Abstract
Coronary artery disease, cerebrovascular disease, pulmonary artery hypertension, and Alzheimer's disease all lead to substantial morbidity and mortality, and we currently lack effective treatments for these vascular diseases. Since the discovery, decades ago, that atherosclerotic lesions display clonal growth, atherosclerosis and other vascular diseases have been postulated to be neoplastic processes, arising by a series of critical somatic mutations. There is conflicting evidence to support this, but studies of DNA damage and mutagenesis, both genomic and mitochondrial, in atherosclerotic and vascular lesions, has yielded evidence that somatic mutations are involved in atherogenesis and vascular disease development. The roles of mitochondrial DNA damage, oxidative stress, and signaling by members of the transforming growth factor-beta receptor family are implicated. With the increasing convenience and cost-effectiveness of genome sequencing, it is feasible to continue to seek specific genetic targets in the pathogenesis of these devastating diseases, with the hope of developing personalized genomic medicine in the future.
Keywords: Somatic mutation, human genome project, atherosclerosis, pulmonary artery hypertension, mitochondrial DNA
Introduction
Ischemic heart disease is the leading cause of death worldwide, followed by cerebrovascular disease, and this is expected to continue in the upcoming decades (WHO health statistics). Both of these diseases are due to atherosclerosis, which is implicated as well in limb ischemia leading to infection and loss. Atherosclerosis is a complex process about which our understanding is continually evolving. Over time, atherosclerosis distorts normal luminal arterial anatomy: the wall thickens as a plaque forms, and this leads to luminal narrowing and decreased blood flow to the target organ. Meanwhile, plaque rupture may lead to distal embolic occlusion or the resultant ulceration may expose thrombogenic material in the plaque, leading to in situ thrombosis and acute ischemia. Plaques are thought to begin as low density lipoproteins (LDL) enter the intima, where they aggregate, become oxidized and are then taken up by macrophages. These lipid-laden macrophages, or “foam cells,” compose the grossly and microscopically visible fatty streak [1]. While traditionally considered to be a process related to ageing, atherosclerosis and its deadly complications occur also in younger people, and these early lesions may even be seen in infants [2] [3], which has been linked to maternal smoking [4]. This early stage lesion is clinically silent, but as smooth muscle cells (SMCs) secrete collagen and extracellular matrix components, fat retention increases. T cells are recruited and create an inflammatory environment, and remodeling occurs [1]. As the plaque progresses, this advanced lesion can potentially rupture [5].
The inciting event in atherogenesis has historically been hotly debated. For decades, the initiation of plaque formation was thought to be a response to injury to the arterial wall, leading subsequently to inflammation, the “response to injury” hypothesis [6]. However, starting with the discovery that SMCs are clonal in origin, a paradigm shift has occurred linking plaque formation to a neoplastic process; plaques may develop by a series of somatic mutations which allow selective and uncontrolled growth, while environmental and heritable factors may continue to play a role [7]. Meanwhile, the inflammatory nature of the atherosclerotic lesion has initiated considerable interest in the field of atherosclerosis research. There are many inflammatory, proliferative, and apoptotic mediators involved in atherogenesis, and investigations of evidence of DNA damage due to oxidative byproducts of inflammation have been myriad.
Thus, atherosclerosis is a complex multifocal arterial disease involving interactions of multiple genetic and environmental factors. Several current reviews have described many candidate genes, germline mutations, genetic polymorphisms and susceptibility genomic loci that have been identified to be associated with atherosclerosis [8–11]. However, there are no updated reviews on the impact of somatic mutations on the atherogenesis although many studies have been reported over many years. In this review, we focus on some of the critical somatic DNA damage and mutations at both genomic and mitochondrial levels that are involved in atherogenesis and vascular disease development. With advances in techniques of DNA sequencing and molecular genetics, it is feasible to continue to seek specific genetic targets in the pathogenesis of atherosclerosis-related diseases for the development of new strategies of disease prevention, diagnosis and treatment.
Vascular disease as a neoplastic process
It has been suggested that somatic mutations are involved in the development of vascular disease. Unlike mutations that occur in the germline, and are thus present in every cell, somatic mutations occur in an individual's genome at some point after development has begun. While a background level of genomic mutation may be present normally, a somatic mutation occurring in a gene that has an important role in the cell may lead to either cell death or to disease. As such, while somatic mutations may occur in every human being, it is the somatic mutation that results in a mutated viable cell that can potentially cause complications to the organism [12, 13].
The sequence by which somatic mutation leads to disease has been well established in carcinogenesis; however, a vast body of research has demonstrated that, in fact, atherogenesis and carcinogenesis share several important features. For one, atherosclerosis and cancer share several risk factors, including obesity, age, family history, and toxic exposures like cigarette smoking [14, 15] and even exposure to low-dose ionizing radiation, which has been known to cause cancer risk due to mutagenesis in DNA [12, 13].
Furthermore, chronic inflammation has been implicated in both atherogenesis and carcinogenesis. Importantly, pathways of oxidative stress and mitochondrial damage have been implicated, and in fact, the common risk factor of tobacco smoke is not only associated with elevated levels of reactive oxygen species (ROS), but with increased levels of mitochondrial DNA (mtDNA) damage in atherosclerotic aortic lesions in mice, especially when combined with alcohol consumption [16].
Our understanding of the role of DNA damage and somatic mutations in the development and potential treatment of vascular disease continues to evolve. Pulmonary artery hypertension (PAH) is a disease where, like in atherosclerosis, lesions that develop in arteries lead to insufficiency of vital target organs. In PAH, germline, heritable mutations have been described, yet there is increasing evidence of DNA damage and somatic mutation in lesions. Similarly, our understanding of the role of oxidative stress and mtDNA deletion in the pathogenesis of Alzheimer's disease (AD) holds promise for new therapeutic and diagnostic approaches to this devastating disease [17–19].
The role of smooth muscle cells and monoclonality in atherosclerotic plaques
In 1973, Benditt and Benditt challenged the conventional “response to injury” theory of atherogenesis in their paper “evidence for a monoclonal origin of human atherosclerotic plaques.” They investigated patterns of expression of glucose-6-phosphate dehydrogenase isoenzymes in females. As an X-linked enzyme, one isoenzyme is singularly expressed in each cell and its progeny, the gene encoding the other isoenzyme having been inactivated by X-chromosome inactivation. They found that atherosclerotic plaques demonstrated a “marked preponderance” or sole presence of one or the other cell type, unlike the patches of normal control arterial wall they sampled, where expression of the two isoenzymes was more balanced. Thus, the SMC proliferation in the plaques was clonal. This suggested that the cell capable of clonal proliferation in this situation had acquired a “selectional advantage,” likely through mutation secondary to viral infection or toxins; this would be supported by the fact that areas of high endothelial turnover, and thus increased proliferation, had been shown to be more prone to plaque development [7]. As such, their “monoclonal hypothesis” was really a “neoplastic hypothesis” [20].
However, while subsequent assays of an X-linked cytosine-adenine-guanine (CAG) repeat polymorphism in the human androgen receptor confirmed the previous findings that most atherosclerotic plaques contain a monoclonal population of SMCs, normal arterial wall was found to also demonstrate “relatively large clonal patches” both in the tunica media and in areas of diffuse intimal thickening (DIT). This raises the question of whether the monoclonality of atherosclerotic plaque SMCs is significant. While it is possible that the SMC proliferation occurs because a single cell mutates and becomes advantaged, the clonal SMCs might represent hyperplasia within a pre-existing clone. Specific assays for somatic mutations in atheromatous plaques would be needed [20, 21].
Meanwhile, Penn et al. demonstrated that DNA from atherosclerotic plaques, when transfected into a fibroblast cell line which was then injected into nude mice, led to tumorogenesis in the mice; furthermore, the tumors contained human DNA sequences. This evidence further supported the monoclonal hypothesis of atherogenesis [22] and further generated interest in the neoplastic aspects of atherogenesis. Further studies demonstrated that, in cultured SMCs from two human atherosclerotic plaques, the c-myc protooncogene implicated in proliferation and malignant transformation is overexpressed compared with healthy aortic SMCs, and plaque SMCs again possessed transforming capacity when injected into nude mice [23, 24]. Actually, much of the interest in a neoplastic theory of atherogenesis based on vascular SMC proliferation was initiated based on the similarities with the monoclonal nature of uterine leiomyomata in women, which are, like atherosclerosis, almost ubiquitous in adults [20]. Similar to how uterine leiomyoma have been found to have chromosomal rearrangements in the cells, chromosome 7 trisomy and tetrasomy have been observed in atherosclerotic plaques deemed “unstable” in adults [25], while these alterations were absent in infant and juvenile plaques [2]; however, no causal relationship has been established.
