Abstract
Rational
AMP-activated protein kinase (AMPK) is an energy sensor and ubiquitously expressed in vascular cells. Recent studies suggest that AMPK activation improves endothelial function by counteracting oxidative stress in endothelial cells. How AMPK suppresses oxidative stress remains to be established.
Objective
The aim of this study is to examine the effects of AMPK in regulating NAD(P)H oxidase, oxidative stress and endothelial function.
Methods and Results
AMPK activity, the markers of oxidative stress, NAD(P)H oxidase subunit expression (gp91phox, p47phox, p67phox, NOX1-4), NAD(P)H oxidase-mediated superoxide production, 26S proteasome activity, IκBα degradation, and nuclear translocation of NF-κB (p50 and p65) were examined in cultured human umbilical vein endothelial cells (HUVEC) and mouse aortas isolated from AMPKα2 deficient mice. Compared to the wild type, acetylcholine (Ach)-induced endothelium-dependent relaxation was significantly impaired in parallel with increased production of oxidants in AMPKα2−/− mice. Further, pretreatment of aorta with either superoxide dismutase or Tempol or apocynin significantly improved Ach-induced endothelium-dependent relaxation in AMPKα2−/− mice. Analysis of aortic endothelial cells from AMPKα2−/− mice and human umbilical vein endothelial cells (HUVECs) expressing dominant negative AMPK or AMPK α2-specific siRNA revealed that loss of AMPK activity increased NAD(P)H oxidase subunit expression (gp91phox, p47phox, p67phox, NOX1-4), NAD(P)H oxidase-mediated superoxide production, 26S proteasome activity, IκBα degradation, and nuclear translocation of NF-κB (p50 and p65), whereas AMPK activation by AICAR or over-expression of constitutively active AMPK had the opposite effect. Consistently, we found that genetic deletion of AMPKα2 in LDL receptor knockout (LDLr−/−) strain markedly increased 26S proteasome activity, IκB degradation, NF-κB transactivation, NAD(P)H oxidase subunit overexpression, oxidative stress, endothelial dysfunction, and atherosclerosis, all of which were largely suppressed by chronic administration of MG132, a potent cell permeable proteasome inhibitor..
Conclusion
We conclude that AMPKα2 functions as a physiological suppressor of NAD(P)H oxidase and ROS production in endothelial cells. In this way AMPK maintains the non-atherogenic and non-inflammatory phenotype of endothelial cells.
Keywords: AMPK, NAD(P)H Oxidase, NF-κB, Proteasome
Introduction
AMPK-activated protein kinase (AMPK) is a serine/threonine kinase consisting of α, β, and γ subunits, each of which has at least two isoforms. The α subunit possesses catalytic activity, while the β and γ regulatory subunits maintain the stability of the heterotrimer complex. AMPK phosphorylates multiple targets, in vivo and in vitro. These targets include several biosynthetic enzymes such as acetyl-CoA carboxylase, hydroxymethylglutaryl-CoA reductase, and glycogen synthase. The importance of AMPKα is illustrated by the fact that dual deficiency of AMPK α1 and α2, the two catalytic subunits of AMPK, is embryonic lethal1. The major isoform of AMPK in endothelial cells is AMPKα1β1γ1, while AMPK α2 is a minor form2. However, both AMPK α1 and AMPK α2 might be equally important in maintaining endothelial function. AMPK α12–4 and α25 increase NO release by phosphorylating both Ser1177 and Ser635 of eNOS in endothelial cells. In addition to its key role in endothelial cell biology, AMPK regulates the functions of vascular cells and monocytes/macrophages. For example, AMPK activation inhibits the vascular smooth muscle cell hypertrophy induced by angiotensin-II6. AMPK α1, which is the predominant isoform of AMPK in human and mouse macrophages, also suppresses the proinflammatory response7. Thus, AMPK is a logical therapeutic target for the treatment of diseases rooted in cellular proliferation, including atherosclerosis and cancer.
Recent studies suggest that AMPK activation improves endothelial function by counteracting oxidative stress in endothelial cells. Studies from Ido8 have shown that incubation of HUVECs with high glucose significantly increases apoptosis related to oxidative stress. However, co-incubation with the AMPK activator, AICAR, completely prevents this change, suggesting that AMPK is important for the protection of endothelial cells against the adverse effects of sustained hyperglycemia. Interestingly, Ouslimani et al. have reported9 that another AMPK activator, metformin, decreases ROS production in aortic endothelial cells, an effect partially attributed to a decrease in ROS derived from the mitochondrial respiratory chain. More direct evidence that activation of AMPK reduces hyperglycemia-induced mitochondrial ROS production has been provided by Kukidome et al.10, who showed that induction of MnSOD and promotion of mitochondrial biogenesis occurs through the activation of the AMPK-PGC1α pathway in HUVECs. Recently, Zang et al11 reported that treatment with the polyphenol S17834, augments hepatic AMPK and acetyl-CoA carboxylase phosphorylation and thereby decreases hepatic and serum lipids, suppressing the acceleration of atherosclerosis caused by diabetes in LDL receptor knockout (LDLr−/−) mice. These studies support the notion that AMPK promotes endothelial function by suppressing NAD(P)H oxidase-derived ROS production. At present, the relationship between AMPK, endothelial function, and NAD(P)H oxidase has not been fully defined. Thus, the aim of this study was to determine the effect and molecular mechanisms of AMPK α2 deletion in endothelial function. Our data suggest that AMPKα2 protects vascular endothelial cells from oxidative stress by suppressing the NF-κB-mediated expression of NAD(P)H oxidase.
Materials and Methods
Animals
Male AMPKα2−/− mice were generated, as previously described12. Their genetic controls (C57BL/6 WT mice) were obtained from the Jackson Laboratory (Bar Harbor, ME) and were 8 ~ 12 weeks of age, with a weight of 20 ~ 25 g. Mice were housed in temperature-controlled cages under a 12 h light-dark cycle and given free access to water and normal chow. The mice were euthanized with inhaled isoflurane. Aortas were then removed and immediately frozen in liquid nitrogen or incubated with different agents. AMPKα2−/− mice that had been backcrossed to a C57BL/6 background were crossed with LDLr−/− mice of C57BL/6 background, to generate LDLr−/−/AMPKα2−/− mice. LDLr−/−/AMPKα+/+ control mice served as controls. The mice aged 5 weeks old were fed western diet (WD) containing 0.21% cholesterol and 21% fat (Research Diets Inc, D12079B) for 8 weeks. Two weeks after western diets, an MG132 osmotic pump (delivered at rate of 0.72 mg/kg/day, DURECT corporation, CA, Model 2006) or the inhibitor-diluent (DMSO), as a negative control, was implanted subcutaneously in LDLr−/−/AMPKα2−/− or LDLr−/− control mice for 6 weeks. The animal protocol was reviewed and approved by the University of Oklahoma Institute Animal Care and Use Committee.
