Abstract
Background and Purpose
Prenatal glucocorticoids prevent germinal matrix hemorrhage in premature infants. The underlying mechanism, however, is elusive. Germinal matrix (GM) is enriched with angiogenic vessels exhibiting paucity of pericytes and GFAP-positive astrocye endfeet. Therefore, we asked whether glucocorticoid treatment would suppress angiogenesis, and enhance periendothelial coverage by pericytes and GFAP-positive endfeet in the GM microvasculature.
Methods
We treated pregnant rabbits with intramuscular betamethasone and delivered pups prematurely by C-section at E29 (Term=32d). Endothelial turnover, vascular density, pericyte coverage, GFAP-positive endfeet, cell-death and growth factors orchestrating angiogenesis, including vascular endothelial growth factor (VEGF), angiopoietins, transforming growth factor-β (TGF-β), platelet derived growth factor-B(PDGF-B), were compared between betamethasone-treated and untreated pups. Similar comparisons were done between autopsy-materials from premature infants exposed and unexposed to prenatal glucocorticoids.
Results
Antenatal glucocorticoid treatment reduced endothelial proliferation, vascular density and VEGF expression in the GM of both rabbits and humans. The pericyte coverage was greater in glucocorticoid-treated rabbit pups and human infants than in controls, but not the GFAP(+) endfeet coverage. TGF-β, but not angiopoietins and PDGF-B, were elevated in glucocorticoid-treated rabbit pups compared to controls. Betamethasone treatment induced apoptosis, neuronal degeneration and gliosis in rabbits pups. However, there was no evidence of increased cell-death in glucocorticoid-exposed human infants.
Conclusions
Prenatal glucocortiocoid suppresses VEGF and elevates TGF-β levels, which results in angiogenic inhibition, trimming of neovasculature and enhanced pericyte coverage. These changes contribute to stabilizing the GM vasculature, thereby reducing its propensity to hemorrhage. Prenatal glucocorticoid exposure does not induce neural cell-death in humans, unlike rabbits.
Keywords: Germinal matrix hemorrhage-intraventricular hemorrhage, glucocorticoids, betamethasone, pericyte, germinal matrix, vasculature, VEGF, TGF-β
Introduction
Prenatal glucocorticoids (GCs) prevent respiratory distress syndrome and intraventricular hemorrhage (IVH) in preterm infants.1, 2 Indeed, NIH Consensus Development Panel on the “Effect of corticosteroids for fetal maturation on perinatal outcomes” has recommended use of prenatal GC in preterm labor.3 In the USA, the preterm birth rate is 12.5%, and 75% of women in preterm labor with gestational age of 34 weeks or less are treated with GC.4 As ~13 million babies are born premature worldwide every year, a huge number of preterm infants (about 5–6 millions) are exposed to prenatal steroid. This increases their survival and reduces both the incidence and severity of IVH.5,6 Yet, the molecular mechanism by which GCs prevent IVH is elusive. Therefore, we asked how prenatal GC would reduce the incidence of IVH.
IVH typically initiates in the germinal matrix (GM). This periventricular region, located on the head of caudate nucleus and underneath ventricular ependyma, is a richly vascularized collection of neural precursor cells and is selectively vulnerable to hemorrhage. IVH is attributed to intrinsic fragility of the GM vasculature and disturbance in cerebral blood flow. Our previous work has shown that a rapid angiogenesis in the GM, induced by high vascular endothelial growth factor (VEGF) and angiopoietin (ANGPT)-2 levels, contributes to increased vascular fragility and vulnerability to hemorrhage, and that angiogenic inhibition reduces the occurrence of IVH in rabbit pup model.7 Furthermore, angiogenic vessels of the GM exhibit paucity of pericytes, deficiency of fibronectin in the basal lamina, and reduced perivascular coverage by GFAP(+) astrocyte endfeet.8–10 Hence, the fragility of GM microvasculature is attributed to immature basal lamina and reduced perivascular coverage by pericytes as well as GPAP(+) endfeet.
Prenatal GC--betamethasone and its stereo-isomer, dexamethasone--are used in preterm labor. They exhibit a wide range of pharmacological effects and toxicities on the brain of premature infants.11–13 However, little is known about the effects of GCs on the morphology and molecular components of the developing cerebral vasculature. The GC downregulates VEGF in an in vitro model of the blood brain barrier and cultured cells of various origin,14,15 and accordingly, the GC treatment effectively suppresses angiogenesis in various disease models.16, 17 The blockade of VEGF signaling prunes the nascent, immature and pericyte-deficient microvasculature of tumors.18 In addition, this remodels the remaining vasculature which results in less dilated blood vessels exhibiting enhanced pericyte coverage.18 Other than VEGF, growth factors angiopioetin-1, PDGF-B and TGF-β play key role in maturation of the vasculature, particularly in the assembly of pericytes around the immature blood vessels.19 Therefore, we hypothesized that prenatal GC would suppress angiogenesis by downregulation of angiogenic growth factors including VEGF and ANGPT-2, and enhance pericyte recruitment by inducing distinctive changes in the regulating growth factors--angiopioetin-1, PDGF-B and TGF-β.
There is increasing evidence that the GC treatment affect the phenotype and function of astrocytes. For example, the dexamethasone treatment in the astrocytes cultures and triamcinolone intravitreal injection in mice model of laser retinal photocoagulation enhance GFAP levels in the astrocytes.20,21 Importantly, high dose of dexamethasone and methylprednisolone induces apoptotic cell death in rats raising safety concerns with prenatal GC treatment.22 Thus, we postulated that prenatal GC treatment might mature the cerebral vasculature by increasing GFAP+ perivascular endfeet, but might cause undesirable adverse effects---neural cell death and gliosis.