As mentioned, the clonality of plaque SMC does not necessarily signify pathologic growth. Monoclonal growth in other tissues besides plaques includes the SMC of the leiomyomata, normal arterial wall (as above) and in hair follicles and intestinal crypts. However, plaque SMCs do express a different phenotype than those of the tunica media. Significant gene expression differences exist for 208 genes, including proteases, protease inhibitors, extracellular matrix and matrix-modifying genes, and membrane adhesive genes between SMCs from the fibrous cap of atherosclerotic plaques and SMCs from the tunica media. As such, either a somatic mutational event, as in neoplasia, or a series of epigenetic differentiation events leads to the phenotypic switch [26]. Meanwhile, whether plaque SMCs differentiate due to a specific mutation is an area for further investigation.
Evidence for DNA damage in atherosclerotic plaques
The inflammatory nature of atherosclerotic lesions has generated considerable interest in the link between inflammation, mutagenesis and atherogenesis. While DNA damage, like background mutation levels, occurs with aging and normal physiology, there is evidence that the cells of atherosclerotic plaques contain increased levels of DNA damage relative to normal vascular tissue.
Several investigations have focused on myeloperoxidase, which is secreted by activated macrophages and is seen in association with lipid-laden macrophages in atherosclerotic lesions. This enzyme, in the presence of hydrogen peroxide, generates hypochlorous acid (HOCl), which in turn is a powerful chlorinating agent. 3-chlorotyrosine is a marker for LDL oxidation by myeloperoxidase, and in human atherosclerotic plaques, levels of both 3-chlorotyrosine bound to proteins and 3-chlorotyrosine in LDL were substantially higher compared with normal intima and circulating LDL, respectively, by a factor of 6 and 30, respectively [27].
Macrophage myeloperoxidase can also lead to production of 5-chlorouracil in vitro, which is detectable using gas chromatography/mass spectrometry. This production is enhanced 9-fold in the presence of nicotine, and inhibited by antioxidants such as GSH. In vivo, the 5-chlorouracil, after conversion to deoxynucleotide, may become incorporated into DNA, and can lead to mispairing with guanine, leading to GC to AT or AT to GC mutations; also, these chlorinated compounds may potentially have additional roles in modulating gene expression. This species has been found at increased rates in the intima and media of atherosclerotic lesions relative to normal aorta. [28]. However, again, while a correlation is clear, causality has been difficult to establish.
Markers of oxidative stress
Oxidative stress occurs in cells when the balance between antioxidants and oxidants is disrupted; even in normal cells, this can occur secondary to ROS produced by respiration and inflammation. This oxidative DNA damage, in turn, plays an important role in atherosclerosis [15]. When DNA is exposed to oxidants, damage to bases can occur, and the oxidation products, such as 7,8-dihydro-8-oxoguanine (8-oxoG) may be used as biomarkers for cellular stress [29]. Meanwhile, this base itself is mutagenic; it may mispair with adenine, leading to a G-to-T point mutation during replication. For this reason, an elaborate system of mismatch repair has evolved. Defects in the genes involved in the 8-oxoG repair pathway have been found in families with polypsosis and predisposition to colon cancer [29].
In comparison with SMCs from normal mammary arteries, the nuclear immunoreactivity for 3-oxo-DG has been found to be elevated by a factor of 55 in all cell types of atherosclerotic carotid artery plaques: macrophages, SMCs, and endothelial cells. DNA strand breaks were also elevated in plaques, and interestingly, strand breaks were localized in portions of the plaque that demonstrated oxidized lipid deposits. Also, DNA base excision repair enzymes such as Ref-1 and PARP-1 are upregulated in plaques [30], all convincing evidence that these mutagenic oxidative DNA species play a role in atherosclerosis. Meanwhile, evidence of increased oxidative stress, as determined by an elevated level of 8-oxoG have been found in circulating cells of patients with coronary artery disease (CAD), and this tended to correlate with the severity of disease [31].
When ROS interact with polyunsaturated fatty acids, lipid peroxidation (LPO) occurs. LPO-end products, such as 4-hydroxy-2-nonenal (HNE), can eventually react with DNA bases to form ethno-DNA adducts, or “etheno-bridged base modifications,” which are highly mutagenic species/miscoding lesions. These species have been implicated in carcinogenesis, have been found in association with a specific mutational hotspot in the p53 gene associated with cancer and, importantly, have also been implicated in reduction of cells' DNA repair capacity [32]. While they are themselves promutagenic, they are also markers for oxidative stress-derived DNA damage [33].
These adducts are also associated with chronic inflammation and are found in pancreases of patients with chronic pancreatitis, which again is in keeping with the inflammatory nature of atherosclerotic plaques [34]. They are also found in elevated levels in the livers of patients with chronic inflammatory conditions such as Wilson's disease and primary hemochromatosis and are also present at increased levels in the chronic inflammatory states of cirrhosis, which has a marked predisposition to carcinogenesis [33].
Meanwhile, these bulky aromatic DNA adducts are found in significantly higher levels in the SMCs of the tunica media of atherosclerotic lesions in human aortas than in normal controls [14, 34, 35]. Specifically, there are elevated levels of 1,N6–ethenodeoxyadenine (ε dA) and 3,N4–ethenodeoxycytosine (εdC), which are thought to arise from exposure polycyclic aromatic hydrocarbons in tobacco smoke and the environment. And, in fact, the level of the “bulky” DNA adducts was found to significantly correlated with the patient's smoking status [34]. It is not surprising to find that cigarette smoking contributes to lipid peroxidation considering the well-established link between atherosclerosis and smoking [14, 15].
Similar findings have been demonstrated in apolipoprotein E knockout (ApoE−/−) mice, which are deficient in apoE and prone to atherosclerosis regardless of diet; after treatment with benzo[a]pyrene (B[a]P), a genotoxic oxidant found in cigarette smoke and grilled meat and known to enhance atherosclerotic plaque progression in experimental animals, benzo[a]pyrene-diolepoxide (BPDE)-DNA and εdA levels were significantly higher in the aortas of ApoE−/− mice compared to unexposed controls [36]. Additionally, an increase in size of atherosclerotic lesions and inflammatory cell content of the lesions in ApoE−/− mice treated with the carcinogenic B[a]P are seen compared with no treatment [37]. However, there was no change in number or location, suggesting a promoting rather than initiating role, and in fact, subsequent investigations by the same group led them to conclude that there was no significantly increased levels of the DNA adducts in the aortas of young ApoE−/− mice before the development of atherosclerotic lesions. The levels actually tended to be lower, suggesting, rather than a causative, an early protective effect; this surprising theory was strengthened by the finding of overexpression of base excision repair enzymes in the plaques of the ApoE−/− mice [38].
Thus, like other markers of oxidative damage, the presence of polycyclic aromatic hydrocarbons-DNA adducts have been well established in atherogenesis without establishment of a convincing causative role [39]. In fact, by comparing B[a]p to the structural isoform benzo[e]pyrene (B[e]p), which is non-mutagenic and noncarcinogenic, it has been demonstrated that while exposure to both polycyclic aromatic hydrocarbons—B[e]p and B[a]p—leads to an increase in plaque size in ApoE−/− mice, along with a significant increase in inflammatory cells like T lymphocytes, DNA-adducts are not detectable in the B[e]p treated mice, suggesting the increase in atherogenesis was not mediated by polycyclic aromatic hydrocarbons-DNA adduct formation [39].
While it has not been proven that the adducts themselves lead to atherogenesis, the fact that DNA damage is significantly increased in atherosclerotic specimens has been well established. Another piece of evidence that supports this is the presence of micronuclei (MN). MN are small membrane bound DNA fragments in the cytoplasm of interphase cells, which may represent chromosome fragments resulting from double-strand breaks or entire chromosomes unable to migrate effectively. They are biomarkers for chromosomal damage and genome instability, and MN in the circulating lymphocytes of peripheral blood are predictive of increased cancer risk [40]. And, these levels appear to correlate with the severity of atherosclerotic CAD [41]. Baseline MN levels are significantly higher in patients with CAD who subsequently go on to have major adverse cardiac events, including death, myocardial infarction, need for revascularization procedures, stroke, or peripheral arterial occlusion disorder [42]. Such a “mutator-type” phenotype seems to increase the risk of atherosclerosis.
Microsatellite instability in atherosclerotic plaques
For over a decade, it has been known that atherosclerotic plaques demonstrate microsatellite instability (MSI) and loss of heterozygosity (LOH) [43, 44]. MSI occurs when a cell has defective post-replicative DNA mismatch repair; this leads to somatic deletions and insertions in “microsatellite” regions of the genome, which are stretches of repeating DNA, typically cytosines and adenines. These microsatellites can be highly polymorphic, and for this reason they are often used to genotype individuals or tumors [45]. Yet, within an individual, under normal conditions, a particular locus would be expected to maintain the same length throughout life; cells that display microsatellite instability have a “mutator phenotype:” a higher rate of mutagenesis.
