Abstract
Purpose
The availability of a variety of immune response modifiers creates an opportunity for improved efficacy of immunotherapy, but it also leads to uncertainty in how to combine agents and how to assess those combinations. We sought to assess the impact of the addition of GM-CSF to vaccination with a melanoma vaccine.
Experimental Design
Ninety-seven patients with resected melanoma (Stage II-IV) were enrolled, stratified by stage and randomized to receive a cellular melanoma vaccine with or without GM-CSF. The primary endpoint was delayed-type hypersensitivity (DTH) response to melanoma cells. Antibody responses, peripheral leukocyte counts and survival were also examined.
Results
The GM-CSF arm demonstrated enhanced antibody responses with an increase in IgM titer against the TA90 antigen and increased TA90 immune complexes. This arm also had diminished anti-melanoma cell DTH response. Peripheral blood leukocyte profiles showed increases in eosinophils and basophils with decreased monocytes in the GM-CSF arm. These immune changes were accompanied by an increase in early melanoma deaths and a trend toward worse survival with GM-CSF.
Conclusion
These data suggest that GM-CSF is not helpful as an immune adjuvant in this dose and schedule, and raise concern that it may be harmful. Based upon the discordant findings of an immune endpoint and clinical outcome, use of such surrogate endopoints in selecting treatments for further evaluation must be done with a great deal of caution.
Translational Relevance.
The trial provides randomized data in an active immunotherapy with a relatively large sample size for a cancer vaccine trial. The data demonstrate changes in immune response and leukocyte milieu induced by the addition of GM-CSF to a previous standard vaccine regimen. More importantly, the report demonstrates that while the immune results were relatively consistent with expectations, the clinical outcomes clearly were not. Although antibody titers improved with GM-CSF, cellular responses were diminished, accompanied by a strong trend toward worse survival. This suggests GM-CSF should not be used in similar fashion in the future, and that using a surrogate immunologic endpoint to select the most promising immunotherapy is potentially hazardous.
Introduction
The arrival of numerous immunomodulatory agents for use in clinical trials has renewed hope that the dramatic and durable regressions seen occasionally with immunotherapy might be experienced by a larger number of patients. The list of these new tools includes cytokines, toll-like receptor (TLR) agonists, anti-regulatory agents, such as anti-CTLA-4 antibodies, and changes to the immunologic milieu through modifications of the host such as lymphodepletion. Due to redundant systems of control and regulation in the immune system, combinations of stimuli or modulators will likely be required to deliver consistent clinical benefit. However, rational strategies for designing and evaluating such combinations are not yet mature.
During the time period that Canvaxin, an allogeneic whole-cell melanoma vaccine, was undergoing phase III trial evaluation, additional research was conducted in an attempt to enhance immune responses. Several trials evaluated the impact of the addition of various immune modulators and adjuvants on immune endpoints. Here we report the results of a randomized, open-label trial of the standard vaccine protocol with or without the addition of granulocyte macrophage-colony stimulating factor (GM-CSF). These randomized data contribute to our understanding of the clinical and immunological impact of GM-CSF on active immunotherapy.
GM-CSF is a leukocyte growth factor approved for use in leukopenic cancer patients and has been incorporated into numerous tumor vaccines. Its use is supported by a significant body of pre-clinical studies 1-4. In addition, GM-CSF has been used as a single agent in the adjuvant setting in melanoma and showed improved outcomes relative to historical controls.5 However, despite its common inclusion as a vaccine component randomized trials examining the impact of GM-CSF on the immunological and clinical effects of vaccines in cancer patients are sparse.
Patients and Methods
Study Design
The study was a randomized, open-label assessment of a whole-cell, allogeneic vaccine (Canvaxin, CancerVax Corporation) with or without GM-CSF (Leukine, Immunex, Seattle, WA). Bacille Calmette-Guerin (BCG) was given with the first two vaccine doses in both study arms. Dosing of BCG, GM-CSF and vaccine was determined after a previously reported pilot trial was completed.6 The final dosages were: 1) vaccine: 25 × 106 cells/dose, 2) GM-CSF: 200 mcg/m2/day starting on the day of vaccine administration and daily thereafter for a total of 5 days during the first 4 months, 3) BCG: 3 × 106 cfu intradermally with the first vaccination and 1.5 × 106 cfu with the second if PPD skin test negative. (PPD positive subjects received half those amounts.)