Material and Methods
Animal experiment
Animal protocol was approved by Institutional Animal Care and Use Committee of New York Medical College, Valhalla, NY. We obtained 8 timed pregnant New Zealand rabbits from Charles River Laboratories (Wilmington, MA, USA). The rabbits were sequentially assigned to receive either intramuscular betamethasone (n=4) or saline (n=4). The dose of betamethasone in pregnant women is 12.5 mg once daily for 2 days; and average weight of pregnant women is about 60 kg.23 On this basis, we calculated a dose of 0.2 mg/kg (12.5/60= 0.2) daily for 2 days in pregnant rabbits. Thus, betamethasone (celestone; Schering Corporation, Kenilworth, NJ) was administered 0.2 mg/kg/dose every 24 hours on gestational day 27 and 28 for a total of 2 doses.
C-section was performed at day 29 of gestational age to deliver rabbit pups prematurely (term=32days). Pups were dried immediately and were kept warm in an infant incubator at 35°C. After stabilization of their conditions, they were weighed and fed with puppy formula (Esbilac, Petag, Hampshire, IL, USA). Pups were sacrificed at 3 epochs--2, 6 and 48 h of age. Brain was then dissected and cut into 2 mm coronal slices on brain matrix. All the histological evaluations were done from coronal sections taken at the level of midseptal nucleus. The comparison groups were balanced with respect to the body weight and gender of rabbit pups.
Laser capture microdissection (LCM)
LCM is described in Online Supplemental Methods.
Human tissue collection and processing
The Institutional Review Board of New York Medical College approved the use of human autopsy materials for this study. Women in preterm labor receive either betamethasone (12.5 mg once daily for 2 days) or dexamethasone (6 mg twice daily for 2 days) to prevent respiratory distress syndrome in premature infants. The preterm infants included in the present study delivered within few hours to 3 days after completion of GC treatment to their mothers; and infants died at 6–72h postnatal age (Supplementary table). The wall of cerebral hemisphere in fetuses consists of ventricular zone, subventricular zone, intermediate zone, cortical plate and marginal zone, as described by the Boulder Committee.24 In this study, we described intermediate zone embryonic white matter synonymously with white matter and cortex for cortical plate for the sake of simplicity of presentation. Brain samples were processed as described.8 About 2–3 mm thick coronal slices were taken at the level of thalamostriate groove from the frontal lobe. The coronal blocks included frontal lobe cortex, white matter and GM. The samples were fixed in 4% paraformaldehyde in phosphate buffer saline for 18 h and then were cryoprotected by immersing into 20% sucrose in PBS buffer. The tissues were frozen after embedding them into optimum cutting temperature compound. Frozen coronal blocks were cut into 15 μm sections using cryostat and saved at −80 °C until use.
Immunohistochemistry, Neuronal degeneration (Fluoro-Jade B), Fluorescent in situ detection of DNA fragmentation (TUNEL), Western Blot Analyses and Quantitative real-time polymerase chain reaction (PCR)
The techniques are illustrated in Online supplemental methods.
Quantification of vascular density, endothelial proliferation, cell death, pericyte and astrocyte coverage
We described in Supplemental methods.
Statistics and Analysis
To determine differences in the endothelial proliferation, vascular density, vessel area and cell degeneration between GC-exposed and unexposed human infants, two-way analysis of variance (ANOVA) with repeated measures was used. The repeated factor was applied to the three brain regions--cortex, white matter and germinal matrix. To assess differences in endothelial proliferation, vessel density, vessel area, pericyte coverage and growth factors in rabbit pups, two way ANOVA was used for each of the brain regions (cortex, white matter and germinal matrix) separately. The independent factors in two way ANOVA were--postnatal age (2h vs. 48h) and treatment (betamethasone vs. no treatment). All post-hoc comparisons to test for differences between means were done using Tukey multiple comparison test at 0.05 significance level. Student t-test was used to compare between 2 groups (Western blot analyses data).
Results
Glucocorticoids reduce endothelial proliferation in both rabbits and humans
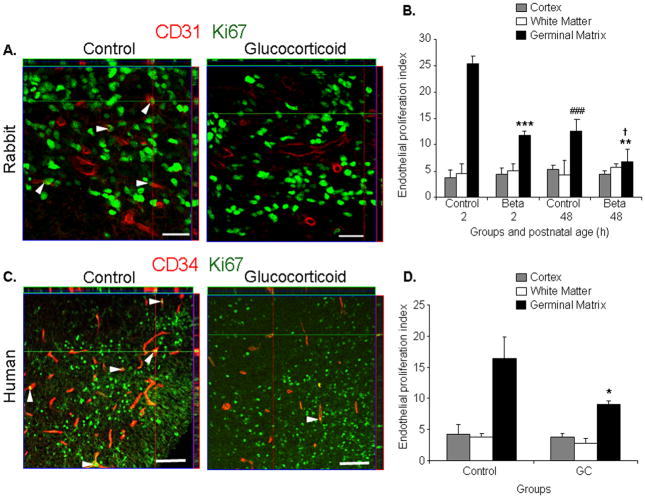
As GC suppresses angiogenesis in various disease models,16,17 we asked whether GC would reduce endothelial proliferation in the GM. To this end, we double labeled the brain sections with Ki67 (proliferation marker) and CD31 (endothelium in rabbit) or CD34 (endothelium in human) specific antibodies and evaluated x–z and y–z (orthogonal views) reconstruction of stacks of confocal images to verify Ki67 immunoreactivity embedded into CD34 (Fig.1A,C). The endothelial proliferation index was significantly lower in the GM of betamethasone-treated rabbit pups compared with untreated controls at both 2 and 48 h age (P<0.001 and 0.007, n=5 each group at each epoch, Fig. 1B). The endothelial proliferation in the GM was also less abundant at 48h age than at 2h age in both control and betamethasone-treated pups (P<0.001 and 0.017). The endothelial turnover in the cortex and white matter was significantly fewer relative to the GM at 2h age (data not shown).
Fig.1. GC suppresses endothelial proliferation.