In cancer, this phenotype predisposes to malignant transformation. Mutations in the mismatch repair genes human MutS homolog 2 (hMSH2) and hMLH1 are present in colon cancers that display MSI [45]. Meanwhile, several genes that are important in cell cycle and differentiation, including BAX and Transforming growth factor-beta type II receptor (TGFBR2), contain microsatellite regions within their coding regions that, when mutation due to defective mismatch repair leads to a frame shift, may lead to inactivation or defunctionalization of the gene; this may further contribute to tumorgenesis [46].
TGF-β1 has been postulated to be an antiproliferative factor in human atherosclerotic plaques, and acquired mutations in its receptor proposed as a contributing factor for localized unstable atheroma formation. In mice, interruption of TGF-β1 signaling has been shown to increase plaque inflammation and decrease fibrosis, characteristics which may indicate a less stable plaque phenotype [47]. Somatic mutations have been detected in the A10 microsatellite of the TGFBR2 in atherosclerotic plaques of human coronary arteries and carotid arteries after percutaneous and open surgical procedures, respectively [48]. One- and two-base pair deletions were identified within the A10 microsatellite region of the TGFBR2 in carotid artery plaques, which were absent in the DNA of normal, internal mammary arterial tissue from similar patients. This deletion led to a premature stop codon. Meanwhile, although this mutation has been described in colorectal cancer in association with mutated mismatch repair genes, in the plaques no mismatch repair mutation was found. Within a single carotid lesion, it was also noted that the overlying fibrous cap had a higher mutational rate than the corresponding media and plaque; this could then be traced into the “plaque core”, where an even higher mutation rate was observed. Thus, these acquired somatic mutations in the TGFBR2 leading to mutant cell proliferation were thought to be the cause of the monoclonal nature of some atherosclerotic lesions. And, the elegance of this argument was that the “instability can occur at a microsatellite region that offers a selective advantage to the cell: the Type II receptor for TGF-β1” [48].
A subsequent investigation by Clark et al., however, suggested that the mutation is not a major contributor to the pathogenesis of atherosclerosis. In an analysis of 22 coronary arteries and 9 aortas from patients with CAD and/or status post heart explantation for transplant, only one coronary artery sample showed a significant level of TGFBR2 polyA tract mutation. While the two studies did sample different arteries (carotid versus coronaries/aortas), the authors concluded that monoclonality of atherosclerotic plaques is unlikely to be due to mutations in the TGFBR, as the frequency of the mutation does not approach the frequency of the monoclonality seen in atheromas [49]. Furthermore, TGFBR2 has emerged as having an even more complex role in plaque formation and stability than being simply antiproliferative [50]. In fact, by knocking out the TGFBR2 in transgenic mice, with a T-cell specific promoter, atherosclerotic plaque size in the aortic sinus was seen to be decreased, suggesting T-cell specific TGF-β activity may actually contribute to plaque formation [51].
Other authors have investigated the presence of genomic rearrangements in atherosclerotic specimens by assessing for microsatellite mutations in DNA from endothelial cells and SMCs from aortic atheroma specimens compared to corresponding blood. If allelic imbalances were generated, and detected as loss of heterozygosity (LOH), this would indicate the presence of extensive rearrangements and may even indicate clonal expansion. In comparison with normal aortic control, a relationship between LOH at several loci and the atherosclerosis risk has been identified at 1p32-p31, 1q22-q25, 2q35 and 6p21.3, which have been mapped to vascular cell adhesion molecule-1 (VCAM-1), selectin E (SELE), aortic-preferentially-expressed gene-1 (APEG-1), and allograft inflammatory factor-1 (AIF-1), respectively. VCAM1 and SELE, expressed by cytokine-stimulated endothelial cells, are involved in leukocyte adhesion to the vessel wall, while APEG-1 is related to vascular SMC differentiation [52]. AIF1, meanwhile, has been implicated in atherosclerosis development when overexpressed in ApoE−/− mice, through a mechanism of enhanced macrophage phagocytotic activity as well as potential vascular SMC modulatory effects [53].
Mitochondrial DNA somatic mutations and atherosclerotic vascular disease
mtDNA has emerged as an area of interest in the study of somatic mutation in aging and age-related disease because of the inherent mutagenicity of the mitochondrial genome and its role in ROS production through oxidative phosphorylation. mtDNA is a maternally-derived, multicopy ring of double-stranded DNA which contains 37 genes encoding tRNA, rRNA, and respiratory chain enzymes. Translation occurs in the organelle; in addition, several other proteins of the respiratory chain as well as other maintenance agents are encoded by the nuclear genome and sorted accordingly [54]. Mitochondria lack the extensive DNA repair and protection systems that protect the nuclear genome [55]. Because of the large number of mitochondrial genome copies within each cell, it is the ratio of mutated to wild-type mtDNA that is a significant determinant of phenotype [56].
Somatic mutations in mtDNA have been implicated in the physiologic changes that occur with aging. In fact, a widely accepted theory of aging purports that a vicious cycle exists, whereby ROS normally produced by mitochondrial respiration cause oxidative damage to the mitochondria and cytosolic elements, leading to dysfunction and further production of ROS and an increase in mtDNA mutation [57]. Trifunovic et al. designed a homozygous “knock-in” mouse expressing a mutated DNA polymerase gamma with a consequently functionally defective proofreading function of the catalytic subunit. These “mtDNA mutator mice” accumulated substantially more somatic mutations in the mitochondrial genome than controls and did have increased evidence of degenerative changes associated with aging, as well as a reduced lifespan. However, in further experiments the production of ROS and evidence of increased oxidative damage in the mutator mice was underwhelming, suggesting that the aging seen in the mutator mice was due to somatic mtDNA damage, but was unlikely to be resulting from increased ROS production. Subsequently, they have shown that the increase in point mutations in mtDNA in the mtDNA mutator mice primarily affect protein coding genes, while protein synthesis itself continues unabated. The proteins implicated are the respiratory chain subunits, whose synthesis was affected by amino acid substitutions, leading to reduced steady-state levels of complex I, III, and IV and respiratory chain deficiency. This was thought to drive the premature aging [58–60].
The detection of a specific somatic mutation in the mtDNA of SMCs in the tunica media of atherosclerotic plaques has also been undertaken. The 4977bp “common deletion”, present in the mtDNA of patients with a variety of degenerative diseases, has been detected in the SMCs of atherosclerotic lesions of the aorta of patients undergoing surgery for aneurysm disease as well as diseased aortas from autopsy. However, while the mtDNA4977 deletion was consistently present in the mtDNA of the SMCs of each diseased aorta, it was present in low levels. There was no correlation between the deletion level of mtDNA4977 and the levels of adducts and oxidative damage to nuclear DNA for each patient, which had been previously determined. There was, however, a significant increase in mtDNA4977 in subjects older than 72 years versus younger than 72 [61].
The mtDNA4977 deletion has also been detected in the peripheral circulation of living patients with atherosclerosis. There was a significantly higher incidence of the mtDNA4977 deletion in the blood of patients with CAD undergoing angiography compared with healthy subjects. However, when the presence of mtDNA4977 in the SMCs of atherosclerotic plaques obtained during coronary endarterectomy of 23 patients was assessed, in contrast to the previous study, only 21.7 % of the lesions contained the deletion. The reason that the deletion was not substantially elevated was thought to be the nature of the cells themselves; as arterial wall cells, they did not have the energy demands/proliferation rate of cells such as cardiac myocytes, in which tissue the “common deletion” has been historically detected at higher frequency [61, 62]. However, a quantitative assay of overall mtDNA mutations in atherosclerotic lesions compared with normal intima revealed ten mutations in mtDNA associated with atherosclerotic lesions; of these, a G13513A substitution was found to be the one most frequently found in liprofibrosis plaques [63].
Diabetes mellitus is a well-known risk factor for the development of atherosclerotic cardiovascular and cerebrovascular disease. The A3243G mtDNA mutation is thought to be a genetic cause for diabetes when occurring in the germline, and has also been implicated in the MELAS syndrome (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes) [64]. In diabetics, the mutational rate of mtDNA has been observed to be four times higher than in healthy patients, which was duration-of diabetes-dependent rather than age dependent. Both hyperglycemia and hyperinsulinemia may contribute to this increased oxidative stress and mtDNA damage [64].
An examination of the intima-media thickness of the carotid artery, an indicator of atherosclerosis, compared to the rate of somatic A3243G mtDNA mutation revealed a positive relationship between the two, making the A3243G a potential marker for atherosclerosis in diabetics. Meanwhile, in healthy subjects, as predicted by previous studies, there is an age-related increase in mtDNA A3243 [65].