The vaccine was administered intradermally in 8 injections distributed to sites adjacent to axillary and inguinal nodal basins. GM-CSF was given intradermally adjacent to the vaccination sites.
The primary aim was to determine whether the addition of GM-CSF to Canvaxin/BCG could enhance DTH responses. Secondary endpoints included antibody responses, adverse events and PPD DTH tests. Clinical outcomes and white blood counts were also examined.
Our Institutional Review Board approved the protocol, and all subjects provided informed consent.
Patient Eligibility
Subjects enrolled in the studies were at least 18 years of age and had a diagnosis of stage II-IV melanoma. Normal laboratory parameters were required, and immunocompromised patients were excluded. All subjects were without evidence of disease at the time of enrollment by physical exam, chest x-ray (stage II), CT scan of the chest/abdomen/pelvis (Stage III/IV), whole-body PET (Stage III/IV), and brain MRI or CT (Stage III/IV).
Preparation and Administration of Canvaxin Vaccine
Canvaxin was an irradiated allogeneic whole-cell vaccine composed of cells from three melanoma cell lines.7 Its preparation has been previously described.8 Briefly, melanoma cell lines were grown, harvested, washed, and pooled (8.3×106 cells/line, 25×106 total cells). The cells were irradiated with 150 Gy and cryopreserved until administration.
The first two doses of Canvaxin were admixed with the induction doses of BCG. Canvaxin was given every two weeks × 5, and then monthly × 4 to complete 6 months of immunization.
A 50% dose reduction was employed for GM-CSF if patients experienced an absolute granulocyte count of >20,000/mm3. GM-CSF was held at the next dose if granulocyte counts increased above 50,000/mm3. Toxicity was recorded using the NCI Common Toxicity Criteria.
Assessment of Immunologic Response
Delayed-type Hypersensitivity Testing
Immunologic monitoring consisted of delayed-type hypersensitivity (DTH) skin tests and serum antibody measurements. DTH was performed immediately prior to initiation of treatment and at the time of each vaccine dose. One tenth of the therapeutic dose of vaccine cells was used for DTH testing. Induration was determined at 48 hours and read as the mean of the widest diameter of induration and the perpendicular diameter thereof. Control DTH response to non-melanoma antigens was monitored by administering a PPD skin test to PPD-negative patients at monthly intervals until the patient became PPD positive or the seventh treatment. Blood samples were collected at baseline; at weeks 2, 4, 6, and 8; and at months 3, 4, 5, and 6 just prior to receiving vaccine for antibody and immune complex measurement.
Anti-TA90 IgG and IgM Titers
Serum samples were analyzed prospectively for IgG and IgM antibodies to TA90 glycoprotein antigen. TA90 was purified from urine of a melanoma and ELISAs were performed according to standard procedures reported elsewhere.9-11 Briefly, TA90 was adsorbed to 96-well ELISA plates at 120 ng/well, and serum sample dilutions added. Subsequently, the bound immunoglobulins were reacted with the alkaline phosphatase-conjugated Fab fragment of goat anti-human IgG or IgM (Sigma Chemicals, Co., St. Louis, MO). Absorbance at 405 nm was assessed, and the antibody titer was defined as the reciprocal of the highest dilution resulting in an absorbance of 0.05 optical density (OD) at 405 nm after subtracting the absorbance values of the controls.
TA90 Immune Complex Assay
Serum was assayed for TA90 immune complex (IC) as previously described.9 Briefly, patient serum was incubated on ELISA microtiter plates coated with murine monoclonal antibody to TA90. After washing, plates were incubated with goat anti-human IgG. An optical density of 0.41 was the upper limit of normal. Interassay variability has been previously measured at less than 15%.9
Statistical Analysis
With a sample size of 96, the study had 80% power to detect a 30% difference in DTH response. Comparison of group mean values for laboratory correlates was performed by T-test or Fisher's exact test. For comparison of immune response parameters, log-transformation was used to normalize distributions. Comparisons between groups during the vaccination period were done using mixed procedure (SAS 9.1.3) for longitudinal analysis. Specific time points were compared using T-test. However, these latter evaluations are considered exploratory only, due to multiple comparisons. Survival was estimated using the Kaplan-Meier method and compared using logrank. All statistical analyses were two-tailed.