A) Representative immunofluorescence of cryosections from E29 rabbit pups stained with Ki67 and CD31 antibodies. Note CD31 staining vessels with Ki67 signals indicating endothelial proliferation (arrowhead). Vessels with Ki67 labeling were fewer in the betamethasone-treated pups than controls. Above and right to the image are orthogonal views in x–z and y–z planes of a composite of z-stack of a series of confocal images taken 0.6μm apart. Scale bar 20μm. B) Data are mean±s.e.m (n=5 at each point). Endothelial proliferation was lower in betamethasone-treated pups than in controls at both 2 and 48 h age, (***P<0.001, **P<0.01); and this decreased at 48h than at 2h age in both control and treated pups (###P<0.001, †P<0.05). C) Cryosections from 23 week premature infants exposed and unexposed to GC labeled for Ki67 and CD34 antibodies. Note endothelial proliferation (Ki67 overlapping CD31, arrowhead) more frequent in GC-treated infants than in controls. Scale bar 50μm. D) Data are mean±s.e.m (n=5 at each point). Endothelial proliferation was less in GM of GC-treated infants relative to untreated controls (*P<0.05).
We next compared endothelial proliferation between human premature infants exposed and unexposed to prenatal GC betamethasone or dexamethasone (Supplementary Table1). The infants in the two groups were of comparable gestational (23–25 weeks) and postnatal age (<72h). Similar to rabbit pups, prenatal GC exposure significantly reduced the endothelial proliferation index in the GM (P=0.019, n=5 each, Fig.1D) of premature infants. In the cortex and white matter, the endothelial proliferation was significantly less compared with the GM in both GC-treated and untreated group (data not shown), and did not reduce on exposure to prenatal GC. Together, prenatal GC exposure diminished endothelial proliferation in the GM of both the premature rabbit pups and human infants.
Glucocorticoids prune the germinal matrix vasculature in both rabbits and humans
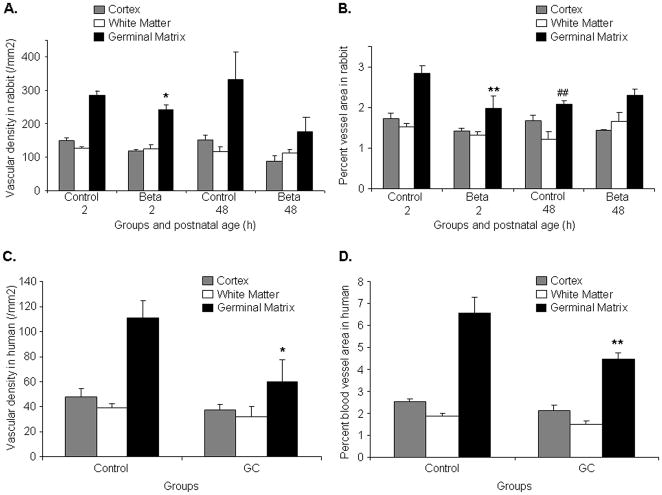
Because GC suppresses VEGF expression in the culture experiments14 and since VEGF inhibitors destroy the angiogenic vasculature in tumors,18 we compared vascular density and percent blood vessel area between coronal sections (midseptal nucleus level) of betamethasone-treated pups and untreated controls. We found that the vascular density was significantly reduced in the GM, but not in the cortex or white matter, of the GC-exposed rabbit pups compared with the unexposed controls at 2 age (P<0.05), but not at 48h age (Fig.2A). The percent area of the blood vessel profiles in the GM were also significantly less in betamethasone-treated pups at 2h age than in untreated controls (P<0.002), but not at 48h age (Fig.2B). The percent blood vessel area was also lesser at 48h than at 2h age among control pups, but not in betamethasone-exposed pups (P<0.004). In the cortex and white matter, the percent blood vessel area was significantly smaller than in the GM at 2h of age (Data not shown).
Fig.2. GC reduces vascularity of the germinal matrix.
A, B) Data are mean±s.e.m (n=5 at each point). Vascular density was less in the GM of GC-exposed pups compared with unexposed controls at 2h, but not at 48h age (*P<0.05 each). The percent blood vessel area in the GM was smaller in betamethasone-treated pups at 2h age than in untreated controls (**P<0.01), but not at 48 h age. The percent blood vessel area is also lesser at 48h age than at 2h age in control pups (##P<0.01). C,D) Data are mean±s.e.m (n=5 at each point). Both the vascular density and percent vessel area in the GM were less in GC-treated infants than in untreated control infants (*P<0.05,**P<0.01).Beta, betamethasone
Accordingly, in human premature infants, both the vascular density and percent blood vessel area in the GM were significantly less in the GC-treated infants than in untreated controls (P<0.04 and 0.008, Fig.2C,2D). In the cortex and white matter, these metrics were comparable between the treatment and control groups. Hence, GC trims the angiogenic GM vasculature in the premature rabbit pups and human infants.
Betamethasone suppresses VEGF, not ANGPT-2
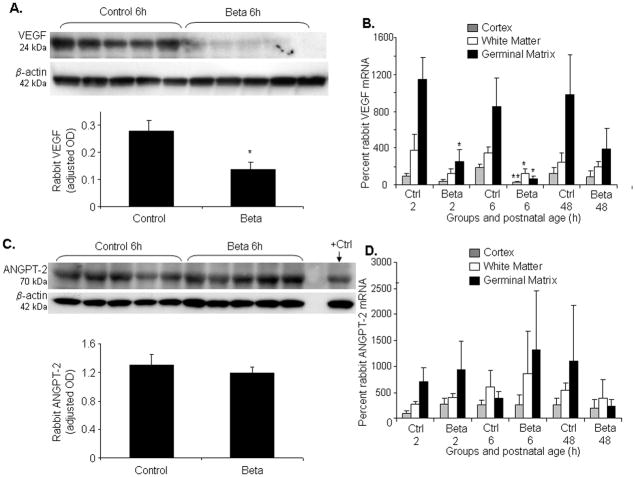
Since prenatal GC pruned GM neovasculature in our experiments, we asked whether prenatal betamethasone would suppress the angiogenic growth factors, VEGF and ANGPT-2, in the GM. To this end, we measured protein levels of these two growth factors in homogenates from a coronal slice taken at midseptal nucleus level; and we assayed mRNA expression in the laser dissected samples from the 3 brain regions--GM, cerebral cortex and white matter. Western blot analysis revealed that 24kDa VEGF was significantly less abundant in betamethasone-treated rabbit pups compared to untreated controls (P=0.04, Fig.3A). Accordingly, real-time PCR showed that betamethasone treatment reduced VEGF mRNA level in the GM (P=0.02, Fig.3B) but not in the cortex and white matter at 2h age. At 6h age, VEGF mRNA expression was significantly reduced in all the three brain regions cortex, white matter and GM of the betamethasone-treated pups compared to controls (P=0.01, 0.04 and 0.02 respectively). However, prenatal betamethasone treatment did not affect VEGF mRNA levels at 48h age in any of the brain regions. Importantly, ANGPT-2 protein and mRNA accumulation were similar in the treated and control pups (Fig. 3C,D). Together, betamethasone treatment suppressed VEGF, not ANGPT-2
Fig.3. Prenatal betamethasone downregulates VEGF, not ANGPT-2.