Cell senescence in atherosclerosis
Advanced age is a ubiquitous risk factor for almost every disease, and is a commonly cited shared risk for cancer and atherosclerosis. Like people, human cells age and have a finite lifespan. Senescence refers to the replicative limit of human cells; a senescent cell has entered terminal growth arrest and undergoes morphologic changes. In vascular cells, this may involve the decreased production of nitric oxide, which may lead to arterial hardening [66]. Also, senescent cells express increased activity of p53, a well known tumor suppressor gene, and in senescent SMCs of atherosclerotic plaques, p53 appears to be involved in the proinflammatory state, which involves ICAM-1 overexpression [67]. In this way, cellular senescence is thought to represent an innate anti-tumor defense in cells with damaged DNA which might otherwise undergo tumorgenesis. Just as atherosclerotic plaques display elevated levels of oxidative DNA damage, so are higher levels of DNA damage foci detectable in senescent cells [68]. Meanwhile, oxidative damage to DNA has been seen to induce senescence in vascular cells, which may be alleviated with antioxidant therapy or competent DNA repair system pathways [66]. Furthermore, human atherosclerotic plaque cells have been found to have a senescent phenotype based on expression of senescence-associated β-galactosidase (SAβG) and presence of shortened telomeres [68]
Telomeres and telomerase, meanwhile, are important players in our understanding of aging and senescence. Telomeres are the DNA-protein complexes located at the ends of every chromosome. Because DNA replication is semi-conservative, these telomeres, over time, are chipped away with each replication cycle. When they reach a certain shortness, cellular senescence is triggered [66]. This is thought to be mediated by p53 activation, and, as described above, represent a mechanism whereby the aging cell, which has undoubtedly accumulated DNA damage elsewhere on the chromosome as well, can avoid cell cycle dysregulation and oncogenesis [69]. This theory is supported by the fact that cancer tends to develop in proliferating tissues [70]. Telomerase, meanwhile, is a reverse transcriptase enzyme complex that can synthesize the repeating telomere sequences and prevent excessive erosion of telomeres with each subsequent replicative cycle. As such, telomeres and telomerase act in concert to determine the lifespan and entry into senescence of vascular cells, and as such a somatic mutation occurring in telomerase could potentially lead to detrimental premature aging phenotype and atherosclerosis. In fact, plaque SMCs in the fibrous cap of the lesion demonstrate markedly shorter telomeres than those in the media of the same lesion. Also, ROS, which as discussed may lead to atherogenesis through vascular cell DNA damage, can also promote the nuclear export of telomerase in endothelial cells, leading to telomere attrition and senescence, and ultimately atherogenesis [71, 72].
Mitochondrial DNA damage and vessel-mediated brain lesions
Alzheimer's Disease (AD) is a devastating cause of dementia that affects aging individuals worldwide. In addition to infarction, it is associated with vascular lesions such as cerebral amyloid angiopathy, cerebrovascular atherosclerosis, endothelial degeneration, and periventicular white matter lesions [18]. Oxidative damage from ROS leading to endothelial cell damage, neuronal damage, mtDNA mutation and atherosclerosis have been implicated in the pathogenesis of AD. Chronic hypoperfusion and sensitive brain tissue hypoxia is also implicated: as perfusion of glucose and oxygen decreases, metabolic changes occur that include increased oxidative stress and mitochondrial dysfunction, eventually leading to perterbations in the blood-brain barrier, further damage, and ultimately the anatomic pathology seen in association with AD: senile plaques and neurofibrillary tangles [17–19,66].
Clearly, oxidative DNA damage plays a role. In areas where vessel lesions are present, increased levels of 8-hydroxy-2-guanosine (8OHG), a marker for oxidative DNA damage, has been found [18]. Meanwhile, clusters of mitochondria-derived lysosomes and necrotic changes have been observed in the endothelial cells of brain microvessels [17]. Such abnormalities, associated with mitochondria derived lysosomes and lipofuscin, are also seen in damaged neurons in the brains of patients with AD [73]. Mitochondria in the cytoplasm, containing various lesions, including edema, membrane disruptions, and broken cristae [19] are not only themselves abnormal-appearing in the vessels and parenchymal cells, there has been evidence of LPO induced damage associated with injured mitochondria, as evidenced by increased levels of RNA oxidation products. These correlate to the degree of mitochondrial abnormalities [17]. Also, mtDNA deletions have been found in the endothelial cells of vessels with atherosclerotic lesions, in addition to perivascular cells and neurons [17, 19]. And, in an AD mouse model overexpressing a mutated form of the amyloid beta precursor protein (ABPP), there was a significantly increased amount of deleted mtDNA compared to controls; this assessment included an assay for the common deletion, which was 3-fold higher in the AD mice [17]. These mice also demonstrated an increased rate of atherosclerotic lesion formation, in which endothelial and perivascular cells contained mtDNA deletions [73].
Somatic mutations and pulmonary artery hypertension
Pulmonary Artery Hypertension (PAH) is a rare, often fatal disease, which is characterized by proliferation and remodeling. The intima and media are affected; intimal and medial cell proliferation is present, as well as muscularization of normally thin-walled structures. Peripheral vascular resistance is elevated, in part because of an imbalance and overproduction of endothelial-derived vasoconstrictors, but primarily because of the anatomic remodeling [74]. Vasoconstrictive elements, combined with vascular remodeling and phenotypic switching, appears to lead to medial smooth muscle hypertrophy which is found in all forms of PAH.
While the importance of the SMC changes is clear, the endothelial cell has emerged as the “key pathobiologic feature” of severe disease. These cells at times obliterate the lumen of pulmonary arteries as “the law of the endothelial monolayer” is broken, contributing to the disruption of pulmonary blood flow; on the other hand, in concentric lesions, medial hypertrophy predominates [75]. However, even as the endothelial cell is recognized as an important player in PAH, with the proclivity for clonal expansion, the role of the extracellular matrix in the balance between proliferative and apoptotic events is under investigation [76].
PAH is classified into idiopathic PAH (IPAH), heritable PAH (HPAH) and PAH associated with other conditions (APAH), such as collagen vascular disease, drugs, congenital heart disease or HIV. Historically, IPAH occurrs apparently sporadically; however, because germline mutations that are associated with familial forms of PAH have recently been identified as occurring in these apparently sporadic cases, the term HPAH is now used instead of “familial PAH” to include these patients; it describes patients who have a familial pattern of disease and/or germline mutations in the bone morphogenetic protein receptor type 2 (BMPR2) gene, activin receptor-like kinase 1 (ALK1) gene, or endoglin gene [77].
In the same way that the paradigm is shifting in the examination of atherosclerotic disease, it has been suggested that PAH might best be viewed as a “neoplastic process of angiogenesis” [78]. There is an obliteration of pulmonary arteries by conglomerates of cells, with tumorlike intraluminal growth, rather than a purely vasoconstrictive process. TGF-β signaling is implicated in the pathogenesis of PAH (the BMPR2 is a TGF-family receptor); again, TGF-β is an example of a mediator and controller of endothelial cell growth which is impaired in the pathogenesis of PAH [78].
In vitro studies of human pulmonary artery endothelial cells (HPAECs) from plexiform lesions in the lungs of patients with IPAH and HPH, accordingly, have shown phenotypic characteristics of “an aberrant apoptosis-resistant, highly proliferative, pulmonary vascular endothelium”, compared to HPAECs of healthy controls [79]. Also, the media and intima of pulmonary arteries in patients with PAH express the survivin gene, which is known to be an apoptosis-inhibiting gene expressed by most human cancer, but not normal tissue; survivin was absent in control pulmonary arteries [74]. This is further support for the model of PAH as a neoplastic process.
Like the SMCs of atherosclerotic plaques, the SMCs of IPAH have been observed to be monoclonal [75]. In an examination of patterns of X-chromosome methylation, using the human androgen receptor gene on the X-chromosome as a marker, it has been demonstrated that endothelial cell proliferation in plexiform lesions in patients with IPAH and HPAH are monoclonal, while the lesions of patients with APAH, or secondary PAH, are actually polyclonal [80]. This is further support for a model based on a succession of somatic mutations providing a growth advantage to a specific cell line, at least in IPAH and HPAH. SMCs in the lesions with medial hypertrophy, on the other hand, did not significantly demonstrate “unbalanced methylation”, highlighting again the importance of the endothelium in the pathogenesis of PAH, whose clonality strongly suggests a somatic genetic alteration [80]. Meanwhile, in a separate study of two patients who succumbed to PAH secondary to anorixigen use, an example of APAH, monoclonal expansion was found in the endothelial cells of the plexiform lesions as well [81].
The question, then, is whether specific somatic mutations are present in the plexiform lesions of PAH. The TGFBR2 has been of special interest due to the presence of microsatellites. In the BAT-26 region of the hMSH2 repair enzyme, which is itself a microsatellite, lesions from patients with IPAH had mutation, while none of the normal lungs or secondary PAH specimens demonstrated microsatellite instability there. Likewise, whole lung and kidney DNA from the same patients did not contain the mutation, confirming the somatic nature of the alteration in the hMSH2 repair gene microsatellite instability in the IPAH lung specimen [82].