Results
Patient Population
Ninety-seven patients were enrolled. Three were screen failures and did not receive vaccine. Demographic characteristics of the 94 patients eligible for analysis were similarly distributed between the two treatment arms (Table 1).
Table 1. Demographics of Population.
| GM-CSF | No GM-CSF | |
|---|---|---|
| Gender | ||
| Male | 31 (67) | 29 (60) |
| Female | 15 (33) | 19 (40) |
| Age | ||
| <60 years | 29 (63) | 32 (67) |
| >60 years | 17 (37) | 16 (33) |
| AJCC stage | ||
| II | 9 (20) | 10 (21) |
| Median Breslow Thickness | 3.25 mm | 4.25 mm |
| III | 26 (57) | 28 (58) |
| Nodal | 24 | 28 |
| Intransit | 2 | 0 |
| Mean # Lymph Node + | 2 | 2.25 |
| IV | 11 (24) | 10 (21) |
| M1A | 5 | 3 |
| M1B | 4 | 4 |
| M1A+B | 2 | 3 |
| Prior Treatment* (All Stages) | 5 | 6 |
| Radiation | 2 | 4 |
| Chemotherapy | 2 | 1 |
| Biotherapy | 2 | 3 |
| Immunotherapy | 1 | 0 |
| Other | 1 | 2 |
Some patients underwent more than one treatment modality.
DTH response to Canvaxin immunotherapeutic
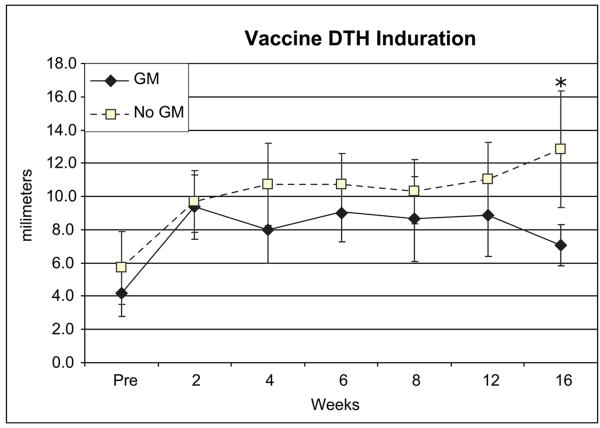
There was no significant difference in mean induration to baseline DTH testing with the vaccine (GM 4.2 ± 4.8 mm, No GM 5.7 ± 7.7 mm, p=0.25 (Figure 1)). However, there was a trend toward increased DTH in the non-GM-CSF arm by week 4, which persisted and became significant at 16 weeks (GM 7.1 ± 4.3 mm, No GM 12.8 ± 12.3 mm, T-test p = 0.01). By longitudinal analysis DTH values after initiating vaccination were significantly greater in the non-GM-CSF arm (overall mean 8.4mm vs. 10.9mm, p=0.006, Table 2). There was no significant difference in maximal DTH response.
Figure 1.
Delayed-type hypersensitivity (DTH) testing. Mean diameter of induration of each treatment group at baseline and during vaccination. * p<0.05 in exploratory T test for each timepoint.
