Abstract
Background
Pooled data were analyzed from the NCI Pancreatic Cancer Cohort Consortium (PanScan) to study the association between pre-diagnostic anthropometric measures and risk of pancreatic cancer.
Methods
PanScan applied a nested case-control study design and included 2,170 cases and 2,209 controls. Odds ratios (OR) and 95% confidence intervals (CI) were estimated using unconditional logistic regression for cohort-specific quartiles of body mass index (BMI), weight, height, waist circumference, and waist-to-hip ratio (WHR), as well as conventional BMI categories: underweight (<18.5 kg/m2), normal (18.5-24.9 kg/m2), overweight (25.0-29.9 kg/m2), obese (30.0-34.9 kg/m2), and severely obese (≥35.0 kg/m2). Models were adjusted for potential confounders.
Results
Among all subjects, a positive association between increasing BMI and risk of pancreatic cancer was observed (adjusted OR for the highest vs. lowest BMI quartile = 1.33, 95% CI = 1.12-1.58, Ptrend < 0.001). Among men, the adjusted OR for pancreatic cancer for the highest vs. lowest quartile of BMI was 1.33 (95% CI = 1.04-1.69, Ptrend <0.03). Among women, the adjusted OR for pancreatic cancer for the highest quartile of BMI was 1.34 (95% CI = 1.05-1.70, Ptrend = 0.01). Increased WHR was associated with increased risk of pancreatic cancer among women (adjusted OR for the highest vs. lowest quartile = 1.87, 95% CI = 1.31-2.69, Ptrend = 0.003) but less so in men.
Conclusion
The findings provide strong support for a positive association between BMI and pancreatic cancer risk. In addition, centralized fat distribution may increase pancreatic cancer risk, especially in women.
Keywords: Anthropometry, body mass index, cohort consortium, obesity, pancreatic cancer
INTRODUCTION
Pancreatic adenocarcinoma is the fourth leading cause of cancer death in the United States 1 and is responsible for about 227,000 deaths per year worldwide 2. Because of the lack of effective screening tests for pancreatic cancer, it is often diagnosed at an advanced stage, contributing to a five-year survival rate that is less than 5% 3. The incidence of pancreatic cancer is higher in men compared with women, and within the United States, it is higher in Blacks compared to Caucasians 3. Smoking, diabetes, and family history of pancreatic cancer are known risk factors 4,5 but these factors do not account for all the cases of pancreatic cancer.
Obesity and high body mass index (BMI) have been proposed as additional risk factors for pancreatic cancer. Prospective studies have yielded conflicting results concerning the association between BMI and risk of pancreatic cancer. A majority of prospective epidemiological studies 6-15 have found that a high body mass index and/or a lack of physical activity are associated with an increased risk of pancreatic cancer incidence or mortality, independently of prior history of diabetes. However, several prospective studies have not confirmed a significant role of BMI in pancreatic cancer 16-23 or found that effect of BMI varied according to smoking status 24,25 or gender 26-28.
The purpose of the current study was to examine the association between BMI, other anthropometric factors, and pancreatic cancer risk by pooling data from nested case-control studies included in the NCI Pancreatic Cancer Cohort Consortium (PanScan). With 2,170 cases, this is one of the largest analyses to date of BMI and pancreatic cancer.
METHODS
Study Population
PanScan is an initiative that was funded jointly by the National Cancer Institute’s Division of Cancer Control and Population Sciences and the Division of Cancer Epidemiology and Genetics in 2006. PanScan includes investigators from 12 prospective epidemiologic cohorts and one case-control study and was created to identify genetic markers of susceptibility through a genome-wide association scan and to investigate environmental, lifestyle, and genetic causes of pancreatic cancer.
Studies in the pooled analysis included: The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Trial (ATBC) 29, CLUE II 30, Cancer Prevention Study II (CPS II) 31, European Prospective Investigation into Cancer and Nutrition (EPIC) 32, the Health Professionals Follow-up Study (HPFS) 33, the Mayo Clinic study (MAYO) 34, the New York University Women’s Health Study (NYUWHS) 35, the Nurses’ Health Study (NHS) 36, the Physicians’ Health Study (PHS I) 37, the Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial (PLCO) 38, Shanghai Men’s and Women’s Health Studies (SMWHS) 39,40, the Women’s Health Initiative (WHI) 41, and the Women’s Health Study (WHS) 42. A total of 2,170 cases and 2,209 controls were eligible for the current study (Table 1).
Table 1. Characteristics of the Cohorts Included in the PanScan Pooled Analysis.
| Cohort | Center | Location | Enrollment yearsa |
Mean follow-up (years) |
Race (%) | Age range |
Available anthropometric data |
Cases/Controls 2170/2209 |
Matching |
|---|---|---|---|---|---|---|---|---|---|
| The Alpha- Tocopherol, Beta- Carotene Cancer Prevention Trial (ATBC) |
National Cancer Institute, National Institute for Health and Welfare |
Finland | 1985-1988 | 11.8 | 100% Caucasian | 57-85 | BMI | 210/211 | Race, age at randomization (1-5 years), month, year of baseline blood draw (+30 days) |
| CLUE II | John Hopkins Bloomberg School of Public Health |
USA | 1989 | 8.3 | 100% Caucasian | 42-94 | BMI | 83/83 | Race, gender, age |
| Cancer Prevention Study (CPS II) |
American Cancer Society |
USA | 1992-1993 | 10.0 | 97.6% Caucasian 1.2% African-American 0.6% Asian 0.6% Other |
64-90 | BMI | 165/165 | Race, self-reported ethnicity, gender, date of birth (± 6 months), DNA source (blood or buccal), DNA sample provided during the same season and year |
| European Prospective Investigation Into Cancer and Nutrition (EPIC) |
International Agency Research on Cancer and Imperial College London |
Europe | 1992-2000 (varied by center) |
6.8 | 100% Caucasian | 37-84 | BMIb, WHRc | 440/459 | Gender, center, age at recruitment (± 1 month), date of blood donation (± 1 month), time of blood draw (± 1 hour), hours between blood draw and last food or drinks (<3, 3-6, >6) |
| Health Professionals Follow-up Study (HPFS) |
Harvard University |
USA | 1986 | 12.7 | 100% Caucasian | 55-87 | BMI, WHR | 55/55 | Race, gender, year of birth (+/− 5 years) smoking status (never/former/current) fasting status, month and hour of blood draw |
| Mayo Clinic study (MAYO) |
Mayo Clinic | USA | 2000-2006 | 0 | 99.3% Caucasian 0.5% African-American 0.3% Asian |
39-86 | BMI | 400/400 | Clinic-based controls, frequency matched to cases on age, race, gender, and residence |
| The New York University Women’s Health Study (NYUWHS) |
New York University |
USA | 1985-1991 | 11.6 | 76.7% Caucasian 7.7% Other 15.4% Missing |
48-82 | BMI, WHR | 13/13 | Age at enrollment (± 6 months), date of enrollment (± 3 months), menopausal status at enrollment, race/ethnicity |
| Nurses’ Health Study (NHS) |
Harvard University |
USA | 1976-2003 | 21.6 | 85.2% Caucasian 1.1% Other 13.6% Missing |
47-80 | BMI, WHR | 88/88 | Race, gender, year of birth (+/− 5 years) smoking status (never/former/current) fasting status, month and hour of blood draw |
| Physicians’ Health Study (PHS I) |
Brigham and Women’s Hospital |
USA | 1982-1983 | 13.6 | 61.2% Caucasian 1.6% African-American 37.1% Missing |
49-88 | BMI | 62/62 | Race, year of birth (+/− 5 years) smoking status (never/former/current) fasting status, month and hour of blood draw |
| Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial (PLCO) |
National Cancer Institute |
USA | 1993-2001 | 6.2 | 90.9% Caucasian 4.7% Asian 3.2% African-American 1.2% Other |
56-84 | BMI | 253/271 | Race, gender, ethnicity, center, frequency samples by calendar year of birth (5 years block), gender, broad categories of race, source of DNA (blood or buccal cell), study arm, study center. For intervention arm additionally stratified sampled by age |
| Shanghai Men’s and Women’s Health Study (SMWHS) |
Vanderbilt University |
China | 1996 (F) 2001 (M) |
3.6 | 100% Asian | 43-77 | BMI, WHR | 78/79 | Race, ethnicity, gender, year of birth (<2 years), menopausal status at baseline, date of sample collection (<30 days), time of sample collection (am/pm), time interval after the last meal (<2 hours) |
| Women’s Health Initiative (WHI) |
WHI Clinical Centers |
USA | 1992-1998 | 3.8 | 85.5% Caucasian 7.4% African-American 4.2% Asian 1.8% Other 1.0% Missing |
53-88 | BMI, WHR | 283/283 | Gender, center, race, ethnicity, age at screening, enrollment date, study component, hysterectomy status, menopausal status |
| Women’s Health Study (WHS) |
Harvard University |
USA | 1992-1993 | 5.1 | 95% Caucasian 2.5% African-American 2.5% Missing |
47-82 | BMI | 40/40 | Race, year of birth (+/− 5 years) smoking status (never/former/current) fasting status, month and hour of blood draw |
NOTE: M, male; F, Female; NA, not available
Study years refer to years of study included in this nested case-control study. Some studies have ongoing recruitment.