The susceptibility of the TGFBR2 to microsatellite instability leading to premature stop codon and truncated protein has been discussed previously in this review. Yeager et al. found that 89% of the PAH lesions did not express TGFBR2, suggesting the possibility that these clonal lesions contained a nonsense mutation in the TGFBR2 gene, leading to decreased protein expression. Decreased expression of TGFBR2, which may serve important functions as a growth suppressor, may then allow tumor-like growth to proceed unchecked.
Somatic mutations have been described in BAX, a proapoptotic gene which displays MSI in colon cancer, conferring a growth advantage to mutated cells which can escape apoptosis. Again, mutation was present in PAH lesions as measured by microsatellite analysis, and all normal controls and secondary PAH lesions were wild type. All of these mutations were in the lesions of patients who had taken anorexigen drugs, but by the study group were characterized as PAH. Again, they convincingly demonstrated that the mutations present in these genes did not represent germline mutations or polymorphisms, as they were absent in the non-lesion tissues examined, including liver and kidney from affected patients with PAH [82].
Yeager concludes that similar to colon cancer, patients who demonstrate MSI may be more prone to somatic mutation and development of the plexiform endothelial lesions present in PAH [82]. However, in a subsequent study of patients with FPAH, evidence of MSI is only found in one sample out of 37 plexiform endothelial lesions. This was suspected to be due to a variation in study design, because their optimized DNA extraction protocol reduced artifact; however, it is noted that the subsequent study involved FPAH, with known BMPR2 mutations, while the study by Yeager et al. did not focus exclusively on FPAH; the fact that MSI is uncommon among FPAH does not necessarily contradict the previous observations [83].
The mutation most commonly associated with PAH is actually a germline mutation. Mutations in BMPR-2, a TGF-β family protein, have been implicated in both IPAH and HPAH, and over a hundred distinct mutations of all types have been detected [84]. Due to the low penetrance of PAH in patients with germline BMPR2 mutations, Machado et al. investigated the possibility that the heritable BMPR2 mutation leads to disease via a somatic mechanism in the other allele, acting as a “second hit” phenomenon. In this way, BMPR2 would actually act as a tumor suppressor gene [83]. A similar mechanism has been implicated in the pathogenesis of the rare, inherited venous anomaly syndrome known as mucocutaneous venous malformation, where a somatic “second hit” in the tyrosine endothelial kinase (TEK) gene encoding the endothelial cell tyrosine kinase receptor Tie2 has been found in lesions [85].
This “second hit” mechanism, however, does not appear to function in the pathogenesis of PAH. The DNA of microdissected vascular lesions of patients with FPAH and previously identified germline BMPR2 mutations was compared with normal lung parenchyma. Maintenance of heterozygosity at loci in the BMPR gene among patients with FPAH and controls was found; and, only one of seven FPAH specimens displayed microsatellite instability, suggesting a “second hit” was not present in the second BMPR2 allele [83]. Epigenetic factors could still play a role, however, such as hypermethylation of the BMPR2 promoter or somatic mutation of the promoter or other upstream factors; the authors had previously shown a reduction of the BMPR2 protein and mRNA in the lungs of patients with severe PAH which was most prominent in patients with two heterozygous germline mutations [83, 86]
TGF-β signaling molecule expression has been found to be reduced in the core endothelial cells of plexiform lesions, in contrast to preserved expression in the luminal endothelial cells, reinforcing the theory that loss of TGF-β signaling might contribute to disordered differentiation, endothelial proliferation and plexiform lesion formation [87]. Meanwhile, Hong et al. showed, in knockout mouse, that a homozygous Bmpr2 deletion, while increasing the susceptibility to development of clinical PAH, was in itself not sufficient to cause PAH, suggesting the importance of environmental factors and/or additional genetic hits in the development of the disease [88]. As the BMPR2 mutations appear to not be sufficient to cause disease, it is likely that multiple defects between SMCs and endothelial cells in the pulmonary artery combine to cause the disordered, dedifferentiated growth, like the “phenotypic switch” described in the SMC of atherosclerotic systemic arteries [89],[90].
Expert commentary
Somatic mutations are suspected to play a role in several vascular diseases, namely atherosclerosis, PAH, and AD. These specific pathologic lesions involve inflammation, disordered growth, and genomic damage, features reminiscent of neoplasia. While establishing causality between oxidative DNA damage and atherogenesis or plexiform lesion formation has been elusive, oxidative damage is nevertheless strongly implicated in the pathogenesis of atherosclerosis and cellular senescence, and the role of antioxidant therapy may prove important.
Throughout a review of the literature on this subject, multiple discrepancies exist, even dating back to the seminal work describing clonality of atherosclerotic plaques. Subsequent investigations have both challenged the importance of this finding, considering the presence of clonality in “normal” tissue. Likewise, while some researchers are able to identify specific mutations in important genes, for example, the mutations seen in the TGFBR2 in atherosclerotic plaque cells, other further investigations suggest a lower, less significant rate of these same mutations. Such findings permeate this field, and lead to ambiguity in our understanding of atherogenesis and disease development. It is encouraging that as our understanding of the human genome and techniques of DNA extraction and isolation from specific tissue fractions advance, we will be able to more precisely genotype diseased cells, leading to fewer of these discrepancies.
Many genes with germline mutations and/or genetic polymorphisms may be associated with atherosclerosis [8–11, 91–94] and cardiomyopathy [95, 96]. However, it is not known whether these germline predispositions contribute to somatic mutation occurence in the vascular cells, leading to vascular disease. Meanwhile, the frequency of somatic mutations in the vascular cells in the diseased vessel is not presently known. Thus, further sequence analyses and functional studies are warranted to address this important issue.
Meanwhile, the convenience and cost-effectiveness of sequencing of an individual's entire genome is quickly approaching levels that make it a realistic undertaking. A model of Personalized Genomic Medicine and Surgery (PGMS) has been proposed that involves a pathway of genomic profiling of individuals, identification of molecular targets involved in cancer, and use of molecular imaging, all of which would ultimately affect therapy with both standard therapies of chemotherapy and surgery. Meanwhile, novel therapies, using small molecule vehicles such as gene silencing, immunotherapy, viral therapy, and gene delivery systems, would emerge as cancer treatments through this model [97]. Considering the similarities to cancer between the pathogenesis of atherosclerosis and other vascular diseases that have been addressed in this review, a model of PGMS may be applicable to vascular disease as well.
Five-year view
The most important change that has occurred in the field of genetic research in recent years is the reduced time and cost involved in gene sequencing. We anticipate that in the upcoming years, it will be feasible to either sequence the entire genome of individual atherosclerotic plaque cells, or identify candidate genes for sequencing from various individuals' plaques compared to control genomic material from their own circulating cells in a reliable and reproducible manner. These genes will undoubtedly include genes involved in inflammation and signaling, such as those in the TGFBR family, as well as genes involved in replication and cell cycle, such as tumor suppressor p53 and telomerase. At our institution, a pipeline exists whereby a selected sequence, and all of its splice variants, can be used to generate a primer, which, after testing in cell lines, can be applied to tissue of interest. Specific alterations from the reference sequence are detected, and any discrepancy between the single-nucleotide polymorphism (SNP) analysis of a candidate gene found in a plaque and the sequence of that gene in the blood will represent a LOH or a somatic mutation. If rare SNPs are found in abundance in all genetic material of patients with atherosclerotic disease, these may represent germline “risk factors” for development of disease. Meanwhile, with the availability of programs that predict the functional consequences of specific mutations on the protein product, specific mutations will be targeted in functional studies using cell culture and mice models. Ultimately, once the molecular targets have been determined using this model, the goal will be translation of this information into clinical practice.
Key issues.
-
·
Atherosclerotic plaques and plexiform lesions in pulmonary artery hypertension display clonal growth.
-
·
Atherosclerotic plaques display evidence of increased oxidative stress and mutagenic DNA species relative to normal vessel tissue
-
·
Atherosclerotic plaques display microsatellite instability and loss of heterozygosity, evidence that defective DNA repair occurs in these regions
-
·
Mitochondrial DNA damage and mutagenesis is implicated in the formation of atherosclerotic lesions
-
·
Vessel lesions in Alzheimer's disease display evidence of oxidative DNA damage and mitochondrial DNA deletions
-
·
Incomplete penetrance of germline mutations known to be present in familial pulmonary artery hypertension may be due to coexisting somatic mutations
-
·
The advent of faster, less expensive DNA sequencing technology will enhance the ability to identify target genes affected by somatic mutations in vascular diseases.
