Abstract
Background
Although HPV infections are common in young women, the rate of and risk for repeated new infections are not well documented. We examined the rate of and risks for new HPV detection in young women.
Methods
We used data from an ongoing study of HPV, initiated in 1990. Sexually active women aged 12–22 years were eligible. Interviews on behaviors and HPV testing were performed at 4-month intervals; sexually transmitted infection (STI) testing was annual or if symptomatic. Starting with 1st HPV detection, time to the next (2nd) visit (event) with detection of new HPV types and then the 2nd event to time to 3rd event was calculated. Risks were determined using Cox Proportional hazard model.
Results
Sixty-nine percent of 1,125 women had a 2nd event and of those with a 2nd event, 63% had a 3rd event by 3 years, respectively. Women with HPV persistence from initial visit to 2nd event [Hazard ratio (H.R.) = 4.51 (3.78 – 5.37)], an STI (H.R. = 1.47 (1.00 – 2.17), bacterial vaginosis (H.R. = 1.60 (1.07 – 2.39), and number of new sex partners (H.R. = 1.10 (1.05 – 1.15 per partner/month) were independent associations for HPV. Risks for 3rd event were similar.
Conclusion
This study documents the repeated nature of HPV infections in young women and their association with sexual risk behaviors.
Impact
This finding underscores the lack of clinical utility of HPV testing in young women. Further studies are needed to examine host factors that lead to HPV acquisition and persistence.
Keywords: HPV infections, adolescents, risk behavior, sexually transmitted infections
INTRODUCTION
Prevalence and incidence studies have repeatedly demonstrated the common nature of human papillomavirus (HPV) infections in young women with prevalence rates averaging 20% and incident rates reaching 50% within 3–4 years after the initiation of sexual activity. (1–3) Most of these infections belong to HPV’s high risk category (i.e. those associated with cancer). Risks for acquisition of cervical infections are almost exclusively those associated with sexual behavior with a recent new sexual partner reflecting the strongest risk. (2–5) Although a marker of sexual risk, associations with sexually transmitted infections (STIs) including HIV have been less consistent. In part, the inconsistency may be due to the lack of documented STIs preceding HPV infection. Since STI’s induce inflammation, they may reflect a biologic risk in that they allow access to basal epithelial cells, the key portal for viral entry. Using serologic evidence, we previously showed that a previous herpes simplex virus (HSV) infection was an important risk for acquisition. (2) Although C. trachomatis has been shown to be a risk for invasive cervical cancer (6), most prospective studies of incident HPV either have not measured past history or have not found an association with C. trachomatis and subsequent incident of HPV. (2–4, 7) In contrast there have been a few cross-sectional studies showing a higher rate of C. trachomatis in women with HPV than without. (8)
It has been proposed that repeated infections with new HPV types in young women are equally common as initial infections, hence, new guidelines for cervical cytology screening have precluded HPV DNA testing in young women. (9, 10) However, the actual rates of these “new” infections have not been well documented. In a lower socioeconomic Brazilian population aged 18 to 60 years, Roussea et al (11) found that 25–35% of women acquired a new infection within 12 months of a previously documented HPV infection.
As described above, most studies have focused on risk for first detected infection, whether prevalent or incident. In contrast, no studies to date have examined behavioral risks for repeated infections. There has been some thought that frequent detection reflects recurrence of latent infections, specifically as a women ages. Overall, the rates of HPV have been shown to decline with age. (1, 12) This would be expected since clearance of HPV in a young women leads to type specific immunity protecting her from future infections of these types. The remaining types which she has not been exposed to as a young woman are likely to be less common types helping to explain the lower incidence as a woman ages. (13) On the other hand, some studies have demonstrated a rise in HPV prevalence in perimenopausal women.(1) In addition, there have been reports of high rates of abnormal Pap smears in women over 65 years of age suggesting the recurrence of a latent infections. (14)
The aim of the study was to examine the rate of acquiring new HPV type infections after the first detected infection in a cohort of adolescents and young women and to examine risks for these repeated infections.