A) Representative Western blot analyses of VEGF from coronal slice of the forebrain. VEGF levels normalized for β actin. Data are mean±s.e.m (n=5 at each point). VEGF was significantly less abundant in betamethasone-treated rabbit pups compared with controls (*P<0.05). B) Data are mean ± s.e.m (n=5 at each point). Betamethasone treatment reduced VEGF mRNA level in the GM at both 2 and 6h age (*P<0.05 each). This also reduced VEGF level in the cortex and white matter at 6h age (**P=0.01,*P<0.05). C) Representative Western blot analyses of ANGPT-2 from coronal slice of the forebrain. ECV 304 cell lysate (Santa Cruz, CA, USA) was used as positive control. Data are mean±s.e.m (n=5 at each point). ANGPT-2 are similar between betamethasone-treated and control pups. D) Data are mean±s.e.m (n=5 at each point). Betamethasone did not affect ANGPT-2 mRNA levels.
Betamethasone enhances pericyte coverage in the germinal matrix vasculature
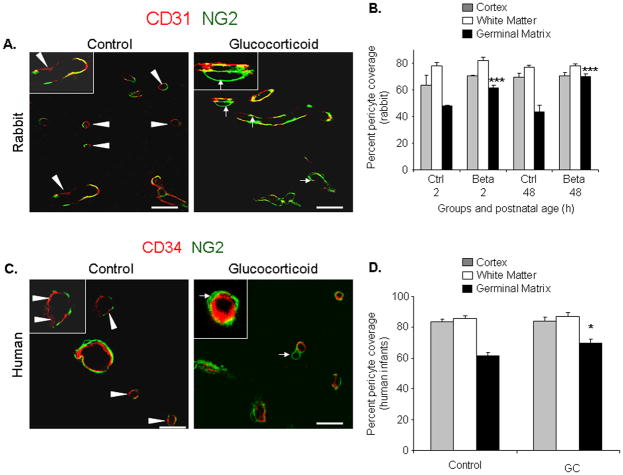
There is paucity of pericytes in the GM vasculature;8 and VEGF inhibition prunes the immature vessels lacking pericytes.18 Therefore, we postulated that betamethasone treatment would enhance pericyte coverage in the GM vasculature. Thus, we assessed coronal brain sections double labeled with NG2 (pericyte marker) and CD31 antibodies. In rabbits, we found that betamethasone enhanced pericyte vascular coverage at both 2 and 48h age (P<0.001each, Fig.4A,B) in the GM, but not in the cortex or white matter. However, the pericyte coverage remained significantly less in the GM than in the other brain regions in the treated pups.
Fig.4. Betamethasone enhances pericyte coverage.
A) Representative immunofluorescence of cryosections from GM of E29 rabbit pups stained with CD31 and NG2 antibodies. Note coverage of endothelia by NG2+ pericyte is interrupted (arrowhead) in untreated and relatively complete in treated pups (arrow). Inset, vessel under high magnification. B) Data are mean ± s.e.m (n=5 at each point). Betamethasone enhanced pericyte coverage at both 2 and 48 h age in the GM (***P=0.001each). C) Cryosections from 23 week premature infants stained for CD34 and NG2. Note relatively compete coverage in GC-treated pups. Inset, vessel under high magnification. D) Data are mean ± s.e.m (n=5 at each point). Coverage was greater in GC-treated pups than untreated controls (*P<0.05). Scale bar, 20μm (A,C).
We next evaluated pericyte coverage in human autopsy materials from premature infants and obtained similar findings as in rabbit pups. The pericyte coverage was higher in the GM of premature infants exposed to prenatal GC compared with untreated infants (P=0.016, Fig.4C,D). GC exposure did not affect pericyte coverage in the cortex and white matter vasculature. Collectively, GC treatment enhanced pericyte coverage in the GM vasculature.