In EPIC: BMI correction for differences in clothing for people with direct measurements of weight or prediction of BMI from self-reports for the Oxford health conscious group.
In EPIC: WHR was available in EPIC-IARC and EPIC-Denmark subcohorts.
Case Ascertainment and Data Collection
Cases included all incident primary pancreatic adenocarcinoma (ICD-O-3 codes C25.0-C25.3, C25.7-C25.9). Endocrine pancreatic tumors (ICD-O-3 code C25.4, histology types 8150, 8151, 8153, 8155, 8240, and 8246) were excluded because the etiology of these cancers is thought to be different from that of exocrine tumors, which account for the vast majority of pancreatic tumors. Case ascertainment varied between studies but included linking participants to cancer registries and national death indices, and self and next of kin report. Most cases were histologically confirmed (ATBC, CLUE II, EPIC, NYUWHS, SMWHS, WHI) or confirmed through cancer registries (ATBC, CPS II, EPIC, SMWHS), death certificates (CPS II, EPIC), or review of medical records by medical personnel (ATBC, CPS II, EPIC, PLCO, SMWHS).
Controls were incidence density-sampled with a 1-to-1 control-to-case ratio and were alive and cancer-free on the date of diagnosis of the matched case. At a minimum, controls were matched to cases on calendar year of birth (±5 years), gender, race and ethnicity. Some cohorts employed more stringent matching on age and, additionally, on other relevant factors (for comparisons of blood levels of analytes of interest) such as age at baseline or age at blood draw (±5 years), date/time of day of blood draw, fasting blood draw, and length of follow-up (Table 1).
Data on anthropometry, demographics and possible confounders were collected through self-administered written questionnaires or in-person interviews. Detailed descriptions of data collection methods have been published previously by the individual studies 29,30,32,33,35-44. From each study, baseline information on body mass index (BMI), weight, height, waist circumference, waist-to-hip ratio (WHR), history of cigarette smoking, gender, age, race, family history of pancreatic cancer, alcohol consumption, pancreatitis, and history of diabetes was requested. Individual datasets were checked for consistency with previously published results. A total of forty cases and forty six controls had missing data on BMI, resulting in 2,130 cases and 2,163 controls available for the main analyses.
The Special Studies Institutional Review Board (SSIRB) of the National Cancer Institute approved the pooled PanScan study. Each study also was approved by its local IRB.
Statistical Analysis
Odds ratios (OR) and 95% confidence intervals (95% CI) for pancreatic cancer risk were calculated using unconditional logistic regression, adjusting for cohort, age (categorical), gender, BMI source (self-reported, measured), and smoking (never, former, current) (Model 1). Several multivariate models were assessed to control the effects of potential confounders. Model 2 was additionally adjusted for diabetes history (yes, no). In model 3, cases diagnosed within the first 2 years of follow-up were excluded to address the possibility of an effect of early undiagnosed disease. In model 4, current smokers (at baseline) were excluded, and in model 5, both cases diagnosed within the first 2 years of follow-up and current smokers were excluded. Furthermore, models including waist circumference and waist-to-hip ratio were additionally adjusted for height to remove extraneous variation due to body size. There was no adjustment for family history of pancreatic cancer as few cohorts had this information. Trend tests were conducted using cohort-specific quartiles of BMI, weight, height, waist circumference, and WHR, as well as descriptive BMI categories: underweight (<18.5 kg/m2), normal (reference, 18.5-<25.0 kg/m2), overweight (25.0-<30.0 kg/m2), obese (30.0-<35.0 kg/m2), and severely obese (≥35.0 kg/m2). To test for heterogeneity BMI quartile categories were modeled as a continuous variable and the risk estimates and standard errors from the cohort-specific models were used to generate the Q statistic.
The association between BMI and time of onset for pancreatic cancer was also examined using logistic regression modeling. Differences in time of onset were examined for normal versus overweight versus obese categories of BMI as well as in a combined category of overweight and obese. The analyses were conducted using the SAS program version 9.1.3.
RESULTS
The study included 2,170 pancreatic cancer cases and 2,209 controls aged between 37 and 94 years (Table 1). Of the 2,170 pancreatic cancer cases, 1,059 were males and 1,111 were females. Cases and controls were similar in terms of age and racial distribution (Table 2). The majority of subjects were Caucasian, and 86% of the study population was 60 years of age or older. Compared to the controls, cases had a higher prevalence of current smoking (18% and 25%, respectively), diabetes (7% and 14%, respectively), history of pancreatitis (0.4% and 11%, respectively), and family history of pancreatic cancer (2% and 6%, respectively) based on data from cohorts with available information. The average age of pancreatic cancer onset among cases was 68.3 years and the average lag time between cohort enrollment and diagnosis of pancreatic cancer among cases was 6.3 years.
Table 2. Participants’ Characteristics, the PanScan Consortium.
| Characteristic | Cases | Controls | P-valueb |
|---|---|---|---|
| N | 2170 | 2209 | |
|
| |||
| Gender, n (%) | |||
| Male | 1059 (49%) | 1080 (49%) | |
| Female | 1111 (51%) | 1129 (51%) | 0.95 |
|
| |||
| Race, n (%) | |||
| European | 1979 (91.2%) | 2046 (92.6%) | |
| African | 35 (1.6%) | 34 (1.5%) | |
| Asian | 104 (4.8%) | 108 (4.9%) | |
| Other | 11 (0.5%) | 8 (0.4%) | |
| Unknown | 41 (1.9%) | 13 (0.6%) | 0.30 |
|
| |||
| Age categories, n (%) | |||
| <55 | 150 (7%) | 119 (5%) | |
| 55-59 | 188 (9%) | 154 (7%) | |
| 60-64 | 338 (16%) | 325 (15%) | |
| 65-69 | 443 (20%) | 473 (21%) | |
| 70-74 | 491 (23%) | 552 (25%) | |
| 75-79 | 368 (17%) | 399 (18%) | |
| ≥80 | 192 (9%) | 187 (8%) | <0.05 |
|
| |||
| Cigarette smoking status, n (%)a | |||
| Never smoker | 829 (39%) | 970 (45%) | |
| Former Smoker | 767 (36%) | 812 (37%) | |
| Current Smoker | 530 (25%) | 397 (18%) | <0.0001 |
|
| |||
| Diabetes mellitus, n (%)a | |||
| No | 1762 (86%) | 1973 (93%) | |
| Yes | 288 (14%) | 157 (7%) | <0.0001 |
|
| |||
| History of pancreatitis, n (%)a | |||
| No | 862 (89%) | 963 (99.6%) | |
| Yes | 109 (11%) | 4 (0.4%) | <0.0001 |
|
| |||
| Family history of pancreatic cancer, n (%)a | |||
| No | 1107 (94%) | 1162 (98%) | |
| Yes | 76 (6%) | 43 (2%) | <0.006 |
|
| |||
| Age at diagnosis of pancreatic cancer, years | |||
| Mean | 68.3 | ||
| SD | 8.8 | -- | |
| Median | 69 | ||
| Range | 37-93 | ||
|
| |||
| Lag time between diagnosis and enrollment, years | |||
| Mean | 6.3 | ||
| SD | 5.7 | -- | |
| Median | 6.0 | ||
| Range | 0-28 | ||
Data were missing for smoking status (44 cases, 30 controls), diabetes status (120 cases, 79 controls), history of pancreatitis (1199 cases, 1242 controls), family history of pancreatic cancer (974 cases, 991 controls).
Chi square test.
Table 3 describes baseline anthropometric characteristics of cases and controls. Weight, height, and corresponding BMI were self-reported in about 50% of subjects, measured in 29% of subjects, and measured and subsequently adjusted for difference in clothing in about 20% of subjects. Thirty-six percent of cases and 39% of controls had BMI in the normal range, 41% of cases and 39% of controls were overweight, and 21% of cases and 19% of controls were obese (Table 3). Cases had slightly higher mean weight compared to controls (76.8 kg and 75.5 kg, respectively), and larger mean waist circumference (86.9 and 85.7, respectively). Mean WHR and height were similar.
