Abstract
Recently, 2 small molecule kinase inhibitors (TKIs), targeting epidermal growth factor receptor (EGFR), have proven effective in the treatment of non-small cell lung cancer. However, it is unknown whether the EGFR double activating mutation of L858R in exon 21 and the in-frame deletion in exon 19 is a predictor of the effectiveness of EGFR-TKIs. We report for the first time a case of non-small cell lung cancer with central nervous system metastases harboring a rare EGFR double activating mutation who showed a good clinical response to erlotinib, regardless of his poor performance status, as swallowing is not possible. Therefore, we suggest that erlotinib may become a therapeutic choice in cases of central nervous system metastases even with poor performance status.
Key Words: Epidermal growth factor receptor inhibitor, Erlotinib, Squamous cell lung cancer, Pharmacokinetics, Pleural effusion
Introduction
Lung cancer is a major cause of cancer-related mortality worldwide and is expected to remain a major health problem for the foreseeable future. Chemotherapy is the cornerstone of the management of the disease; however, its therapeutic impact on patient survival has been modest. Recent discoveries have provided greater understanding of the molecular basis of the disease, and the success of 2 small molecule kinase inhibitors (TKIs), gefitinib (Iressa(r), AstraZeneca, London, UK) and erlotinib (Tarceva(r), OSI Pharmaceuticals, Melville City, N.Y., USA), against epidermal growth factor receptor (EGFR) in the treatment of non-small cell lung cancer (NSCLC) has provided evidence for the effectiveness of the strategy. Erlotinib and gefitinib inhibit the tyrosine kinase activity of EGFR and have been studied extensively. Since Lynch et al. have identified specific activating mutations within the tyrosine kinase domain of EGFR [1], the missense mutation L858R in exon 21 and the in-frame deletion in exon 19, nested around the amino acid residues 747 to 750 of the EGFR polypeptide, account for >85% of all clinically important mutations related to TKI sensitivity [2]. The detection of EGFR mutations in tumor tissues has been applied for predicting the response of TKI treatment and hence guiding the treatment for advanced NSCLC. A panel of 30 EGFR kinase domain mutations that were recently reported in NSCLC patients was cloned and expressed for analysis of kinase activity, transforming potential, and drug sensitivity. These mutations affect the N-lobe (exons 18-20) and the C-lobe (exon 21) of the EGFR kinase domain [3], however it is uncertain whether the EGFR double activating mutation is a predictor of the effectiveness of EGFR-TKIs.
Patients with central nervous system (CNS) metastases in general suffer from deterioration of performance status (PS) and do not have a long survival time. The primary treatment for CNS metastases in patients with NSCLC has traditionally consisted of whole-brain radiotherapy, surgery, or radiosurgery, while systemic chemotherapy has been thought to play a limited role because of the belief that the brain is a pharmacologic sanctuary site [4, 5]. Several articles in which CNS metastases were improved by erlotinib have been reported [6,7,8,9,10,11]; however, it remains unknown whether CNS metastases are improved by erlotinib in a NSCLC case harboring EGFR double activating mutation with a poor PS.
We report, for the first time, a case of NSCLC with CNS metastases harboring an EGFR double activating mutation that showed a good clinical response to erlotinib even with a poor PS.
Case Report
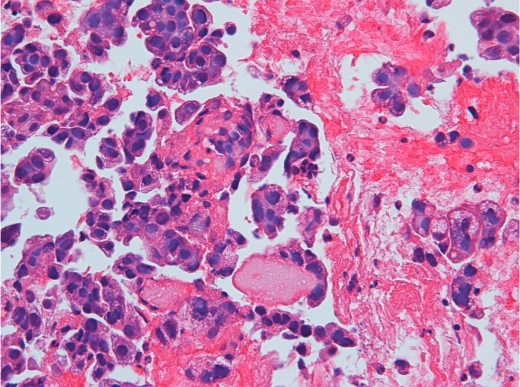
The patient was a 60-year-old Japanese man with no smoking history and PS 1 (table 1). He suffered from prolonged left chest pain on our 1st visit. Evaluation at another facility revealed a nodule in the left lung measuring 3.5 cm in diameter, multiple mediastinal contralateral lymph node metastases, multiple brain metastases, and left pleural effusion and disseminations. Pathologic examination of specimens obtained by transbronchial biopsy from the left lung mass revealed adenocarcinoma (fig. 1) with a disease stage of cT4N3M1. To choose a therapeutic strategy, we investigated the EGFR gene mutation status of the transbronchial biopsy specimen with the PNA-LNA clamp method [12,13,14] and detected an EGFR gene double activating mutation; L747-S752 deletion in exon 19 and L858R in exon 21.