MATERIALS AND METHODS
Subject population
Women in this study were recruited into the Teen HPV natural history study starting in 1990. Recruitment of these women has been detailed previously. (15–18) In brief, sexually active women from 1990 to 1994 were recruited from a state university medical clinic and Planned Parenthood clinic. Women were screened for HPV DNA. (16) If positive, women were contacted for eligibility. Inclusion criteria included being between 13 and 21 years of age and having had less than 5 years of sexual experience. Women were excluded if they were immunosuppressed, currently pregnant or had a history of ablative or surgical therapy of the cervix. A smaller group of HPV negative women were randomly identified and recruited. A total of 908 women were recruited into this study. In 1999, women who were still actively participating and had become HPV negative for over two years (a minimum of 7 consecutive negative tests at 4 month intervals) were exited; 125 (31%) continued in the study after 1999. This cohort is referred to as the old cohort. Between 2000 and 2004, a second wave of recruitment (referred to as new cohort) was initiated from the same sites, however, women were randomly approached and not recruited based on HPV status. Other inclusion criteria as described above were applied. Six-hundred fifty one women were recruited into the new cohort. Although the cohort is ongoing, the data was censored as of September, 2007.
There were some differences in cohorts at baseline: the older cohort had more subjects that were white compared to the new cohort (54% vs 27%, respectively; p<0.0001); were more likely to report a past pregnancy (32% vs 18%, respectively; p<0.0001) and report a past history of C. trachomatis (23% vs 9%, respectively; p<0.0001). The older cohort also had more number of lifetime sexual partners (6.9 vs 4.5; p<0.0001). The differences were likely due to the recruitment strategies for the older cohort as well as sexual and screening practices within the intervening decade. No differences were found in regards to age, condom use, past history of other STIs and age of first intercourse.
This study was approved by the University of California, San Francisco and San Francisco State. University institutional review boards. Women were seen at baseline and 4-month intervals for interview on demographics including race and ethnicity and detailed sexual and substance use behaviors as detailed previously. (2, 16, 17) Examinations included samples for HPV DNA testing, cytology, and wet mounts for diagnosis of T. vaginalis, yeast, and bacterial vaginosis. (2, 15, 17, 18) Samples for C. trachomatis and N. gonorrhoeae were obtained at annual visits or if symptomatic and tested using amplification techniques. For one year, all samples were screened for T. vaginalis using wet mount and culture (InPouchTV, Biomed Diagnostics, White City, Oregon). Since no cases were missed by wet mount, cultures were discontinued. Subjects were encouraged to use the study clinic for all lower genital tract symptoms in order to document infections. Lesions suggested of HSV were tested by standard culture and direct fluorescent antibody. Only those lesions positive for HSV were considered HSV infected.
HPV testing
All samples were processed in the same laboratory. HPV typing for the old and new cohorts were previously described using the PGMY09/11 primer system. (17, 18) Briefly, denatured biotin-labeled PCR product was hybridized to an array of immobilized oligonucleotides: HPV types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 55, 56, 58, 59, 68, 82, 83, 73, 6, 11, 40, 42, 53, 54, 57, 66, 84 and two β-globin controls for monitoring sample adequacy. An enhanced chemiluminescence (ECL) system in a dot blot format tested for HPV 67, 70 and 72. In 2003, HPV types 61, 62, 64, 67, 70, 71, 72, 81 and 89 were added to the set of immobilized oligonucleotides and the ECL was discontinued. Samples with negative beta-globin or positive for three or more HPV types were re-prepped and re-amplified by PCR. Only those types matching in both amplifications were considered positive. Five percent of all samples were chosen at random and run in duplicate on each plate.