Table 2. Immune Parameters.
| Pre-Vaccination | During Vaccination | |||||
|---|---|---|---|---|---|---|
| GM-CSF | No GM-CSF | p-value* | GM-CSF | No GM-CSF | p-value* | |
| DTH to Vaccine | 4.2 | 5.7 | 0.25 | 8.4 | 10.9 | 0.006 |
| IgM | 350 | 337 | 0.88 | 583 | 464 | 0.086 |
| IgG (adsorbed) | 477 | 388 | 0.33 | 526 | 496 | 0.72 |
| IgG (non-adsorbed) | 471 | 506 | 0.73 | 1188 | 866 | 0.059 |
| Immune Complex | 0.33 | 0.25 | 0.26 | 1.05 | 0.71 | 0.01 |
| White Blood Count | 5.7 | 6.2 | 0.26 | 6.9 | 6.3 | 0.23 |
| Hemoglobin | 14.0 | 14.0 | 0.88 | 14.0 | 14.2 | 0.23 |
| Platelets | 238 | 236 | 0.87 | 237 | 239 | 0.18 |
| Absolute Neutrophil | 3.5 | 3.8 | 0.29 | 4.5 | 4.0 | 0.19 |
| Absolute Lymphocyte | 1.59 | 1.54 | 0.69 | 1.61 | 1.56 | 0.65 |
| Absolute Monocyte | 0.39 | 0.44 | 0.28 | 0.37 | 0.47 | 0.008 |
| Absolute Eosinophil | 0.161 | 0.149 | 0.69 | 0.267 | 0.152 | 0.014 |
| Absolute Basophil | 0.046 | 0.038 | 0.46 | 0.047 | 0.034 | 0.13 |
p-values are by T-test for pre-treatment and by longitudinal analysis during treatment.
PPD response
Mean PPD induration showed a trend toward a disproportionate increase in at week 4 in the non-GM-CSF arm (GM 6.3 ± 8.0 mm, vs. No GM 11.4 ± 8.0, T-test p =0.03). Since subjects were no longer PPD tested after becoming positive, there are few datapoints after week four. By longitudinal and logrank analyses, the increase in PPD response was not statistically significant.
Anti-TA90 antibody response
Three antibody titers were measured: anti-TA90 IgM, anti-TA90 IgG, and an adsorbed anti-TA90 IgG. The last was performed due to possible retention of bovine serum albumen (BSA) in the vaccine preparation. Adsorption of serum had a significant impact on IgG values, but not in IgM. Both IgG assays are presented due to differences between groups seen in the non-adsorbed samples even though these responses are likely due to vaccine-specific but not tumor-specific antigens.
There were no significant differences in any antibody titer at baseline. The GM-CSF arm showed increased IgG responses in the non-adsorbed assay (significant at 8, 12, and 20 weeks.) By the longitudinal analysis of log-transformed values this difference was a strong trend (p=0.059). (Figure 2A)There were no differences in the adsorbed assay values.(Figure 2B). During treatment, IgM titers were generally higher in the GM-CSF arm (Figure 2C). Both maximal IgM (GM 803 ± 623 vs. No GM 565 ± 549, p=0.015) and mean values at 8 weeks (GM 662 ± 588 vs. No GM 415 ± 509, p =0.047) were higher with GM-CSF. Using longitudinal analysis of the log-transformed values, comparison of all on-treatment IgM values showed a trend toward increase in the GM-CSF arm (p=0.086).
Figure 2.
Antibody response measures: A) Mean titer of anti-TA90 IgG, B) Mean titer of anti-TA90 IgG in the absence of adsorption with bovine serum albumen, C) mean titer of anti-TA90 IgM, D)Mean measure (OD: optical density) of TA90-IgG immune complex. * p<0.05, ** p=0.06 for T-test at each timepoint.
The TA90 immune complex assay showed the most marked difference between groups with a rapid and significant increase in TA90 IC levels by week 4 which persisted throughout the study period (overall mean GM 1.1 vs. No GM .71,(Figure 2D)). Longitudinal analysis showed the on-treatment comparison to be significant (p=0.01).
Peripheral Leukocyte Counts
White blood cell counts and differentials were measured prior to each vaccine administration after the acute increase following GM-CSF dosing had abated. Hemoglobin and platelet counts were similar between groups throughout (not shown). Total WBC and profiles were similar at baseline (p>0.05 for all baseline values (Figure 3).) There was an increase in total WBC in the GM-CSF arm at weeks 2 and 8 and an increase in the non-GM-CSF arm at week 16 (p=0.04) but no significant difference by longitudinal analysis (p=0.23). There was an increase in mean absolute neutrophil count (ANC) in the GM-CSF arm which was most marked at week 2 (GM 4.8 vs. No GM 3.1), but that was not statistically significant (p=0.19). Mean absolute lymphocyte counts were similar between arms (p=0.59). The GM-CSF arm also had lower monocyte and higher eosinophil counts than the non-GM-CSF arm (p=0.008, p=0.014 respectively). Basophil counts trended higher in the GM-CSF arm (p=0.13).