Table 3. Baseline Anthropometric Characteristics by Gender, the PanScan Consortium.
| Characteristic | Females | Males | ||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
| N | 1111 | 1129 | 1059 | 1080 |
|
| ||||
| BMI source, n (%) | ||||
| Self-reported | 533 (48%) | 518 (46%) | 544 (51%) | 559 (52%) |
| Measured | 384 (35%) | 396 (35%) | 250 (24%) | 251 (23%) |
| Adjusted | 178 (16%) | 187 (17%) | 241 (23%) | 252 (23%) |
| Unknown | 16 (1%) | 28 (2%) | 24 (2%) | 18 (2%) |
|
| ||||
| BMI Mean | 26.8 | 26.2 | 27.0 | 26.7 |
| SD | 5.3 | 4.9 | 4.1 | 3.9 |
| Median | 25.8 | 25.4 | 26.6 | 26.3 |
| Range | 14.0-67.5 | 15.0-54.6 | 16.8-53.4 | 15.4-51.5 |
|
| ||||
| BMI, quartiles, cohort- and gender-specific | ||||
| Q1 (low) | 251 (23%) | 286 (25%) | 251 (24%) | 285 (26%) |
| Q2 | 257 (23%) | 269 (24%) | 247 (23%) | 257 (24%) |
| Q3 | 273 (25%) | 279 (25%) | 251 (24%) | 261 (24%) |
| Q4 (high) | 314 (28%) | 267 (24%) | 286 (27%) | 259 (24%) |
| Unknown | 16 (1%) | 28 (2%) | 24 (2%) | 18 (2%) |
|
| ||||
| BMI, categories, n (%) | ||||
| Underweight (<18.5 kg/m2) | 14 (1%) | 21 (2%) | 5 (0.5%) | 4 (0.4%) |
| Normal (18.5-24.9 kg/m2) | 445 (40%) | 507 (45%) | 327 (31%) | 356 (33%) |
| Overweight (25.0-29.9 kg/m2) | 381 (34%) | 339 (30%) | 505 (48%) | 524 (49%) |
| Obese (30.0-34.9 kg/m2) | 175 (16%) | 175 (16%) | 153 (14%) | 141 (13%) |
| Severely obese (≥35.0 kg/m2) | 80 (7%) | 59 (5%) | 45 (4%) | 37 (3%) |
| Unknown | 16 (1%) | 28 (2%) | 24 (2%) | 18 (2%) |
|
| ||||
| Weight source, n (%) | ||||
| Self-reported | 537 (48%) | 526 (47%) | 622 (59%) | 626 (58%) |
| Measured | 561 (51%) | 581 (51%) | 428 (40%) | 440 (41%) |
| Unknown | 13 (1%) | 22 (2%) | 9 (1%) | 14 (1%) |
|
| ||||
| Weight, kg | ||||
| Mean | 70.0 | 68.5 | 83.8 | 82.7 |
| SD | 14.7 | 13.5 | 14.6 | 13.3 |
| Median | 67.5 | 66.5 | 81.9 | 81.0 |
| Range | 41.4-167.5 | 38.0-134.1 | 43.9-172.4 | 48.0-171.0 |
|
| ||||
| Weight, quartiles, cohort- and gender-specific | ||||
| Q1 (low) | 270 (24%) | 291 (26%) | 260 (25%) | 292 (27%) |
| Q2 | 244 (22%) | 284 (25%) | 253 (24%) | 257 (24%) |
| Q3 | 286 (26%) | 274 (24%) | 237 (22%) | 262 (24%) |
| Q4 (high) | 298 (27%) | 258 (23%) | 300 (28%) | 255 (24%) |
| Unknown | 13 (1%) | 22 (2%) | 9 (1%) | 14 (1%) |
|
| ||||
| Height source, n (%) | ||||
| Self-reported | 541 (49%) | 536 (47%) | 613 (58%) | 629 (58%) |
| Measured | 560 (50%) | 581 (51%) | 428 (40%) | 441 (41%) |
| Unknown | 10 (1%) | 12 (1%) | 18 (2%) | 10 (1%) |
|
| ||||
| Height, cm | ||||
| Mean | 162 | 162 | 176 | 176 |
| SD | 6.6 | 6.8 | 7.0 | 6.8 |
| Median | 162 | 163 | 176 | 176 |
| Range | 132-184 | 140-198 | 136-199 | 152-201 |
|
| ||||
| Height, quartiles, cohort- and gender-specific | ||||
| Q1 (low) | 349 (31%) | 340 (30%) | 290 (27%) | 309 (29%) |
| Q2 | 251 (23%) | 266 (24%) | 300 (28%) | 305 (28%) |
| Q3 | 256 (23%) | 272 (24%) | 249 (24%) | 251 (23%) |
| Q4 (high) | 245 (22%) | 239 (21%) | 202 (19%) | 205 (19%) |
| Unknown | 10 (1%) | 12 (1%) | 18 (2%) | 10 (1%) |
|
| ||||
| Waist circumference at baseline, cm | ||||
| Mean | 83.4 | 81.4 | 96.2 | 96.8 |
| SD | 16.4 | 14.8 | 10.8 | 9.6 |
| Median | 83.3 | 80.0 | 96.0 | 96.0 |
| Range | 38.1-129.0 | 38.1-134.0 | 64.0-144.8 | 65.2-131.0 |
| Unknown | 515 | 523 | 836 | 846 |
|
| ||||
| Waist-to-hip ratio | ||||
| Mean | 0.83 | 0.82 | 0.95 | 0.95 |
| SD | 0.09 | 0.09 | 0.06 | 0.06 |
| Median | 0.82 | 0.80 | 0.95 | 0.95 |
| Range | 0.43-1.25 | 0.65-1.73 | 0.76-1.12 | 0.78-1.15 |
| Unknown, n | 577 | 586 | 836 | 846 |
Table 4 displays odds ratios (ORs) and 95% confidence intervals (CIs) for pancreatic cancer according to baseline anthropometric factors for all subjects in the study. Among all subjects, a positive association between increasing BMI and risk of pancreatic cancer was observed (adjusted OR for the highest vs. lowest BMI quartile = 1.33, 95% CI = 1.12-1.58, Ptrend < 0.001 in model 1). Statistically significant trends of increasing risk of pancreatic cancer with increasing BMI (both quartiles and clinical categories) were observed in all five models analyzed.
Table 4. Odds ratios and 95% CIs of Pancreatic Cancer according to Baseline Anthropometric Factors, the PanScan Consortium, All Subjects.
| Characteristic | Cases | Controls | OR (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| 2095 | 2141 | Model 1a | Model 2b | Model 3c | Model 4d | Model 5e | |
| BMI, kg/m2, cohort-specific quartiles | |||||||
| Q1 | 500 | 563 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 496 | 523 | 1.09 (0.92-1.30) | 1.09 (0.92-1.31) | 1.09 (0.90-1.33) | 1.12 (0.91-1.36) | 1.04 (0.82-1.32) |
| Q3 | 515 | 534 | 1.13 (0.95-1.34) | 1.08 (0.90-1.29) | 1.13 (0.93-1.38) | 1.11 (0.91-1.35) | 1.03 (0.81-1.31) |
| Q4 | 584 | 521 | 1.33 (1.12-1.58) | 1.21 (1.01-1.44) | 1.29 (1.06-1.57) | 1.43 (1.18-1.74) | 1.39 (1.10-1.77) |
| P trend | <0.001 | 0.049 | 0.008 | <0.001 | 0.004 | ||
|
| |||||||
| BMI, kg/m2, categories | |||||||
| Underweight (<18.5 kg/m2) | 19 | 24 | 0.83 (0.45-1.55) | 0.84 (0.44-1.59) | 0.65 (0.31-1.35) | 0.71 (0.33-1.50) | 0.48 (0.18-1.24) |
| Normal (≥18.5 and <25.0 kg/m2) | 759 | 854 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Overweight (≥25.0 and <30.0 kg/m2) | 868 | 853 | 1.18 (1.03-1.35) | 1.15 (1.00-1.33) | 1.22 (1.04-1.42) | 1.19 (1.02-1.40) | 1.15 (0.95-1.39) |
| Obese (≥30.0 and <35.0 kg/m2) | 325 | 315 | 1.20 (1.00-1.44) | 1.13 (0.93-1.37) | 1.22 (0.98-1.51) | 1.25 (1.02-1.55) | 1.28 (0.99-1.67) |
| Severely obese (≥35.0) | 124 | 95 | 1.55 (1.16-2.07) | 1.26 (0.93-1.71) | 1.32 (0.94-1.87) | 1.62 (1.19-2.21) | 1.53 (0.99-2.36) |
| P trend | <0.001 | 0.047 | 0.008 | <0.001 | 0.003 | ||
|
| |||||||
| Weight, kg, cohort-specific quartiles | |||||||
| Q1 | 522 | 575 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 485 | 530 | 1.03 (0.86-1.22) | 1.02 (0.85-1.22) | 1.06 (0.87-1.29) | 1.01 (0.83-1.23) | 1.02 (0.80-1.29) |
| Q3 | 512 | 528 | 1.10 (0.92-1.30) | 1.08 (0.91-1.29) | 1.10 (0.90-1.34) | 1.15 (0.95-1.40) | 1.10 (0.87-1.40) |
| Q4 | 576 | 508 | 1.30 (1.09-1.54) | 1.19 (1.00-1.42) | 1.34 (1.10-1.63) | 1.32 (1.09-1.60) | 1.34 (1.05-1.71) |
| P trend | 0.002 | 0.035 | 0.003 | 0.002 | 0.01 | ||
|
| |||||||
| Height, cm, cohort-specific quartiles | |||||||
| Q1 | 622 | 635 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 543 | 556 | 0.98 (0.83-1.16) | 0.99 (0.84-1.18) | 1.01 (0.84-1.23) | 0.96 (0.79-1.15) | 0.92 (0.73-1.17) |
| Q3 | 495 | 514 | 0.98 (0.83-1.16) | 1.00 (0.84-1.18) | 1.04 (0.86-1.27) | 0.94 (0.78-1.13) | 0.96 (0.76-1.21) |
| Q4 | 435 | 436 | 0.99 (0.83-1.18) | 1.02 (0.85-1.22) | 1.06 (0.87-1.30) | 0.95 (0.78-1.16) | 0.93 (0.72-1.18) |
| P trend | 0.93 | 0.81 | 0.41 | 0.58 | 0.65 | ||
|
| |||||||
| Waist circumference, cm, cohort-specific quartilesf | |||||||
| Q1 | 215 | 224 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 172 | 208 | 0.87 (0.66-1.15) | 0.89 (0.67-1.18) | 0.82 (0.61-1.10) | 0.88 (0.65-1.20) | 0.82 (0.58-1.16) |
| Q3 | 200 | 198 | 1.10 (0.83-1.45) | 1.08 (0.81-1.44) | 1.04 (0.77-1.40) | 1.09 (0.81-1.47) | 1.05 (0.75-1.48) |
| Q4 | 225 | 200 | 1.23 (0.94-1.62) | 1.21 (0.91-1.60) | 1.21 (0.90-1.61) | 1.20 (0.89-1.61) | 1.14 (0.81-1.59) |
| P trend | 0.04 | 0.09 | 0.07 | 0.10 | 0.22 | ||
|
| |||||||
| Waist-to-hip ratio, cohort-specific quartilesf | |||||||
| Q1 | 186 | 206 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 172 | 196 | 1.01 (0.75-1.35) | 1.07 (0.79-1.44) | 1.01 (0.74-1.37) | 1.07 (0.79-1.47) | 1.05 (0.74-1.49) |
| Q3 | 167 | 207 | 0.90 (0.67-1.21) | 0.88 (0.65-1.19) | 0.89 (0.65-1.22) | 0.87 (0.63-1.20) | 0.87 (0.60-1.24) |
| Q4 | 225 | 158 | 1.71 (1.27-2.30) | 1.69 (1.24-2.30) | 1.62 (1.18-2.22) | 1.83 (1.32-2.53) | 1.57 (1.09-2.26) |
| P trend | 0.001 | 0.004 | 0.007 | 0.001 | 0.06 | ||
Adjusted for cohort, age (categorical), gender, anthropometry source (self-reported, measured), and smoking (never, former, current).
