Abstract
The straightforward protonation of lactams by treatment with acid and the full structural characterization of three resulting N-protonated lactams are disclosed. This work provides experimental evidence that N-protonation of amide bonds results in a dramatic increase of non-planarity about the C-N amide bond. The resulting compounds are discussed in structural, spectroscopic, and reactivity terms. The data suggests that ca. 50° distortion of these amide bonds suffices for their efficient N-activation.
Although most amides usually protonate on oxygen, amide N-protonation has been nonetheless proposed in a number of biologically relevant mechanisms,1 including cis–trans peptide bond isomerization,2 amide bond hydrolysis,3 and protein splicing.4 Although fundamentally different reactivity patterns are expected – and observed – to result from amide subsitution,5 the study of N-protonated amides has been hampered by the unavailability of molecules that contain them.
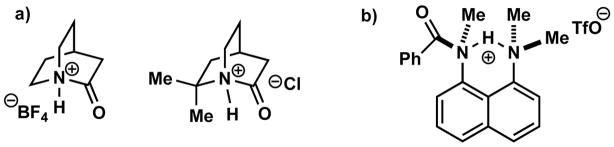
The characterization of N-protonated amides is challenging because such species are thermodynamically disfavored relative to their O-protonated isomers by ca. 40 kcal/mol.1 To date, examples of isolated and otherwise unadorned N-protonated amides are severely limited to perpendicularly twisted 2-quinuclidone derivatives (Figure 1a).6a–f In addition, Lectka reported an elegantly designed N-protonated amide that was stabilized by the proximity of an additional tertiary amine (Figure 1b).6g To date, only two N-protonated amides have been characterized by X-ray crystallography.7 Herein, we demonstrate that a series of medium-bridged N-protonated lactams can be readily prepared by acid treatment. This streamlined access to multiple lactam conjugate acids has permitted the full structural characterization of three N-protonated amides and provided the first experimental evidence showing that the N-protonation of amide bonds results in a dramatic increase of pyramidalization around the C-N amide bond.8 Our data also suggest that ca. 50° distortion of amide bonds (where 0° would correspond to planar amide, 90° to a fully orthogonal amide bond) suffices for efficient N-protonation or alkylation of lactams.
Figure 1.
Previously reported examples of isolated N-protonated amides.
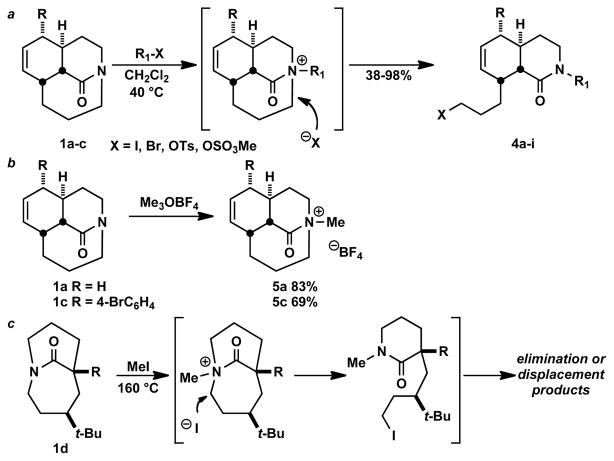
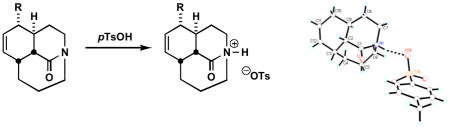
Recent efforts in this laboratory have provided ready access to a family of bridged lactams that are characterized by moderate amide bond distortion (twist values ca. 40–50°).9 Such compounds display decidedly non-traditional amide bond reactivity patterns that include unusual cleavage reactions of a C-N σ bond adjacent to the amide bond.9d–f We hypothesized that these reactions take place via initial N-alkylation made possible by the increased basicity of the non-planar amide bond nitrogen. The facility of these reactions suggested it might be possible to isolate the responsible species by straightforward protonation or alkylation. Indeed, when tricyclic amides 1a–c were exposed to mild acids, protonation took place at nitrogen to afford the corresponding stable salts in excellent yields (Scheme 1). The tosylates 3a–c were crystalline, and their structures could be confirmed by X-ray crystallography (Figure 2). Table 1 summarizes Winkler-Dunitz distortion parameters τ, χC, and χN (describing magnitude of rotation around the N-C(O) bond, pyramidalization at carbon and pyramidalization at nitrogen, respectively)10 and bond lengths of the N-protonated lactams.
