabetes-specific, has met with some success. For example, oxidative stress contributes to neuron and glial degeneration in DPN (Obrosova, 2009) and some small molecule antioxidants have shown efficacy in reversing clinical and electrophysiological deficits associated with the disease (Pop-Busui et al., 2006). A common theme in the above approaches has been the pharmacological targeting of one specific biochemical pathology associated with DPN. An alternative and relatively unexplored paradigm for treating DPN is to up-regulate a broad cytoprotective response.
Hsp90 (heat-shock protein 90) is the master regulator of the HSR (heat-shock response) since it binds HSF1 (heat-shock factor 1). Disruption of the Hsp90–HSF1 complex by cellular stress induces the transcriptional up-regulation of antioxidant genes and molecular chaperones, such as Hsp70, that characterize the cytoprotective HSR (Peterson and Blagg, 2009). The induction of molecular chaperones can minimize the accumulation of damaged proteins by enhancing their refolding and interfering with pro-apoptotic pathways (Brown, 2007). Since small-molecule N-terminal Hsp90 inhibitors can mimic cell stress and promote the release of HSF1 from Hsp90 (Blagg and Kerr, 2006), their ability to decrease protein aggregation has been proposed for treating neurodegenerative diseases whose aetiology is linked to the accumulation of specific misfolded or aggregated proteins (Luo et al., 2007; Luo et al., 2008). Although the pathogenesis of DPN is unlikely to result from accumulation of any one specific misfolded or aggregated protein, hyperglycaemia can increase oxidative modification of proteins (Akude et al., 2009). This can damage protein structure, impair protein folding, decrease refolding of damaged protein or induce protein aggregation. Thus chaperone induction by Hsp90 inhibitors may help to minimize hyperglycaemic stress; however, their use is potentially complicated by the dual role of Hsp90 in regulating protein folding.
In the presence of co-chaperones, the folding of nascent and damaged polypeptides into their biologically active structures is dependent on Hsp90 and Hsp70. ‘Client proteins’ form a stabilized complex with an Hsp90 homodimer, and ATP hydrolysis by the chaperone's intrinsic N-terminal ATPase provides the energy necessary for conformational maturation of the client. Inhibiting the chaperone activity of Hsp90 blocks protein folding and leads to client protein degradation (Powers and Workman, 2007). Since many oncoproteins are Hsp90 clients, N-terminal Hsp90 inhibitors promote oncoprotein degradation and cytotoxicity (Bishop et al., 2007; Taldone et al., 2008). Thus Hsp90 inhibitors can be useful as both chemotherapeutic and neuroprotective agents, and their primary biological outcome is dictated by the therapeutic window that dissociates client protein degradation and cytotoxicity from the cytoprotective induction of an HSR (Peterson and Blagg, 2009). Although many N-terminal Hsp90 inhibitors have been designed (Pacey et al., 2006), we sought novel Hsp90 analogues that effectively promote neuroprotection in the absence of cytotoxicity.
Novobiocin is an inhibitor of bacterial DNA gyrase that also exhibits weak affinity for the C-terminal ATP-binding domain of Hsp90. Since novobiocin promotes client protein degradation at concentrations that also induce an HSR (Marcu et al., 2000a, 2000b; Figure 1A), a systematic modification of novobiocin was undertaken to identify structure–activity relationships that yielded high-affinity C-terminal analogues that diverged client protein degradation from Hsp70 induction (Burlison et al., 2006; Donnelly et al., 2008). KU-32 (N-{7-[(2R,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyl-tetrahydro-2H-pyran-2-yloxy]-8-methyl-2-oxo-2H-chromen-3-yl}acetamide; Figure 1A) emerged from this library as a potent inducer of Hsp70, but a poor inducer of client protein degradation. Importantly, KU-32 was found to be minimally cytotoxic to primary cortical neurons and protected against neuronal death induced by amyloid β-peptide (Ansar et al., 2007; Lu et al., 2008).
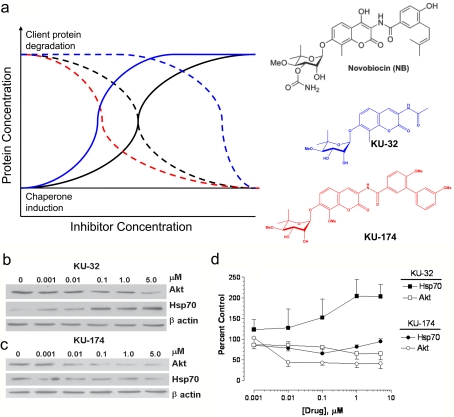
Figure 1. Structure–activity relationships between novobiocin, KU-32 and KU-174 that diverge client protein degradation from induction of Hsp70.
(a) Structure of analogues and theoretical representation of concentrations affecting client protein degradation (dotted lines) and chaperone induction (solid lines) for novobiocin (black), KU-32 (blue) and KU-174 (red). (b) KU-32 induces Hsp70 expression but shows limited degradation of the Hsp90 client protein Akt. (c) KU-174 promotes Akt degradation but does not increase Hsp70 levels. MCF7 cells were treated with the indicated concentration of inhibitors for 24 h and immunoblot analysis was performed. (d) Band intensities were normalized to β-actin and expressed as a percentage of the control (n = 3).
Previous work has suggested that Hsp70 can improve the survival of motor and sensory neurons after axotomy of neonatal sciatic nerves (Tidwell et al., 2004). However, it is unknown whether pharmacologically manipulating molecular chaperones in adult animals is sufficient to improve clinically relevant indices of unmyelinated and myelinated fibre function. Since KU-32 inhibits Hsp90 and increases Hsp70 levels, we examined whether it decreased neurodegeneration of non-myelinated or myelinated sensory neurons in vitro and attenuated the pathophysiological progression of DPN in mice. Our results provide compelling evidence that inhibiting Hsp90 can protect against sensory neuron death and demyelination and reverse the sensory deficits associated with DPN. Moreover, Hsp70 expression is critical to this neuroprotection since its genetic ablation abolished drug efficacy. These results establish proof-of-principle that pharmacological modulation of molecular chaperones may be useful toward decreasing neurodegeneration associated with the onset of DPN.