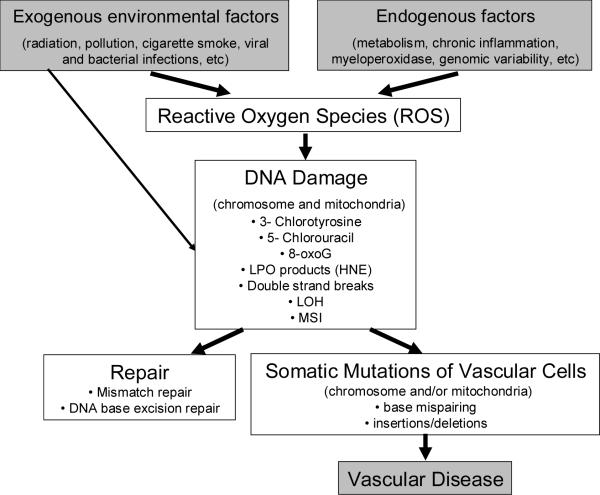
Figure 1.
Potential mechanisms of somatic mutation in vascular disease. Reactive Oxygen Species (ROS) occur in human cells due to toxic environmental exposures, such as radiation and exposure to compounds such as polycyclic aromatic hydrocarbons (PAH) and the carcinogenic benzo[a]pyrene (B[a]P) found in tobacco smoke. These species are also formed by normal metabolism and in states of increased production of oxidants, such as during chronic inflammation or in patients with genetic predisposition. These ROS can produce damage to DNA, and the resulting byproducts can be mutagenic. Production of 5-chlorouracil, in particular, when incorporated into DNA as a deoxynucleotide, leads to mispairing with Guanine and GC to AT or AT to GC mutation. Meanwhile, the oxidative byproduct of base damage, such as 8-oxoG, may itself mispair with adenine, leading to G-to-T mutation during replication. Meanwhile, ROS may interact with polyunsaturated fatty acids, and lipid peroxidation (LPO) may occur, this leads to buildup of endproducts such as 4-hydroxy-2-nonenal (HNE), which can lead to ethno-DNA base modifications which are highly mutagenic/miscoding. If the change is not repaired, a somatic mutation occurs. These somatic mutations, which may occur in mitochondrial or chromosomal DNA, may lead have deleterious effects on the organism. If they occur in a gene which is critical for cell survival, the cell may undergo apoptosis; however, should the cell be able to survive with the mutation, important changes in cell phenotype occur, depending on the cell type. Uncontrolled proliferation may lead to atherogenesis or vascular lesion formation.
Acknowledgments
Financial & competing interests disclosure This work was partially supported by grants (R01HL083471, T32HL083774, and K08HL076345) from the National Institutes of Health and by the Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, TX, USA.
Footnotes
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
Papers of special notes have been highlighted as:
* of interest
** considerable interest
- 1.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451(7181):904–13. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 2.Lavezzi AM, Ottaviani G, Matturri L. Biology of the smooth muscle cells in human atherosclerosis. Apmis. 2005;113(2):112–21. doi: 10.1111/j.1600-0463.2005.apm1130204.x. [DOI] [PubMed] [Google Scholar]
- 3.Milei J, Ottaviani G, Lavezzi AM, et al. Perinatal and infant early atherosclerotic coronary lesions. Can. J. Cardiol. 2008;24(2):137–41. doi: 10.1016/s0828-282x(08)70570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matturri L, Lavezzi AM, Ottaviani G, Rossi L. Intimal preatherosclerotic thickening of the coronary arteries in human fetuses of smoker mothers. J. Thromb. Haemost. 2003;1(10):2234–8. doi: 10.1046/j.1538-7836.2003.00409.x. [DOI] [PubMed] [Google Scholar]
- 5.Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler. Thromb. Vasc. Biol. 2000;20(5):1177–8. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 6.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J. Am. Coll. Cardiol. 2005;46(6):937–54. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 7.Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc. Natl. Acad. Sci. U S A. 1973;70(6):1753–6. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This seminal paper describes the monoclonal nature of atherosclerotic plaques and proposes the mechanism of somatic mutation in the development of these lesions.
- 8.Roy H, Bhardwaj S, Yla-Herttuala S. Molecular genetics of atherosclerosis. Hum. Genet. 2009;125(5–6):467–91. doi: 10.1007/s00439-009-0654-5. [DOI] [PubMed] [Google Scholar]
- 9.Andreassi MG. Metabolic syndrome, diabetes and atherosclerosis: influence of gene-environment interaction. Mutat. Res. 2009;667(1–2):35–43. doi: 10.1016/j.mrfmmm.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Hamsten A, Eriksson P. Identifying the susceptibility genes for coronary artery disease: from hyperbole through doubt to cautious optimism. J. Intern. Med. 2008;263(5):538–52. doi: 10.1111/j.1365-2796.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JL, Carlquist JF, Horne BD, Hopkins PN. Progress in unraveling the genetics of coronary artery disease and myocardial infarction. Curr. Atheroscler. Rep. 2007;9(3):179–86. doi: 10.1007/s11883-007-0017-4. [DOI] [PubMed] [Google Scholar]
- 12.Little MP, Tawn EJ, Tzoulaki I, et al. A systematic review of epidemiological associations between low and moderate doses of ionizing radiation and late cardiovascular effects, and their possible mechanisms. Radiat. Res. 2008;169(1):99–109. doi: 10.1667/RR1070.1. [DOI] [PubMed] [Google Scholar]
- 13.Little MP, Tawn EJ, Tzoulaki I, et al. Review and meta-analysis of epidemiological associations between low/moderate doses of ionizing radiation and circulatory disease risks, and their possible mechanisms. Radiat. Environ. Biophys. 2009 doi: 10.1007/s00411-009-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binkova B, Strejc P, Boubelik O, et al. DNA adducts and human atherosclerotic lesions. Int. J. Hyg. Environ. Health. 2001;204(1):49–54. doi: 10.1078/1438-4639-00072. [DOI] [PubMed] [Google Scholar]
- 15.Izzotti A, Piana A, Minniti G, et al. Survival of atherosclerotic patients as related to oxidative stress and gene polymorphisms. Mutat. Res. 2007;621(1–2):119–28. doi: 10.1016/j.mrfmmm.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Cakir Y, Yang Z, Knight CA, et al. Effect of alcohol and tobacco smoke on mtDNA damage and atherogenesis. Free Radic. Biol. Med. 2007;43(9):1279–88. doi: 10.1016/j.freeradbiomed.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Aliev G, Gasimov E, Obrenovich ME, et al. Atherosclerotic lesions and mitochondria DNA deletions in brain microvessels: implication in the pathogenesis of Alzheimer's disease. Vasc. Health. Risk Manag. 2008;4(3):721–30. doi: 10.2147/vhrm.s2608. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This group of authors likens the development of Alzheimer's Disease to atherosclerotic lesions formation, ischemia, and local oxidative stress; they describe mitochondrial damage and mtDNA deletions.
- 18.Aliev G, Seyidova D, Lamb BT, et al. Mitochondria and vascular lesions as a central target for the development of Alzheimer's disease and Alzheimer disease-like pathology in transgenic mice. Neurol. Res. 2003;25(6):665–74. doi: 10.1179/016164103101201977. [DOI] [PubMed] [Google Scholar]
- 19.Aliev G, Seyidova D, Neal ML, et al. Atherosclerotic lesions and mitochondria DNA deletions in brain microvessels as a central target for the development of human AD and AD-like pathology in aged transgenic mice. Ann. N. Y. Acad. Sci. 2002;977:45–64. doi: 10.1111/j.1749-6632.2002.tb04798.x. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz SM, Murry CE. Proliferation and the monoclonal origins of atherosclerotic lesions. Annu. Rev. Med. 1998;49:437–60. doi: 10.1146/annurev.med.49.1.437. [DOI] [PubMed] [Google Scholar]
- 21.Chung IM, Schwartz SM, Murry CE. Clonal architecture of normal and atherosclerotic aorta: implications for atherogenesis and vascular development. Am. J. Pathol. 1998;152(4):913–23. [PMC free article] [PubMed] [Google Scholar]
- 22.Penn A, Garte SJ, Warren L, Nesta D, Mindich B. Transforming gene in human atherosclerotic plaque DNA. Proc Natl. Acad. Sci. U. S. A. 1986;83(20):7951–5. doi: 10.1073/pnas.83.20.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Describes how genetic material obtained from atherosclerotic plaques led to tumor formation when injected into mice.