Scheme 1.
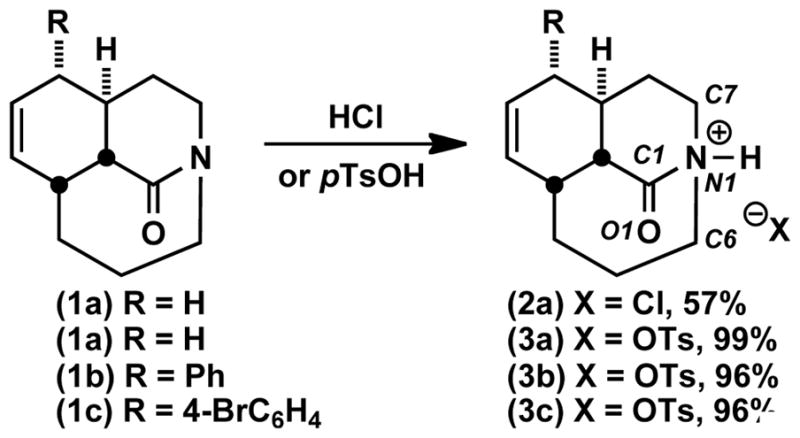
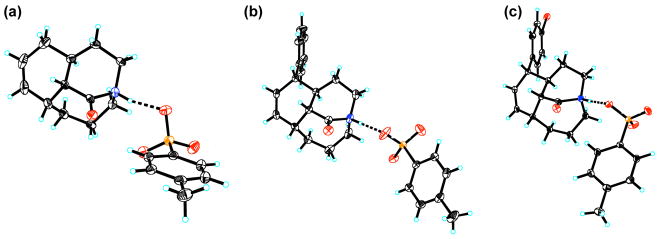
Figure 2.
Crystal structures of (a) 3a, (b) 3b, and (c) 3c. Selected bond lengths [Å] and angles [°]: (a) N1-C1, 1.492(3), C1-O1, 1.202(4), C1-C2, 1.489(4), N1-H1, 0.93, C7-N1-C1-O1, −121.5(5)°; C6-N1-C1-O1, 111.1(5)°; C7-N1-C1-C2, 59.3(3)°; C6-N1-C1-C2, −68.2(3)°. (b) N1-C1, 1.491(3), C1-O1, 1.197(3), C1-C2, 1.487(4), N1-H1, 0.86(4), C7-N1-C1-O1, −126.3(3)°; C6-N1-C1-O1, 106.2(3)°; C7-N1-C1-C2, 56.1(3)°; C6-N1-C1-C2, −71.5(3)°. (c) N1-C1, 1.502(2), C1-O1, 1.192(2), C1-C2, 1.488(3), N1-H1, 0.89(3), C7-N1-C1-O1, −124.84(18)°; C6-N1-C1-O1, 107.21(19)°; C7-N1-C1-C2, 56.58(19)°; C6-N1-C1-C2, −71.38(19)°.
Table 1.