MATERIALS AND METHODS
Materials
STZ (streptozotocin) was obtained from Sigma–Aldrich (St. Louis, MO, U.S.A.). KU-32 and KU-174 (Figure 1A) were synthesized and structural purity was verified as described previously (Burlison et al., 2006; Donnelly et al., 2008). The antibodies used and their sources were: SMI-94R (Covance, Princeton, NJ, U.S.A.); compact myelin protein zero (P0), ubiquitin C-terminal hydrolase (PGP 9.5; Chemicon, Temecula, CA, U.S.A.); monoclonal Hsp70 C92F3A-5 (Stressgen, Ann Arbor, MI, U.S.A.); Akt (also called protein kinase B), β-actin and horseradish-peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.); Alexa Fluor® 488 rabbit anti-mouse and Alexa Fluor® 568 goat anti-rabbit antibodies (Molecular Probes, Eugene, OR, U.S.A.). MCF7 cells were maintained in DMEM (Dulbecco's modified Eagle's medium)-F12 medium containing 10% (v/v) FCS (fetal calf serum) and 100 units/ml penicillin and 100 μg/ml streptomycin.
Preparation of non-myelinated and myelinated DRG (dorsal root ganglion) neurons
DRG neurons were dissected from embryonic day 15–18 rat pups (Zanazzi et al., 2001) and ganglia were collected into L15 medium and sedimented at 1000 g for 5 min. After dissociation, the cells were resuspended in serum-free neurobasal medium containing 2 mM glutamate, B27 supplement, 100 units/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamicin and 50 ng/ml NGF (nerve growth factor; Harlan Biosciences, Indianapolis, IN, U.S.A.) and seeded at a density of (2–3)×104 cells per well. Mitotic cells were partially depleted by treating the neurons with 10 μM each of fluorodeoxyuridine and cytosine β-d-arabinoside for 2 days. The cells were switched to neurobasal medium containing 50 ng/ml NGF and were pretreated for 6 h with the indicated concentration of KU-32. Hyperglycaemia was induced by the addition of 20 mM excess glucose (final glucose concentration 45 mM), and cell viability was assessed after 24 h using calcein AM (acetoxymethyl ester) and propidium iodide as previously described (Li et al., 2003).
Schwann cells were isolated from postnatal day 3 rat pups, and myelinated rat SC-DRGs (Schwann cell DRGs) neuron co-cultures were prepared as described previously (Yu et al., 2008). At 3 weeks after initiating myelination, the cultures were treated with vehicle or 0.1–1 μM KU-32 for 6 h, followed by 100 ng/ml of NRG1 (human recombinant neuregulin-1-β1 epidermal growth factor domain; amino acids 176–246; R&D Systems, Minneapolis, MN, U.S.A.). After 48 h, the cultures were fixed and stained for MBP (myelin basic protein). Degenerated myelin segments were quantified as previously described (Yu et al., 2008).
Myelinated mouse neuron cultures were prepared using DRGs isolated from 1-day-old mouse pups by collecting the ganglia into L15 medium and dissociating the tissue with 0.25% trypsin at 37°C for 30 min. The cells were resuspended in DMEM containing 25 mM glucose and 10% FCS (Atlas Biologicals, Fort Collins, CO, U.S.A.), triturated with a fire-polished glass pipette and plated in maintenance medium (DMEM containing 25 mM glucose, 10% FCS, antibiotics as above and 50 ng/ml NGF) in the centre of collagen-coated glass coverslips. Proliferating cells were removed by treating the neurons with the antimitotics for 3 days. After 1 week in culture, myelination was induced by the addition of 50 μg/ml ascorbic acid in maintenance medium. The cells were maintained for 15–18 days with medium replenishment every 2 to 3 days. Demyelination was induced by the addition of 100–200 ng/ml NRG1 for 2–4 days. Some cultures were treated overnight with vehicle or the indicated concentration of KU-32 prior to the addition of NRG1. The cultures were co-stained for MBP and PGP9.5 and nuclei were visualized with DAPI (4′,6-diamidino-2-phenylindole). Degeneration of the myelin segments was quantified with the aid of the open source imaging software, Cell Profiler (http://www.cellprofiler.org). Individual myelin internodes were identified using Otsu's method for thresholding and segmentation (Otsu, 1979). Segmentation was visually inspected for errors or regions where segments were closely apposed and manually edited where necessary. The length was computed for each identified myelin internode. In cases where segments intersected and a minimum minor axis width was exceeded, lengths were not included in the average of the population of segments surveyed. However, total area of coverage for myelin segments did include the intersecting regions. In some experiments, cell lysates were prepared and immunoblot analyses were performed as previously described (Yu et al., 2008; McGuire et al., 2009).
Induction of diabetes
WT (wild-type) C57Bl/6 and Hsp70.1/70.3 double KO mice (Hunt et al., 2004) were obtained from Harlan Laboratories (Indianapolis, IN, U.S.A.) and the Mutant Mouse Resource Center (San Diego, CA, U.S.A.) respectively. The absence of Hsp70.1 and 70.3 was confirmed by genotyping and immunoblot analysis. Eight-week-old WT and Hsp70 mice were rendered diabetic with three daily doses of STZ (85, 70 and 55 mg/kg; McGuire et al., 2009) and 3 days after the last injection, mice with FBG (fasting blood glucose)>290 mg/dl were deemed diabetic. After 12 weeks of diabetes, animals were given a once per week intraperitoneal injection of 5% Captisol (CyDex Pharmaceuticals, Lenexa, KS, U.S.A.) or 20 mg/kg KU-32 in 5% Captisol. All animals were maintained on a 12 h light/12 h dark cycle with ad libitum access to water and Purina diet 5001 rodent chow. FBG (One-Touch Ultra glucometer) and HbA1c levels (Act1) were determined prior to killing the animals. Plasma insulin levels were determined by ELISA using a commercial kit from Mercodia AB (Uppsala, Sweden). All animal procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee and in compliance with standards and regulations for the care and use of laboratory rodents set by the National Institutes of Health.
Measures of NCV (nerve conduction velocity), mechanical and thermal sensitivity
MNCV (motor NCV) and SNCV (sensory NCV) measurements were performed using a TECA™ Synergy N2-EMG monitoring system (McGuire et al., 2009). Mechanical sensitivity was assessed using a Dynamic Plantar aesthesiometer (Stoelting, Wood Dale, IL, U.S.A.) fitted with a stiff monofilament that was delivered at an upward force of 8 g. Thermal stimuli were delivered using a Hargreaves analgesiometer (Hargreaves et al., 1988). Responses from each animal were measured three or four times on alternate feet and averaged (McGuire et al., 2009). After measuring NCV, the animals were killed, and the sciatic nerves were dissected and flash frozen.
In vivo pharmacokinetics
KU-32 (2 mg/ml) was administered intraperitoneally to 60 Balb/c mice and blood was collected from six mice at the indicated time via cardiac puncture while the mice were under isoflurane anaesthesia. The animal was perfused with saline to remove any residual blood from the organs and the brain tissue was harvested.