Adjusted for cohort, age (categorical), gender, anthropometry source (self-reported, measured), smoking (never, former, current), and diabetes history (no, yes).
Adjusted for cohort, age (categorical), gender, anthropometry source, smoking, and excluding the first 2 years of follow-up.
Adjusted for cohort, age (categorical), gender, anthropometry source, and excluding current and former smokers.
Adjusted for cohort, age (categorical), gender, anthropometry source, and excluding the first 2 years of follow-up, current and former smokers, and people with diabetes.
Models for waist circumference and waist-to-hip ratio were additionally adjusted for height.
NOTE: Statistically significant results are in bold.
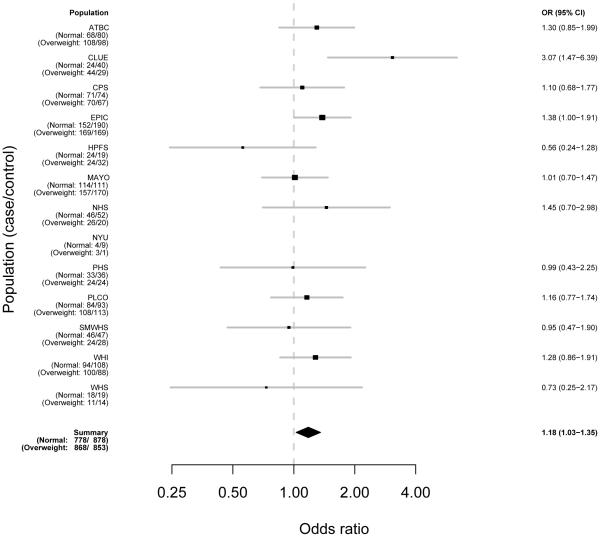
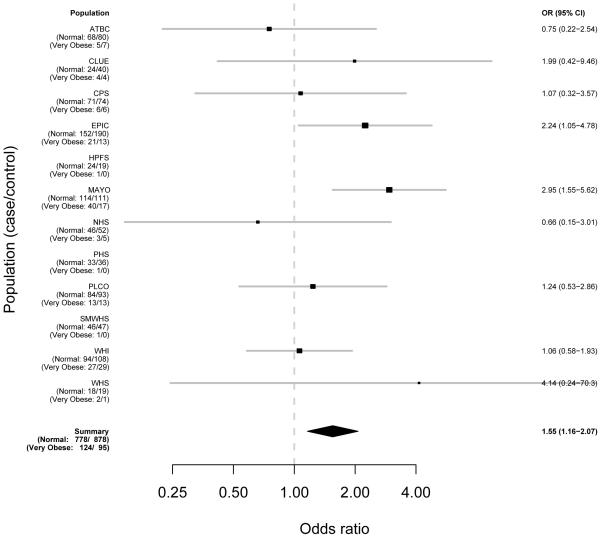
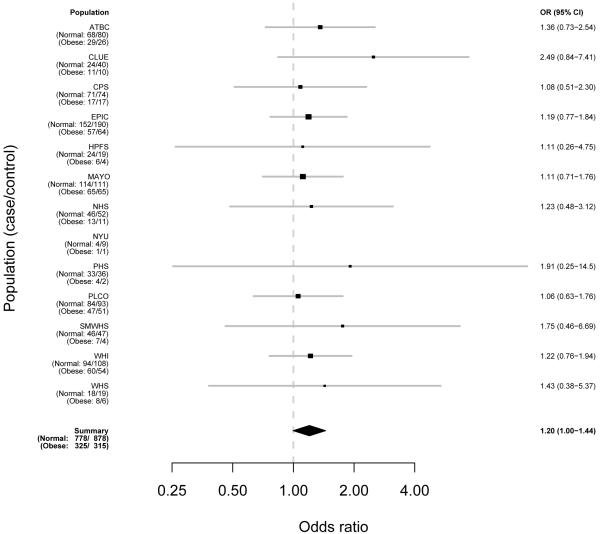
Figures 1-3 demonstrate the individual study results (model 1) and pooled risk estimates for overweight, obese, and severely obese individuals, respectively.
Figure 1. Risk Estimates for Pancreatic Cancer Associated with BMI by Study for Overweight People (25–<30 kg/m^2) As Compared to Normal (<=25 kg/m^2).
Figure 3. Risk Estimates for Pancreatic Cancer Associated with BMI by Study for Very Obese People (35+ kg/m^2) As Compared to Normal (<=25 kg/m^2).
Further adjustment for diabetes history (model 2) resulted in attenuation of risk estimates compared to model 1 but P values for trend were statistically significant for BMI quartiles and categories (Table 4). In addition, waist circumference and waist-to-hip ratio were positively associated with risk of pancreatic cancer among all subjects with top versus bottom quartile ORs = 1.23 (95% CI = 0.94-1.62) and 1.71 (95% CI = 1.27-2.30), respectively (Table 4). Stratification by BMI source (self-reported, measured) resulted in similar risk estimates: ORs (95% CIs) for obese vs. normal BMI were 1.24 (0.92-1.68) for measured BMI and 1.21 (0.95-1.53) for self-reported BMI. The OR per 5 kg/m2 increase in BMI was 1.13 (95% CI = 1.11-1.14).
The risk estimates did not change significantly in the sensitivity analysis excluding the Mayo Clinic case-control study (data not shown), therefore we decided to include the Mayo subjects in the final analyses. There was no evidence of significant heterogeneity between different cohorts for the BMI-pancreatic cancer results (P heterogeneity = 0.36).
Tables 5 and 6 show ORs and 95% CIs of pancreatic cancer among males and females, respectively. Among men, adjusted risk estimate (model 1) for the top versus bottom quartile of BMI was 1.33 (95% CI = 1.04-1.69). Higher risk estimates were observed after exclusion of current smokers (model 4). Among males who never smoked, there was a statistically significant trend of increasing risk with increasing BMI (P trend = 0.007) with the top versus bottom quartile OR = 1.51 (95% CI = 1.13-2.03). Height, waist circumference, and waist-to-hip ratio were not significantly associated with pancreatic cancer among males (Table 5).
Table 5. Odds ratios and 95% CIs of Pancreatic Cancer according to Baseline Anthropometric Factors by Gender, the PanScan Consortium, Males.