A full description of materials and methods used, including cell culture, adenovirus and siRNA transfection to cells, preparation of subcellular fractions, western blot analysis, 26S proteasome activity assay, detection of ROS, assays for endothelium-dependent relaxation, measurement of serum cholesterol, triglyceride, and blood glucose levels, immunohistochemistry, cytokine assay, assays of SOD and catalase activity, and statistical analysis can be found in the online-only Data Supplement.
Results
AMPK α2 deletion impairs endothelium-dependent vasorelaxation
In endothelial cells, AMPK regulates the bioactivity of NO, an essential factor for vascular homeostasis2–4. We first determined whether AMPKα2 deletion affects endothelial function in isolated mouse aortas. Mouse aortas that were pre-constricted with 9,11-dideoxy-11-,9-epoxymethanoprostaglandin F2 (U46619, 30 nM) were stimulated with acetylcholine (Ach, 1 × 10−8 to 1 × 10−4 M) to assay the endothelium-dependent vasorelaxation. The maximal endothelium-dependent relaxation induced by Ach (1 × 10−4 M) was less in AMPKα2−/− aortas (60.9 ± 5.5%) than in C57BL6 WT aortas (85.5 ± 10.5%, P<0.05) (Figure 1A). However, the endothelium-independent vasorelaxation induced by sodium nitroprusside (SNP, 1 × 10−10 ~ 1 × 10−6 M) did not differ between AMPKα2−/− and WT mice (Figure 1B).
Figure 1. AMPKα2 deletion induces endothelial dysfunction in an NAD(P)H oxidase- and ROS- dependent manner.
Ach-induced endothelium-dependent relaxation in WT and AMPKα2−/− aortas after 12 h incubation with (A) tempol (10 µM), (C) PEG-SOD (100 Units/ml), and (D) apocynin (100 µM). (B & E) SNP-induced endothelium-independent relaxation in WT or AMPKα2−/− aortas. *P<0.05 vs. WT, #P<0.05 vs. AMPKα2−/−, two-way ANOVA followed by student's t-test. n=6. (F) Western blot analysis of aortic NAD(P)H oxidase subunits (p47phox, p67phox, and gp91phox), phosphorylated eNOS at Ser1177 (peNOS), and total eNOS expressions in WT and AMPKα2−/− mice. The blot is a representative of blots obtained with five mice. *P<0.05 vs. WT. (G) NAD(P)H oxidase activity in WT and AMPKα2−/− mice aortas. *P<0.05 vs. WT. n=5. (H) Superoxide production in aortas from WT and AMPKα2−/− mice, as assessed by DHE/HPLC. Aortas were incubated with allopurinol (50 µM), tempol (10 µM), or apocynin (100 µM) for 24 h. *P<0.05 vs. WT, #P<0.05 vs. AMPKα2−/− control. n=5.
Impaired endothelium-dependent relaxation in the AMPKα2−/− aortas is ROS dependent
Because increased ROS production is thought to cause endothelial dysfunction, we determined whether scavenging ROS improves Ach-induced relaxation in aortas from AMPKα2−/− mice. Aortic rings from WT and AMPKα2−/− mice were incubated with tempol (10 µM), a SOD mimetic, or polyethene glycated superoxide dismutase (PEG-SOD, 100 Units/mL) for 12 h in culture medium. Tempol, which didn’t alter Ach-induced relaxation in WT aortas, increased Ach-induced endothelium-dependent relaxation in aortas from AMPKα2−/− mice (73.8 ± 9.50% vs. 60.9 ± 5.5%, P<0.05, Figure 1A). However, tempol failed to alter SNP-induced endothelium-independent relaxation in aortas from AMPKα2−/− mice (Figure 1B). Further, the addition of PEG-SOD normalized Ach-induced endothelium-dependent relaxation in AMPKα2−/− mice without affecting SNP-induced endothelium-independent vasorelaxation (Figure 1C).
Inhibition of NAD(P)H oxidase abolishes endothelial dysfunction in AMPKα2−/− mice
To determine whether NAD(P)H oxidase is a source of ROS in AMPKα2−/− mice, aortas isolated from these animals were incubated with apocynin (100 µM), a selective NAD(P)H oxidase inhibitor for 24 h. Apocynin partially but significantly normalized Ach-induced endothelium-dependent relaxation in AMPKα2−/− aortas (75.5 ± 13.1% vs. 60.9 ± 5.5%, P<0.05, Figure 1D), but had no effect on SNP-induced endothelium-independent relaxation (Figure 1E). Taken together, these results suggest that NAD(P)H oxidase is the primary source of ROS in AMPKα2−/− mice.
AMPKα2 deletion increases the expression of NAD(P)H oxidase subunits, NAD(P)H oxidase activity, and ROS production
NAD(P)H oxidase is an enzyme complex consisting of Nox1, Nox2, Nox4, Nox5, p22phox, p47phox, p67phox, and the small G-protein, Rac113, 14. As apocynin partially inhibited endothelial dysfunction in AMPKα2−/− mice (Figure 1D), we measured the expression of each NAD(P)H oxidase subunit in AMPKα2−/− mice. As expected, AMPKα2 protein was absent in the aortas of AMPKα2−/− mice. However, aortic expression of the essential subunits, p47phox, p67phox, and gp91phox (Figure 1F), as well as the catalytic subunit, both NOX1 and NOX415, 16, was increased in AMPKα2−/− mice compared to their WT counterparts (Online Figure IA), accompanied with the increased NAD(P)H oxidase activity (Figure 1G).
AMPK is reported to phosphorylate eNOS at Ser1177 or 633 resulting in increased NO release5, 17, 18. Next, we determined if AMPKα2 deletion altered the expression and phosphorylation of eNOS. As shown in Figure 1F, the levels of total eNOS and phospho-eNOS were similar between WT and AMPKα2−/−, suggesting that impaired endothelium-dependent relaxation observed in mouse aortas from AMPKα2−/− mice was not due to abnormal expression or altered phosphorylation status of eNOS.
Next, we studied whether the increased NAD(P)H oxidase expression is due to macrophage infiltration of the vascular wall by immunohistochemical staining of CD68 and F4/80, two well characterized macrophage markers. As depicted in Online Figure IB, weak stainings of CD68 were found in the aortic roots from WT and AMPKα2−/− mice. Further, there was no difference between WT and AMPKα2−/− mice. Similarly, there was no difference in the stainings of F4/80 between WT and AMPKα2−/− mice (data not shown). These results are consistent with a recent report that AMPKα2 is only minimally present in mouse and human macrophages and monocytes7. Taken together, our results suggest that increased expression of NOX-2 and other subunits of NAD(P)H oxidase is unlikely due to increased infiltration of leukocytes.