Summary of structural parameters of amides and N-protonated amides.
| entry | amide (notes) | N-C(O) [Å] | C=O [Å] | χN [deg] | χC [deg] | τ [deg] |
|---|---|---|---|---|---|---|
| 1a | 1c (tricyclic) | 1.387 | 1.218 | 36.1 | 12.8 | 51.5 |
| 2b | 1d (bicyclic) | 1.367 | 1.227 | 34.1 | 16.5 | 42.8 |
| 3 | 3a (N-protonated) | 1.492 | 1.202 | 52.6 | 0.7 | 85.2 |
| 4 | 3b (N-protonated) | 1.491 | 1.197 | 52.5 | 2.4 | 81.2 |
| 5 | 3c (N-protonated) | 1.502 | 1.192 | 52.1 | 1.4 | 81.9 |
| 6c | 3d (N-protonated) | 1.526 | 1.192 | 59.5 | 0.2 | 90.9 |
| 7d,e | 1e (formamide) | 1.349 | 1.193 | 0.0 | 0.0 | 0.0 |
Notably, the availability of crystal structures of both 1c and 3c allowed the neutral amide and its conjugate acid to be easily compared. As expected, N-protonation of amide bonds enhances the pyramidal character at nitrogen (χN). The resulting change is substantial: from a moderately pyramidal to practically sp3 hybridized nitrogen (in series c χN increases from 36.1° to 52.1°). This is accompanied by the flattening of the C=O carbon (in series c χC drops from 12.8° to 1.4°) and a dramatic decrease in C-N bond planarity (in 2c τ is 81.9°, cf. 51.5° for 1c). The bond lengths were also influenced by N-protonation. The N-C(O) bond experiences significant lengthening (in 1c by 0.115 Å), while the C=O bond was moderately shortened (in 1c by 0.026 Å). Overall, these structural changes indicate substantial rehybridization upon N-protonation and C-N bond rotation, even in the fairly rigid tricyclic ring system of 1.8a, b
Furthermore, the X-ray structures reveal that the N-protons are stabilized by hydrogen bonding to tosylate oxygens (Figure 2). The O1A-H1 (O3A-H1) distances of 1.79–1.82 Å and the N1-H1-O1A (N1-H1-O3A) angles of 163–177° are consistent with the presence of moderately strong hydrogen bonds. The bond distances between the amide bond nitrogens and tosylate oxygens in salts 3a–3c (2.68–2.72 Å) also support the formation of medium-strength hydrogen bonds engaging the amide nitrogen atoms.11
We earlier reported that tricyclic lactams react with MeI to form the corresponding amidinium salts followed by regioselective SN2 displacement with iodide.9e As expected, similar chemistry was observed upon exposure of tricyclic amides to related electrophiles under mild conditions (Scheme 2a, see SI for details). In contrast, treatment with Meerwein’s reagent, lacking a nucleophilic counterion, permitted isolation of two rare examples of N-alkylated amides (Scheme 2b).12 Unfortunately, we were not able to obtain high-resolution crystal structures of these compounds.
Scheme 2.
Our attempts to similarly protonate or alkylate [4.3.1] bicyclic lactams like 1d revealed differences in reactivity between these species and their tricyclic cousins 3a–c (Scheme 2c). For example, 1d did not readily form a salt upon room-temperature treatment with acid or MeI However, under forcing conditions 1d did afford products resulting from N-methylation followed by I− displacement (Scheme 2c, R = Ph, see SI for details). Control reactions confirmed that both the bridged structure and the alkylating agent were necessary for this reaction. Comparison of twist parameters for the bicyclic and tricyclic lactams suggests that a twist angle of ≥50° is required for efficient N-protonation and N-alkylation of amide bonds8a, b although a twist angle of ca. 40° may be sufficient to result in N-alkylation under suitably vigorous conditions.
In summary, this work demonstrates that the N-protonation of amide bonds results in a dramatic increase of distortion around the N-C(O) bond. Furthermore, even moderately distorted amide bonds participate in electrophilic N-activation of amides. Studies on general activation of amide bonds are underway.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of General Medical Sciences (GM-49093). We thank Dr. Kevin Frankowski for helpful suggestions.