Figure 3.
Mean peripheral blood counts of each treatment arm at the indicated time points. * p<0.05, ** p=0.06 for T-test at each time point.
Adverse event profile
Adverse event profiles were similar between the two arms, although grade 1 or 2 fatigue and injection site reaction/pain were more common in the GM-CSF arm (Supplemental Table).
Survival
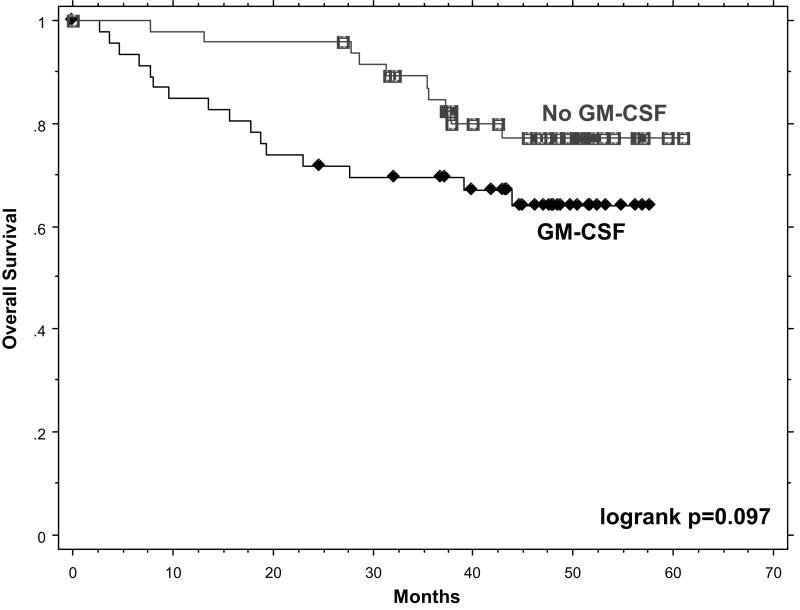
The study was not powered to definitively assess survival differences, but examination of these data revealed a surprising result. There was an excess of early recurrences and deaths in the GM-CSF arm resulting in a significantly decreased survival in that group when the results were analyzed at two years (p=0.002). With longer follow up, the curves have become closer and only a trend remains (p=0.097.) (Figure 4)
Figure 4.
Kaplan-Meier plot of overall survival of the GM-CSF (black) and non-GM-CSF (gray) arms. Logrank test demonstrated a significant difference when examined at two years, but with additional follow up this difference is now a trend, p=0.097.
Discussion
GM-CSF has been explored as an adjuvant to numerous vaccines. Strategies include co-administration of recombinant GM-CSF and transfection of vaccine or bystander cells for in vivo cytokine production and are supported by pre-clinical studies demonstrating improved immunogenicity and plausible biological mechanisms of antitumor activity. 1-4
Our data demonstrate effects of the cytokine on the immunologic milieu of the host including increased eosinophils and basophils and decreased monocytes in the peripheral blood. These changes were accompanied by enhanced humoral and diminished cellular responses, and both IgM and IgG seem to have been affected. These immune endpoints were accompanied by a troubling trend toward reduced survival in the GM-CSF arm. All of these findings are in keeping with the limited randomized clinical trial data preceding this report.
Randomized trials of GM-CSF have been conducted in non-cancer vaccines, such as hepatitis B vaccination.12,13 Consistent with our data, these studies showed enhanced humoral responses with the addition of GM-CSF.