Table 1.
Patient's background
| EGFR mutation |
Age | Gender | Histology | Smoking status | PS | Stage | Metastatic lesion | |
|---|---|---|---|---|---|---|---|---|
| exon 19 | exon 21 | |||||||
| L747-S752del | 2573T>G:L858R | 60 | male | adenocarcinoma | never smoker | 1 | CTN3M1/IV | brain |
Fig. 1.
High-power magnification of a tumor specimen of the left lung nodule shows adenocarcinoma.
Cancer cells were obtained from paraffin-embedded biopsy specimens by manual microdissection. The definition of a small specimen is that the quantity of the specimen is sufficient to make a pathologic diagnosis; at most several hundred tumor cells are necessary. Formalin-fixed paraffin-embedded tissue was cut in 6-8 μm sections, and mounted on pre-treated glass slides. Noncancer cells and necrotic parts were manually removed from the slide under the microscope. The slides were deparaffinized, then DNA was extracted with phenol-chloroform and ethanol precipitation. A PNA-LNA PCR clamp, which was designed to detect 11 different EGFR mutations, was used for determination of the EGFR gene mutation status [12,13,14]. In this study, we adopted the PNA-LNA PCR clamp for analysis of the EGFR somatic mutations. In the PNA-LNA PCR clamp, other types of EGFR mutations are used to control for amplification by the clamp primer. In this case, a direct sequencing method was employed to seek other types of EGFR mutations.
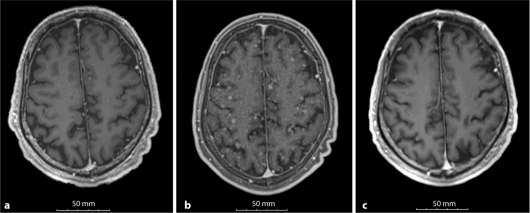
On the basis of the results of molecular analysis, the patient received carboplatine (area under the concentration-time curve 5) and paclitaxel (180 mg/m2) intravenously on day 1 of a 3-week cycle. Both the pulmonary lesion and the left pleural effusion and disseminations showed stable disease, as assessed by response evaluation criteria in solid tumors [15]. After he was on medication for 3 courses with stable disease, he developed seizures and multiple brain lesions (fig. 2a, b, c). He then received the oral EGFR-TKI erlotinib as the 2nd-line treatment. Upon administration of erlotinib, he could not swallow because of disturbance of consciousness so we administered erlotinib dissolved in water from a nasal tube.
Fig. 2.
Brain MRI of the current case. a On our 1st visit. b The signs of CNS metastases were revealed before administration of erlotinib. c 4 weeks after initiation of erlotinib, the signs had improved.
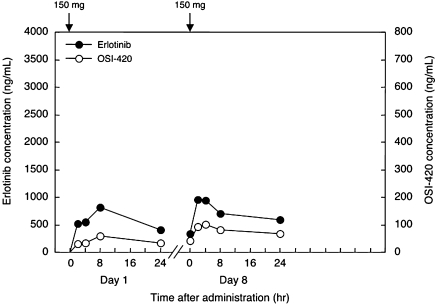
He was also administered hydantoin as an anticonvulsant which induced cytochrome CYP3A4 and might promote metabolism of erlotinib. Therefore, we performed pharmacokinetic analyses of erlotinib and OSI-420 on days 1 and 8 after starting its administration. Blood samples were obtained at 5 points a day: just before administration (C0), 2 h (C2), 4 h (C4), 8 h (C8), and 24 h (C24) after administration. The concentrations were determined by high-performance liquid chromatography with ultraviolet detection, as previously reported [16]. Before analysis, we got written informed consent from the patient. Surprisingly, although the serum concentration of erlotinib was quite low (Cmax 925 ng/ml on day 8, fig. 3 and table 2), the signs of brain metastases improved dramatically (fig. 2) and his PS recovered from 4 to 1. He has remained on erlotinib and has been without evidence of disease progression for about 2 months.