- 23.Parkes JL, Cardell RR, Hubbard FC, Jr., et al. Cultured human atherosclerotic plaque smooth muscle cells retain transforming potential and display enhanced expression of the myc protooncogene. Am. J. Pathol. 1991;138(3):765–75. [PMC free article] [PubMed] [Google Scholar]
- 24.Napoli C, Lerman LO, de Nigris F, Sica V. c-Myc oncoprotein: a dual pathogenic role in neoplasia and cardiovascular diseases? Neoplasia. 2002;4(3):185–90. doi: 10.1038/sj.neo.7900232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matturri L, Cazzullo A, Turconi P, et al. Chromosomal alterations in atherosclerotic plaques. Atherosclerosis. 2001;154(3):755–61. doi: 10.1016/s0021-9150(00)00488-3. [DOI] [PubMed] [Google Scholar]
- 26.Mulvihill ER, Jaeger J, Sengupta R, et al. Atherosclerotic plaque smooth muscle cells have a distinct phenotype. Arterioscler. Thromb. Vasc. Biol. 2004;24(7):1283–9. doi: 10.1161/01.ATV.0000132401.12275.0c. [DOI] [PubMed] [Google Scholar]
- 27.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Invest. 1997;99(9):2075–81. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeshita J, Byun J, Nhan TQ, et al. Myeloperoxidase generates 5-chlorouracil in human atherosclerotic tissue: a potential pathway for somatic mutagenesis by macrophages. J. Biol. Chem. 2006;281(6):3096–104. doi: 10.1074/jbc.M509236200. [DOI] [PubMed] [Google Scholar]
- 29.David SS, O'Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447(7147):941–50. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106(8):927–32. doi: 10.1161/01.cir.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- 31.Botto N, Masetti S, Petrozzi L, et al. Elevated levels of oxidative DNA damage in patients with coronary artery disease. Coron. Artery Dis. 2002;13(5):269–74. doi: 10.1097/00019501-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Feng Z, Hu W, Tang MS. Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells: a possible mechanism for lipid peroxidation-induced carcinogenesis. Proc. Natl. Acad. Sci. U. S. A. 2004;101(23):8598–602. doi: 10.1073/pnas.0402794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartsch H, Nair J. Oxidative stress and lipid peroxidation-derived DNA-lesions in inflammation driven carcinogenesis. Cancer Detect. Prev. 2004;28(6):385–91. doi: 10.1016/j.cdp.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Nair J, De Flora S, Izzotti A, Bartsch H. Lipid peroxidation-derived etheno-DNA adducts in human atherosclerotic lesions. Mutat. Res. 2007;621(1–2):95–105. doi: 10.1016/j.mrfmmm.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Binkova B, Smerhovsky Z, Strejc P, et al. DNA-adducts and atherosclerosis: a study of accidental and sudden death males in the Czech Republic. Mutat. Res. 2002;501(1–2):115–28. doi: 10.1016/s0027-5107(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 36.Godschalk R, Curfs D, Bartsch H, Van Schooten FJ, Nair J. Benzo[a]pyrene enhances lipid peroxidation induced DNA damage in aorta of apolipoprotein E knockout mice. Free Radic. Res. 2003;37(12):1299–305. doi: 10.1080/10715760310001621333. [DOI] [PubMed] [Google Scholar]
- 37.Curfs DM, Lutgens E, Gijbels MJ, et al. Chronic exposure to the carcinogenic compound benzo[a]pyrene induces larger and phenotypically different atherosclerotic plaques in ApoE-knockout mice. Am. J. Pathol. 2004;164(1):101–8. doi: 10.1016/S0002-9440(10)63101-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godschalk RW, Albrecht C, Curfs DM, et al. Decreased levels of lipid peroxidation-induced DNA damage in the onset of atherogenesis in apolipoprotein E deficient mice. Mutat. Res. 2007;621(1–2):87–94. doi: 10.1016/j.mrfmmm.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Curfs DM, Knaapen AM, Pachen DM, et al. Polycyclic aromatic hydrocarbons induce an inflammatory atherosclerotic plaque phenotype irrespective of their DNA binding properties. Faseb J. 2005;19(10):1290–2. doi: 10.1096/fj.04-2269fje. [DOI] [PubMed] [Google Scholar]
- 40.Iarmarcovai G, Bonassi S, Botta A, Baan RA, Orsiere T. Genetic polymorphisms and micronucleus formation: a review of the literature. Mutat. Res. 2008;658(3):215–33. doi: 10.1016/j.mrrev.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Botto N, Rizza A, Colombo MG, et al. Evidence for DNA damage in patients with coronary artery disease. Mutat. Res. 2001;493(1–2):23–30. doi: 10.1016/s1383-5718(01)00162-0. [DOI] [PubMed] [Google Scholar]
- 42.Federici C, Botto N, Manfredi S, et al. Relation of increased chromosomal damage to future adverse cardiac events in patients with known coronary artery disease. Am. J. Cardiol. 2008;102(10):1296–300. doi: 10.1016/j.amjcard.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 43.Hatzistamou J, Kiaris H, Ergazaki M, Spandidos DA. Loss of heterozygosity and microsatellite instability in human atherosclerotic plaques. Biochem. Biophys. Res. Commun. 1996;225(1):186–90. doi: 10.1006/bbrc.1996.1151. [DOI] [PubMed] [Google Scholar]
- 44.Spandidos DA, Ergazaki M, Arvanitis D, Kiaris H. Microsatellite instability in human atherosclerotic plaques. Biochem Biophys Res Commun. 1996;220(1):137–40. doi: 10.1006/bbrc.1996.0370. [DOI] [PubMed] [Google Scholar]
- 45.Laghi L, Bianchi P, Malesci A. Differences and evolution of the methods for the assessment of microsatellite instability. Oncogene. 2008;27(49):6313–21. doi: 10.1038/onc.2008.217. [DOI] [PubMed] [Google Scholar]
- 46.de la Chapelle A. Microsatellite instability. N. Engl. J. Med. 2003;349(3):209–10. doi: 10.1056/NEJMp038099. [DOI] [PubMed] [Google Scholar]
- 47.Lutgens E, Gijbels M, Smook M, et al. Transforming growth factor-beta mediates balance between inflammation and fibrosis during plaque progression. Arterioscler. Thromb. Vasc. Biol. 2002;22(6):975–82. doi: 10.1161/01.atv.0000019729.39500.2f. [DOI] [PubMed] [Google Scholar]
- 48.McCaffrey TA, Du B, Consigli S, et al. Genomic instability in the type II TGF-beta1 receptor gene in atherosclerotic and restenotic vascular cells. J. Clin. Invest. 1997;100(9):2182–8. doi: 10.1172/JCI119754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark KJ, Cary NR, Grace AA, Metcalfe JC. Microsatellite mutation of type II transforming growth factor-beta receptor is rare in atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2001;21(4):555–9. doi: 10.1161/01.atv.21.4.555. [DOI] [PubMed] [Google Scholar]
- 50.Singh NN, Ramji DP. The role of transforming growth factor-beta in atherosclerosis. Cytokine Growth Factor Rev. 2006;17(6):487–99. doi: 10.1016/j.cytogfr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Gojova A, Brun V, Esposito B, et al. Specific abrogation of transforming growth factor-beta signaling in T cells alters atherosclerotic lesion size and composition in mice. Blood. 2003;102(12):4052–8. doi: 10.1182/blood-2003-05-1729. [DOI] [PubMed] [Google Scholar]
- 52.Arvanitis DA, Flouris GA, Spandidos DA. Genomic rearrangements on VCAM1, SELE, APEG1and AIF1 loci in atherosclerosis. J. Cell. Mol. Med. 2005;9(1):153–9. doi: 10.1111/j.1582-4934.2005.tb00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mishima T, Iwabuchi K, Fujii S, et al. Allograft inflammatory factor-1 augments macrophage phagocytotic activity and accelerates the progression of atherosclerosis in ApoE−/− mice. Int. J. Mol. Med. 2008;21(2):181–7. [PubMed] [Google Scholar]
- 54.Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 2005;6(5):389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu J, Sharma LK, Bai Y. Implications of mitochondrial DNA mutations and mitochondrial dysfunction in tumorigenesis. Cell Res. 2009;19(7):802–15. doi: 10.1038/cr.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krishnan KJ, Reeve AK, Samuels DC, et al. What causes mitochondrial DNA deletions in human cells? Nat. Genet. 2008;40(3):275–9. doi: 10.1038/ng.f.94. [DOI] [PubMed] [Google Scholar]
- 57.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ. Res. 2007;100(4):460–73. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 58.Edgar D, Shabalina I, Camara Y, et al. Random point mutations with major effects on protein-coding genes are the driving force behind premature aging in mtDNA mutator mice. Cell Metab. 2009;10(2):131–8. doi: 10.1016/j.cmet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Trifunovic A, Hansson A, Wredenberg A, et al. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc. Natl. Acad. Sci. U. S. A. 2005;102(50):17993–8. doi: 10.1073/pnas.0508886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429(6990):417–23. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 61.Bogliolo M, Izzotti A, De Flora S, et al. Detection of the `4977 bp' mitochondrial DNA deletion in human atherosclerotic lesions. Mutagenesis. 1999;14(1):77–82. doi: 10.1093/mutage/14.1.77. [DOI] [PubMed] [Google Scholar]
- 62.Botto N, Berti S, Manfredi S, et al. Detection of mtDNA with 4977 bp deletion in blood cells and atherosclerotic lesions of patients with coronary artery disease. Mutat. Res. 2005;570(1):81–8. doi: 10.1016/j.mrfmmm.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Sazonova M, Budnikov E, Khasanova Z, et al. Studies of the human aortic intima by a direct quantitative assay of mutant alleles in the mitochondrial genome. Atherosclerosis. 2009;204(1):184–90. doi: 10.1016/j.atherosclerosis.2008.09.001. [DOI] [PubMed] [Google Scholar]; ** Describes the use of pyrosequencing technology to identify mutations in the mitochondrial DNA in atherosclerotic plaques.