Footnotes
Supporting Information Available: Experimental details, characterization data for new compounds and .cif files of 1d, 3a–c. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Greenberg A, Breneman CM, Liebman JF. Amide Linkage: Selected Structural Aspects in Chemistry, Biochemistry, and Materials Science. Wiley; New York: 2000. [Google Scholar]
- 2.(a) Harrison RK, Stein RL. Biochemistry. 1990;29:1684–1689. doi: 10.1021/bi00459a003. [DOI] [PubMed] [Google Scholar]; (b) Liu J, Albers MW, Chen CM, Schreiber SL, Walsh CT. Proc Natl Acad Sci U S A. 1990;87:2304–2308. doi: 10.1073/pnas.87.6.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Fischer G. Chem Soc Rev. 2000;29:119–127. [Google Scholar]; (d) Cox C, Lectka T. Acc Chem Res. 2000;33:849–858. doi: 10.1021/ar990165g. [DOI] [PubMed] [Google Scholar]; (e) Eakin CM, Berman AJ, Miranker AD. Na Struct Mol Biol. 2006;13:202–208. doi: 10.1038/nsmb1068. [DOI] [PubMed] [Google Scholar]
- 3.(a) Williams A. J Am Chem Soc. 1976;98:5645–5651. [Google Scholar]; (b) Somayaji V, Brown RS. J Org Chem. 1986;51:2676–2686. [Google Scholar]; (c) Perrin CL. Acc Chem Res. 1989;22:268–275. [Google Scholar]; (d) Brown RS, Bennet AJ, Slebocka-Tilk H. Acc Chem Res. 1992;25:481–488. [Google Scholar]; (e) Mujika JI, Mercero JM, Lopez X. J Am Chem Soc. 2005;127:4445–4453. doi: 10.1021/ja044873v. [DOI] [PubMed] [Google Scholar]; (f) Mujika JI, Formoso E, Mercero JM, Lopez X. J Phys Chem B. 2006;110:15000–15011. doi: 10.1021/jp0604037. [DOI] [PubMed] [Google Scholar]
- 4.(a) Poland BW, Xu MQ, Quiocho FA. J Biol Chem. 2000;275:16408–16413. doi: 10.1074/jbc.275.22.16408. [DOI] [PubMed] [Google Scholar]; (b) Romanelli A, Shekhtman A, Cowburn D, Muir TW. Proc Natl Acad Sci U S A. 2004;101:6397–6402. doi: 10.1073/pnas.0306616101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shemella P, Pereira B, Zhang YM, Van Roey P, Belfort G, Garde S, Nayak SK. Biophys J. 2007;92:847–853. doi: 10.1529/biophysj.106.092049. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Johansson DGA, Wallin G, Sandberg A, Macao B, Aqvist J, Hard T. J Am Chem Soc. 2009;131:9475–9477. doi: 10.1021/ja9010817. [DOI] [PubMed] [Google Scholar]
- 5.(a) Shimada T, Nakamura I, Yamamoto Y. J Am Chem Soc. 2004;126:10546–10547. doi: 10.1021/ja047542r. [DOI] [PubMed] [Google Scholar]; (b) Ito M, Sakaguchi A, Kobayashi C, Ikariya T. J Am Chem Soc. 2007;129:290–291. doi: 10.1021/ja067777y. [DOI] [PubMed] [Google Scholar]; (c) Ueno S, Chatani N, Kakiuchi F. J Am Chem Soc. 2007;129:6098–6099. doi: 10.1021/ja0713431. [DOI] [PubMed] [Google Scholar]; (d) Kajita Y, Matsubara S, Kurahashi T. J Am Chem Soc. 2008;130:6058–6059. doi: 10.1021/ja7114426. [DOI] [PubMed] [Google Scholar]; (e) Yoshino Y, Kurahashi T, Matsubara S. J Am Chem Soc. 2009;131:7494–7495. doi: 10.1021/ja900805y. [DOI] [PubMed] [Google Scholar]