Next, we determined whether upregulation of these NAD(P)H oxidase subunits results in increased ROS production. Aortic ROS levels measured by dihydroethidium (DHE)/HPLC were greater in AMPKα2−/− mice than WT mice (Figure 1H), and this elevation in ROS was inhibited by either tempol or apocynin. In contrast, allopurinol (50 µM) did not alter ROS production in AMPKα2−/− aortas. Increased ROS production in AMPKα2−/− mice aortic endothelium was further confirmed by DHE/vWF double staining (Online Figure IIA). In addition, the aortic expression and activity of SOD and catalase, two important antioxidant enzymes, were similar in AMPKα2−/− and WT mice (Online Figure IIB and IIB). These results suggest that upregulation of NAD(P)H oxidase contributes to the increased aortic ROS levels observed in AMPKα2−/− mice.
AICAR-induced AMPK activation inhibits the expression of NAD(P)H oxidase subunits in endothelial cells
In human neutrophils, AMPK activation with AICAR significantly attenuates phorbol 12-myristate 13-acetate-stimulated O2.- release through the suppression of NAD(P)H oxidase activity19. We analyzed the effect of AMPK activation on NAD(P)H oxidase expression by incubating HUVECs with varying concentrations of AICAR (0.25 – 2 mM) for 24 h and then measuring p47phox and p67phox levels by western blotting. AICAR activated AMPK, as evidenced by an increase in AMPK phosphorylation at threonine 172, an essential phosphorylation site for AMPK activation. In addition, exposure of HUVECs to AICAR decreased both p47phox and p67phox in a time-dependent (Figure 2A) and dose-dependent manner (Figure 2B). Dose-dependent decreases in these subunits were mirrored by both time- and dose-dependent increases in AMPK Thr172 phosphorylation (Online Figure III).
Figure 2. AICAR inhibits the expression of the NAD(P)H oxidase subunits, p47phox and p67phox, in HUVECs in an AMPKα2-dependent manner.
Western blot analysis of p47phox and p67phox expression in HUVECs (A) treated with AICAR (2 mM) for the indicated times or (B) treated with varying concentrations of AICAR for 24 h. The blot is a representative of blots obtained in three independent experiments. *P<0.05 vs. control. AICAR-induced changes in p47phox and p67phox expression were also examined in HUVECs infected with (C & D) ad-GFP or AMPKα2-DN or (E & F) control siRNA or AMPKα2 siRNA. 24 h after infection/transfection, cells were incubated with AICAR (2 mM) or vehicle for 8 h. The blots are representative of blots obtained from three independent experiments. *P<0.05 vs. control; #P<0.05 vs. GFP or control siRNA; $P<0.05 vs. GFP or control siRNA plus AICAR. (D & F) NAD(P)H oxidase-dependent superoxide production in AMPKα2-DN-infected or AMPKα2 siRNA-transfected HUVECs. Superoxide production was assayed in HUVECs with/without apocynin (100 µM, 24 h) by DHE/HPLC, as decribed in Methods. *P<0.05 vs. GFP or control siRNA, #P<0.05 vs. ad-AMPK-DN or AMPKα2 siRNA. n=3.
AICAR-induced suppression of NAD(P)H oxidase subunit expression is AMPKα2-dependent
To determine whether inhibition of NAD(P)H oxidase by AICAR is AMPKα2-dependent, we infected cells with adenovirus encoding dominant negative AMPK (Ad-AMPK-DN) in HUVECs. As shown in Figure 2C, AICAR inhibited the expression of p47phox and p67phox in both non-infected cells and cells expressing adenoviral GFP (Ad-GFP). In contrast, over-expression of Ad-AMPK-DN bypassed the effect of AICAR on both p47phox and p67phox. In addition, the over-expression of Ad-AMPK-DN led to a marked increase in ROS levels (Figure 2D).
The ability of AICAR-induced AMPKα2 activation to inhibit NAD(P)H oxidase was confirmed by transfecting HUVECs with AMPKα2 siRNA. Suppression of AMPKα2 expression by AMPKα2-specific siRNA was confirmed by western blotting (Figure 2E). AMPKα2 siRNA, but not control siRNA, blocked the effect of AICAR on p47phox and p67phox expression (Figure 2E). Accordingly, AMPKα2 siRNA, but not control siRNA, significantly increased ROS production in HUVECs (Figure 2F), and this increase was partially inhibited by apocynin (Figure 2F).
AMPKα2 deletion increases IκBα degradation, NF-κB translocation, and expressions of NAD(P)H oxidase subunits in endothelial cells
Next, we compared the expression of NAD(P)H oxidase subunits between mouse aortic endothelial cells (MAECs) isolated from WT and AMPKα2−/− mice. Compared to WT MAECs, AMPKα2−/− MAECs exhibited elevated expression of p47phox, p67phox, and gp91phox (Figure 3A). Since NF-κB regulates the expression of NAD(P)H oxidase subunits20, 21 and is anchored and inactivated in the cytoplasm by association with members of IκBα in quiescent cells, we next examined the levels and phosphorylation of the NF-κB inhibitor, IκBα, as well as the subcellular localization of the NF-κB subunits, p65 and p50, which trigger gene transcription upon translocation to the nucleus22. As shown in Figure 3B, AMPKα2−/− MAECs contained reduced IκBα levels and exhibited increased IκBα phosphorylation, which is required for proteasome-dependent degradation of IκBα. Further, nuclear levels of p65 and p50 were significantly higher in AMPKα2−/− MAECs than in WT MAECs (Figure 3C).
Figure 3. AMPKα2 deletion increases NAD(P)H oxidase expression via NF-κB activation in MAECs.
Lysates from WT and AMPKα2−/− MAECs were subjected to western blot analysis for (A) p47phox, p67phox, and gp91phox, as well as (B) IκBα and phosphorylated IκBα. The blots are representative of blots obtained from three independent experiments. *P<0.05 vs. WT. (C) Western blot analysis of the NF-κB subunits, p50 and p65, in nuclear fraction of MAECs (n=3). *P<0.05 vs. WT. (D) The expressions of p47phox and p67phox in AMPKα2−/− MAECs treated with an NF-κB translocation inhibitor (peptide sequence: AAVALLPAVLLALLAPVQRKRQKLMP, 50 mg/ml) for 24 h (n=3). *P<0.05 vs. control. (E) p47phox and p67phox expressions in MAECs (WT and AMPKα2−/−) incubated with the proteasome inhibitor, MG132 (0.5 µM), for 4 h (n=3). *P<0.05 vs. WT control, #P<0.05 vs. AMPKα2−/− control.