DATA ANALYSIS
Subjects for this analysis must have had at least one visit with a positive HPV test (whether prevalent or incident) and at least one follow-up visit following the positive HPV test. Sexual behaviors and reported sexually transmitted infections (STI) were calculated as recent (reported on the 4 month questionnaire as “since your last visit, have you had”) or cumulative. Most variables were time-dependent except race/ethnicity, age at menarche, first cigarette use, and first sexual intercourse. Baseline data for both groups has been previously reported. (2, 16, 18) Since the study has been ongoing for several years, characteristics from the last study visit (recent and/or cumulative) is shown in Table 1. Race/ethnicity was examined since disparaties among race/ethnicity have been noted for many of the STIs.
Table 1.
Demographics and Behavioral Characteristics of the Population N = 1125
| Characteristics | N (%) |
|---|---|
| Race (N %) | |
| White | 475 (42.2) |
| African-American | 157 (14.0) |
| Asian | 166 (14.8) |
| Hispanic | 264 (23.5) |
| Mixed/other | 63 (5.6) |
| Weekly alcohol use1 | 441 (39.2) |
| Weekly marijuana use1 | 180 (16.0) |
| Weekly drug2 use1 | 19 (1.7) |
| Currently smokes cigarettes1 | 251 (22.3) |
| Ever engaged in anal sex3 | 577 (51.6) |
| History of pregnancy3 | 568 (50.7) |
| History of douching3 | 790 (70.5) |
| History of genital warts3 | 276 (24.8) |
| History of reported C. trachomatis 3 | 318 (28.5) |
| History of reported N. gonorrhoeae 3 | 62 (5.6) |
| History of reported T. vaginalis 3 | 99 (8.9) |
| History of reported STI, but can’t remember the name3 | 36 (3.2) |
| History of reported genital herpes simplex3 | 146 (13.0) |
| Ectopy noted at least at one visit | 299 (26.6) |
| N. gonorrhoeae infection4 | 28 (2.5) |
| C. trachomatis infection4 | 144 (12.8) |
| T. vaginalis infection4 | 46 (4.1) |
| Herpes simplex virus4 | 25 (2.2) |
| Bacterial vaginosis4 | 253 (22.5) |
| Yeast infection4 | 290 (25.8) |
| Mean (± S. D.) | |
| Mean Age at entry (years) | 18.97 (± 2.13) |
| Mean years of sexual activity (at entry) | 2.93 (± 1.82) |
| Mean time in study (months) | 57.65 (± 47.28) |
| Mean age at menarche (years) | 12.6 (± 1.35) |
| Mean months of combined hormonal contraceptive use3, 5 | 32.5 (± 32.00) |
| Mean months of medroxyprogesterone use3, 5 | 17.5 (± 17.60) |
| Mean number of lifetime sexual partners3 | 11.63 (± 10.97) |
Reported behavior at last visit.
Other than alcohol or marijuana
Cumulative reporting by subject up to last visit
Cumulative reporting of laboratory documented infection up to last visit.
Among those using hormonal contraceptives: 19% reported ever using hormonal contraception
Kaplan-Meier estimates of the distribution of time to reinfection were based on the visit of the first detected infection (incident or prevalent) and the first visit for which at least one new HPV type was detected (termed the 2nd event). Actual event times were imputed as the midpoint of the intervals between these two visits. Separate estimates were made for groups of women defined by number of HPV types detected at the visit of first infection, including one, two, three, and four or more types. Occurrence of a new type was based on the first visit where a type distinct from the type(s) detected at the visit of first infection.