| Characteristic | Cases | Controls | OR (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| 1031 | 1055 | Model 1a | Model 2b | Model 3c | Model 4d | Model 5e | |
| BMI, kg/m2, cohort- and gender specific quartiles | |||||||
| Q1 | 251 | 283 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 247 | 256 | 1.13 (0.88-1.45) | 1.15 (0.89-1.48) | 1.09 (0.82-1.45) | 1.20 (0.89-1.63) | 1.01 (0.70-1.47) |
| Q3 | 249 | 259 | 1.12 (0.88-1.44) | 1.07 (0.83-1.38) | 1.04 (0.78-1.38) | 1.12 (0.83-1.52) | 0.88 (0.60-1.29) |
| Q4 | 284 | 257 | 1.33 (1.04-1.69) | 1.23 (0.96-1.58) | 1.22 (0.92-1.62) | 1.51 (1.13-2.03) | 1.27 (0.88-1.84) |
| P trend | <0.03 | 0.16 | 0.19 | 0.007 | 0.21 | ||
|
| |||||||
| BMI, kg/m2, categories | |||||||
| Underweight (<18.5 kg/m2) | 5 | 4 | 1.45 (0.37-5.68) | 1.89 (0.42-8.48) | 0.90 (0.14-5.60) | 0.92 (0.15-5.66) | 0.73 (0.06-8.33) |
| Normal (≥18.5 and <25.0 kg/m2) | 326 | 354 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Overweight (≥25.0 and <30.0 kg/m2) | 503 | 520 | 1.09 (0.89-1.33) | 1.06 (0.86-1.30) | 1.08 (0.87-1.35) | 1.08 (0.85-1.38) | 0.96 (0.71-1.29) |
| Obese (≥30.0 kg/m2) | 152 | 141 | 1.23 (0.93-1.63) | 1.13 (0.85-1.51) | 1.21 (0.87-1.68) | 1.26 (0.89-1.77) | 1.29 (0.82-2.03) |
| Severely obese (≥35.0) | 45 | 36 | 1.48 (0.92-2.39) | 1.26 (0.77-2.06) | 1.07 (0.58-1.97) | 1.65 (0.96-2.84) | 0.90 (0.40-2.02) |
| P trend | 0.07 | 0.33 | 0.32 | 0.047 | 0.54 | ||
|
| |||||||
| Weight, kg, cohort- and gender specific quartiles | |||||||
| Q1 | 257 | 290 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 250 | 254 | 1.14 (0.89-1.46) | 1.12 (0.87-1.44) | 1.28 (0.96-1.69) | 1.10 (0.82-1.49) | 1.29 (0.89-1.87) |
| Q3 | 233 | 259 | 1.05 (0.82-1.34) | 1.04 (0.81-1.35) | 1.03 (0.77-1.38) | 1.12 (0.83-1.51) | 1.06 (0.72-1.56) |
| Q4 | 291 | 252 | 1.36 (1.07-1.74) | 1.27 (0.99-1.63) | 1.42 (1.07-1.88) | 1.43 (1.07-1.91) | 1.36 (0.93-1.98) |
| P trend | 0.02 | 0.09 | 0.046 | 0.015 | 0.21 | ||
|
| |||||||
| Height, cm, cohort- and gender-specific quartiles | |||||||
| Q1 | 284 | 305 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 298 | 301 | 1.05 (0.83-1.32) | 1.08 (0.85-1.37) | 1.13 (0.87-1.48) | 1.05 (0.79-1.38) | 1.10 (0.78-1.57) |
| Q3 | 247 | 249 | 1.07 (0.84-1.37) | 1.08 (0.85-1.39) | 1.15 (0.87-1.52) | 1.00 (0.75-1.34) | 0.90 (0.63-1.30) |
| Q4 | 202 | 200 | 1.05 (0.81-1.36) | 1.08 (0.83-1.41) | 1.19 (0.88-1.60) | 1.02 (0.74-1.41) | 1.04 (0.69-1.56) |
| P trend | 0.63 | 0.54 | 0.23 | 0.92 | 0.98 | ||
|
| |||||||
| Waist circumference, cohort- and gender specific quartilesf | |||||||
| Q1 | 68 | 62 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 55 | 65 | 0.79 (0.47-1.34) | 0.78 (0.45-1.34) | 0.82 (0.47-1.42) | 0.94 (0.50-1.74) | 0.96 (0.49-1.88) |
| Q3 | 47 | 50 | 1.04 (0.60-1.80) | 0.98 (0.55-1.76) | 0.98 (0.54-1.78) | 1.05 (0.55-2.01) | 0.84 (0.40-1.74) |
| Q4 | 52 | 53 | 1.04 (0.61-1.79) | 1.11 (0.63-1.96) | 1.02 (0.58-1.80) | 1.09 (0.58-2.06) | 1.08 (0.55-2.14) |
| P trend | 0.72 | 0.61 | 0.84 | 0.72 | 0.86 | ||
|
| |||||||
| Waist-to-hip ratio, cohort- and gender specific quartilesf | |||||||
| Q1 | 67 | 63 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 52 | 65 | 0.76 (0.45-1.29) | 0.83 (0.47-1.44) | 0.79 (0.45-1.38) | 0.74 (0.40-1.38) | 0.76 (0.39-1.48) |
| Q3 | 41 | 53 | 0.80 (0.46-1.39) | 0.78 (0.43-1.40) | 0.78 (0.44-1.41) | 0.76 (0.39-1.46) | 0.64 (0.31-1.35) |
| Q4 | 62 | 49 | 1.41 (0.83-2.40) | 1.46 (0.83-2.56) | 1.39 (0.79-2.44) | 1.57 (0.85-2.89) | 1.50 (0.77-2.93) |
| P trend | 0.20 | 0.19 | 0.24 | 0.12 | 0.29 | ||
Adjusted for cohort, age (categorical), anthropometry source (self-reported, measured), and smoking (never, former, current).
Adjusted for cohort, age (categorical), anthropometry source (self-reported, measured), smoking (never, former, current), and diabetes history (no, yes).
Adjusted for cohort, age (categorical), anthropometry source, smoking, and excluding the first 2 years of follow-up.
Adjusted for cohort, age (categorical), anthropometry source, and excluding current and former smokers.
Adjusted for cohort, age (categorical), anthropometry source, and excluding the first 2 years of follow-up, current and former smokers, and people with diabetes.
Models for waist circumference and waist-to-hip ratio were additionally adjusted for height.
NOTE: Statistically significant results are in bold.
Table 6.
Odds ratios and 95% CIs of Pancreatic Cancer according to Baseline Anthropometric Factors by Gender, the PanScan Consortium, Females
| Characteristic | Cases | Controls | OR (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| 1064 | 1086 | Model 1a | Model 2b | Model 3c | Model 4d | Model 5e | |
| BMI, kg/m2, cohort- and gender specific quartiles | |||||||
| Q1 | 249 | 280 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 249 | 267 | 1.07 (0.83-1.36) | 1.06 (0.82-1.36) | 1.11 (0.84-1.48) | 1.07 (0.82-1.39) | 1.08 (0.79-1.47) |
| Q3 | 266 | 275 | 1.13 (0.89-1.44) | 1.08 (0.85-1.39) | 1.24 (0.94-1.64) | 1.10 (0.85-1.43) | 1.16 (0.85-1.57) |
| Q4 | 300 | 264 | 1.34 (1.05-1.70) | 1.19 (0.93-1.53) | 1.37 (1.03-1.81) | 1.39 (1.08-1.80) | 1.52 (1.11-2.10) |
| P trend | 0.01 | 0.17 | 0.02 | 0.008 | 0.007 | ||
|
| |||||||
| BMI, kg/m2, categories | |||||||
| Underweight (<18.5 kg/m2) | 14 | 20 | 0.75 (0.37-1.51) | 0.72 (0.35-1.48) | 0.65 (0.29-1.44) | 0.68 (0.29-1.55) | 0.45 (0.16-1.29) |
| Normal (≥18.5 and <25.0 kg/m2) | 433 | 500 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Overweight (≥25.0 and <30.0 kg/m2) | 365 | 333 | 1.31 (1.07-1.60) | 1.27 (1.03-1.55) | 1.40 (1.12-1.76) | 1.30 (1.05-1.61) | 1.34 (1.04-1.72) |
| Obese (≥30.0 kg/m2) | 173 | 174 | 1.19 (0.93-1.54) | 1.12 (0.87-1.46) | 1.23 (0.92-1.64) | 1.25 (0.96-1.63) | 1.29 (0.93-1.79) |
| Severely obese (≥35.0) | 79 | 59 | 1.61 (1.12-2.33) | 1.29 (0.88-1.89) | 1.50 (0.98-2.30) | 1.65 (1.13-2.40) | 1.98 (1.17-3.36) |
| P trend | 0.003 | 0.08 | 0.01 | 0.002 | 0.001 | ||
|
| |||||||
| Weight, kg, cohort- and gender specific quartiles | |||||||
| Q1 | 265 | 285 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 235 | 276 | 0.92 (0.72-1.18) | 0.93 (0.73-1.20) | 0.89 (0.67-1.18) | 0.94 (0.72-1.21) | 0.86 (0.62-1.18) |
| Q3 | 279 | 269 | 1.14 (0.90-1.46) | 1.12 (0.87-1.43) | 1.16 (0.88-1.52) | 1.18 (0.91-1.52) | 1.14 (0.84-1.54) |
| Q4 | 285 | 256 | 1.23 (0.96-1.56) | 1.12 (0.87-1.44) | 1.27 (0.96-1.68) | 1.24 (0.96-1.61) | 1.34 (0.98-1.84) |
| P trend | 0.03 | 0.22 | 0.03 | 0.03 | 0.02 | ||
|
| |||||||
| Height, cm, cohort- and gender specific quartiles | |||||||
| Q1 | 338 | 330 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 245 | 255 | 0.91 (0.72-1.16) | 0.89 (0.70-1.14) | 0.89 (0.67-1.17) | 0.89 (0.69-1.15) | 0.79 (0.57-1.09) |
| Q3 | 248 | 265 | 0.89 (0.71-1.13) | 0.92 (0.72-1.17) | 0.95 (0.73-1.24) | 0.89 (0.69-1.14) | 0.98 (0.73-1.32) |
| Q4 | 233 | 236 | 0.92 (0.72-1.17) | 0.95 (0.74-1.21) | 0.95 (0.72-1.25) | 0.89 (0.69-1.16) | 0.85 (0.63-1.16) |
| P trend | 0.45 | 0.69 | 0.80 | 0.38 | 0.52 | ||
|
| |||||||
| Waist circumference, cohort- and gender specific quartilesf | |||||||
| Q1 | 147 | 162 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 117 | 143 | 0.89 (0.64-1.25) | 0.92 (0.66-1.30) | 0.81 (0.56-1.16) | 0.85 (0.60-1.22) | 0.77 (0.51-1.14) |
| Q3 | 153 | 148 | 1.14 (0.83-1.58) | 1.14 (0.82-1.58) | 1.08 (0.76-1.54) | 1.12 (0.80-1.57) | 1.14 (0.77-1.68) |
| Q4 | 173 | 147 | 1.31 (0.95-1.80) | 1.26 (0.91-1.75) | 1.28 (0.91-1.80) | 1.24 (0.88-1.73) | 1.17 (0. 79-1.73) |
| P trend | 0.04 | 0.09 | 0.06 | 0.10 | 0.19 | ||
|
| |||||||
| Waist-to-hip ratio, cohort- and gender specific quartilesf | |||||||
| Q1 | 119 | 143 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 120 | 131 | 1.14 (0.80-1.63) | 1.21 (0.84-1.74) | 1.15 (0.78-1.68) | 1.20 (0.83-1.75) | 1.20 (0.80-1.82) |
| Q3 | 126 | 154 | 0.96 (0.67-1.36) | 0.94 (0.66-1.34) | 0.96 (0.66-1.39) | 0.92 (0.63-1.33) | 0.97 (0.64-1.47) |
| Q4 | 163 | 109 | 1.87 (1.31-2.69) | 1.85 (1.27-2.69) | 1.77 (1.20-2.61) | 1.95 (1.33-2.86) | 1.61 (1.03-2.50) |
| P trend | 0.003 | 0.008 | 0.01 | 0.005 | 0.11 | ||
Adjusted for cohort, age (categorical), anthropometry source (self-reported, measured), and smoking (never, former, current).