Consistent with these results, an NF-κB inhibitor significantly reduced aortic p47phox and p67phox levels in MAECs isolated from AMPKα2−/− mice (Figure 3D), suggesting that AMPK α2 deficiency induces NAD(P)H oxidase subunit expression through activation of IκBα/NF-κB. Like the NF-κB inhibitor, MG132 (a potent 26S proteasome inhibitor) reduced p47phox and p67phox levels in AMPKα2−/−mice (Figure 3E). This effect was also seen in WT mice.
AICAR increases IκBα protein levels via AMPKα2-dependent manner
Under normal conditions, NF-κB is bound to IκBα, which retains NF-κB in the cytoplasm and prevents its activation. Thus, increasing IκBα expression or decreasing its proteasomal degradation might suppress NF-κB activation. Since AMPK activation suppressed the expression of both p47phox and p67phox, we determined whether AICAR increased the levels of IκBα in endothelial cells. Treatment of HUVECs with AICAR led to a progressive increase in IκBα protein levels (Figure 4A). Interestingly, AICAR suppressed the expression of p47phox and p67phox in HUVECs transfected with control but not IκBα siRNA (Figure 4B), implying that IκBα is required for p47phox and p67phox expression by AICAR.
Figure 4. Activation of AMPK by AICAR increases IκBα protein levels.
(A) Western blot analysis of IκBα in HUVECs treated with AICAR (2 mM) for the indicated time. The blot is a representative of blots obtained from three independent experiments. *P<0.05 vs. control. (B) Genetic inhibition of IκBα by transfection of IκBα-specific siRNA abolishes AICAR-induced reduction of p47phox and p67phox in HUVECs. The blot is a representative of three individual experiments. (C) IκBα protein levels in MAECs (WT and AMPKα2−/−) incubated with AICAR (2 mM) for 6 h (n=3). *P<0.05 vs. WT control.
We also tested whether AICAR suppresses IκBα degradation in MAECs from AMPKα2−/− mice. We found that AICAR significantly increased the levels of IκBα in MAECs from WT mice, but not in AMPKα2−/− mice (Figure 4C). Taken together, these results suggest that AMPKα2 is required for AICAR-induced elevation of IκBα in endothelial cells.
AMPK activation inhibits 26S proteasome activity in endothelial cells
The ubiquitination and consequent proteasome-dependent degradation of IκBα is critical for NF-κB transactivation in endothelial cells. Since MG132 countered the stimulatory effect of AMPKα2 deletion on p47phox and p67phox expression, we determined whether AMPK suppresses NF-κB activation by inhibiting proteasome-dependent IκBα degradation. We discovered that 26S proteasome activity was greater in MAECs from AMPKα2−/− mice than those from WT mice (Figure 5A), and that AICAR suppressed 26S proteasome activity only in WT MAECs. Another potent AMPK activator, metformin, also inhibited 26S proteasome activity in HUVECs (Figure 5B). In addition, AICAR inhibited 26S proteasome activity, in a time-dependent manner (Figure 5C). Over-expression of Ad-AMPK-DN, but not of GFP, in these cells increased 26S proteasome activity (Figure 5D). Further, adenoviral over-expression of Ad-AMPK-CA but not of GFP, significantly suppressed the activity of the 26S proteasome in HUVECs. Taken together, these results imply that inhibition of 26S proteasome by AICAR is dependent on AMPK.
Figure 5. AMPK activation suppresses 26S proteasome activity.
(A) 26S proteasome activity in WT and AMPKα2−/− MAECs treated with or without AICAR (2 mM) for 8 h (n=3) proteasome activity was assayed using fluorescent proteasome substrates. *P<0.05 vs. WT without AICAR, #P<0.05 vs. WT plus AICAR. 26S proteasome activity was also measured in HUVECs treated with (B) AICAR (2 mM) or metformin (2 mM) for 8 h (n=3) or with (C) AICAR (2 mM) for the indicated times (n=3). *P<0.05 vs. control. (D) 26S proteasome activity in HUVECs infected with Ad-GFP or Ad-AMPK-DN or ad-AMPK-CA for 24 h. *P<0.05 vs. GFP. n=3.
AMPKα2 deletion enhances oxidative stress in LDLr−/− mice
We next determined whether AMPK deletion affects the development of oxidative stress. To determine the effects of AMPKα2 deletion in HFD-induced oxidative stress, both LDLr−/−/AMPKα2−/− and LDLr−/− mice were fed an 8-week WD. Positive staining of three stable markers of reactive nitrogen species, 3-nitrotyrosine (3-NT), 4-hydroxy-2-nonenal (HNE), and malondialdehyde (MDA), were mainly found in the vascular endothelial cells of the aortic arches from both LDLr−/− and LDLr−/−/AMPKα2−/− mice (Figure 6A). Importantly, 3-NT, MDA and HNE stainings were greater in LDLr−/−/AMPKα2−/− mice than in the LDLr−/− control mice. The increased oxidative stress in LDLr−/−/AMPKα2−/− mice was further confirmed by increased ROS productions assayed by DHE/HPLC (Figure 6B), and 3-NT by Western blot (Figure 6C). Collectively, these results suggest that AMPKα2 depletion heightens oxidative stress in LDLr−/− mice.
Figure 6. Chronic administration of MG132 suppresses AMPKα2 deletion-enhanced oxidative stress, in vivo.
(A) Representatives of immunohistochemical staining and quantifications for 3-NT, MDA, and HNE positive proteins (original magnification ×400) in aortas from LDLr−/− and LDLr−/−/AMPKα2−/− mice. *P<0.05 vs. LDLr−/− control mice, #P<0.05 vs. LDLr−/−/AMPKα2−/− control mice. (B) The ROS productions were detected by DHE/HPLC in LDLr−/− and LDLr−/−/AMPKα2−/− mice aortas. *P<0.05 vs. LDLr−/− control mice. (C) The levels of 3-NT were measured by Western blot. *P<0.05 vs. LDLr−/− control mice, #P<0.05 vs. LDLr−/−/AMPKα2−/− control mice. (D) Effects of MG132 on endothelium-dependent vasorelaxation in LDLr−/− and LDLr−/−/AMPKα2−/− mice aortas. *P<0.05 vs. LDLr−/− control mice, #P<0.05 vs. LDLr−/−/AMPKα2−/− control mice. n=5–6 per group.
AMPKα2 deletion in LDLr−/− background does not affect body weight, serum lipid, or blood glucose
Next, we compared metabolic parameters between LDLr−/− and LDLr−/−/AMPKα2−/− mice. No differences in body weight, blood glucose, serum cholesterol, or triglyceride were observed between LDLr−/−/AMPKα2−/− and LDLr−/− mice (Suppl. Table I). MG132 treatment did not affect body weight, blood glucose, cholesterol, or triglyceride levels in either group (Online Table I).