Separate estimates were also made for women with persistent first infections and those who had cleared the first infection at the time of detection of a second infection. Persistence was defined as continued detection of the first type at the 2nd event. For those with persistence, the median time of persistence prior to 2nd event was 5.4 months (interquartile range (IQR) = 4.2 – 9.8). Clearance of a particular type was defined as occurring on the first of two consecutive negative tests for that type. At least 3 consecutive follow-up visits were required for a woman to be eligible for estimates of clearance distributions. Women whose observed tests ended with a single HPV negative visit and no subsequent negative confirmatory visit were right-censored at the last positive visit. Time from the 2nd event to a 3rd event for new HPV types was defined using the same procedure just described. The median time of persistence between the 2nd and 3rd event was 8.0 months (IQR = 4.4 – 13.0). Between-group differences in estimated distributions of time to re-infection were evaluated using log-rank tests.
To assess sensitivity of Kaplan-Meier estimates of the distribution of time to the 2nd event to the inclusion of prevalent initial infections and midpoint imputation of reinfection event times, we made alternate estimates for these distributions using methods for doubly censored event time data. This approach avoids assumptions about the exact time of occurrence of the initial infection and reinfections, and uses the information about possible event times contained within the visit intervals in which the events occurred. Rather than imputing possible event times within these intervals, the estimate is based on semiparametric maximum likelihood techniques that average over possible event times in producing a final estimate. (19). Intervals for the initial infection extended from the date of first reported sexual activity to the date of the first prevalent HPV test. The interval for the 2nd event extends from the last date the person was known to be negative for the new type, to the first time observed positive with that type. The resulting estimates were compared to the Kaplan-Meier estimates at specific distribution times (1 and 3 years). We did not perform doubly censored estimates for time to 3rd event from 2nd event since all 2nd events by definition were incident.
Cox proportional hazards regression models were used to evaluate the effects of both fixed and time-dependent covariates. For the 2nd event analysis, covariates displaying marginal associations significant at 0.10 level or below were considered in multivariate regression models. Final models included variables significant at 0.05 or below, as well as those that were considered a priori as important to control for including source cohort (new vs. old) and prevalent vs. incident 1st detected HPV. The models for the 3rd event were selected in the same fashion, except that we also included the variables significant in the models for the 2nd event as potential candidate variables. Overall statistical significance of the models was measured by the likelihood ratio chi-square statistic. We examined each STI separately in the model as well as combined. HSV was examined using the cumulative lab documented data only since too few cases were available for visit specific analysis (previous or current) using lab data. For reported history, we used HSV since last visit and cumulative. We excluded HSV from the combined STI variable since HSV is considered a latent infection with known recurrences and hence would not reflect necessarily new STI exposure. HSV serology was not available. We also compared relative hazards for the effects of fixed covariates in Cox models for the 2nd event analyses to corresponding relative hazards estimated from proportional hazards models for doubly censored event times, to assess sensitivity of results to inclusion of prevalent infections. These analyses did not extend to evaluation of covariate effects for time varying covariates because regression methods for doubly censored outcomes including such covariates have not been developed.
As mentioned, in 2003, HPV types 61, 62, 64, 71, 81 and 89 were added to the set of olignonucleotides for HPV testing. Since we did not have information on these types previous to this date, the chance of acquiring HPV increased after 2003. To examine the impact these types had on the analysis, we made additional Kaplan-Meier estimates excluding these 6 types. Since the effects on resulting estimates were minimal (see text), and exclusion could also lead to bias assessment of covariate effects, we chose not to exclude them in regression models. The new types did not affect the old cohort since most had their 3rd event prior to 2003. For women with follow-up pre-and post-2003 and for whom at least one of the 6 types was detected as an event, we pulled the previous samples and retested them for the 6 types to determine if the infection was truly new. Five women were excluded from the analysis since we were unable to determine exactly when this type appeared.
RESULTS
One thousand one hundred twenty-five women, reflecting 13,775 visits, were eligible for this analysis; 635 women from the old cohort and 490 from the new cohort. Table 1 describes the demographics at baseline and sexual and substance use behavior at the last available study visit for both recent and cumulative behaviors. The mean age at end of follow-up was 24.01 years (range 14.5–39.25). The rate of reported C. trachomatis, N. gonorroeae and T. vaginalis were approximately twice as high as the laboratory documented rate. The majority of the reported infections that were not verified were from the baseline visit.