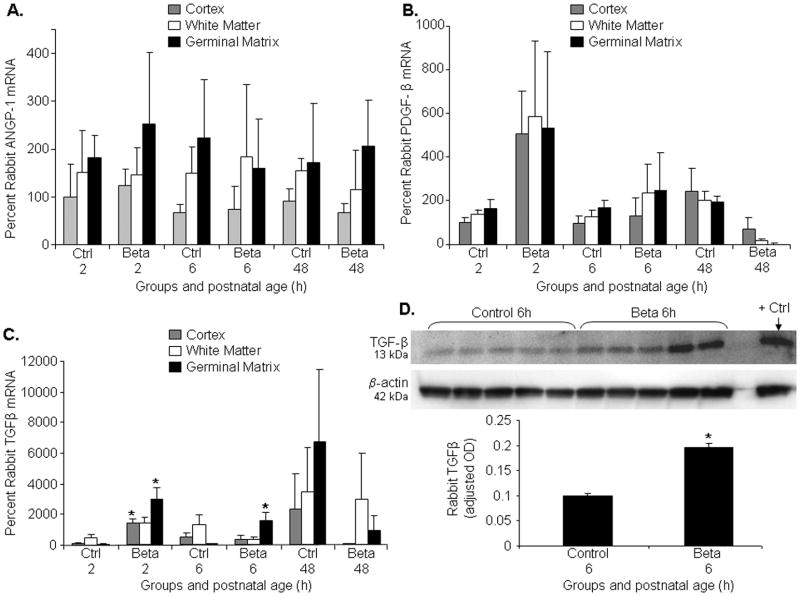
Betamethasone elevates TGF-β, but not ANGPT-1, PDGF-B levels
The ligand-receptor systems that recruit pericytes include, TGF-β, ANGPT-1, PDGF-B and their receptors. Since betamethasone treatment augmented pericyte coverage in the GM vasculature, we determined whether TGF-β, ANGPT-1, PDGF-B levels were higher in the GM of GC-treated pups compared to untreated controls. Real-time PCR showed that ANGPT-1 and PDGF-B levels were comparable between betamethasone-treated and control groups at all epochs (Fig. 5A,B). However, TGF-β mRNA expression was elevated in the rabbit GM exposed to betamethasone compared with unexposed controls at both 2 and 6h (P=0.04, 0.03), but not at 48h age. TGF-β was also higher in cortex of treated pups compared to controls at 2h (P<0.05), but not at 6 and 48h age. (Fig.5C). To confirm elevation in TGF-β levels, we measured its protein expression by Western blot analyses and found that TGF-β protein level was higher in betamethasone-treated rabbit pups compared with untreated controls (P<0.05; Fig.5D). Hence, betamethasone treatment upregulates TGF-β levels, but not ANGPT-1, PDGF-B expression.
Fig.5. Betamethasone elevates TGF-β, not ANGPT-1 or PDGF-B.
A,B,C) ANGPT-1 or PDGF-B and TGF-β assayed by real-time PCR in rabbit forebrain. Data are mean±s.e.m (n=5 at each point). There is no substantial difference in ANGPT-1 and PDGF-B between GC-treated pups and controls at any time point. TGF-β mRNA expression was elevated in the rabbit GM exposed to betamethasone- compared to unexposed controls at 2 and 6h age (*P<0.05 each), but not at 48h age. D) Representative Western blot analyses of TGF-β in the forebrain of GC-treated and control pups. Breast cancer tissue lysate was used as positive control. Data are mean±s.e.m (n=5 at each point). TGF-β levels normalized for β actin. TGF-β levels were higher in the betamethasone-treated pups than in controls (*P<0.05).
We also assessed the receptors of VEGF, ANGPT and PDGF-B by real-time PCR. We found no significant difference in mRNA expression of VEGFR2, Tie-2 and PDGFRβ receptors between betamethasone-treated and control groups (data not shown).
Betamethasone enhances GFAP-positive astrocytes in the germinal matrix
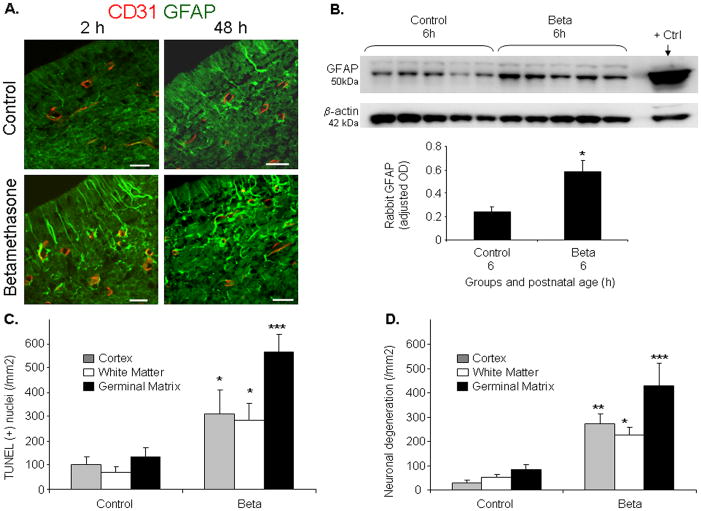
As GC treatment enhances GFAP in astrocytes,20,21 we assessed GFAP expression in the GM using immunohistochemistry. Immuolabeling revealed that GFAP-positive astrocytes were more abundant in the GM of betamethasone-treated pups compared to untreated controls at both 2 and 48h of age (Fig.6A). We next performed Western blot analyses on the homogenates from 1 mm thick brain slice at the level of midseptal nucleus. GFAP protein expression was significantly greater in the betamethasone-treated pups compared with untreated controls (P<0.05, n=5 in each group, Fig.6b).
Fig.6. Betamethasone induces cell death in rabbit pups.
A) Representative immunofluorescence of cryosections from GM of E29 rabbit pups stained with CD31 and GFAP antibodies. GFAP immunoreactivity was higher in betamethasone-treated pups compared to controls at both 2 and 48h age. Scale bar, 20μm. B) Representative Western blot analyses of GFAP from coronal slice of the forebrain. GFAP levels normalized for β actin. Rat brain lysate was used as positive control. Data are mean±s.e.m (n=5 at each point). GFAP levels were greater in betamethasone-treated pups than controls (*P<0.05). C, D) Data are mean±s.e.m (n=5 at each point). TUNEL- and Fluoro-Jade B positive cells were in significantly larger number in the GM, cortex and white matter in the betamethasone-treated pups than controls. *P<0.05,**P<0.01 and***P<0.001 for the comparison between GC-treated and control pups.
We then compared GFAP+ perivascular endfeet coverage in the GC-exposed and unexposed human infants. The percent GFAP+ astrocyte endfeet was 1.5 fold greater in the betamethasone-treated pups compared to untreated controls (32.5±9.6 vs. 20.2±4.3%). The difference, however, was not statistically significant. In conclusion, prenatal GC significantly enhances GFAP expression in the astrocytes of the rabbit GM, but not GFAP+ endfeet coverage in the GM of premature infants.