- 6.(a) Levkoeva EI, Nikitskaya ES, Yakhontov LN. Dokl Akad Nauk. 1970;192:342–345. [Google Scholar]; (b) Levkoeva EI, Nikitskaya ES, Yakhontov LN. Khim Geterotsikl Soedin. 1971:378–384. [Google Scholar]; (c) Pracejus H. Ber. 1959;92:988–998. [Google Scholar]; (d) Pracejus H. Ber. 1965;98:2897–2905. [Google Scholar]; (e) Pracejus H, Kehlen M, Kehlen H, Matschin H. Tetrahedron. 1965;21:2257–2270. [Google Scholar]; (f) Tani K, Stoltz BM. Nature. 2006;441:731–734. doi: 10.1038/nature04842. [DOI] [PubMed] [Google Scholar]; (g) Cox C, Wack H, Lectka T. Angew Chem, Int Ed. 1999;38:798–800. doi: 10.1002/(SICI)1521-3773(19990315)38:6<798::AID-ANIE798>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.(a) See, references 6f and 6g. (b) Note that Kirby et al. described protonated 2-azaadamantanone in its hydrated form. Kirby AJ, Komarov IV, Feeder N. J Chem Soc, Perkin Trans. 2001;2:522–529.
- 8.(a) Greenberg A, Venanzi CA. J Am Chem Soc. 1993;115:6951–6957. [Google Scholar]; (b) Greenberg A, Moore DT, DuBois TD. J Am Chem Soc. 1996;118:8658–8668. [Google Scholar]; (c) Laidig KE, Cameron LM. Can J Chem. 1993;71:872–879. [Google Scholar]; (d) Mo YR, Schleyer PV, Wu W, Lin MH, Zhang Q, Gao JL. J Phys Chem A. 2003;107:10011–10018. [Google Scholar]; (e) Kemnitz CR, Loewen MJ. J Am Chem Soc. 2007;129:2521–2528. doi: 10.1021/ja0663024. [DOI] [PubMed] [Google Scholar]
- 9.Yao L, Aubé J. J Am Chem Soc. 2007;129:2766–2767. doi: 10.1021/ja068919r.Szostak M, Yao L, Aubé J. Org Lett. 2009;11:4386–4389. doi: 10.1021/ol901771b.Szostak M, Yao L, Aubé J. J Org Chem. 2009;74:1869–1875. doi: 10.1021/jo802192v.Szostak M, Aubé J. Org Lett. 2009;11:3878–3881. doi: 10.1021/ol901449y.Lei Y, Wrobleski AD, Golden JE, Powell DR, Aubé J. J Am Chem Soc. 2005;127:4552–4553. doi: 10.1021/ja050214m.Szostak M, Aubé J. Chem Commun. 2009:7122–7124. doi: 10.1039/b917508c.Szostak M, Yao L, Aubé J. J Am Chem Soc. 2010;132:2078–2084. doi: 10.1021/ja909792h.For reviews on bridged amides, see: Hall HK, Elshekeil A. Chem Rev. 1983;83:549–555.Clayden J, Moran WJ. Angew Chem, Int Ed. 2006;45:7118–7120. doi: 10.1002/anie.200603016.
- 10.Winkler FK, Dunitz JD. J Mol Biol. 1971;59:169–182. doi: 10.1016/0022-2836(71)90419-0. [DOI] [PubMed] [Google Scholar]
- 11.Formation of the N-protonated amides is also evident from changes in the 13C NMR and IR spectra. See Table B in the SI for details.
- 12.To the best of our knowledge only three examples of isolated N-alkylated amides have been reported to date (Ref. 6a, 6e and 7b). In contrast to the current study, all of these lactams contained perfectly perpendicular amide bonds. For an elegant study on the methylation of quinuclidone derivatives, see: Werstiuk NH, Brown RS, Wang Q. Can J Chem. 1996;74:524–532.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.