Time to 2nd and 3rd event with new HPV types
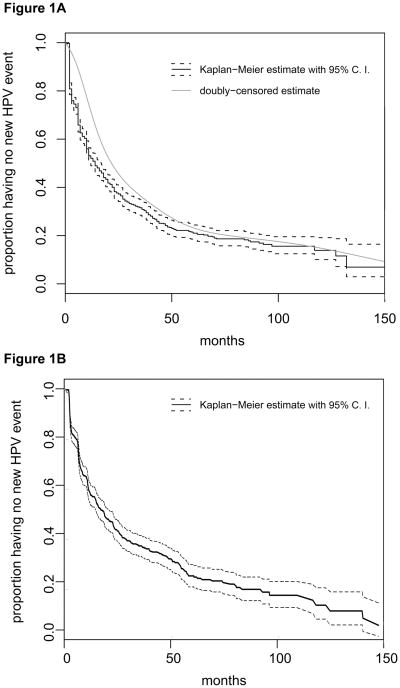
Forty-eight percent had a 2nd event with new HPV types detected by one year follow-up and 69% by three years. Of the women with a 2nd event, 43% had a 3rd event with new HPV types detected within the following year and 63% by three years. Figures 1a and 1b show the Kaplan-Meier curves for time to 2nd and 3rd event. When we ignored the 6 HPV types introduced in 2003, rates were relatively unchanged: 45% and 67% had a 2nd event by one and three years, respectively, and 42% and 62% had a 3rd event by one and three years, respectively. Because the prevalent cases may differ in risk for HPV acquisition, we made alternate estimates for these distributions for the 2nd event using methods for doubly censored event time data as described above. Using this method, we found that 28% had a 2nd event by one year and 64% by three years. This showed that the prevalent cases may bias early estimates of 2nd HPV events but that this difference lessens over time. Comparison of the two estimates of 2nd HPV events is shown in Figure 1a.
Figure 1. Time to 2nd and 3rd event with new HPV infections.
Figure A is time to 2nd event with new HPV types among those with a 1st event (prevalent or incident). The thick black line represents the Kaplan-Meier curve estimates and the dark dashed line is the 95% confidence intervals (C.I.). The thin black line represents the interval-based curve estimated using methods for doubly-censored outcomes. Figure B is time to 3rd event with new HPV types among those with a 1st and 2nd event. Only the Kaplan-Meier curve estimate and 95% C.I. is shown since the methods for double-censored outcomes is not relevant to the estimate for time to 3rd event.
Multiple vs Single HPV type infections
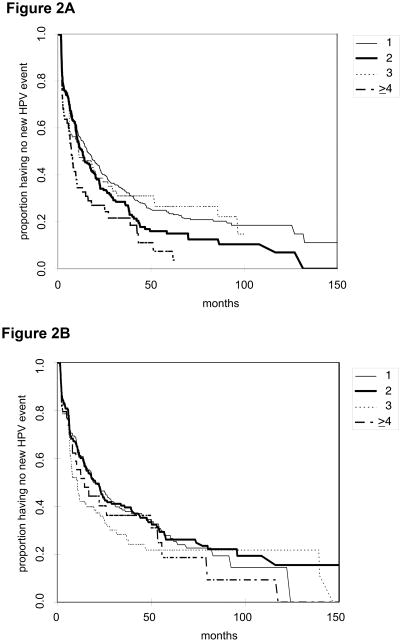
Forty-four percent of women with a single HPV type detected at the 1st event had a 2nd event by one year of follow-up and 67% by three years. The corresponding percentages for one and three years are 49% and 73% for women with two HPV types, 53% and 69% for women with three types, and 65% and 78% for women with four or more types. Overall, the difference between two types and single type is statistically significant (p=0.04), as well as the difference between four or more types and single type (p=0.001), two types (p=0.03), and three types (p=0.02). Figure 2 shows the Kaplan-Meier curves for multiple vs single types.