Plasma (0.05 ml) or 0.25 ml brain homogenate was spiked with trideutero KU-32 as the internal standard, the protein was precipitated with acetonitrile, KU-32 was extracted from the supernatant with t-butyl methyl ether and the solvent was evaporated. Samples were reconstituted with 0.1 ml of CH3CN/water (20:80) and 0.01 ml was used for LC-MS (liquid chromatography MS) analysis. Chromatographic separation was performed using a 5 μm Agilent Zorbax SB 2.1 mm×50 mm column and a linear gradient of CH3CN/water/formic acid (5:95:0.2) to CH3CN/water/formic acid (95:5.0:0.1) at a flow rate of 0.20 ml/min. The effluent was introduced to a Sciex API3200 Linear Ion Trap detector using turbo ion spray in the positive mode. Assay response was linear (r2>0.997) and validated over the range of 1–1000 ng/ml for plasma samples and 1–500 ng/ml for mouse brain samples. Recoveries ranged from 65 to 75%, and PK Solutions software (Summit Research Services, Montrose, CO, U.S.A.) was used for data analysis.
Statistical analyses
Results are presented as means±S.E.M. After verifying equality of variance, differences between treatments and genotypes were determined using a one-way or a two-way ANOVA. Differences between group means were ascertained using Tukey's test.
RESULTS
KU-32 decreases cell death and demyelination in culture models of unmyelinating and myelinating sensory neurons
KU-32 is a novobiocin-based Hsp90 inhibitor containing an acetamide substitution on the coumarin ring (Figure 1A). KU-32 significantly increased the expression of Hsp70 in MCF7 cells at 10 nM, which is consistent with the induction of an HSR. Although inhibiting Hsp90 can destabilize its association with client proteins such as Akt and lead to their degradation, 5 μM KU-32 only induced a 35% decrease in the Akt client (Figures 1B and 1D). In contrast, KU-174 is a novobiocin analogue in which the acetamide is replaced with a biaryl ring system (Figure 1A). This substitution abolished Hsp70 induction and shifted the potency for promoting a 50% decrease in the Akt client protein from >5 μM for KU-32 to approx. 10 nM for KU-174 (Figures 1C and 1D). Thus substitution of the prenylated benzamide of novobiocin with the simplified acetamide is responsible for differentiating client protein degradation from Hsp70 induction.
Since DPN affects both non-myelinated and myelinated sensory fibres, we assessed whether KU-32 could protect cultured sensory neurons against death and demyelination. We first determined whether KU-32 could protect unmyelinated embryonic primary sensory neurons from glucose-induced cell death (Vincent et al., 2005). Rat embryonic DRG neurons were cultured for 3 days and hyperglycaemia was induced by the addition of 20 mM excess glucose (45 mM final glucose concentration) to the medium. Cell death was assessed by the uptake of propidium iodide. Hyperglycaemia increased neuronal death by ∼1.5-fold, but a 6 h pretreatment with either 0.1 or 1 μM KU-32 prevented glucose-induced cell death (Figure 2A). Regardless of any arguments surrounding the physiological relevance of the glucose concentration, these in vitro data support that targeting molecular chaperones can protect unmyelinated sensory neurons from acute glucotoxicity.
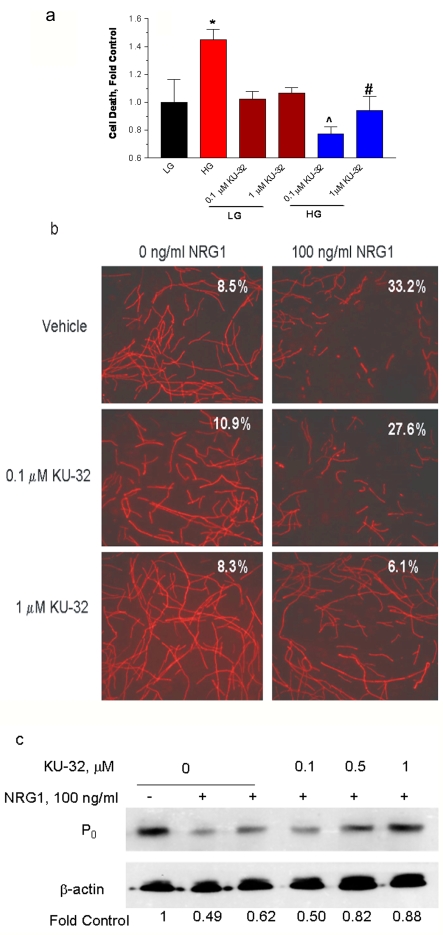
Figure 2. KU-32 protects rat sensory neurons against glucose-induced death and neuregulin-induced demyelination.
(a) Embryonic sensory neurons were treated for 6 h with 1% DMSO or 0.1–1 μM KU-32 in a medium containing 25 mM glucose (LG). The glucose concentration was raised to 45 mM to induce hyperglycaemia (HG) and the cells were incubated for an additional 24 h. Cell death was assessed using calcein AM and propidium iodide. *P< 0.05 versus LG control; ∧P<0.003 versus HG; #P<0.02 versus HG (n = 3). (b) Myelinated rat SC-DRG neuron co-cultures were treated overnight with 1% DMSO, 0.1 or 1 μM KU-32 and the cultures treated with PBS or 100 ng/ml NRG1 for 48 h. The myelin segments were visualized by staining for MBP. Numbers show percentage degenerated segments in each culture. Results are from a single experiment performed twice with similar outcomes. (c) Myelinated rat SC-DRG neuron co-cultures were treated as above and immunoblot analysis for the P0 compact myelin protein was performed. Band intensities were normalized to β-actin and expressed as a percentage of the control. Results are from one experiment performed twice with similar outcomes.
To ascertain whether KU-32 could protect myelinated axons against degeneration, we assessed its ability to prevent NRG1-induced demyelination. Neuregulin-1 is a family of EGF (epidermal growth factor)-like ligands that bind ErbB receptors and can induce demyelination of myelinated rat or mouse SC-DRG neurons (Syed et al., 2010). Myelin degeneration is readily visualized and quantified by staining the SC-DRG co-cultures for MBP and assessing the number of damaged myelin segments (Zanazzi et al., 2001). At 2 days after treating rat SC-DRG co-cultures with 100 ng/ml NRG1, the percentage of damaged myelin segments was approx. 33% (Figure 2B). Overnight pretreatment with 1 μM KU-32 was sufficient to prevent this damage. Similarly, KU-32 also prevented the NRG1-induced decrease in the expression of the compact myelin protein zero, P0 (Figure 2C). Importantly, any alterations in client protein levels by KU-32 were not sufficient to affect viability since 1 μM KU-32 neither promoted the death of the unmyelinated sensory neurons (Figure 2A) nor increased the number of damaged myelin segments in the SC-DRG co-cultures (Figure 2B).