Fig. 3.
Plasma concentration-time plots of erlotinib and OSI-420 in case 1 on days 1 and 8. Arrows indicate administration of 150 mg erlotinib.
Table 2.
The pharmacokinetics parameters and serum concentrations of erlotinib and OSI-420
| Erlotinib |
OSI-420 |
|||||||
|---|---|---|---|---|---|---|---|---|
| AUCo-24 ng × h/ml | Cmax ng/ml | tmax h | tl/2 h | AUC0-24 ng x h/ml | Cmax ng/ml | tmax h | tl/2 h | |
| Day 1 | 14,178 | 824 | 8 | 15.7 | 1,029 | 59 | 8 | 20.1 |
| Day 8 | 16,936 | 925 | 2 | 64.6 | 1,895 | 101 | 4 | 69.4 |
Cmax = Maximal plasma concentration; tmax = time to Cmax t1/2 = half period.
Discussion
In this case, the Cmax of erlotinib on day 8 was 956 ng/ml, which was remarkably lower than the plasma concentration previously reported [17]. The low plasma concentration may be due to CYP3A4 induced by hydantoin. In spite of the low plasma concentration, the patient showed a good clinical response of his multiple brain metastases and his PS recovered dramatically from 4 to 1.
Erlotinib inhibits the activity of the EGFR tyrosine kinase in intact tumor cells, with 50% inhibitory concentration values of 20 nmol/l (7.9 ng/ml) [18]. In a study of athymic mice with human carcinomas, the plasma concentration of erlotinib associated with a 50% inhibition of EGFR tyrosine kinase was estimated at 8-12 μmol/l (3.1-4.7 μg/ml) [19]. According to these reports, although the plasma concentration was relatively low compared to previously reported results [17], 956 ng/ml on day 8 is above the IC50. Therefore, this may be the 1st reason for the patient's good clinical response.
Erlotinib is metabolized in the liver, mainly by the cytochrome CYP3A4 (approximately 80%). Erlotinib and its metabolites are excreted predominantly via the feces [20]. Although the administration of erlotinib dissolved in water is expected to yield a good absorption, the plasma concentration of erlotinib was quite low. In our opinion, the reason may be cytochrome CYP3A4. Thus in administration of erlotinib, coadministration with medicine that might induce cytochrome CYP3A4 may affect the plasma concentration of erlotinib more than we expected. Such a coadministration may contribute to a poor clinical response to erlotinib.
In terms of EGFR-TKI sensitivity, Kancha et al. have identified 4 sets of mutations based on their drug sensitivity profiles: (a) mutations that are very sensitive to all 3 drugs tested with IC50 values in the low nanomolar range (L858R and Del 747-753insS), (b) mutations that are less sensitive to gefitinib (IC50 >100 nmol/l) but sensitive to both erlotinib and AEE788 (G719S, V742A, and R776C; IC50 < 100 nmol/l), (c) mutations that are less sensitive to both gefitinib and erlotinib but sensitive to AEE788 (D761N, S768I, S784F, L838V, and L861Q), and (d) mutations that are resistant to all three drugs tested (N826S and T790M) [3]. To our knowledge, reports concerning cases with an EGFR double activating mutation are few. Sriuranpong et al. reported one case with an EGFR double activating mutation in Thailand [21]. Zhang et al. described 5 cases with EGFR double activating mutation in Chinese patients [22]. In the Zhang report, exon 19 deletion + L858R double mutations were located on the same allele and the double mutant had a higher sensitivity to TKIs than the other two single activating mutation in the in vitro study. Although it remains unknown whether the double mutant shows good clinical response to erlotinib, this may be the 2nd reason of the patient's good clinical response. To our knowledge, this is the first case with a good clinical response to erlotinib harboring this EGFR double activating mutation.