Figure 2. Time to 2nd (Figure A) and 3rd (Figure B) event with new HPV types by number of HPV types detected previously.
1, 2, 3, and greater than 4 refer to the number of HPV types detected at 1st and 2nd events in figures A and B, respectively.
For time from 2nd event to 3rd event, 39% of women with one HPV type at a 2nd event had a 3rd event by one year and 62% by 3 years. The corresponding percentages for one and three years are 39% and 60% for women with two HPV types at a 2nd event, 58% and 72% for women with three types, and 45% and 64% for women with four or more types. Overall, there is no significant difference between number of types in time to the 3rd event.
Persistence vs clearance
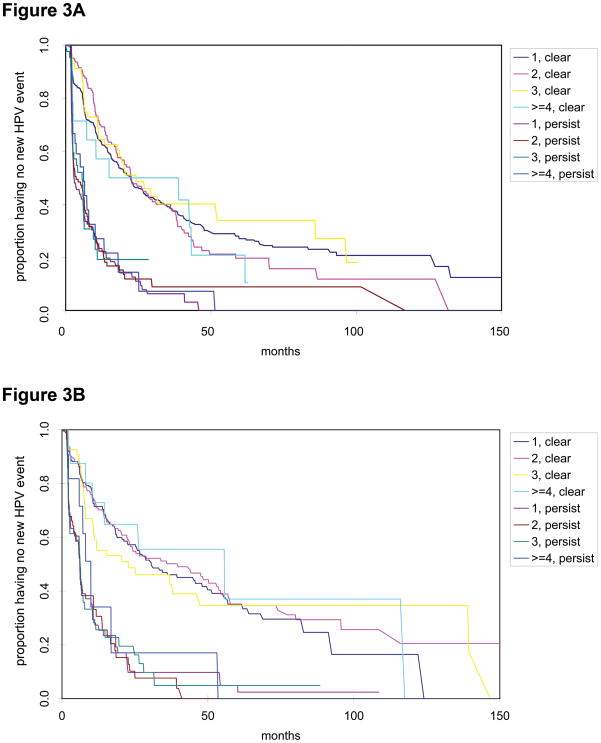
We next examined rate of acquisition by whether any of the types detected at the 1st event persisted and by presence of multiple types. Figure 3 shows that persistence of any of the HPV types detected at the 1st event highly influenced the rate of acquisition independent of whether there were multiple or single types present. Overall, for those with a 1st event, 35% of women acquired a new HPV type within one year and 60% within 3 years if the initial infection was cleared. In comparison, 78% of women acquired a new HPV type within one year and 93% within 3 years if one HPV type from the 1st event persisted (p<0.0001). When we examined the incident cases only for first detected HPV visit, the results were consistent (data not shown). Among those with a 2nd event, 30% of women had a 3rd visit with a new HPV type within one year and 52% had a 3rd visit within 3 years if the previous infection cleared. In comparison, 72% and 91% had a 3rd visit with new HPV types within one and three years, respectively, if an HPV type from the 2nd event continued to persist to 3rd event (p<0.0001).
Figure 3. Time to acquisition of a 2nd and 3rd event with HPV by number of HPV types and history of persistence vs. clearance.
Clear refers to those who clear their HPV types from the 1st event prior to 2nd event (A) or 2nd to 3rd event (B). Persist refers to those with a persistent HPV type from 1st event to the 2nd event (A) or 2nd to 3rd event (B). 1, 2, 3, and greater than 4 refers to number of types at the 1st and 2nd event in figures A and B, respectively.