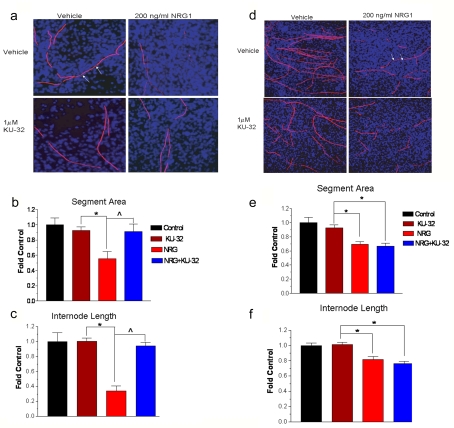
Although KU-32 induces the expression of Hsp70, it remains unclear whether this protein may be central to its neuroprotective efficacy. Therefore SC-DRG co-cultures were prepared from C57BL/6 (WT) and Hsp70.1/70.3 double KO mice. These two genes represent the inducible forms of Hsp70, while a related chaperone that is constitutively expressed, Hsc70 (heat-shock cognate 70 stress protein), is present at normal levels in these mice (Hunt et al., 2004). WT cells treated with 200 ng/ml NRG1 for 4 days exhibited a 40% decrease in the area occupied by myelinated segments (Figures 3A and 3B). Additionally, the segments showed a significant shortening of internode length, indicative of an increased number of breaks due to the ongoing degeneration of the myelin segments (Figure 3C). As above, pretreatment with 1 μM KU-32 prevented the damage to the myelin segments formed in cultures from WT mice, and this correlated with an increase in Hsp70 levels in the KU-32-treated cultures (results not shown). On the other hand, KU-32 was ineffective in preventing NRG1-induced demyelination in co-cultures prepared from Hsp70 KO mice (Figures 3D–3F). As expected, KU-32 did not increase Hsp70 levels in these cultures (results not shown). These results support the notion that Hsp70 expression is a key component of the neuroprotective efficacy of KU-32.
Figure 3. Hsp70 is required for KU-32 to protect against neuregulin-induced demyelination.
Myelinated mouse SC-DRG neuron co-cultures were prepared from WT (a–c) or Hsp70 KO (d–f) mice and treated overnight with vehicle or 1 μM KU-32. The cultures were treated with PBS or 200 ng/ml NRG1 for 4 days and myelin segments were visualized by staining the cultures for MBP. Total cell number was assessed by staining nuclei with DAPI. Cell profiler was used to calculate the total segment area and internode length from six fields per six coverslips per treatment. Arrows show examples of internode length. The results are expressed as fold of the untreated control and are the average of three experiments per genotype. *P< 0.05 versus KU-32, ∧P<0.05 versus NRG1.
KU-32 attenuates the pathophysiological progression of DPN
The onset of DPN in rodents (3–12 weeks) is characterized by early metabolic alterations in the conduction velocity of peripheral nerves and the development of altered sensory thresholds to noxious thermal (unmyelinated fibres) or mechanical (myelinated fibres) stimuli presented to the foot. Since our in vitro readouts support the notion that KU-32 protects unmyelinated and myelinated nerves from death and degeneration, we rationalized that it may be similarly protective in a rodent model of DPN.
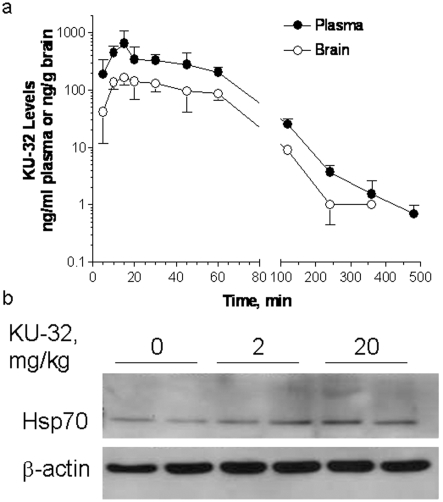
KU-32 is highly bioavailable and has a systemic clearance of 71.4 ml/min per kg. The drug was detectable to similar levels in both brain tissue and plasma after intraperitoneal administration in mice and had a mean half-life (t½) of 105.6 and 106.9 min in plasma and brain respectively (Figure 4A). Although the accumulation of KU-32 in peripheral nerve has not been assessed, the level of Hsp70 was modestly increased in sciatic nerve at 1 week after drug administration (Figure 4B). Preliminary studies indicated that more frequent dosing at these concentrations did not increase the expression of Hsp70. Weekly treatment of KU-32 for 6 weeks at both doses did not alter body weight and showed no confounding affects on electrophysiological and psychophysical measures that are diagnostic for the onset of DPN (results not shown). No overt toxicity was seen at 60 mg/kg, which was the highest dose tested.
Figure 4. Pharmacokinetic profile of KU-32 uptake in plasma and brain after intraperitoneal administration.
(a) KU-32 (2 mg/ml) was injected intraperitoneally in 5% Captisol, and plasma and brain samples were taken at the indicated time. KU-32 levels were quantified by LC-MS. Plasma AUC0-∞, 27.4 μg/min per ml. Results are the means±S.E.M. for six mice per time point. (b) Effect of KU-32 on expression of Hsp70 in sciatic nerve. Mice were injected with KU-32 and sciatic nerve was harvested after 1 week. Hsp70 and β-actin levels were determined by immunoblotting. The level of Hsp70 was normalized to β-actin, and KU-32 increased Hsp70 expression by 1.2- and 1.35-fold at 2 and 20 mg/kg respectively.