In addition, several articles in which CNS metastases were improved by erlotinib have been reported [6,7,8,9,10,11] and Katayama et al. reported that erlotinib was a reasonable option for the treatment of CNS diseases that appeared after a good initial response of extra CNS lesions to gefitinib [9]. However, very little is known about the CNS penetration of and exposure to this drug [23,24,25]. In our case, CNS metastases were improved by erlotinib regardless of low plasma concentration, poor PS, and disturbance of consciousness. Therefore, in cases with CNS metastases, even if they suffer from deterioration of PS, erlotinib may become a therapeutic choice when the cytotoxic agents are not appropriate.
References
- 1.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba, Fong KM, Lee H, Toyooka S, Shimizu N, Fujisawa T, Feng Z, Roth JA, Herz J, Minna JD, Gazdar AF. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 3.Sheu JJ, Hua CH, Wan L, Lin YJ, Lai MT, Tseng HC, Jinawath N, Tsai MH, Chang NW, Lin CF, Lin CC, Hsieh LJ, Wang TL, Shih IeM, Tsai FJ. Functional genomic analysis identified epidermal growth factor receptor activation as the most common genetic event in oral squamous cell carcinoma. Cancer Res. 2009;69:2568–2576. doi: 10.1158/0008-5472.CAN-08-3199. [DOI] [PubMed] [Google Scholar]
- 4.Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–1304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 5.Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol. 2005;23:6207–6219. doi: 10.1200/JCO.2005.03.145. [DOI] [PubMed] [Google Scholar]
- 6.Altavilla G, Arrigo C, Santarpia MC, Galletti G, Picone G, Marabello G, Tomasello C, Pitini VV. Erlotinib therapy in a patient with non-small-cell lung cancer and brain metastases. J Neurooncol. 2008;90:31–33. doi: 10.1007/s11060-008-9623-4. [DOI] [PubMed] [Google Scholar]
- 7.Dhruva N, Socinski MA. Carcinomatous meningitis in non-small-cell lung cancer: Response to high-dose erlotinib. J Clin Oncol. 2009;27:e31–e32. doi: 10.1200/JCO.2008.21.0963. [DOI] [PubMed] [Google Scholar]
- 8.Fekrazad MH, Ravindranathan M, Jones DV., Jr Response of intracranial metastases to erlotinib therapy. J Clin Oncol. 2007;25:5024–5026. doi: 10.1200/JCO.2007.13.3751. [DOI] [PubMed] [Google Scholar]
- 9.Katayama T, Shimizu J, Suda K, Onozato R, Fukui T, Ito S, Hatooka S, Sueda T, Hida T, Yatabe Y, Mitsudomi T. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol. 2009;4:1415–1419. doi: 10.1097/JTO.0b013e3181b62572. [DOI] [PubMed] [Google Scholar]
- 10.Lai CS, Boshoff C, Falzon M, Lee SM. Complete response to erlotinib treatment in brain metastases from recurrent NSCLC. Thorax. 2006;61:91. doi: 10.1136/thx.2005.052233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popat S, Hughes S, Papadopoulos P, Wilkins A, Moore S, Priest K, Meehan L, Norton A, O'Brien M. Recurrent responses to non-small cell lung cancer brain metastases with erlotinib. Lung Cancer. 2007;56:135–137. doi: 10.1016/j.lungcan.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Nagai Y, Miyazawa H, Huqun, Tanaka T, Udagawa K, Kato M, Fukuyama S, Yokote A, Kobayashi K, Kanazawa M, Hagiwara K. Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res. 2005;65:7276–7282. doi: 10.1158/0008-5472.CAN-05-0331. [DOI] [PubMed] [Google Scholar]