Proteasome inhibition with MG132 attenuates oxidative stress and improves the endothelium-dependent vasorelaxation in LDLr−/−/AMPKα2−/−
We next determined whether the proteasome activation caused by AMPKα2 deletion contributes to increased oxidative stress in LDLr−/−/AMPKα2−/− mice. Both LDLr−/−and LDLr−/−/AMPKα2−/− mice were infused with MG132 for 6 weeks while being fed a WD. As depicted in Figure 6A, MG132 administration significantly attenuated the expression of 3-NT, MDA, and HNE in LDLr−/−/AMPKα2−/− mice, but had no effect on LDLr−/− mice, implying that chronic proteasome inhibition suppresses oxidative stress in LDLr−/−/AMPKα2−/− mice.
We then tested whether proteasome inhibition alters endothelial function in LDLr−/−/AMPKα2−/− and LDLr−/− mice. When fed a normal diet (ND), the aortas of LDLr−/−/AMPKa2−/− mice exhibited impaired endothelium-dependent vasorelaxation, compared to LDLr−/− mice (data not shown). WD decreased the endothelium-dependent response to Ach in both LDLr−/−/AMPKα2−/− and LDLr−/− mice (Figure 6D). Further, MG132 administration markedly improved the endothelium-dependent vasorelaxation of both LDLr−/− and LDLr−/−/AMPKα2−/− mice.
MG132 ablates abnormal expressions and activity of the NAD(P)H oxidase in vivo
Since apocynin partially restored Ach-induced endothelium-dependent relaxation in the aortas isolated from AMPKα2−/− and increased expression of NAD(P)H oxidase subunits in cultured MAECs from AMPKα2−/−, we proceeded to measure the expression of the NAD(P)H subunits in the aortas from LDLr−/−/AMPKα2−/− and LDLr−/− mice. As shown in Figure 7A, immunohistochemical stainings for NOX4, p47phox and p67phox subunits were noticeably increased in LDLr−/−/AMPKα2−/− mice compared to LDLr−/− mice. MG132 treatment suppressed the expressions of NOX4, p47phox and p67phox subunits in LDLr−/−/AMPKα2−/− mice. The upregulation of p47phox and NOX4, and the effect of MG132 were confirmed by western blot analysis (Figure 7B). Consistent with these observations, we found increased activity of NAD(P)H oxidase in LDLr−/−/AMPKα2−/− compared to LDLr−/− counterparts (Figure 7C). Importantly, MG132 suppressed NAD(P)H oxidase activity in both LDLr−/− and LDLr−/−/AMPKα2−/− mice (Figure 7C). Collectively, our results suggest that proteasome inhibition suppresses abnormal expression and activity of NAD(P)H oxidase in LDLr−/−/AMPKα2−/− mice.
Figure 7. MG132 abrogates AMPKα2 deletion-increased expression of NAD(P)H oxidase subunits and NAD(P)H oxidase activity, in vivo.
(A) Representatives of immunohistochemical staining and quantifications for NOX4, p47phox, and p67phox in the aortas of LDLr−/− and LDLr−/−/AMPKα2−/− mice (original magnification ×400). *P<0.05 vs. LDLr−/− control mice, #P<0.05 vs. LDLr−/−/AMPKα2−/− control mice. (B) Western blot analysis of NOX4 and p47phox in LDLr−/− and LDLr−/−/AMPKα2−/− mice aortas. *P<0.05 vs. LDLr−/− control mice, #P<0.05 vs. LDLr−/−/AMPKα2−/− control mice. n=4 mice in each group. (C) Quantitative analysis of NAD(P)H oxidase activity in LDLr−/− and LDLr−/−/AMPKα2−/− mice aortas. *P<0.05 vs. LDLr−/− ND control mice, #P<0.05 vs. LDLr−/− WD control mice, $P<0.05 vs. LDLr−/−/AMPKα2−/− WD control mice. n=4 mice in each group.
MG132 suppresses the reduction of IκBα and NF-κB translocation in LDLr−/−/AMPKα2−/− mice aortas
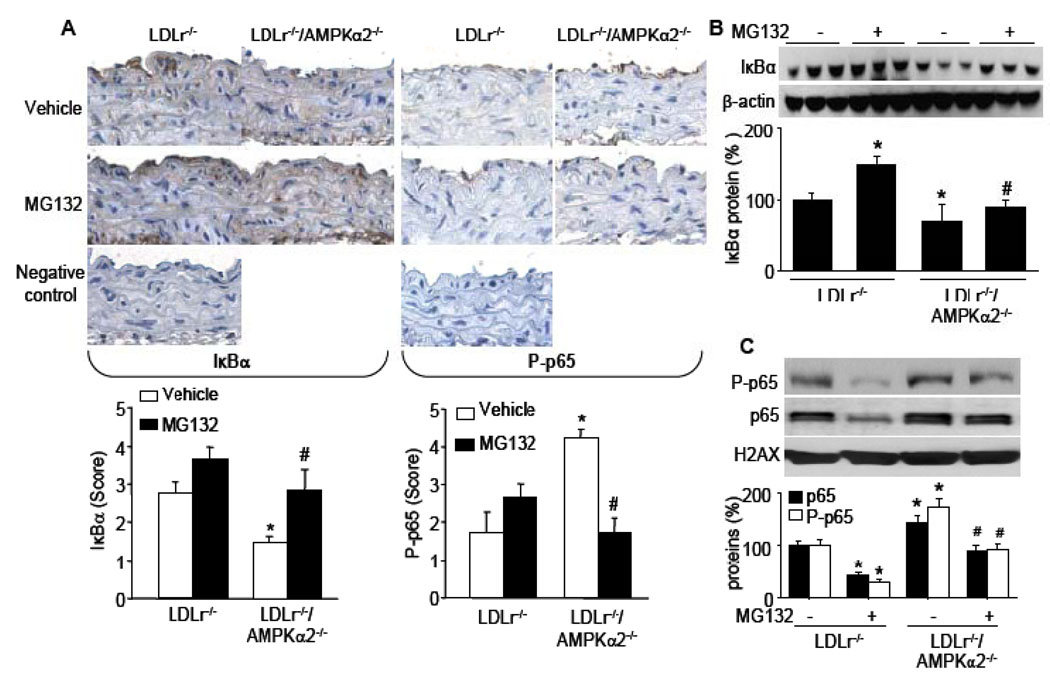
NF-κB is anchored and inactivated in the cytoplasm by association with IκBα23. Thus, it was important to examine the levels of IκBα in aortas of LDLr−/− and LDLr−/−/AMPKα2−/− mice. As expected, immunohistochemical staining of IκBα was mainly found in endothelial cells, although weak staining was also found in other cell types. Importantly, IκBα staining in endothelial cells was reduced in LDLr−/−/AMPKα2−/− mice compared to LDLr−/− mice (Figure 8A). Further, MG132 treatment increased the expression IκBα in LDLr−/−/AMPKα2−/− mice, as demonstrated by immunohistochemical staining. The change of IκBα protein was further confirmed by Western blot (Figure 8B).