Betamethasone induces apoptosis, neuronal degeneration and growth retardation in rabbits
Because betamethasone treatment can induce cell death,22 we assessed apoptosis and neuronal degeneration in rabbit pups treated with prenatal betamethasone compared to untreated controls at 2h postnatal age. TUNEL-positive cells were more abundant in the GM, cortex and white matter of the treated pups compared with untreated controls (P< 0.001, 0.025, 0.026 respectively; Fig. 6 and Supplemental Fig.1). Accordingly, Fluoro-Jade B positive neurons were in larger number in the GM, cortex and white matter of pups treated with prenatal betamethasone compared with untreated controls (P=0.001,0.022,0.003, Fig.6D, Supplemental Fig. 1). We next assessed apoptosis and neuronal degeneration in human premature infants exposed and unexposed to antenatal GC. In contrast to rabbits, density of TUNEL-positive neural cells and Fluoro-Jade B positive neurons were remarkably comparable between the two groups of human infants (Supplemental Fig.1).
The betamethasone-treated pups were markedly smaller in weight (29.3±1.6 g vs. 47.1± 0.9 g; P<0.001, n=12 pups each) compared to untreated controls. Hence, prenatal GC induces cell death in premature rabbit pups, but not in preterm infants.
DISCUSSION
IVH is the most common neurological complication of prematurity affecting about 12,000 infants each year in the USA alone.25 Of note, prematurity rate is escalating;26 survival of premature infants has remarkably increased with advances in the medical care; and the IVH rate among preterm infants has remained almost stationary during the last 10 years.27 Thus, IVH and the attendant complications, including cerebral palsy, post-hemorrhagic hydrocephalus and cognitive deficits, have emerged as global health problems. No treatment of IVH is currently available. The only widely practiced preventive strategy is the use of prenatal GC in women in preterm labor, which reduces the occurrence of IVH in preterm infants by more than 50%.5 In this study, we determined the mechanistic basis of the use of prenatal GC to prevent IVH in preterm infants. We found that the prenatal GC suppressed angiogenesis, pruned the neovasculature, and enhanced pericyte coverage, thereby stabilizing the GM vasculature. We then observed that prenatal GC increased apoptotic neural cell death and neuronal degeneration in premature rabbit pups, but not in human premature infants.
In the present study, the GC suppressed VEGF expression in the forebrain, particularly in the GM; and accordingly, endothelial proliferation was diminished in the GM of GC-treated pups compared with untreated controls. Consistent with our findings, the GC downregulates VEGF in culture model of the blood brain barrier as well as in other cell types; and GC also suppresses tumor angiogenesis in animal models.14–17 Importantly, our previous study has shown that GM has high VEGF and angiopoietin-2 levels inducing rapid endothelial proliferation in the microvasculature, and that the suppression of VEGF by celecoxib, a COX-2 inhibitor, or ZD6474, a VEGFR2 blocker, minimizes both the incidence and severity of IVH.7 Hence, this is plausible that the prenatal GC confers protection against IVH by downregulation of VEGF and suppression of angiogenesis.
Another key finding in our study was that the GC treatment reduced vascular density and enhanced pericyte coverage in the GM microvasculature. Because angiogenic inhibitors suppress VEGF levels resulting in apoptosis of endothelial cells not protected by pericytes, the increase in pericyte coverage on GC exposure could be secondary to selective loss of neovasculature lacking in pericytes18. Importantly, trimming of GM vasculature might impair oxygenation in this brain region that could adversely affect its development. To determine an alternate mechanism that might augment pericyte coverage in the vasculature, we assessed levels of growth factors involved in pericyte recruitment. We observed elevation in TGF-β levels on GC treatment, but not in PDGF-B, angiopoietin or their receptors--Tie-2 and PDGFRβ. TGF-β promotes stabilization of the neovasculature by differentiation of pericytes from mesenchymal cells and by recruitment of pericytes around the angiogenic blood vessels.19 TGF-β is generally antiangiogenic, but could be proangiogenic in low concentration.19 Therefore, we speculate that elevation of TGF-β in the GM with GC exposure might assist in suppression of angiogenesis and contribute to pericyte recruitment in the GM vasculature.
Our previous work has shown that perivascular coverage by GFAP(+) end-feet was decreased in the GM compared with the cerebral cortex and white matter in premature infants 23–34 wk9. We expected that GC exposure will increase GFAP (+) endfeet in the GM. However, although GFAP (+) endfeet perivascular coverage tended to be elevated in the GM of infants exposed to GC, the difference was not statistically significant. These studies performed on human autopsy material of short postmortem interval are invaluable. Nevertheless, the limitations of such studies are exposure of infants to a number of prenatal and postnatal variables including mechanical ventilation, medications and others, which can potentially confound the results. We also found elevation in GFAP levels in the rabbit brain, demonstrated by immunolabeling and Western blot analyses. The increase in GFAP on GC treatment might be attributable to an elevation in TGF-β levels. Several other studies have also shown an elevation in GFAP levels in astrocytes on steroid treatment.20, 21 Together, GC treatment enhances GFAP levels in the astrocytes, but this elevation may not be significant in the perivascular endfeet.
Of note, we observed abundance of apoptotic neural cells and neuronal degeneration in the rabbit pups exposed to prenatal GC. High doses of dexamethasone and methylprednisolone also induce apoptotic cell death in hippocampal culture experiments and in rats and monkeys.22,28,29 The apoptosis is typically mediated by GC receptors via genomic or non genomic pathways, and these effects differ with respect to GC preparation, dose and duration of treatment as well as with the stage of neural cell maturation.29 In contrast to rabbit pups, cell death was comparable in premature infants exposed and unexposed to prenatal GC. Similar to humans, prenatal dexamethasone exposure does not affect neural cell death in ovine fetuses at 90% gestation.30 However, at 70% gestational age, prenatal dexamethasone reduces apoptosis and caspase activity in the ovine fetal cerebral cortex.30 This marked discrepancy in the effect of GC on cell death between the human, rabbit and sheep fetuses could be attributed to distinctive maturation and susceptibility of neural cells to GC as well as to the differences in the pharmacokinetics of GC among the species. All the mothers of the infants included in the present study completed the GC course within 72 hours of the delivery of their infants; and these premature infants died at 6–72 h postnatal age. Therefore, it is less likely that we missed the window of apoptotic cell death and neuronal degeneration following prenatal steroid treatment. In this context, it is important to link intrauterine cerebral development of rabbits to humans. The E29 rabbit pups (term=32d, E29=85–90% gestation) could be considered equivalent to 33 week premature infant. However, previous studies indicate that cortical and non-cortical development of E29 rabbits equates to ~20 weeks of gestational age in humans and myelination initiates in the early 3rd trimester in humans and at postnatal day 4–7 in rabbits.31 Thus, E29 pups might be similar to premature infants of 30±4 weeks gestational age.