In contrast to the infectious disease results, there are few published randomized studies of GM-CSF in cancer immunotherapy. Non-randomized GM-CSF data have appeared promising in comparisons to historical controls5, but this comparison has been questioned due to large potential confounders between the compared populations. Small trials have been reported, but have not demonstrated consistent immune or significant clinical effects.14-16 Hamid and colleagues performed a randomized, three-arm trial of peptide vaccination. Two arms received a sustained-release formulation of IL-12 and a third arm received soluble IL-12 and GM-CSF. 17 They demonstrated significantly increased cellular immune responses by DTH and ELISPOT in the arms not receiving GM-CSF. Interestingly the risk of relapse was greatest in the GM-CSF arm as well, though not by a statistically significant amount. These immune and clinical differences were attributed to the IL-12 formulation rather than GM-CSF because there was no pre-clinical data supporting an adverse effect of GM-CSF.
Accrual to one large cooperative group randomized trial using GM-CSF and peptide vaccination has been completed, but final clinical results have not been reported.
Over the last several years data have emerged to suggest potential mechanisms for an adverse effect of GM-CSF on tumor immunity. GM-CSF receptors are present in vascular endothelial cells suggesting the possibility of facilitated tumor growth18-20. Another potential mechanism is induction and activation of myeloid derived suppressor cells (MDSC). In mice, these cells are fairly well characterized as CD11b+GR1+. In humans, several candidate marker profiles have been identified including Lineage-HLA-DR-, and CD11b+CD14-CD15+ cells.21,22 Such cells may produce immunosuppressive factors including TGF-β, and lead to activation of regulatory T cells. MDSC also appear to have a role in inducing vascular endothelial growth factor (VEGF) secretion, and this role depends at least in part on the presence of GM-CSF.23 Increases in MDSC have been linked to diminished anti-melanoma T cell responses. The frequency of circulating MDSC correlates directly with stage in solid tumors and increases in the setting of GM-CSF treatment.24,25 Together these findings suggest a mechanism connecting GM-CSF with diminished DTH response and early recurrence.
GM-CSF dose appears to be critical in determining the immunologic effect. Several trials using lower doses of GM-CSF (<80mcg/day) have shown improvements in immune T-lymphocyte responses,26-29 while higher dose trials have shown either no effect or a decreased response.29-33 As reviewed by Parmiani et al, the threshold for an adverse effect appears to be approximately 100mcg/day.29 Above this dose MDSC may be recruited in substantial numbers. Our trial, which was designed well before this potential adverse impact was known, utilized a dose well above the threshold, at approximately 400 mcg/day (mean BSA 1.97 m2). Route of administration and dose interval are also important in determining the area under the curve for GM-CSF plasma concentration, which can be variable even with consistent dosing.34 If future trials use GM-CSF, this important dose-response relationship needs to be taken into account.
Immunotherapy trials in the adjuvant setting have generally relied on surrogate immunological endpoints including antibody titers, DTH skin testing, and in vitro cellular response assays. The antibody and DTH responses used here have been in use for many years and enjoy a high level of correlation to clinical outcomes.11,35 Numerous phase II trials have demonstrated not only an impact of vaccination on immune measures, but a correlation of immune response to survival. Such clinical correlation is relatively uncommon for surrogate endpoints, many of which have either mixed or no demonstrated correlation with survival. However, despite this prior record, in this trial a positive impact on one surrogate endpoint was accompanied by an increase in early recurrence and a concerning survival trend. This raises general questions about the interpretation of immunologic data and their use in directing development of immunotherapies. The current trial also suggests the utility of a functional cellular response assay, DTH, as an endpoint. While this endpoint may be considered outdated, it is both in vivo and functional and has a well established track record in experienced hands. Not only has DTH been correlated with survival, but changes in DTH have now been correlated with changes in survival. This is the first report of a randomized trial demonstrating such a linkage of immunological and clinical endpoints with the addition of an immunomodulator. Alternative surrogate immune endpoints are certainly reasonable and necessary, but interpretation of such measures should be done cautiously.
Supplementary Material
Acknowledgments
Supported in part by grants CA87071, CA12582, and CA76489 from the National Cancer Institute and by funding from Nancy and Carol O'Connor (Los Angeles, CA). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institutes of Health.