- 64.Nomiyama T, Tanaka Y, Hattori N, et al. Accumulation of somatic mutation in mitochondrial DNA extracted from peripheral blood cells in diabetic patients. Diabetologia. 2002;45(11):1577–83. doi: 10.1007/s00125-002-0893-7. [DOI] [PubMed] [Google Scholar]
- 65.Nomiyama T, Tanaka Y, Piao L, et al. Accumulation of somatic mutation in mitochondrial DNA and atherosclerosis in diabetic patients. Ann. N. Y. Acad. Sci. 2004;1011:193–204. doi: 10.1007/978-3-662-41088-2_20. [DOI] [PubMed] [Google Scholar]
- 66.Minamino T, Komuro I. Vascular aging: insights from studies on cellular senescence, stem cell aging, and progeroid syndromes. Nat. Clin. Pract. Cardiovasc. Med. 2008;5(10):637–48. doi: 10.1038/ncpcardio1324. [DOI] [PubMed] [Google Scholar]
- 67.Gorgoulis VG, Pratsinis H, Zacharatos P, et al. p53-dependent ICAM-1 overexpression in senescent human cells identified in atherosclerotic lesions. Lab. Invest. 2005;85(4):502–11. doi: 10.1038/labinvest.3700241. [DOI] [PubMed] [Google Scholar]
- 68.Costopoulos C, Liew TV, Bennett M. Ageing and atherosclerosis: Mechanisms and therapeutic options. Biochem. Pharmacol. 2008;75(6):1251–61. doi: 10.1016/j.bcp.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Hezel AF, Bardeesy N, Maser RS. Telomere induced senescence: end game signaling. Curr. Mol. Med. 2005;5(2):145–52. doi: 10.2174/1566524053586563. [DOI] [PubMed] [Google Scholar]
- 70.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell. Biol. 2007;8(9):729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 71.Matthews C, Gorenne I, Scott S, et al. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ. Res. 2006;99(2):156–64. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- 72.Andreassi MG. DNA damage, vascular senescence and atherosclerosis. J. Mol. Med. 2008;86(9):1033–43. doi: 10.1007/s00109-008-0358-7. [DOI] [PubMed] [Google Scholar]
- 73.Aliyev A, Chen SG, Seyidova D, et al. Mitochondria DNA deletions in atherosclerotic hypoperfused brain microvessels as a primary target for the development of Alzheimer's disease. J. Neurol. Sci. 2005:229–230. 285–92. doi: 10.1016/j.jns.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 74.McMurtry MS, Archer SL, Altieri DC, et al. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J. Clin. Invest. 2005;115(6):1479–91. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tuder RM, Cool CD, Yeager M, et al. The pathobiology of pulmonary hypertension. Endothelium. Clin. Chest. Med. 2001;22(3):405–18. doi: 10.1016/s0272-5231(05)70280-x. [DOI] [PubMed] [Google Scholar]
- 76.Zaiman A, Fijalkowska I, Hassoun PM, Tuder RM. One hundred years of research in the pathogenesis of pulmonary hypertension. Am. J. Respir. Cell. Mol. Biol. 2005;33(5):425–31. doi: 10.1165/rcmb.F307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2009;54(1 Suppl):S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 78.Rai PR, Cool CD, King JA, et al. The cancer paradigm of severe pulmonary arterial hypertension. Am. J. Respir. Crit. Care. Med. 2008;178(6):558–64. doi: 10.1164/rccm.200709-1369PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masri FA, Xu W, Comhair SA, et al. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2007;293(3):L548–54. doi: 10.1152/ajplung.00428.2006. [DOI] [PubMed] [Google Scholar]
- 80.Lee SD, Shroyer KR, Markham NE, et al. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J. Clin. Invest. 1998;101(5):927–34. doi: 10.1172/JCI1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tuder RM, Radisavljevic Z, Shroyer KR, Polak JM, Voelkel NF. Monoclonal endothelial cells in appetite suppressant-associated pulmonary hypertension. Am. J. Respir. Crit. Care. Med. 1998;158(6):1999–2001. doi: 10.1164/ajrccm.158.6.9805002. [DOI] [PubMed] [Google Scholar]
- 82.Yeager ME, Halley GR, Golpon HA, Voelkel NF, Tuder RM. Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ. Res. 2001;88(1):E2–E11. doi: 10.1161/01.res.88.1.e2. [DOI] [PubMed] [Google Scholar]
- 83.Machado RD, James V, Southwood M, et al. Investigation of second genetic hits at the BMPR2 locus as a modulator of disease progression in familial pulmonary arterial hypertension. Circulation. 2005;111(5):607–13. doi: 10.1161/01.CIR.0000154543.07679.08. [DOI] [PubMed] [Google Scholar]
- 84.Machado RD, Aldred MA, James V, et al. Mutations of the TGF-beta type II receptor BMPR2 in pulmonary arterial hypertension. Hum. Mutat. 2006;27(2):121–32. doi: 10.1002/humu.20285. [DOI] [PubMed] [Google Scholar]
- 85.Limaye N, Wouters V, Uebelhoer M, et al. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat. Genet. 2009;41(1):118–24. doi: 10.1038/ng.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Atkinson C, Stewart S, Upton PD, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105(14):1672–8. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 87.Richter A, Yeager ME, Zaiman A, et al. Impaired transforming growth factor-beta signaling in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2004;170(12):1340–8. doi: 10.1164/rccm.200311-1602OC. [DOI] [PubMed] [Google Scholar]
- 88.Hong KH, Lee YJ, Lee E, et al. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation. 2008;118(7):722–30. doi: 10.1161/CIRCULATIONAHA.107.736801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yuan JX, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation. 2005;111(5):534–8. doi: 10.1161/01.CIR.0000156326.48823.55. [DOI] [PubMed] [Google Scholar]
- 90.Wamhoff BR, Hoofnagle MH, Burns A, et al. A G/C element mediates repression of the SM22alpha promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis. Circ. Res. 2004;95(10):981–8. doi: 10.1161/01.RES.0000147961.09840.fb. [DOI] [PubMed] [Google Scholar]
- 91.Roberts R. A customized genetic approach to the number one killer: coronary artery disease. Curr. Opin. Cardiol. 2008;23(6):629–33. doi: 10.1097/HCO.0b013e32830e6b4e. [DOI] [PubMed] [Google Scholar]
- 92.Dandona S, Roberts R. Creating a genetic risk score for coronary artery disease. Curr. Atheroscler. Rep. 2009;11(3):175–81. doi: 10.1007/s11883-009-0028-4. [DOI] [PubMed] [Google Scholar]
- 93.Jarinova O, Stewart AF, Roberts R, et al. Functional analysis of the chromosome 9p21.3 coronary artery disease risk locus. Arterioscler. Thromb. Vasc. Biol. 2009;29(10):1671–7. doi: 10.1161/ATVBAHA.109.189522. [DOI] [PubMed] [Google Scholar]
- 94.Dandona S, Roberts R. Genomic view of factors leading to plaque instability. Curr. Cardiol. Rep. 2009;11(4):282–7. doi: 10.1007/s11886-009-0041-9. [DOI] [PubMed] [Google Scholar]
- 95.Soranzo N, Spector TD, Mangino M, et al. A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nat. Genet. 2009;41(11):1182–90. doi: 10.1038/ng.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Daw EW, Chen SN, Czernuszewicz G, et al. Genome-wide mapping of modifier chromosomal loci for human hypertrophic cardiomyopathy. Hum. Mol. Genet. 2007;16(20):2463–71. doi: 10.1093/hmg/ddm202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brunicardi FC, Gibbs RA, Fisher W, Chen C. Overview of the Molecular Surgeon Symposium on Personalized Genomic Medicine and Surgery. World J. Surg. 2009;33(4):612–4. doi: 10.1007/s00268-008-9861-9. [DOI] [PubMed] [Google Scholar]; ** Describes the new technologies available for genome sequencing, which make the model of Personalized Genomic Medicine and Surgery a reality.