Univariate associations for new HPV type detection
Univariate associations using Cox proportional hazards model for the 2nd and 3rd acquisitions with new HPV types are summarized in Table 2. In addition to persistent infection, associations for the 2nd HPV event (new incident infection) included cohort (new vs old), illicit drug use, medroxyprogesterone acetate use, irregular menses, number of new sexual partners in past 8 months, total number of lifetime partners, recent history of reported anal sex, African American race (vs white), and Asian/Pacific Islander (vs white). Older age, greater number of years sexually active, and current oral contraceptive use were protective.
Table 2.
Univariate assocations for risk of infection with new HPV types detected at 2nd and 3rd event
| Hazard Ratio (95% confidence interval) | ||
|---|---|---|
| 2nd event1 | 3rd event1 | |
| New cohort2 (vs. old) | 1.76 (1.5 – 2.05) | 2.29 (1.85 – 2.83) |
| Persistent HPV infection3 (vs. cleared) | 3.5 (2.97 – 4.15) | 3.51 (2.85 – 4.33) |
| Age (per year) | 0.92 (0.88 – 0.96) | 0.84 (0.78 – 0.92) |
| Years sexually active (per year) | 0.93 (0.89 – 0.97) | 0.89 (0.84 – 0.95) |
| Weekly drug use | 1.8 (1.01 – 3.2) | NS |
| Currently smoking cigarettes | NS | 1.23 (0.98 – 1.54) |
| Current oral contraceptive use | 0.86 (0.73 – 1.00) | 0.75 (0.61 – 0.94) |
| Months of medroxyprogesterone use (per month) | 1.01 (1.003 – 1.026) | 1.02 (1.009 – 1.032) |
| Irregular mense (greater than 36 days since last menstrual period) | 1.26 (1.02 – 1.56) | 1.38 (1.01 – 1.88) |
| # of new partners per month in last 8 months (per partner) | 1.08 (1.04 – 1.12) | 1.09 (1.05 – 1.13) |
| # of lifetime sexual partners (per partner) | 1.02 (1.007 – 1.03) | NS |
| Recent4 history of anal sex | 1.26 (0.99 – 1.6) | 1.27 (0.94 – 1.7) |
| Recent4 history of douching | NS | 1.26 (0.98 – 1.64) |
| African American (vs White) | 1.69 (1.33 – 2.4) | NS |
| Asian/Pacific Islander (vs White) | 1.35 (1.06 – 1.72) | NS |
| Reported history of infections | ||
| Recent4 Bacterial Vaginosis | 1.5 (1.03 – 2.12) | 1.55 (1.0 – 2.39) |
| Recent4 N. gonorrhoeae | 5.08 (2.40 – 10.8) | 7.87 (1.89 – 32.74) |
| Recent4 C. trachomatis | 1.5 (0.99 – 2.24) | 2.07 (1.23 – 3.48) |
| Recent4 HSV | 2.07 (1.39 – 3.07) | NS |
| Recent4 STI5 | 1.8 (1.3 – 2.6) | 1.74 (1.08 – 2.81) |
| Reported4 history of ever having an STI5 | 1.33 (1.13 – 1.56) | NS |
| Recent4 STI, but cannot remember name | 7.5 (2.35 – 23.89) | NS |
| Recent4 reported genital warts | NS | 1.65 (.98 – 2.77) |
| Laboratory-documented infections | ||
| C. trachomatis (current) | NS | NS |
| C. trachomatis (previous visit)6 | 2.1 (1.4 – 3.11) | 3.02 (1.88 – 4.87) |
| C. trachomatis (cumulative history) | 1.59 (1.24 – 2.03) | 1.72 (1.33 – 2.23) |
| N. gonorrhoeae (current) | NS | 7.79 (1.87 – 32.5) |
| N. gonorrhoeae (previous visit)6 | 4.78 (2.3 – 10.14) | NS |
| N. gonorrhoeae (cumulative history) | 1.68 (0.95 – 2.97) | NS |
| Bacterial vaginosis (current) | 1.53 (1.06 – 2.21) | 1.99 (1.36 – 2.89) |
| Bacterial vaginosis (previous visit)6 | 1.59 (1.1 – 2.31) | 1.69 (1.12 – 2.54) |
| Bacterial vaginosis (cumulative history) | 1.56 (1.24 – 1.95) | 2.25 (1.77 – 2.85) |
| Any STI7 (current) | NS | NS |
| Any STI7 (previous visit)6 | 2.54 (1.79 – 3.61) | 2.62 (1.67 – 4.12) |
| Any STI7 (cumulative history) | 1.76 (1.37 – 2.15) | 1.60 (1.24 – 2.03) |
| Greater than WBC/hpf on wet mount (current) | 0.52 (0.26 – 1.04) | NS |
All subjects begin with a known initial HPV infection. 2 nd event reflect the visit at which an HPV type is detected which was not previously detected at the initial visit. 3 rd event reflects the visit after the 2 nd event in which a new HPV type was detected.