This article presents the mechanistic basis of GC treatment in the prevention of IVH. Obtaining autopsy materials from premature infants of short postmortem interval with comparable demographics for the GC-treatment and control group was a result of our diligently made unremitting effort of several years. The infants in the two groups were of short postnatal age to reflect the effect of prenatal steroid. However, the limitations of human studies are exposure of premature infants to a number of pre- and post-natal variables including mechanical ventilation, exposure to medications and others that can potentially impact the data. The data in both rabbits and humans showed that GC augmented the perivascular pericyte coverage. However, despite the enhancement in pericyte coverage after GC-treatment, this remained less in the GM than in the other brain regions cerebral cortex and white matter. As pericytes are the providers of structural integrity to the vasculature, strategies to further enhance the pericyte coverage might offer greater protection against the development of IVH in premature infants.
In conclusion, prenatal GC suppressed VEGF levels and elevates TGF-β, which resulted in inhibition of angiogenesis, trimming of the neovasculature and enhancement in the pericyte coverage. These morphological and molecular changes would stabilize the GM vasculature, thereby reducing its vulnerability to hemorrhage. Prenatal glucocorticoid exposure did not induce neural cell death in premature human infants, unlike rabbit pups.
Supplementary Material
Supplemental Fig. 1: Prenatal GC induces cell death in rabbit pups, not in human infants. Representative TUNEL and Fluoro-Jade B staining of brain sections from premature rabbit pups (E29) and premature infants (23–24 week gestation) exposed (upper panel) and unexposed (lower panel) to prenatal GC. TUNEL(+) and Fluoro-Jade B(+) cells (arrowhead) were abundant in rabbit pups treated with prenatal GC, whereas these cells were scarce in pups unexposed to GC. In human infants, TUNEL(+) cells were scant and Fluoro-Jade B(+) cells were rare both in premature infants exposed and unexposed to prenatal GC. Scale bar, 50μm. Insets show high power view of TUNEL(+) and Fluoro-Jade B (+) cells in rabbit pups exposed to GC. Scale bar, 20μm.
Supplementary Table: Demographics of premature infants exposed and unexposed to GC.
Acknowledgments
American Heart Association grant-in-aid and NIH/NICHD HD061778 grant (PB). Authors thank Dr. Quihu Shi, PhD for statistical advice.
Footnotes
Conflict of interest: None
References
- 1.Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database Syst Rev. 2000:CD000065. doi: 10.1002/14651858.CD000065. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran S, Bauer CR, Bain R, Wright LL, Zachary J. Relationship between antenatal steroid administration and grades iii and iv intracranial hemorrhage in low birth weight infants. The NICHD neonatal research network. Am J Obstet Gynecol. 1995;173:305–312. doi: 10.1016/0002-9378(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 3.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH consensus development panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 4.Meadow WL, Bell A, Sunstein CR. Statistics, not memories: What was the standard of care for administering antenatal steroids to women in preterm labor between 1985 and 2000? Obstet Gynecol. 2003;102:356–362. doi: 10.1016/s0029-7844(03)00510-6. [DOI] [PubMed] [Google Scholar]
- 5.Elimian A, Garry D, Figueroa R, Spitzer A, Wiencek V, Quirk JG. Antenatal betamethasone compared with dexamethasone (betacode trial): A randomized controlled trial. Obstet Gynecol. 2007;110:26–30. doi: 10.1097/01.AOG.0000268281.36788.81. [DOI] [PubMed] [Google Scholar]
- 6.Sen S, Reghu A, Ferguson SD. Efficacy of a single dose of antenatal steroid in surfactant-treated babies under 31 weeks’ gestation. J Matern Fetal Neonatal Med. 2002;12:298–303. doi: 10.1080/jmf.12.5.298.303. [DOI] [PubMed] [Google Scholar]
- 7.Ballabh P, Xu H, Hu F, Braun A, Smith K, Rivera A, Lou N, Ungvari Z, Goldman SA, Csiszar A, Nedergaard M. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 2007;13:477–485. doi: 10.1038/nm1558. [DOI] [PubMed] [Google Scholar]
- 8.Braun A, Xu H, Hu F, Kocherlakota P, Siegel D, Chander P, Ungvari Z, Csiszar A, Nedergaard M, Ballabh P. Paucity of pericytes in germinal matrix vasculature of premature infants. J Neurosci. 2007;27:12012–12024. doi: 10.1523/JNEUROSCI.3281-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Khoury N, Braun A, Hu F, Pandey M, Nedergaard M, Lagamma EF, Ballabh P. Astrocyte end-feet in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr Res. 2006;59:673–679. doi: 10.1203/01.pdr.0000214975.85311.9c. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Hu F, Sado Y, Ninomiya Y, Borza DB, Ungvari Z, Lagamma EF, Csiszar A, Nedergaard M, Ballabh P. Maturational changes in laminin, fibronectin, collagen iv, and perlecan in germinal matrix, cortex, and white matter and effect of betamethasone. J Neurosci Res. 2008;86:1482–1500. doi: 10.1002/jnr.21618. [DOI] [PubMed] [Google Scholar]
- 11.Aghajafari F, Murphy K, Matthews S, Ohlsson A, Amankwah K, Hannah M. Repeated doses of antenatal corticosteroids in animals: A systematic review. Am J Obstet Gynecol. 2002;186:843–849. doi: 10.1067/mob.2002.121624. [DOI] [PubMed] [Google Scholar]