Adjusted for cohort, age (categorical), anthropometry source (self-reported, measured), smoking (never, former, current), and diabetes history (no, yes).
Adjusted for cohort, age (categorical), anthropometry source, smoking, and excluding the first 2 years of follow-up.
Adjusted for cohort, age (categorical), anthropometry source, and excluding current and former smokers.
Adjusted for cohort, age (categorical), anthropometry source, and excluding the first 2 years of follow-up, current and former smokers, and people with diabetes.
Models for waist circumference and waist-to-hip ratio were additionally adjusted for height.
NOTE: Statistically significant results are in bold.
Among women, statistically significant trends of increasing risk of pancreatic cancer with increasing BMI were observed overall (model 1) and after exclusion of cases diagnosed within the first 2 years of follow-up (model 3) or current and former smokers (model 4) (Table 6). Compared to normal BMI (model 1), the ORs of pancreatic cancer were 1.31 (95% CI = 1.07-1.60) for overweight women and 1.61 (95% CI = 1.12-2.33, P trend = 0.003) for severely obese women. Increasing waist circumference and WHR were significantly associated with pancreatic cancer risk in women. Compared to the reference group, women in the highest quartile of WHR had an OR of 1.87 (95% CI = 1.31-2.69) after adjustment for cohort, age, BMI source, and smoking status. Inclusion of both BMI (categorical) and WHR (quartiles) in the same model suggested that the effect of increasing WHR is stronger (P = 0.006) compared to that of BMI categories (P = 0.44) after adjustment for cohort, age, gender, BMI source, smoking, and diabetes history.
We did not observe clinically meaningful differences in time of onset for pancreatic cancer between normal and overweight/obese individuals. Overweight and obese individuals together were diagnosed approximately 4 months earlier than normal weight individuals (data not shown). When comparing obese individuals only with normal weight individuals, obese subjects were diagnosed on average about one year earlier than normal weight individuals and the difference was statistically significant (p = 0.03).
COMMENT
Results from this large, pooled set of studies support the hypothesis that obesity is associated with an increased risk of pancreatic cancer. The present findings are consistent with the majority of previous epidemiologic studies that found a positive association between BMI and pancreatic cancer risk 45 and support the conclusion from a recent review panel from the World Cancer Research Fund that the strength of the evidence supporting an association between obesity and pancreatic cancer is convincing 45.
Previous studies that did not observe a positive association between body mass index and pancreatic cancer were often limited by use of proxy respondents 46-49 or by inadequate statistical power to examine associations at BMI levels that correspond with obesity (fewer than 10 cases with BMI > 30.0) 7,10,17,50. Controversy regarding the role of smoking in the BMI and pancreatic cancer relationship still remains. Many previous studies that did not observe an association with obesity did not properly control for smoking history 7,47,48,50. It is possible that residual confounding due to improper adjustment for smoking history may have biased the association between body mass index and pancreatic cancer toward the null. When stratifying on smoking status, some previous studies found the relationship between BMI and pancreatic cancer was strongest among never smokers 24,25. Our findings are consistent with previous reports that the association between BMI and pancreatic cancer is stronger among non-smokers (adjusted OR for BMI ≥30 = 1.37, 95% CI = 1.06-1.78) than smokers (adjusted OR for BMI ≥30 = 1.14, 95% CI = 0.91-1.78).
Unlike a recent report where the authors reported that individuals who were overweight or obese from the ages of 20 to 49 had an earlier onset of pancreatic cancer compared to those with normal body weight 51, we did not find a substantial difference in age of diagnosis between normal weight and the combined group of overweight/obese individuals.
In our study, BMI was assessed between the ages 37 and 94 and overweight or obese individuals were diagnosed on average 4 months earlier than normal weight individuals. The previous report by Li et al. 51, using a hospital-based case-control study design, found that overweight or obese patients from the ages 20 to 49 had a median age of pancreatic cancer onset 2 to 6 years earlier than normal weight patients. However, these differences were based on BMI as recalled from early adulthood and may have been subject to recall bias. Nevertheless, as suggested by Li et al., obesity at younger ages might have a more profound effect on risk and age of onset of pancreatic cancer than obesity at older ages.
There are established biologic pathways to support a relationship between excess body weight and the development of pancreatic cancer. Body fatness has a direct linear relationship with insulin production and is related to the development of insulin resistance 52. Furthermore, insulin resistance and abnormal glucose metabolism, even in the absence of diabetes, is associated with pancreatic cancer risk 8,53,54. In vitro studies have also shown that insulin has growth-promoting effects in the pancreas 55. A hyperinsulinemic state allows increased insulin to pass through the pancreas and trigger mitotic activity 8,53,56. Additionally, excess insulin can also down-regulate insulin-like growth factor-I binding proteins, which would result in more bioavailable insulin-like growth factor-I that has been associated with cell proliferation and pancreatic cancer risk 54,57.
The results of this study also support a specific role of central adiposity in pancreatic cancer risk, especially among women. In addition to general body fatness, there is a direct linear relationship between intra-abdominal fat deposits, insulin production, and the development of insulin resistance 52.
There are several strengths of the current study including the very large sample size, the wide range of BMI, and the ability to control for most known or suspected pancreatic cancer risk factors. Additionally, our population was largely a nested sample from various prospective cohort studies, so that BMI was measured prior to pancreatic cancer diagnosis, thus reducing differential reporting of past exposure information. Limitations include the use of some self-reported exposure information; however, adjusting for source of exposure information (self-reported or measured), did not alter the association. Another potential limitation is the wide range of lag periods between BMI measurement (collected at baseline for each cohort) and the date of diagnosis; however, sensitivity analyses examining this lag time by excluding the first 2 years of follow-up did not change the point estimates appreciably, thus arguing against an influence of pre-diagnostic disease-related changes in anthropometric measures (reverse causation). Participating cohorts had different coding systems for physical activity that were not readily comparable; therefore we could not assess whether the association between BMI and pancreatic cancer vary by level of physical activity. To address potential residual confounding by smoking, we have performed the analyses in never smokers and found slightly stronger association between BMI and pancreatic risk. Lastly, only a few cohorts had data available on waist and hip circumference, so that there was limited statistical power to examine these relationships.
In summary, the results of this study provide additional evidence that obesity is associated with increased risk of pancreatic cancer. In addition, the association between waist circumference and pancreatic cancer risk, especially in women, suggests a possible association with the distribution of body fat. These findings, along with those from previous studies, strongly support the role of obesity in pancreatic cancer development.
Figure 2. Risk Estimates for Pancreatic Cancer Associated with BMI by Study for Obese People (30–<35 kg/m^2) As Compared to Normal (<=25 kg/m^2).
Acknowledgment
The authors thank the investigators from the PanScan Cohort Consortium centers and the study participants, without whom this study would have not been possible.
Funding/Grant Support: This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Trial was supported by funding provided by the Intramural Research Program of the National Cancer Institute, and the U.S. Public Health Service contracts [(N01-CN-45165, N01-RC-45035, N01-RC-37004].
CLUE II was supported by National Institute of Aging [5U01AG018033] and National Cancer Institute [CA105069, CA73790].