Figure 8. Chronic administration of MG132 attenuates AMPKα2 deletion-induced reduction of IκBα and NF-κB transactivation, in vivo.
(A) Representatives of immunohistochemical staining and quatifications for IκBα and P-p65 in aortas of LDLr−/− and LDLr−/−/AMPKα2−/− mice (original magnification ×400). *P<0.05 vs. LDLr−/− control mice, #P<0.05 vs. LDLr−/−/AMPKα2−/− control mice. (B) Western blot analysis of IκBα in LDLr−/− and LDLr−/−/AMPKα2−/− mice aortas. *P<0.05 vs. LDLr−/− control mice, #P<0.05 vs. LDLr−/−/AMPKα2−/− control mice. n=4 mice in each group. (C) Western blot analysis of nucleus-localized p65 and P-p65 proteins in LDLr−/− and LDLr−/−/AMPKα2−/− mice aortas. *P<0.05 vs. LDLr−/− control mice, #P<0.05 vs. LDLr−/−/AMPKα2−/− control mice. n=4 mice in each group.
Phosphorylation of the p65 subunit of NF-κB increases its binding affinity to targeted genes and phospho-p65 (P-p65) is a marker for NF-κB activation24. We therefore measured the levels of P-p65 in both LDLr−/−/AMPKα2−/− and LDLr−/− mice. Compared to LDLr−/−, the levels of P-p65 at Ser536 were dramatically increased in the aortas of LDLr−/−/AMPKα2−/− mice (Figure 8A). Consistent with this observation, the levels of p65 and P-p65 in the nuclear extracts were higher in LDLr−/−/AMPKα2−/− mice than LDLr−/− mice (Figure 8C), as assayed by western blot. As expected, MG132 treatment suppressed the phosphorylation of p65 in nucleus and p65 nuclear translocation in LDLr−/−/AMPKα2−/− mice (Figure 8C). Taken together, our data suggest AMPKα2 deletion increases IκBα degradation and NF-κB translocation, all of which are sensitive to MG132.
AMPKα2 depletion increases the levels of cytokines in serum
The proinflammatory cytokines IL-2 and IFNγ in serum were significantly increased in LDLr−/−/AMPKα2−/− mice (Online Table II). After MG132 treatment for 6 weeks, the levels of these cytokines in LDLr−/−/AMPKα2−/− mice were decreased to the levels seen in LDLr−/− mice. Interestingly, the anti-inflammatory cytokines IL-5 and IL-10 were also significantly increased in LDLr−/−/AMPKα2−/− mice and were down-regulated by MG132 treatment (Online Table II).
Discussion
This study demonstrates for the first time that a global deletion of AMPKα2 in vivo causes accelerated oxidative stress and endothelial dysfunction, all of which are abrogated by chronic administration of the proteasome inhibitor, MG132. Mechanistically, this phenotype is attributable to increased activity of the 26S proteasome and accelerated degradation of IκBα, resulting in excessive activation of NF-κB and consequent NAD(P)H oxidase expression and activity. As 26S proteasome-dependent IκBα degradation is a key step in NF-κB activation, these findings suggest that AMPK-dependent suppression of 26S proteasome activity and consequent suppression of NAD(P)H oxidase expression reduces oxidative stress and, in this way, maintains the non-atherogenic and non-inflammatory phenotype of endothelial cells.
One of the major findings of this paper is that AMPKα2 deletion increases oxidative stress by increasing NAD(P)H oxidase-derived ROS in endothelial cells. Importantly, we have identified NAD(P)H oxidase as the main source of ROS and have shown that AMPK depletion increases the expression of NAD(P)H oxidase subunits, in vivo. Accordingly, inhibition of NAD(P)H oxidase abolished ROS production and endothelial dysfunction in AMPKα2−/− mice. Further, scavenging of ROS with tempol, or inhibition of NAD(P)H oxidase with apocynin, significantly reversed the impairments in endothelium-dependent relaxation resulting from AMPKα2 deficiency. Chronic administration of MG132, a potent proteasome inhibitor, suppressed endothelium-dysfunction, inflammation, oxidative stress, and NAD(P)H oxidase expression in LDLr−/−/AMPKα2−/−. Collectively, our results suggest that AMPKα2 functions as a physiological suppressor of NAD(P)H oxidase and ROS production in endothelial cells. This conclusion is also supported by several published studies done in cultured cells 8, 10, 25, which have shown that activation of AMPK inhibits ROS production induced by high glucose. However, two recent studies26, 27 reported that AICAR potentates high glucose-induced mitochondrial ROS production and subsequent β-cell apoptosis through fatty acid oxidation. The reason for this discrepancy might be related to differences in the cell types used. Thus, these results obtained with HUVEC and MAEC should be interpreted with caution as HUVEC and MAEC are not the same and results from these two cell types may not necessarily be interchangeable.
We have also proposed a novel mechanism explaining how AMPK regulates the expression and activity of NAD(P)H oxidase in vivo. Increasing evidence shows that NAD(P)H oxidase subunits are transcriptionally upregulated in response to certain cytokines (TNFα, IFNγ, IL-15), leading to increased or prolonged ROS production in endothelial cells28, 29. NF-κB triggers NAD(P)H oxidase gene transcription in several cell types21. However, to the best of our knowledge, there is no evidence that NF-κB-dependent NAD(P)H oxidase is operating in endothelial cells, in vivo. The data presented here clearly indicate that AMPK regulates NF-κB activation by inhibiting the 26S proteasome-dependent IκBα degradation pathway. The ability of NF-κB inhibition to decrease p67phox and p47phox protein levels in the absence of a stimulus suggests that basal expression of these subunits is regulated by the NF-κB pathway. These results are consistent with several recent reports30, 31 demonstrating that AMPK activation by metformin or AICAR inhibits NF-κB activation by decreasing IKK-dependent IκBα phosphorylation in endothelial cells.