Footnotes
Presented in part at the 44th annual meeting of American Society of Clinical Oncology, May 31, 2008, Chicago, IL.
References
- 1.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mach N, Gillessen S, Wilson SB, et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–46. [PubMed] [Google Scholar]
- 3.Gillessen S, Naumov YN, Nieuwenhuis EE, et al. CD1d-restricted T cells regulate dendritic cell function and antitumor immunity in a granulocyte-macrophage colony-stimulating factor-dependent fashion. Proc Natl Acad Sci U S A. 2003;100:8874–9. doi: 10.1073/pnas.1033098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer HG, Frosch S, Reske K, et al. Granulocyte-macrophage colony-stimulating factor activates macrophages derived from bone marrow cultures to synthesis of MHC class II molecules and to augmented antigen presentation function. J Immunol. 1988;141:3882–8. [PubMed] [Google Scholar]
- 5.Spitler LE, Grossbard ML, Ernstoff MS, et al. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2000;18:1614–21. doi: 10.1200/JCO.2000.18.8.1614. [DOI] [PubMed] [Google Scholar]
- 6.Hsueh E, Essner R, Foshag L, et al. Active specific immunotherapy of melanoma with a polyvalent vaccine and recombinant human GM-CSF: An immunogenicity study. Proc Am Soc Clin Oncol. 2003;22:176. [Google Scholar]
- 7.Morton D, Barth A. Vaccine therapy for malignant melanoma. CA Cancer J Clin. 1996;46:225–44. doi: 10.3322/canjclin.46.4.225. [DOI] [PubMed] [Google Scholar]
- 8.Morton D, Foshag L, Hoon D, et al. Prolongation of survival in metastatic melanoma after active specific immunotherapy with a new polyvalent melanoma vaccine. Ann Surg. 1992;216:463–482. doi: 10.1097/00000658-199210000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta R, Morton D. Monoclonal antibody-based ELISA to detect glycoprotein tumor-associated-antigen-specific immune complexes in cancer. J Clin Lab Analysis. 1992;6:329–336. doi: 10.1002/jcla.1860060514. [DOI] [PubMed] [Google Scholar]
- 10.Euhus DM, Gupta RK, Morton DL. Induction of antibodies to a tumor-associated antigen by immunization with a whole melanoma cell vaccine. Cancer Immunol Immunother. 1989;29:247–54. doi: 10.1007/BF00199212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsueh E, Gupta R, Qi K, et al. Correlation of specific immune responses with survival in melanoma patients with distant metastases receiving polyvalent melanoma cell vaccine. J Clin Oncol. 1998;16:2913–2920. doi: 10.1200/JCO.1998.16.9.2913. [DOI] [PubMed] [Google Scholar]
- 12.Yagci M, Acar K, Sucak GT, et al. Hepatitis B virus vaccine in lymphoproliferative disorders: a prospective randomized study evaluating the efficacy of granulocyte-macrophage colony stimulating factor as a vaccine adjuvant. Eur J Haematol. 2007;79:292–6. doi: 10.1111/j.1600-0609.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- 13.Anandh U, Bastani B, Ballal S. Granulocyte-macrophage colony-stimulating factor as an adjuvant to hepatitis B vaccination in maintenance hemodialysis patients. Am J Nephrol. 2000;20:53–6. doi: 10.1159/000013556. [DOI] [PubMed] [Google Scholar]
- 14.Celis E. Overlapping human leukocyte antigen class I/II binding peptide vaccine for the treatment of patients with stage IV melanoma: evidence of systemic immune dysfunction. Cancer. 2007;110:203–14. doi: 10.1002/cncr.22744. [DOI] [PubMed] [Google Scholar]
- 15.Marshall JL, Gulley JL, Arlen PM, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–31. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 16.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, et al. Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol. 2003;21:4016–26. doi: 10.1200/JCO.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Hamid O, Solomon JC, Scotland R, et al. Alum with interleukin-12 augments immunity to a melanoma peptide vaccine: correlation with time to relapse in patients with resected high-risk disease. Clin Cancer Res. 2007;13:215–22. doi: 10.1158/1078-0432.CCR-06-1450. [DOI] [PubMed] [Google Scholar]