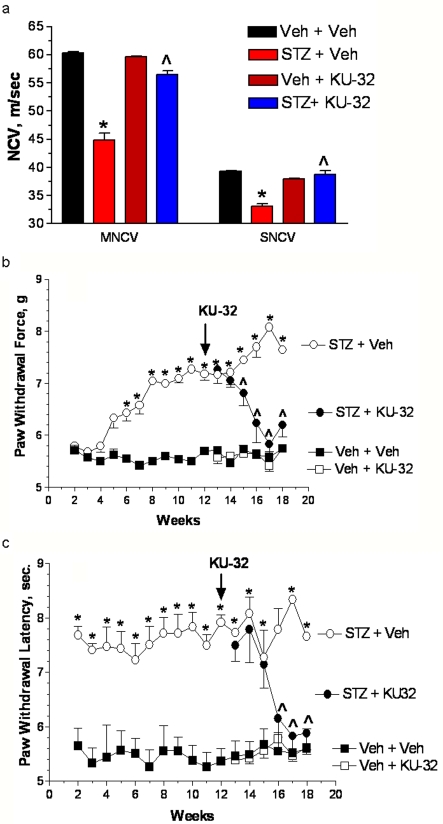
A powerful indicator of the ability of KU-32 to improve neurodegeneration is whether it could reverse pre-existing deficits in SNCV and MNCV, and psychophysical measures of thermal and mechanical sensitivity. Therefore diabetes was induced in 8-week-old C57Bl/6 mice and allowed to progress for 12 weeks prior to drug treatment. Mice then received vehicle or 20 mg/kg KU-32 once a week for 6 weeks. WT mice that were diabetic for 3 months developed significant deficits in MNCV prior to drug treatment (control, 60.4±2.0 m/s; STZ, 49.5±6.3 m/s, P< 0.05, Student's two-tailed t test). While SNCV declined, it did not yet reach significance (control, 39.3±2.0 m/s; STZ, 35.8±1.7 m/s). Importantly, although both MNCV and SNCV continued to significantly decline after 18 weeks of diabetes, KU-32 prevented this degeneration and returned NCV to control levels (Figure 5A). Consistent with the development of a sensory hypoalgesia, diabetic mice also showed decreased responses to mechanical (Figure 5B) and thermal (Figure 5C) stimuli. KU-32 treatment also significantly improved these deficits in a time-dependent manner to near control levels after 6 weeks of treatment. It is important to note that diabetic mice receiving KU-32 showed no changes in FBG, HbA1c or plasma insulin levels compared with the diabetic mice receiving vehicle (Table 1). Thus the improvements in nerve function are not related to decreased hyperglycaemia or an outright increase in insulin levels. However, since STZ does not completely ablate insulin production, we cannot rule out the possibility that KU-32 may improve insulin action (Chung et al., 2008).
Figure 5. KU-32 reversed pre-existing indices of diabetic sensory neuropathy in WT mice.
WT mice were rendered diabetic for 12 weeks and then treated with weekly doses of vehicle or 20 mg/kg KU-32 for 6 weeks. (a) After 18 weeks of diabetes, MNCV and SNCV significantly decreased in untreated mice, but KU-32 treatment for 6 weeks improved the deficits in both MNCV and SNCV. *P<0.01 versus time-matched untreated control; ∧P<0.001 versus 18 week STZ+vehicle. Two weeks after the induction of diabetes, mechanical sensitivity (b) and thermal sensitivity (c) were assessed weekly. Twelve weeks of diabetes produced a significant mechanical and thermal hypoalgesia and weekly treatment with KU-32 induced a time-dependent improvement in both sensory thresholds to near control levels. Since the sensory measures in the diabetic mice became more variable after 14 weeks, this time point was used for statistical comparisons. *P<0.05 compared with the time-matched control. ∧P<0.05 compared with STZ+vehicle at 14 weeks.
Table 1. FBG, HbA1C and insulin levels in WT and Hsp70 KO mice.
*P<0.05 versus vehicle+vehicle; †P<0.05 versus vehicle+KU-32; n.d., not determined.
| Treatment | FBG (mg/dl) | HbA1C (%) | Insulin (nM) | n |
|---|---|---|---|---|
| WT mice | ||||
| Vehicle+vehicle | 136±4 | 4.7±0.1 | 0.25±0.04 | 11 |
| Vehicle+KU-32 | 121±4 | 4.6±0.2 | 0.34±0.06 | 11 |
| STZ+vehicle | 389±83* | 11.4±0.6* | 0.1±0.004* | 3 |
| STZ+KU-32 | 450±13† | 11.6±0.3† | 0.11±0.006† | 6 |
| Hsp70 KO mice | ||||
| Vehicle+vehicle | 115±5 | 4.9±0 | n.d. | 5 |
| Vehicle+KU-32 | 119±11 | 4.8±0.1 | n.d. | 7 |
| STZ+vehicle | 380±45* | 10.9±0.5* | n.d. | 4 |
| STZ+KU-32 | 412±33† | 10.9±0.8† | n.d. | 5 |
The efficacy of KU-32 requires Hsp70
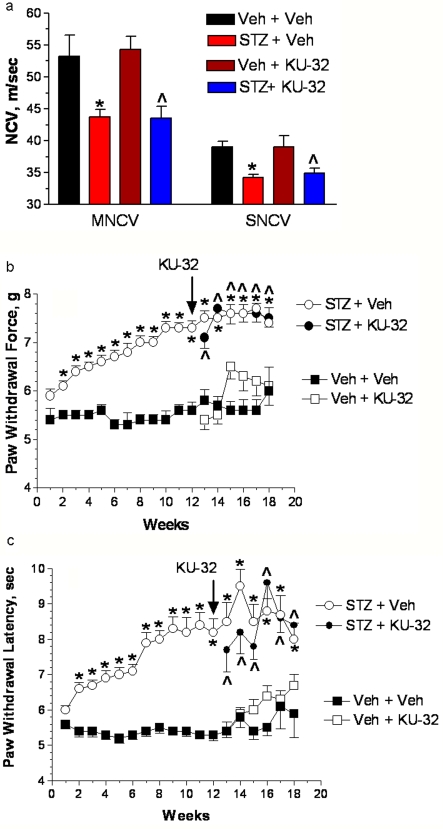
To define the relative contribution of Hsp70 to the efficacy of KU-32, we examined the effect of diabetes and KU-32 treatment on the development of DPN in the Hsp70 KO mice. Hsp70 KO mice (C57Bl/6 background) readily developed diabetes and showed no differences in blood glucose or HbA1c levels after 18 weeks of diabetes compared with diabetic C57Bl/6 mice (Table 1). Diabetic Hsp70 KO mice also developed significant deficits in MNCV and SNCV (Figure 6A) that mirrored those seen in WT mice. Compared with WT mice, genetic deletion of Hsp70 did not alter the severity of the deficits in mechanical (Figure 6B) and thermal sensitivity (Figure 6C) that had developed prior to drug treatment. Thus the Hsp70 KO mice respond to diabetes in a manner very similar to that observed in WT mice. However, weekly dosing of the diabetic Hsp70 KO mice with 20 mg/kg KU-32 was unable to correct the deficits in NCV or mechanical and thermal sensitivities. Consistent with the in vitro data, these results provide strong support that Hsp70 plays a central role in the neuroprotective efficacy of KU-32.