European Prospective Investigation into Cancer and Nutrition was supported by the European Commission: Public Health and Consumer Protection Directorate 1993-2004; Research Directorate-General 2005; Ligue contre le Cancer; Societé 3M; Mutuelle Générale de l’Education Nationale; Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center, Federal Ministry of Education and Research (Germany); Danish Cancer Society (Denmark); Health Research Fund (FIS) of the Spanish Ministry of Health, The participating regional governments and institutions (Spain); Cancer Research UK, Medical Research Council, Stroke Association, British Heart Foundation, Department of Health, Food Standards Agency, the Wellcome Trust (United Kingdom); Greek Ministry of Health and Social Solidarity, Hellenic Health Foundation and Stavros Niarchos Foundation (Greece); Italian Association for Research on Cancer (AIRC) (Italy); Dutch Ministry of Public Health, Welfare and Sports, Dutch Prevention Funds, LK Research Funds, Dutch ZON (Zorg Onderzoek Nederland) (the Netherlands); Swedish Cancer Society, Swedish Scientific Council, Regional Government of Skane and Västerbotten (Sweden); World Cancer Research Fund (WCRF).
The New York University Women’s Health Study is supported by the National Cancer Institute research grants [R01CA034588, R01CA098661, P30CA016087] and the National Institute of Environmental Health Sciences Center grant [ES000260].
The Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial was supported by contracts from the National Cancer Institute [University of Colorado Denver, NO1-CN-25514, Georgetown University NO1-CN-25522, Pacific Health Research Institute NO1-CN-25515, Henry Ford Health System NO1-CN-25512, University of Minnesota, NO1-CN-25513, Washington University NO1-CN-25516, University of Pittsburgh NO1-CN-25511, University of Utah NO1-CN-25524 Marshfield Clinic Research Foundation NO1-CN-25518, University of Alabama at Birmingham NO1-CN-75022, Westat, Inc. NO1-CN-25476, University of California, Los Angeles NO1-CN-25404].
The Shanghai Men’s and Women’s Health Studies were supported by the National Cancer Institute extramural research grants [R01 CA82729, R01 CA70867] and by the Intramural Research Program of National Cancer Institute (Division of Cancer Epidemiology and Genetics).
The Women’s Health Initiative is funded by the National Heart, Lung, and Blood Institute through contracts [N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221].
Role of the Sponsors: This project has been funded with federal funds from the National Cancer Institute, National Institutes of Health. The funding agency had no role in the conduct of the study, the interpretation of the data, or the decision to submit the manuscript for publication.
Abbreviations
- ATBC
Alpha-Tocopherol, Beta-Carotene Cancer Prevention Trial
- BMI
Body Mass Index
- CPS II
Cancer Prevention Study II
- CI
Confidence Interval
- EPIC
European Prospective Investigation into Cancer and Nutrition
- Clue II
Give Us a Clue to Cancer and Heart Disease Study
- HPFS
Health Professionals Follow-up Study
- MAYO
Mayo Clinic study
- NYUWHS
New York University Women’s Health Study
- NHS
Nurses’ Health Study
- OR
Odds Ratio
- PanScan
Pancreatic Cancer Cohort Consortium
- PHS I
Physicians’ Health Study
- PLCO
Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial
- SMWHS
Shanghai Men’s and Women’s Health Studies
- SSIRB
Special Studies Institutional Review Board
- WHI
Women’s Health Initiative
- WHS
Women’s Health Study
- WHR
Waist-to-hip ratio
Footnotes
Author Contributions:
Dr. Arslan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Conception and Design: Patricia Hartge, Demetrius Albanes, Laufey Amundadottir, Stephen J. Chanock, Robert N. Hoover, Amy Hutchinson, Kevin B. Jacobs, Julie B. Mendelsohn, Gilles Thomas, Geoffrey S. Tobias, Kai Yu
Analysis and Interpretation of Data: Alan A. Arslan, Alpa V. Patel, Emily Steplowski, Charles Kooperberg, Kathy J. Helzlsouer, Xiao-Ou Shu
Drafting of the article: Alan A. Arslan, Alpa V. Patel, Kathy J. Helzlsouer, Charles Kooperberg, Xiao-Ou Shu, Emily Steplowski
Critical revision of the article for important intellectual content: Alan A. Arslan, Kathy J. Helzlsouer, Charles Kooperberg, Xiao-Ou Shu, Emily Steplowski, H. Bas Bueno-de-Mesquita, Eric J. Jacobs, Heiner Boeing, Edward L. Giovannucci, Amy Hutchinson, JoAnn E. Manson, Anne McTiernan, Julie B. Mendelsohn, Dominique S. Michaud, Thomas E. Rohan, Geoffrey S. Tobias, Dimitrios Trichopoulos, Jarmo Virtamo, Alpa V. Patel
Final approval of the article: Alan A. Arslan, Kathy J. Helzlsouer, Charles Kooperberg, Xiao-Ou Shu, Emily Steplowski, H. Bas Bueno-de-Mesquita, Charles S. Fuchs, Myron D. Gross, Eric J. Jacobs, Andrea LaCroix, Gloria M. Petersen, Rachael Z. Stolzenberg-Solomon, Wei Zheng, Demetrius Albanes, Laufey Amundadottir, William R. Bamlet, Aurelio Barricarte, Sheila A. Bingham, Heiner Boeing, Marie-Christine Boutron-Ruault, Julie E. Buring, Stephen J. Chanock, Sandra Clipp, J. Michael Gaziano, Edward L. Giovannucci, Susan E. Hankinson, Patricia Hartge, Robert N. Hoover, David J. Hunter, Amy Hutchinson, Kevin B. Jacobs, Peter Kraft, Shannon M. Lynch, Jonas Manjer, JoAnn E. Manson, Anne McTiernan, Robert R. McWilliams, Julie B. Mendelsohn, Dominique S. Michaud, Domenico Palli, Thomas E. Rohan, Nadia Slimani, Gilles Thomas, Anne Tjønneland, Geoffrey S. Tobias, Dimitrios Trichopoulos, Jarmo Virtamo, Brian M. Wolpin, Kai Yu, Anne Zeleniuch-Jacquotte, Alpa V. Patel
Provision of study materials or patients: Demetrius Albanes, Eric J. Jacobs, H. Bas Bueno-de-Mesquita, Aurelio Barricarte, Heiner Boeing, Marie-Christine Boutron-Ruault, Jonas Manjer, Dominique S. Michaud, Domenico Palli, Nadia Slimani, Anne Tjønneland, Dimitrios Trichopoulos, Jarmo Virtamo, Kathy J. Helzlsouer, Charles S. Fuchs, Gloria M. Petersen, Anne Zeleniuch-Jacquotte, Susan E. Hankinson, J. Michael Gaziano, Wei Zheng, Xiao-Ou Shu, Andrea LaCroix
Statistical expertise: Charles Kooperberg, Emily Steplowski, Peter Kraft, Kai Yu
Obtaining of funding: Patricia Hartge, Demetrius Albanes, Laufey Amundadottir, Stephen J. Chanock, Robert N. Hoover, Amy Hutchinson, Kevin B. Jacobs, Julie B. Mendelsohn, Gilles Thomas, Geoffrey S. Tobias, Kai Yu
Administrative, technical, or logistical support: Geoffrey S. Tobias, Julie B. Mendelsohn Collection and assembly of data: Geoffrey S. Tobias, Julie B. Mendelsohn, Emily Steplowski
Financial Disclosure: None reported.