- 18.Mattei S, Colombo MP, Melani C, et al. Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int J Cancer. 1994;56:853–7. doi: 10.1002/ijc.2910560617. [DOI] [PubMed] [Google Scholar]
- 19.Gasson JC. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991;77:1131–45. [PubMed] [Google Scholar]
- 20.Baldwin GC, Golde DW, Widhopf GF, et al. Identification and characterization of a low-affinity granulocyte-macrophage colony-stimulating factor receptor on primary and cultured human melanoma cells. Blood. 1991;78:609–15. [PubMed] [Google Scholar]
- 21.Serafini P, De Santo C, Marigo I, et al. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother. 2004;53:64–72. doi: 10.1007/s00262-003-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–45. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larrivee B, Pollet I, Karsan A. Activation of vascular endothelial growth factor receptor-2 in bone marrow leads to accumulation of myeloid cells: role of granulocyte-macrophage colony-stimulating factor. J Immunol. 2005;175:3015–24. doi: 10.4049/jimmunol.175.5.3015. [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Montero CM, Salem ML, Nishimura MI, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serafini P, Carbley R, Noonan KA, et al. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–43. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 26.Jager E, Ringhoffer M, Dienes HP, et al. Granulocyte-macrophage-colony-stimulating factor enhances immune responses to melanoma-associated peptides in vivo. Int J Cancer. 1996;67:54–62. doi: 10.1002/(SICI)1097-0215(19960703)67:1<54::AID-IJC11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 27.Scheibenbogen C, Schmittel A, Keilholz U, et al. Phase 2 trial of vaccination with tyrosinase peptides and granulocyte-macrophage colony-stimulating factor in patients with metastatic melanoma. J Immunother. 2000;23:275–81. doi: 10.1097/00002371-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Ullenhag GJ, Frodin JE, Mosolits S, et al. Immunization of colorectal carcinoma patients with a recombinant canarypox virus expressing the tumor antigen Ep-CAM/KSA (ALVAC-KSA) and granulocyte macrophage colony-stimulating factor induced a tumor-specific cellular immune response. Clin Cancer Res. 2003;9:2447–56. [PubMed] [Google Scholar]
- 29.Parmiani G, Castelli C, Pilla L, et al. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18:226–32. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 30.Dillman R, Weimann M, Nayak S, et al. Interferon-gamma or granulocyte-macrophage colony-stimulating factor adminstered as adjuvants with a vaccine of irradiated autologous tumor cells from short-term cell line cultures: a randomized phase 2 trial of the cancer biotherapy research group. J Immunother. 2003;26:367–73. doi: 10.1097/00002371-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Weber J, Sondak VK, Scotland R, et al. Granulocyte-macrophage-colony-stimulating factor added to a multipeptide vaccine for resected Stage II melanoma. Cancer. 2003;97:186–200. doi: 10.1002/cncr.11045. [DOI] [PubMed] [Google Scholar]
- 32.Simmons SJ, Tjoa BA, Rogers M, et al. GM-CSF as a systemic adjuvant in a phase II prostate cancer vaccine trial. Prostate. 1999;39:291–7. doi: 10.1002/(sici)1097-0045(19990601)39:4<291::aid-pros10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Belli F, Testori A, Rivoltini L, et al. Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J Clin Oncol. 2002;20:4169–80. doi: 10.1200/JCO.2002.09.134. [DOI] [PubMed] [Google Scholar]
- 34.Hewitt RG, Morse GD, Lawrence WD, et al. Pharmacokinetics and pharmacodynamics of granulocyte-macrophage colony-stimulating factor and zidovudine in patients with AIDS and severe AIDS-related complex. Anitmicrob Agents Chemother. 1993;37:512–22. doi: 10.1128/aac.37.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsueh E, Essner R, Foshag L, et al. Prolonged survival after complete resection of disseminated melanoma and active immunotherapy with a therapeutic cancer vaccine. J Clin Oncol. 2002;20:4549–54. doi: 10.1200/JCO.2002.01.151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.