Figure 6. Hsp70 is required for the in vivo efficacy of KU-32 against indices of diabetic sensory neuropathy.
Hsp70 KO mice were rendered diabetic for 12 weeks and then treated with weekly doses of vehicle or 20 mg/kg KU-32 for 6 weeks. (a) After 18 weeks of diabetes, MNCV and SNCV decreased in vehicle-treated mice and weekly treatment with KU-32 for 6 weeks did not reverse these deficits. *P<0.01 versus untreated control; ∧P<0.01 versus vehicle+KU-32. Two weeks after the induction of diabetes, mechanical sensitivity (b) and thermal sensitivity (b) were assessed weekly. Although 12 weeks of diabetes produced a significant mechanical and thermal hypoalgesia, weekly treatment with KU-32 had no effect on improving the sensory thresholds.*P<0.01 compared with the time-matched untreated control. ∧P<0.01 compared with time-matched vehicle+KU-32.
DISCUSSION
Targeting molecular chaperones has shown promising results in treating both neurodegenerative and diabetes-associated phenotypes. For example, N-terminal Hsp90 inhibitors decreased motor neuron degeneration in a murine model of spinal and bulbar muscular atrophy (Waza et al., 2005) and reduced aggregated or hyperphosphorylated forms of tau protein in JNPL3 mice (Luo et al., 2007) and humanized tau protein transgenic mice (Dickey et al., 2007). Similarly, induction of Hsp70 with HSF1 activators (bimoclomol and related hydroxylamine derivatives) improved insulin resistance (Chung et al., 2008), diabetic wound healing (Atalay et al., 2009; Vigh et al., 1997) and was sufficient to prevent the slowing of NCV in diabetic rats (Biro et al., 1997). In the present study, we provide the first evidence that a novel C-terminal Hsp90 inhibitor that increases Hsp70 expression is similarly effective in preventing death of unmyelinated neurons, demyelination of myelinated sensory neurons and was able to reverse pre-existing sensory deficits associated with the development of DPN in mice.
Hsp70 is a critical mechanistic component necessary for the efficacy of KU-32 in improving a major neurodegenerative complication of diabetes. However, the molecular basis by which Hsp70 intersects with biochemical mediators that contribute to the pathophysiology of DPN is unclear. It is well recognized that induction of the HSR can up-regulate numerous genes that may have a beneficial effect in ameliorating DPN, i.e. Mn superoxide dismutase and haem oxygenase 1. The reliance on Hsp70 expression for neuroprotection suggests that if KU-32 promotes a broader HSR via the release of HSF-1, this is not sufficient for drug efficacy. Previous work has shown that up-regulation of Hsp70 can improve insulin resistance and that this is associated with a decrease in phosphorylation of JNK (c-Jun N-terminal kinase) (Chung et al., 2008). Although increasing the expression of Hsp70 inhibits JNK-dependent apoptosis (Bienemann et al., 2008; Salehi et al., 2006), neuronal apoptosis is not a substantial contributing feature to the pathophysiology of DPN (Cheng and Zochodne, 2003). Indeed, adult neurons are relatively insensitive to JNK-induced apoptosis (Walsh et al., 2004). However, JNK activation does mediate death of embryonic neurons (Bienemann et al., 2008; Salehi et al., 2006; Walsh et al., 2004), and the ability of Hsp70 to block this activity may protect against glucose-induced death of the immature, embryonic DRG neurons. Interestingly, although increased expression of the c-jun transcription factor contributes to NRG1-induced demyelination, its phosphorylation by JNK is not essential to mediate Schwann cell degeneration (Parkinson et al., 2008). These results suggest that any inhibitory effect of Hsp70 on JNK activity would not be critical to the ability of KU-32 to attenuate demyelination. However, an interaction of Hsp70 with JNK is possible in the context of DPN, since JNK activity increases in diabetic nerves (Fernyhough et al., 1999; Purves et al., 2001) and contributes to features of neuropathic pain (Gao and Ji, 2008). Thus up-regulating Hsp70 may be an effective treatment for both insensate and painful diabetic neuropathy.
The ability of Hsp70 to refold aggregated or oxidatively damaged proteins may also contribute to improving nerve function. It is generally accepted that enhanced production of reactive oxygen species and subsequent mitochondrial dysfunction (Verkhratsky and Fernyhough, 2008) and fission (Figueroa-Romero et al., 2009; Leinninger et al., 2006) contribute to DPN. Oxidative modification of the mitochondrial fission protein Drp1 (dynamin-related protein 1) by S-nitrosylation favours the formation of Drp1 tetramers and larger aggregates that display enhanced GTPase activity, which promotes mitochondrial fission (Nakamura and Lipton, 2010). It will be important to determine whether an interaction of Hsp70 with Drp1 may aid its refolding or prevent aggregation to minimize increased GTPase activity and organelle fission. However, Hsp70 mutants that are folding-incompetent, but are able to bind and maintain the solubility of denatured proteins, were as effective as WT Hsp70 in decreasing oxidative stress and the rate of proton leak across the inner mitochondrial membrane in glucose-deprived astrocytes (Ouyang et al., 2006). Thus the folding competence of Hsp70 may not be a critical feature of neuroprotection, at least in transient models of oxidative stress that are not associated with protein aggregation. On the other hand, Hsp70 may also help maintain mitochondrial protein integrity via its interaction with TOM70 (translocase of outer membrane 70), which is important for mitochondrial protein import (Young et al., 2003).
It is notable that the diabetic Hsp70 KO mice developed a similarly severe neuropathy as was observed in the WT mice. This outcome indicates that inducible forms of Hsp70 are not necessary for the pathogenesis and progression of neuropathy. However, since constitutive forms of Hsp70 (Hsc70) may substitute for Hsp70.1 and 70.3, we cannot rule out the possibility that diabetes-induced changes in chaperone activity may contribute to decreased neuronal function. Indeed, the effect of diabetes on Hsp70 protein expression and its role in the development of diabetic complications are not clear. In short-term diabetic rats (1 month), Hsp70 levels did not change in various skeletal muscles, heart, liver or kidney (Najemnikova et al., 2007). However, diabetes may affect Hsp70 in a cell-selective manner since immunoreactivity increased in the kidney outer medulla, but not the glomeruli, of rats rendered diabetic for 4–24 weeks (Barutta et al., 2008). Unfortunately, whether this contributed to the nephropathy that developed in these animals was not determined. Similarly, some reports indicate that a 15 min heat stress enhanced the expression of Hsp70 in diabetic heart relative to levels observed in heat-stressed non-diabetic rats (Najemnikova et al., 2007). In contrast, induction of Hsp70 has been reported to be similar in hearts from heat stressed control and diabetic rats and that Hsp70 induction in diabetic heart was not cardioprotective against an ischaemia/reperfusion injury (Joyeux et al., 1999). These results raise the issue of whether Hsp70 induction may be broadly protective in reversing diabetic complications associated with other organs. However, to the best of our knowledge, the potential efficacy of Hsp90 inhibitors and Hsp70 induction against other diabetic complications has not been adequately examined.