The most important finding of our study is that chronic inhibition of 26S proteasome with MG132 abrogates vascular inflammation and endothelial dysfunction in vivo. The ubiquitin proteosome system acts to fine tune the intracellular levels of these factors to maintain optimal cell division, growth, differentiation, signal transduction, and stress responses. In addition, the ubiquitin proteasome system plays a key role in protein quality control by removing damaged, oxidized, and/or misfolded proteins. Structurally, the 26S proteasome consists of a catalytic core, the 20S proteasome, and a multisubunit regulatory protein, called PA700, which confers ATP/ubiquitin-dependent proteolytic properties to the 26S proteasome32. The 26S proteasome can also degrade proteins in an ATP-dependent and ubiquitin-independent fashion33. Proteasome-dependent degradation of IκBα, in particular, might be essential for the initiation and progression of atherosclerosis, as it unmasks the nuclear localization signal within NF-κB dimers, allowing them to translocate to the nucleus and induce transcription of NAD(P)H oxidase subunits and proinflammatory cytokines. Thus, increased ubiquitin-proteasome activity may enhance cellular expression of NF-κB target genes, an event that may be a crucial step in the pathophysiology of atherosclerosis progression. Our data demonstrates that chronic administration of MG132 abrogates abnormal expression of NAD(P)H oxidase, ROS, and endothelial dysfunction in LDL−/−/AMPKα2−/−. Consistent with this observation, ubiquitin-proteasome systems and NF-κB activation have been associated with coronary and carotid plaque instability34. Currently, there is no evidence suggesting that NAD(P)H oxidase subunits such as p22phox, p67phox, p47phox are subjected to ubiquitination and proteasomal degradation by 26S proteasome. Thus, an increased level of NAD(P)H oxidase is not likely due to their decreased rates of its degradation. Consistently, a recent study report that AMPK activation by AICAR and metformin inhibits 26S proteasome in vitro35.
The 26S proteasome is composed of the 20S catalytic core, where the proteins are degraded, as well as one or two 19S regulatory complexes. In our experiments Western blot analysis revealed that there was no significant difference among protein levels of PA700 and β7 between wild type and AMPK-knockout mice (Xu, et al., unpublished observations). It is likely AMPK might alter 26S proteasome activity by altering the phosphorylation status of proteasome subunits and/or the interaction of 19S and 20S proteasomes. In addition, dysfunctional NO release might also contribute to 26S proteasome activation in AMPKα2−/− mice since NO is reported to suppress the catalytic activity of the 26S proteasome36 and AMPK can upregulate eNOS phosphorylation with increased NO production3, 5. Further studies are warranted.
In summary, we are the first to report that AMPK α2 deletion increases the expression and activity of NAD(P)H oxidase, ROS production and endothelial dysfunction, all of which are abrogated by chronic administration of MG132. Our results suggest AMPK might function as a physiological suppressor of NF-κB and NF-κB-dependent NAD(P)H oxidase, two major players in cardiovascular diseases, including atherosclerosis and diabetes. In line with these observations, metformin, a widely used anti-diabetic drug, was recently shown to exert its therapeutic effect in diabetes by activating AMPK18. In large-scale clinical trials, metformin also improved vascular function and dramatically reduced cardiovascular endpoints and mortality in type 2 diabetic patients37. Thus, the current study might provide rationales for pharmacological inhibition of 26S proteasome or AMPK-activating reagents in treating oxidative stress related cardiovascular diseases.
Novelty and Significance
What Is Known?
NAD(P)H oxidase is a major source of oxidants in cardiovascular diseases.
Aberrant 26S proteasome activation contributes to atherosclerotic lesions.
AMP-activated protein kinase (AMPK), a cellular energy sensor, is reported to inhibit oxidative stress and inflammation.
What New Information Does This Article Contribute?
Deletion of AMPKα2 triggers aberrant oxidative stress and endothelial dysfunction by increasing the expression of NAD(P)H oxidase subunits and NAD(P)H oxidase activity.
Chronic inhibition of 26S proteasome abrogates aberrant expression and activity of NAD(P)H oxidase, oxidative stress, vascular inflammation, and endothelial dysfunction in vivo.
AMPK is identified as a physiological suppressor of both 26S proteasome activity and NAD(P)H oxidase in endothelial cells.
AMPK is a potential therapeutic target for correcting vascular dysfunction associated with common diseases including aging, obesity, diabetes, hypertension, and atherosclerosis
AMPK is a highly conserved kinase that acts as a sensor of cellular energy status. In this manuscript, we report for the first time that genetic deletion of AMPKα2 in LDL receptor knockout (LDLr−/−) mice markedly increased 26S proteasome activity, endothelial dysfunction, aortic lesion area, inflammation, oxidative stress, and NAD(P)H oxidase expression, all of which were largely suppressed by chronic administration of MG132, a potent and cell permeable proteasome inhibitor. Mechanistically, this phenotype is attributable to an increase in the activity of the 26S proteasome and accelerated degradation of IκBα resulting in excessive activation of NF-κB and consequent NAD(P)H oxidase expression and activity. As 26S proteasome-dependent IκBα degradation is a key step in NF-κB activation, these findings suggest that AMPK-dependent suppression of 26S proteasome activity and the consequent suppression of NAD(P)H oxidase expression reduce oxidative stress, and therefore maintains the non-angiogenic, non-inflammatory, and atherosclerotic-resistant phenotype of vascular cells. The observations reported herein uncover a new facet of AMPK, which may link oxidant stress, energy metabolism, and vascular biology. Our novel findings support the notion that AMPK is a potential therapeutic target for combating vascular dysfunction associated with common diseases including aging, obesity, diabetes, hypertension, and atherosclerosis.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by NIH grants (HL079584, HL074399, HL080499, HL089920, and HL096032), as well as by research awards from the American Diabetes Association, Juvenile Diabetes Research Foundation, Oklahoma Center for Advancement of Science and Technology, and Travis Endowed Chair in Endocrinology, University of Oklahoma Health Sciences Center. Dr. M.H. Zou is a recipient of National Established Investigator Award of American Heart Association. Drs. J. Xu and Z. Xie are supported by Scientist Development Award from American Heart Association.