REFERENCES
- 1.American Cancer Society . Cancer Facts & Figures - 2009. American Cancer Society, Inc.; Atlanta: 2009. [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97(19):1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 4.Gapstur SM, Gann P. Is pancreatic cancer a preventable disease? JAMA. 2001;286(8):967–968. doi: 10.1001/jama.286.8.967. [DOI] [PubMed] [Google Scholar]
- 5.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363(9414):1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 6.Friedman GD, van den Eeden SK. Risk factors for pancreatic cancer: an exploratory study. Int J Epidemiol. 1993;22(1):30–37. doi: 10.1093/ije/22.1.30. [DOI] [PubMed] [Google Scholar]
- 7.Shibata A, Mack TM, Paganini-Hill A, Ross RK, Henderson BE. A prospective study of pancreatic cancer in the elderly. Int J Cancer. 1994;58(1):46–49. doi: 10.1002/ijc.2910580109. [DOI] [PubMed] [Google Scholar]
- 8.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283(19):2552–2558. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 9.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286(8):921–929. doi: 10.1001/jama.286.8.921. [DOI] [PubMed] [Google Scholar]
- 10.Isaksson B, Jonsson F, Pedersen NL, Larsson J, Feychting M, Permert J. Lifestyle factors and pancreatic cancer risk: a cohort study from the Swedish Twin Registry. Int J Cancer. 2002;98(3):480–482. doi: 10.1002/ijc.10256. [DOI] [PubMed] [Google Scholar]
- 11.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 12.Patel AV, Rodriguez C, Bernstein L, Chao A, Thun MJ, Calle EE. Obesity, recreational physical activity, and risk of pancreatic cancer in a large U.S. Cohort. Cancer Epidemiol Biomarkers Prev. 2005;14(2):459–466. doi: 10.1158/1055-9965.EPI-04-0583. [DOI] [PubMed] [Google Scholar]
- 13.Rapp K, Schroeder J, Klenk J, et al. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93(9):1062–1067. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsson SC, Permert J, Hakansson N, Naslund I, Bergkvist L, Wolk A. Overall obesity, abdominal adiposity, diabetes and cigarette smoking in relation to the risk of pancreatic cancer in two Swedish population-based cohorts. Br J Cancer. 2005;93(11):1310–1315. doi: 10.1038/sj.bjc.6602868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berrington dG, Spencer EA, Bueno-de-Mesquita HB, et al. Anthropometry, physical activity, and the risk of pancreatic cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2006;15(5):879–885. doi: 10.1158/1055-9965.EPI-05-0800. [DOI] [PubMed] [Google Scholar]
- 16.Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, Virtamo J, Albanes D. A prospective study of medical conditions, anthropometry, physical activity, and pancreatic cancer in male smokers (Finland) Cancer Causes Control. 2002;13(5):417–426. doi: 10.1023/a:1015729615148. [DOI] [PubMed] [Google Scholar]
- 17.Lee IM, Sesso HD, Oguma Y, Paffenbarger RS., Jr. Physical activity, body weight, and pancreatic cancer mortality. Br J Cancer. 2003;88(5):679–683. doi: 10.1038/sj.bjc.6600782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuriyama S, Tsubono Y, Hozawa A, et al. Obesity and risk of cancer in Japan. Int J Cancer. 2005;113(1):148–157. doi: 10.1002/ijc.20529. [DOI] [PubMed] [Google Scholar]
- 19.Sinner PJ, Schmitz KH, Anderson KE, Folsom AR. Lack of association of physical activity and obesity with incident pancreatic cancer in elderly women. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1571–1573. doi: 10.1158/1055-9965.EPI-05-0036. [DOI] [PubMed] [Google Scholar]
- 20.Batty GD, Shipley MJ, Jarrett RJ, Breeze E, Marmot MG, Smith GD. Obesity and overweight in relation to organ-specific cancer mortality in London (UK): findings from the original Whitehall study. Int J Obes (Lond) 2005;29(10):1267–1274. doi: 10.1038/sj.ijo.0803020. [DOI] [PubMed] [Google Scholar]
- 21.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23(21):4742–4754. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 22.Lukanova A, Bjor O, Kaaks R, et al. Body mass index and cancer: results from the Northern Sweden Health and Disease Cohort. Int J Cancer. 2006;118(2):458–466. doi: 10.1002/ijc.21354. [DOI] [PubMed] [Google Scholar]
- 23.Nothlings U, Kolonel LN. Risk factors for pancreatic cancer in the Hawai’i-Los Angeles Multiethnic Cohort Study. Hawaii Med J. 2006;65(1):26–28. [PubMed] [Google Scholar]
- 24.Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF., Jr. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17(7):901–909. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 25.Stolzenberg-Solomon RZ, Adams K, Leitzmann M, et al. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health-AARP Diet and Health Cohort. Am J Epidemiol. 2008;167(5):586–597. doi: 10.1093/aje/kwm361. [DOI] [PubMed] [Google Scholar]
- 26.Nothlings U, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Kolonel LN. Body mass index and physical activity as risk factors for pancreatic cancer: the Multiethnic Cohort Study. Cancer Causes Control. 2007;18(2):165–175. doi: 10.1007/s10552-006-0100-0. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Kikuchi S, Tamakoshi A, et al. Obesity, physical activity and the risk of pancreatic cancer in a large Japanese cohort. Int J Cancer. 2007;120(12):2665–2671. doi: 10.1002/ijc.22614. [DOI] [PubMed] [Google Scholar]
- 28.Luo J, Iwasaki M, Inoue M, et al. Body mass index, physical activity and the risk of pancreatic cancer in relation to smoking status and history of diabetes: a large-scale population-based cohort study in Japan--the JPHC study. Cancer Causes Control. 2007;18(6):603–612. doi: 10.1007/s10552-007-9002-z. [DOI] [PubMed] [Google Scholar]
- 29.The ATBC Cancer Prevention Study Group The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4(1):1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 30.Huang HY, Alberg AJ, Norkus EP, Hoffman SC, Comstock GW, Helzlsouer KJ. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am J Epidemiol. 2003;157(4):335–344. doi: 10.1093/aje/kwf210. [DOI] [PubMed] [Google Scholar]
- 31.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(9):2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 32.Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6B):1113–1124. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 33.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(8765):464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 34.McWilliams RR, Bamlet WR, de Andrade M, Rider DN, Cunningham JM, Petersen GM. Nucleotide excision repair pathway polymorphisms and pancreatic cancer risk: evidence for role of MMS19L. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1295–1302. doi: 10.1158/1055-9965.EPI-08-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst. 1995;87(3):190–197. doi: 10.1093/jnci/87.3.190. [DOI] [PubMed] [Google Scholar]
- 36.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5(5):388–396. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 37.Hennekens CH, Buring JE. Methodologic considerations in the design and conduct of randomized trials: the U.S. Physicians’ Health Study. Control Clin Trials. 1989;10(4 Suppl):142S–150S. doi: 10.1016/0197-2456(89)90053-6. [DOI] [PubMed] [Google Scholar]
- 38.Gohagan JK, Prorok PC, Hayes RB, Kramer BS. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials. 2000;21(6 Suppl):251S–272S. doi: 10.1016/s0197-2456(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 39.Cai H, Zheng W, Xiang YB, et al. Dietary patterns and their correlates among middle-aged and elderly Chinese men: a report from the Shanghai Men’s Health Study. Br J Nutr. 2007;98(5):1006–1013. doi: 10.1017/S0007114507750900. [DOI] [PubMed] [Google Scholar]
- 40.Zheng W, Chow WH, Yang G, et al. The Shanghai Women’s Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162(11):1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 41.The Women’s Health Initiative Study Group Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 42.Buring JE, Hennekens CH. The Women’s Health Study: rationale and background. J Myocard Ischemia. 1992:430–40. [Google Scholar]
- 43.Garfinkel L. Selection, follow-up, and analysis in the American Cancer Society prospective studies. Natl Cancer Inst Monogr. 1985:6749–52. [PubMed] [Google Scholar]
- 44.Haakenson CP, Vickers KS, Cha SS, et al. Efficacy of a simple, low-cost educational intervention in improving knowledge about risks and benefits of screening mammography. Mayo Clin Proc. 2006;81(6):783–791. doi: 10.4065/81.6.783. [DOI] [PubMed] [Google Scholar]
- 45.WCRF. World Cancer Research Fund . Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. 2nd ed. American Institute for Cancer Research; Washington, DC: 2007. pp. 215–216. [Google Scholar]
- 46.Bueno de Mesquita HB, Moerman CJ, Runia S, Maisonneuve P. Are energy and energy-providing nutrients related to exocrine carcinoma of the pancreas? Int J Cancer. 1990;46(3):435–444. doi: 10.1002/ijc.2910460319. [DOI] [PubMed] [Google Scholar]
- 47.Howe GR, Jain M, Miller AB. Dietary factors and risk of pancreatic cancer: results of a Canadian population-based case-control study. Int J Cancer. 1990;45(4):604–608. doi: 10.1002/ijc.2910450405. [DOI] [PubMed] [Google Scholar]
- 48.Zatonski W, Przewozniak K, Howe GR, Maisonneuve P, Walker AM, Boyle P. Nutritional factors and pancreatic cancer: a case-control study from south-west Poland. Int J Cancer. 1991;48(3):390–394. doi: 10.1002/ijc.2910480314. [DOI] [PubMed] [Google Scholar]
- 49.Ghadirian P, Simard A, Baillargeon J, Maisonneuve P, Boyle P. Nutritional factors and pancreatic cancer in the francophone community in Montreal, Canada. Int J Cancer. 1991;47(1):1–6. doi: 10.1002/ijc.2910470102. [DOI] [PubMed] [Google Scholar]
- 50.Nilsen TI, Vatten LJ. A prospective study of lifestyle factors and the risk of pancreatic cancer in Nord-Trondelag, Norway. Cancer Causes Control. 2000;11(7):645–652. doi: 10.1023/a:1008916123357. [DOI] [PubMed] [Google Scholar]
- 51.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301(24):2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.International Agency for Research on Cancer . IARC Handbook of Cancer Prevention: Weight Control and Physical Activity. Vol. 6. IARC Press; Lyon, France: 2002. [Google Scholar]
- 53.Fisher WE, Boros LG, Schirmer WJ. Insulin promotes pancreatic cancer: evidence for endocrine influence on exocrine pancreatic tumors. J Surg Res. 1996;63(1):310–313. doi: 10.1006/jsre.1996.0266. [DOI] [PubMed] [Google Scholar]
- 54.McCarty MF. Insulin secretion as a determinant of pancreatic cancer risk. Med Hypotheses. 2001;57(2):146–150. doi: 10.1054/mehy.2001.1316. [DOI] [PubMed] [Google Scholar]
- 55.Mossner J, Logsdon CD, Goldfine ID, Williams JA. Do insulin and the insulin like growth factors (IGFs) stimulate growth of the exocrine pancreas? Gut. 1987;28(Suppl):51–55. doi: 10.1136/gut.28.suppl.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams JA, Goldfine ID. The insulin-pancreatic acinar axis. Diabetes. 1985;34(10):980–986. doi: 10.2337/diab.34.10.980. [DOI] [PubMed] [Google Scholar]
- 57.Conover CA, Lee PD, Kanaley JA, Clarkson JT, Jensen MD. Insulin regulation of insulin-like growth factor binding protein-1 in obese and nonobese humans. J Clin Endocrinol Metab. 1992;74(6):1355–1360. doi: 10.1210/jcem.74.6.1375600. [DOI] [PubMed] [Google Scholar]