In summary, the present study provides proof-of-principle that pharmacologically targeting molecular chaperones can be sufficiently cytoprotective to reverse pre-existing symptoms associated with insensate DPN in experimental animals. The potential of this approach as a monotherapy or in conjunction with pharmacological agents that antagonize a specific biochemical pathology that contributes to DPN may broaden our translational options toward developing effective therapies for the medical management of DPN.
Footnotes
This work was supported by grants from the Juvenile Diabetes Research Foundation and the National Institutes of Health [NS054847 and DK073594] (to R.T.D.) and [CA120458 and CA109265] (to B.S.J.B.).
REFERENCES
- Akude E, Zherebitskaya E, Roy Chowdhury SK, Girling K, Fernyhough P. 4-Hydroxy-2-nonenal induces mitochondrial dysfunction and aberrant axonal outgrowth in adult sensory neurons that mimics features of diabetic neuropathy. Neurotox Res. 2009;1:28–38. doi: 10.1007/s12640-009-9074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansar S, Burlison JA, Hadden MK, Yu XM, Desino KE, Bean J, Neckers L, Audus KL, Michaelis ML, Blagg BS. A non-toxic Hsp90 inhibitor protects neurons from Aβ-induced toxicity. Bioorg Med Chem Lett. 2007;17:1984–1990. doi: 10.1016/j.bmcl.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Atalay M, Oksala N, Lappalainen J, Laaksonen DE, Sen CK, Roy S. Heat shock proteins in diabetes and wound healing. Curr Protein Pept Sci. 2009;10:85–95. doi: 10.2174/138920309787315202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barutta F, Pinach S, Giunti S, Vittone F, Forbes JM, Chiarle R, Arnstein M, Perin PC, Camussi G, Cooper ME, Gruden G. Heat shock protein expression in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295:F1817–F1824. doi: 10.1152/ajprenal.90234.2008. [DOI] [PubMed] [Google Scholar]
- Bienemann AS, Lee YB, Howarth J, Uney JB. Hsp70 suppresses apoptosis in sympathetic neurones by preventing the activation of c-Jun. J Neurochem. 2008;104:271–278. doi: 10.1111/j.1471-4159.2007.05006.x. [DOI] [PubMed] [Google Scholar]
- Biro K, Jednakovits A, Kukorelli T, Hegedus E, Koranyi L. Bimoclomol (BRLP-42) ameliorates peripheral neuropathy in streptozotocin-induced diabetic rats. Brain Res Bull. 1997;44:259–263. doi: 10.1016/s0361-9230(97)00118-4. [DOI] [PubMed] [Google Scholar]
- Bishop SC, Burlison JA, Blagg BS. Hsp90: a novel target for the disruption of multiple signaling cascades. Curr Cancer Drug Targets. 2007;7:369–388. doi: 10.2174/156800907780809778. [DOI] [PubMed] [Google Scholar]
- Blagg BS, Kerr TD. Hsp90 inhibitors: small molecules that transform the Hsp90 protein folding machinery into a catalyst for protein degradation. Med Res Rev. 2006;26:310–338. doi: 10.1002/med.20052. [DOI] [PubMed] [Google Scholar]
- Brown I. Heat shock proteins and protection of the nervous system. Ann NY Acad Sci. 2007;1113:147–158. doi: 10.1196/annals.1391.032. [DOI] [PubMed] [Google Scholar]
- Burlison JA, Neckers L, Smith AB, Maxwell A, Blagg BS. Novobiocin: redesigning a DNA gyrase inhibitor for selective inhibition of hsp90. J Am Chem Soc. 2006;128:15529–15536. doi: 10.1021/ja065793p. [DOI] [PubMed] [Google Scholar]
- Cheng C, Zochodne DW. Sensory neurons with activated caspase-3 survive long-term experimental diabetes. Diabetes. 2003;52:2363–2371. doi: 10.2337/diabetes.52.9.2363. [DOI] [PubMed] [Google Scholar]
- Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2008;105:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM, Dunmore J, Ash P, Shoraka S, Zlatkovic J, Eckman CB, Patterson C, Dickson DW, Nahman NS, Hutton M, Jr, Burrows F, Petrucelli L. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly AC, Mays JR, Burlison JA, Nelson JT, Vielhauer G, Holzbeierlein J, Blagg BSJ. The design, synthesis, and evaluation of coumarin ring derivatives of the novobiocin scaffold that exhibit antiproliferative activity. J Org Chem. 2008;73:8901–8920. doi: 10.1021/jo801312r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernyhough P, Gallagher A, Averill SA, Priestley JV, Hounsom L, Patel J, Tomlinson DR. Aberrant neurofilament phosphorylation in sensory neurons of rats with diabetic neuropathy. Diabetes. 1999;48:881–889. doi: 10.2337/diabetes.48.4.881. [DOI] [PubMed] [Google Scholar]
- Figueroa-Romero C, Iniguez-Lluhi JA, Stadler J, Chang CR, Arnoult D, Keller PJ, Hong Y, Blackstone C, Feldman EL. SUMOylation of the mitochondrial fission protein Drp1 occurs at multiple nonconsensus sites within the B domain and is linked to its activity cycle. FASEB J. 2009;23:3917–3927. doi: 10.1096/fj.09-136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y-J, Ji R-R. Activation of JNK pathway in persistent pain. Neurosci Lett. 2008;437:180–183. doi: 10.1016/j.neulet.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hunt CR, Dix DJ, Sharma GG, Pandita RK, Gupta A, Funk M, Pandita TK. Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice. Mol Cell Biol. 2004;24:899–911. doi: 10.1128/MCB.24.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyeux M, Faure P, Godin-Ribuot D, Halimi S, Patel A, Yellon DM, Demenge P, Ribuot C. Heat stress fails to protect myocardium of streptozotocin-induced diabetic rats against infarction. Cardiovasc Res. 1999;43:939–46. doi: 10.1016/s0008-6363(99)00185-6. [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Backus C, Sastry AM, Yi YB, Wang CW, Feldman EL. Mitochondria in DRG neurons undergo hyperglycemic mediated injury through Bim, Bax and the fission protein Drp1. Neurobiol Dis. 2006;23:11–22. doi: 10.1016/j.nbd.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Li G, Faibushevich A, Turunen BJ, Yoon SO, Georg G, Michaelis ML, Dobrowsky RT. Stabilization of the cyclin-dependent kinase 5 activator, p35, by paclitaxel decreases α-amyloid toxicity in cortical neurons. J Neurochem. 2003;84:347–362. doi: 10.1046/j.1471-4159.2003.01526.x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Ansar S, Michaelis ML, Blagg BS. Neuroprotective activity and evaluation of Hsp90 inhibitors in an immortalized neuronal cell line. Bioorg Med Chem. 2008;17:1709–1715. doi: 10.1016/j.bmc.