- 13.Sutani A, Nagai Y, Udagawa K, Uchida Y, Koyama N, Murayama Y, Tanaka T, Miyazawa H, Nagata M, Kanazawa M, Hagiwara K, Kobayashi K. Gefitinib for non-small-cell lung cancer patients with epidermal growth factor receptor gene mutations screened by peptide nucleic acid-locked nucleic acid PCR clamp. Br J Cancer. 2006;95:1483–1489. doi: 10.1038/sj.bjc.6603466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka T, Nagai Y, Miyazawa H, Koyama N, Matsuoka S, Sutani A, Huqun, Udagawa K, Murayama Y, Nagata M, Shimizu Y, Ikebuchi K, Kanazawa M, Kobayashi K, Hagiwara K. Reliability of the peptide nucleic acid-locked nucleic acid polymerase chain reaction clamp-based test for epidermal growth factor receptor mutations integrated into the clinical practice for non-small cell lung cancers. Cancer Sci. 2007;98:246–252. doi: 10.1111/j.1349-7006.2006.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Siu LL, Moore MJ, Chen EX. Simultaneous determination of OSI-774 and its major metabolite OSI-420 in human plasma by using HPLC with UV detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;814:143–147. doi: 10.1016/j.jchromb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto N, Horiike A, Fujisaka Y, Murakami H, Shimoyama T, Yamada Y, Tamura T. Phase I dose-finding and pharmacokinetic study of the oral epidermal growth factor receptor tyrosine kinase inhibitor ro50-8231 (erlotinib) in Japanese patients with solid tumors. Cancer Chemother Pharmacol. 2008;61:489–496. doi: 10.1007/s00280-007-0494-8. [DOI] [PubMed] [Google Scholar]
- 18.Moyer JD, Barbacci EG, Iwata KK, Arnold L, Boman B, Cunningham A, DiOrio C, Doty J, Morin MJ, Moyer MP, Neveu M, Pollack VA, Pustilnik LR, Reynolds MM, Sloan D, Theleman A, Miller P. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57:4838–4848. [PubMed] [Google Scholar]
- 19.Pollack VA, Savage DM, Baker DA, Tsaparikos KE, Sloan DE, Moyer JD, Barbacci EG, Pustilnik LR, Smolarek TA, Davis JA, Vaidya MP, Arnold LD, Doty JL, Iwata KK, Morin MJ. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J Pharmacol Exp Ther. 1999;291:739–748. [PubMed] [Google Scholar]
- 20.Hidalgo M, Bloedow D. Pharmacokinetics and pharmacodynamics: Maximizing the clinical potential of erlotinib (tarceva) Semin Oncol. 2003;30:25–33. [PubMed] [Google Scholar]
- 21.Sriuranpong V, Chantranuwat C, Huapai N, Chalermchai T, Leungtaweeboon K, Lertsanguansinchai P, Voravud N, Mutirangura A. High frequency of mutation of epidermal growth factor receptor in lung adenocarcinoma in Thailand. Cancer Lett. 2006;239:292–297. doi: 10.1016/j.canlet.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Zhang GC, Lin JY, Wang Z, Zhou Q, Xu CR, Zhu JQ, Wang K, Yang XN, Chen G, Yang JJ, Huang YJ, Liao RQ, Wu YL. Epidermal growth factor receptor double activating mutations involving both exons 19 and 21 exist in Chinese non-small cell lung cancer patients. Clin Oncol (R Coll Radiol) 2007;19:499–506. doi: 10.1016/j.clon.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Broniscer A, Panetta JC, O'Shaughnessy M, Fraga C, Bai F, Krasin MJ, Gajjar A, Stewart CF. Plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite OSI-420. Clin Cancer Res. 2007;13:1511–1515. doi: 10.1158/1078-0432.CCR-06-2372. [DOI] [PubMed] [Google Scholar]
- 24.Lassman AB, Rossi MR, Raizer JJ, Abrey LE, Lieberman FS, Grefe CN, Lamborn K, Pao W, Shih AH, Kuhn JG, Wilson R, Nowak NJ, Cowell JK, DeAngelis LM, Wen P, Gilbert MR, Chang S, Yung WA, Prados M, Holland EC. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: Tissue analysis from North American Brain Tumor Consortium Trials 01-03 and 00-01. Clin Cancer Res. 2005;11:7841–7850. doi: 10.1158/1078-0432.CCR-05-0421. [DOI] [PubMed] [Google Scholar]
- 25.Meany HJ, Fox E, McCully C, Tucker C, Balis FM. The plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite (OSI-420) after intravenous administration of erlotinib in non-human primates. Cancer Chemother Pharmacol. 2008;62:387–392. doi: 10.1007/s00280-007-0616-3. [DOI] [PubMed] [Google Scholar]