- 12.Scheepens A, van de Waarenburg M, van den Hove D, Blanco CE. A single course of prenatal betamethasone in the rat alters postnatal brain cell proliferation but not apoptosis. J Physiol. 2003;552:163–175. doi: 10.1113/jphysiol.2003.043414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang WL, Beazley LD, Quinlivan JA, Evans SF, Newnham JP, Dunlop SA. Effect of corticosteroids on brain growth in fetal sheep. Obstet Gynecol. 1999;94:213–218. doi: 10.1016/s0029-7844(99)00265-3. [DOI] [PubMed] [Google Scholar]
- 14.Kim H, Lee JM, Park JS, Jo SA, Kim YO, Kim CW, Jo I. Dexamethasone coordinately regulates angiopoietin-1 and vegf: A mechanism of glucocorticoid-induced stabilization of blood-brain barrier. Biochem Biophys Res Commun. 2008;372:243–248. doi: 10.1016/j.bbrc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Iwai A, Fujii Y, Kawakami S, Takazawa R, Kageyama Y, Yoshida MA, Kihara K. Down-regulation of vascular endothelial growth factor in renal cell carcinoma cells by glucocorticoids. Mol Cell Endocrinol. 2004;226:11–17. doi: 10.1016/j.mce.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Yano A, Fujii Y, Iwai A, Kageyama Y, Kihara K. Glucocorticoids suppress tumor angiogenesis and in vivo growth of prostate cancer cells. Clin Cancer Res. 2006;12:3003–3009. doi: 10.1158/1078-0432.CCR-05-2085. [DOI] [PubMed] [Google Scholar]
- 17.Kasselman LJ, Kintner J, Sideris A, Pasnikowski E, Krellman JW, Shah S, Rudge JS, Yancopoulos GD, Wiegand SJ, Croll SD. Dexamethasone treatment and icam-1 deficiency impair VEGF-induced angiogenesis in adult brain. J Vasc Res. 2007;44:283–291. doi: 10.1159/000101450. [DOI] [PubMed] [Google Scholar]
- 18.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 19.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 20.Dot C, Behar-Cohen F, BenEzra D, Doat M, Jonet L, May F, Jeanny JC. Influence of triamcinolone intravitreal injection on retinochoroidal healing processes. Exp Eye Res. 2007;84:1081–1089. doi: 10.1016/j.exer.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Avola R, Di Tullio MA, Fisichella A, Tayebati SK, Tomassoni D. Glial fibrillary acidic protein and vimentin expression is regulated by glucocorticoids and neurotrophic factors in primary rat astroglial cultures. Clin Exp Hypertens. 2004;26:323–333. doi: 10.1081/ceh-120034137. [DOI] [PubMed] [Google Scholar]
- 22.Duksal F, Kilic I, Tufan AC, Akdogan I. Effects of different corticosteroids on the brain weight and hippocampal neuronal loss in rats. Brain Res. 2009;1250:75–80. doi: 10.1016/j.brainres.2008.10.051. [DOI] [PubMed] [Google Scholar]
- 23.Lohninger AK, Bock P, Salzer H, Sevelda P, Lohninger AF. Antenatal betamethasone-dose-effects on fetal rat lung morphology and surfactant. J Perinat Med. 1994;22:319–328. doi: 10.1515/jpme.1994.22.4.319. [DOI] [PubMed] [Google Scholar]
- 24.Embryonic vertebrate central nervous system: Revised terminology. The boulder committee. Anat Rec. 1970;166:257–261. doi: 10.1002/ar.1091660214. [DOI] [PubMed] [Google Scholar]
- 25.Ballabh P. Intraventricular hemorrhage in premature infants: Mechanism of disease. Pediatr Res. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arias E, MacDorman MF, Strobino DM, Guyer B. Annual summary of vital statistics--2002. Pediatrics. 2003;112:1215–1230. doi: 10.1542/peds.112.6.1215. [DOI] [PubMed] [Google Scholar]
- 27.Jain NJ, Kruse LK, Demissie K, Khandelwal M. Impact of mode of delivery on neonatal complications: Trends between 1997 and 2005. J Matern Fetal Neonatal Med. 2009;22:491–500. doi: 10.1080/14767050902769982. [DOI] [PubMed] [Google Scholar]
- 28.Yu S, Patchev AV, Wu Y, Lu J, Holsboer F, Zhang JZ, Sousa N, Almeida OF. Depletion of the neural precursor cell pool by glucocorticoids. Ann Neurol. 2010;67:21–30. doi: 10.1002/ana.21812. [DOI] [PubMed] [Google Scholar]
- 29.Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, Holden J. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- 30.Malaeb SN, Hovanesian V, Sarasin MD, Hartmann SM, Sadowska GB, Stonestreet BS. Effects of maternal antenatal glucocorticoid treatment on apoptosis in the ovine fetal cerebral cortex. J Neurosci Res. 2009;87:179–189. doi: 10.1002/jnr.21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- 32.Georgiadis P, Xu H, Chua C, Hu F, Collins L, Huynh C, Lagamma EF, Ballabh P. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke. 2008;39:3378–3388. doi: 10.1161/STROKEAHA.107.510883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1: Prenatal GC induces cell death in rabbit pups, not in human infants. Representative TUNEL and Fluoro-Jade B staining of brain sections from premature rabbit pups (E29) and premature infants (23–24 week gestation) exposed (upper panel) and unexposed (lower panel) to prenatal GC. TUNEL(+) and Fluoro-Jade B(+) cells (arrowhead) were abundant in rabbit pups treated with prenatal GC, whereas these cells were scarce in pups unexposed to GC. In human infants, TUNEL(+) cells were scant and Fluoro-Jade B(+) cells were rare both in premature infants exposed and unexposed to prenatal GC. Scale bar, 50μm. Insets show high power view of TUNEL(+) and Fluoro-Jade B (+) cells in rabbit pups exposed to GC. Scale bar, 20μm.
Supplementary Table: Demographics of premature infants exposed and unexposed to GC.