Non-standard abbreviations
- ACh
acetylcholine
- Ad-AMPK-CA
adenovirus encoding constitutively active AMPK mutants
- Ad-AMPK-DN
adenovirus encoding AMPK dominant negative mutants
- AICAR
5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside
- BCA
bicinchoninic acid
- DHE
dihydroethidium
- DTT
dithiothreitol
- HNE
4-hydroxy-2-nonenal
- IFN
interferon
- IL
interleukin
- MAECs
mouse aortic endothelial cells
- MDA
malondialdehyde
- ND
normal diet
- NOX
NAD(P)H oxidase
- 3-NT
3-nitrotyrosine
- PEG-SOD
Polyethylene glycol SOD
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- U46619
9,11-dideoxy-11-,9-epoxymethanoprostaglandin F2
- WD
western diet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier L, Hebrard S, Devin-Leclerc J, Beauloye C, Foretz M, Andreelli F, Ventura-Clapier R, Bertrand L. AMPK: Lessons from transgenic and knockout animals. Front Biosci. 2009;14:19, 44. doi: 10.2741/3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulz E, Anter E, Zou MH, Keaney JF., Jr Estradiol-mediated endothelial nitric oxide synthase association with heat shock protein 90 requires adenosine monophosphate-dependent protein kinase. Circulation. 2005;111:3473–3480. doi: 10.1161/CIRCULATIONAHA.105.546812. [DOI] [PubMed] [Google Scholar]
- 3.Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 4.Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA. Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem. 2002;277:32552–32557. doi: 10.1074/jbc.M204512200. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, Zhu Y, DeFea K, Pan S, Tsai MD, Shyy JY. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, Hirata Y. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004;110:444–451. doi: 10.1161/01.CIR.0000136025.96811.76. [DOI] [PubMed] [Google Scholar]
- 7.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5'-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 9.Ouslimani N, Peynet J, Bonnefont-Rousselot D, Therond P, Legrand A, Beaudeux JL. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism. 2005;54:829–834. doi: 10.1016/j.metabol.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55:120–127. [PubMed] [Google Scholar]
- 11.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 12.Viollet B, Andreelli F, Jorgensen SB, Perrin C, Flamez D, Mu J, Wojtaszewski JF, Schuit FC, Birnbaum M, Richter E, Burcelin R, Vaulont S. Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem Soc Trans. 2003;31:216–219. doi: 10.1042/bst0310216. [DOI] [PubMed] [Google Scholar]
- 13.Lassegue B, Griendling KK. NADPH Oxidases: Functions and Pathologies in the Vasculature. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31(Suppl 2):S170–S180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 15.Lee MY, San Martin A, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause KH, Banfi B, Lambeth JD, Lassegue B, Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Xu J, Song P, Viollet B, Zou MH. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes. 2009;58:1893–1901. doi: 10.2337/db09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 19.Alba G, El Bekay R, Alvarez-Maqueda M, Chacon P, Vega A, Monteseirin J, Santa Maria C, Pintado E, Bedoya FJ, Bartrons R, Sobrino F. Stimulators of AMP-activated protein kinase inhibit the respiratory burst in human neutrophils. FEBS Lett. 2004;573:219–225. doi: 10.1016/j.febslet.2004.07.077. [DOI] [PubMed] [Google Scholar]
- 20.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 21.Gauss KA, Nelson-Overton LK, Siemsen DW, Gao Y, DeLeo FR, Quinn MT. Role of NF-kappaB in transcriptional regulation of the phagocyte NADPH oxidase by tumor necrosis factor-alpha. J Leukoc Biol. 2007;82:729–741. doi: 10.1189/jlb.1206735. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima H, Fujiwara H, Furuichi Y, Tanaka K, Shimbara N. A novel small-molecule inhibitor of NF-kappaB signaling. Biochem Biophys Res Commun. 2008;368:1007–1013. doi: 10.1016/j.bbrc.2008.01.166. [DOI] [PubMed] [Google Scholar]
- 23.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 24.Goon Goh F, Sloss CM, Cunningham MR, Nilsson M, Cadalbert L, Plevin R. G-protein-dependent and -independent pathways regulate proteinase-activated receptor-2 mediated p65 NFkappaB serine 536 phosphorylation in human keratinocytes. Cell Signal. 2008;20:1267–1274. doi: 10.1016/j.cellsig.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Ouedraogo R, Wu X, Xu SQ, Fuchsel L, Motoshima H, Mahadev K, Hough K, Scalia R, Goldstein BJ. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes. 2006;55:1840–1846. doi: 10.2337/db05-1174. [DOI] [PubMed] [Google Scholar]
- 26.Cai Y, Martens GA, Hinke SA, Heimberg H, Pipeleers D, Van de Casteele M. Increased oxygen radical formation and mitochondrial dysfunction mediate beta cell apoptosis under conditions of AMP-activated protein kinase stimulation. Free Radic Biol Med. 2007;42:64–78. doi: 10.1016/j.freeradbiomed.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Kim WH, Lee JW, Suh YH, Lee HJ, Lee SH, Oh YK, Gao B, Jung MH. AICAR potentiates ROS production induced by chronic high glucose: roles of AMPK in pancreatic beta-cell apoptosis. Cell Signal. 2007;19:791–805. doi: 10.1016/j.cellsig.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Nwariaku FE, Liu Z, Zhu X, Nahari D, Ingle C, Wu RF, Gu Y, Sarosi G, Terada LS. NADPH oxidase mediates vascular endothelial cadherin phosphorylation and endothelial dysfunction. Blood. 2004;104:3214–3220. doi: 10.1182/blood-2004-05-1868. [DOI] [PubMed] [Google Scholar]
- 29.Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 30.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 31.Giri S, Nath N, Smith B, Viollet B, Singh AK. Singh I. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci. 2004;24:479–487. doi: 10.1523/JNEUROSCI.4288-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka K. Proteasomes: structure and biology. J Biochem. 1998;123:195–204. doi: 10.1093/oxfordjournals.jbchem.a021922. [DOI] [PubMed] [Google Scholar]
- 33.Lam YA, DeMartino GN, Pickart CM, Cohen RE. Specificity of the ubiquitin isopeptidase in the PA700 regulatory complex of 26 S proteasomes. J Biol Chem. 1997;272:28438–28446. doi: 10.1074/jbc.272.45.28438. [DOI] [PubMed] [Google Scholar]
- 34.Marfella R, Siniscalchi M, Portoghese M, Di Filippo C, Ferraraccio F, Schiattarella C, Crescenzi B, Sangiuolo P, Ferraro G, Siciliano S, Cinone F, Mazzarella G, Martis S, Verza M, Coppola L, Rossi F, D'Amico M, Paolisso G. Morning blood pressure surge as a destabilizing factor of atherosclerotic plaque: role of ubiquitin-proteasome activity. Hypertension. 2007;49:784–791. doi: 10.1161/01.HYP.0000259739.64834.d4. [DOI] [PubMed] [Google Scholar]
- 35.Viana R, Aguado C, Esteban I, Moreno D, Viollet B, Knecht E, Sanz P. Role of AMP-activated protein kinase in autophagy and proteasome function. Biochem Biophys Res Commun. 2008;369:964–968. doi: 10.1016/j.bbrc.2008.02.126. [DOI] [PubMed] [Google Scholar]
- 36.Kapadia MR, Eng JW, Jiang Q, Stoyanovsky DA, Kibbe MR. Nitric oxide regulates the 26S proteasome in vascular smooth muscle cells. Nitric Oxide. 2009;20:279–288. doi: 10.1016/j.niox.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Mamputu JC, Wiernsperger NF, Renier G. Antiatherogenic properties of metformin: the experimental evidence. Diabetes Metab. 2003;29:6S71–6S76. doi: 10.1016/s1262-3636(03)72790-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.