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Dou F, Rodina A, Chip S, Kim J, Zhao Q, Moulick K, Aguirre J, Wu N, Greengard P, Chiosis G. Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc Natl Acad Sci USA. 2007;104:9511–9516. doi: 10.1073/pnas.0701055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Rodina A, Chiosis G. Heat shock protein 90: translation from cancer to Alzheimer's disease treatment? BMC Neurosci. 2008;9(Suppl. 2):S7. doi: 10.1186/1471-2202-9-S2-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J Biol Chem. 2000a;275:37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- Marcu MG, Schulte TW, Neckers L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J Natl Cancer Inst. 2000b;92:242–248. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- McGuire JF, Rouen S, Siegfreid E, Wright DE, Dobrowsky RT. Caveolin-1 and altered neuregulin signaling contribute to the pathophysiological progression of diabetic peripheral neuropathy. Diabetes. 2009;58:2677–2686. doi: 10.2337/db09-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najemnikova E, Rodgers CD, Locke M. Altered heat stress response following streptozotocin-induced diabetes. Cell Stress Chaperones. 2007;12:342–352. doi: 10.1379/CSC-292.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Lipton SA. Redox regulation of mitochondrial fission, protein misfolding, synaptic damage, and neuronal cell death: potential implications for Alzheimer's and Parkinson's diseases. Apoptosis. 2010 doi: 10.1007/s10495-010-0476-x. 10.1007/s10495-010-0476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2009;10:931–940. doi: 10.1016/j.bbadis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Sys Man Cyber. 1979;9:62–66. [Google Scholar]
- Ouyang YB, Xu LJ, Sun YJ, Giffard RG. Overexpression of inducible heat shock protein 70 and its mutants in astrocytes is associated with maintenance of mitochondrial physiology during glucose deprivation stress. Cell Stress Chaperones. 2006;11:180–186. doi: 10.1379/CSC-182R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey S, Banerji U, Judson I, Workman P. Hsp90 inhibitors in the clinic. Handb Exp Pharmacol. 2006:331–358. doi: 10.1007/3-540-29717-0_14. [DOI] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R, Jessen KR. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181:625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LB, Blagg BS. To fold or not to fold: modulation and consequences of Hsp90 inhibition. Future Med Chem. 2009;1:267–283. doi: 10.4155/fmc.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006;22:257–273. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- Powers MV, Workman P. Inhibitors of the heat shock response: biology and pharmacology. FEBS Lett. 2007;581:2758–3769. doi: 10.1016/j.febslet.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Purves TD, Middlemas A, Agthong SI, Jude EB, Boulton AJM, Fernyhough P, Tomlinson DR. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. FASEB J. 2001;15:2508–2514. doi: 10.1096/fj.01-0253hyp. [DOI] [PubMed] [Google Scholar]
- Salehi AH, Morris SJ, Ho WC, Dickson KM, Doucet G, Milutinovic S, Durkin J, Gillard JW, Barker PA. AEG3482 is an antiapoptotic compound that inhibits Jun kinase activity and cell death through induced expression of heat shock protein 70. Chem Biol. 2006;13:213–223. doi: 10.1016/j.chembiol.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Syed N, Reddy K, Yang DP, Taveggia C, Salzer JL, Maurel P, Kim HA. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J Neurosci. 2010;30:6122–6131. doi: 10.1523/JNEUROSCI.1681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taldone T, Sun W, Chiosis G. Discovery and development of heat shock protein 90 inhibitors. Bioorg Med Chem. 2008;17:2225–2235. doi: 10.1016/j.bmc.2008.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidwell JL, Houenou LJ, Tytell M. Administration of Hsp70 in vivo inhibits motor and sensory neuron degeneration. Cell Stress Chaperones. 2004;9:88–98. doi: 10.1379/CSC-9R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9:36–45. doi: 10.1038/nrn2294. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Fernyhough P. Mitochondrial malfunction and Ca2+ dyshomeostasis drive neuronal pathology in diabetes. Cell Calcium. 2008;44:112–122. doi: 10.1016/j.ceca.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Vigh L, Literati PN, Horvath I, Torok Z, Balogh G, Glatz A, Kovacs E, Boros I, Ferdinandy P, Farkas B, Jaszlits L, Jednakovits A, Koranyi L, Maresca B. Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat Med. 1997;3:1150–1154. doi: 10.1038/nm1097-1150. [DOI] [PubMed] [Google Scholar]
- Vincent AM, McLean LL, Backus C, Feldman EL. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005;19:638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- Walsh GS, Orike N, Kaplan DR, Miller FD. The invulnerability of adult neurons: a critical role for p73. J Neurosci. 2004;24:9638–9647. doi: 10.1523/JNEUROSCI.1299-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waza M, Adachi H, Katsuno M, Minamiyama M, Sang C, Tanaka F, Inukai A, Doyu M, Sobue G. 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat Med. 2005;11:1088–1095. doi: 10.1038/nm1298. [DOI] [PubMed] [Google Scholar]
- Young JC, Hoogenraad NJ, Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 2003;112:41–50. doi: 10.1016/s0092-8674(02)01250-3. [DOI] [PubMed] [Google Scholar]
- Yu C, Rouen S, Dobrowsky RT. Hyperglycemia and downregulation of caveolin-1 enhance neuregulin-induced demyelination. Glia. 2008;56:877–887. doi: 10.1002/glia.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanazzi G, Einheber S, Westreich R, Hannocks MJ, Bedell-Hogan D, Marchionni MA, Salzer JL. Glial growth factor/neuregulin inhibits Schwann cell myelination and induces demyelination. J Cell Biol. 2001;152:1289–1299. doi: 10.1083/jcb.152.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]