New cohort reflects those women recruited starting in 2000; old cohort reflects women recruited from 1990 – 1994.
For incident 2 nd event, persistence is defined as persistence of a type found at initial HPV event to 2 nd event. For 3 rd event, persistence is defined as persistence of a type found at the 2 nd event to 3 rd event.
Recent is reported to have infection/behavior since the last visit. For HSV, refers to new or recurrent infection
Sexually transmitted infections (STI) include C. trachomatis, N. gonorrhoeae, T. vaginalis, and an infection that the subject cannot remember the name.
Includes any interim visits between previous and current visit but excludes current.
Includes C. trachomatis, N. gonorrhoeae, and T. vaginalis.
NS = not significant
Other variables found not significant (p greater than 0.1) include yeast (history or lab documented), condom use, T. vaginalis (history or lab), herpes simplex virus (lab), alcohol or marijuana use, smoking history, monogamy, menarchael age, reported number of STI at baseline, and pregnancy history.
When looking at reported STI history, self-report of a recent case of N. gonorrhoeae, C. trachomatis, HSV, bacterial vaginosis or an unspecified STI were all associated with acquisition of a new HPV type. A cumulative report of having had a prior STI (included N. gonorrhoeae, C. trachomatis and T. vaginalis) or HSV was also significant. When we examined laboratory documented infections, having a current STI (same visit as incident HPV) was not a risk. However, having a documented STI (inclusive of C. trachomatis, N. gonorrhoeae and T. vaginalis) at the previous visit or prior to the incident visit was a significant risk. More specifically this was true for C. trachomatis and N. gonorrhoeae but not T. vaginalis. Cumulative history was also significant for C. trachomatis and N. gonorrhoeae. Having a current, previous or cumulative history of bacterial vaginosis was also a significant risk for acquiring a new HPV type. The presence of greater than 10 polymorphonuclear cells per high powered field (PMNs/hpf) on wet mount at the incident visit was protective. Relative hazards for the effect of fixed covariates estimated using the doubly censored approach were comparable to corresponding estimates from Cox models (data not shown).
Similar associations were found for 3rd HPV event for the following factors: persistent infection from the 2nd to 3rd event, cohort (new vs old), medroxyprogesterone acetate use, irregular menses, number of new sexual partners in past 8 months, recent history of reported anal sex,, previous history of C. trachomatis, bacterial vaginosis or any STI, current history of bacterial vaginosis, and cumulative history of C. trachomatis, bacterial vaginosis or any STI. Significant associations with reported histories included recent history of bacterial vaginosis, N. gonorrhoeae, C. trachomatis, any STI, and genital warts. Older age, greater number of years sexually active, and current oral contraceptive use were protective. Additional risks for the 3rd event not seen for the 2nd event included a recent history of genital warts, smoking cigarettes, a recent history of douching and a current lab documented N. gonorrhoeae infection.
We also compared persistence of HPV 16/18 to high-risk non-HPV 16/18 and low-risk types. We found that there was no difference in risk for 2nd infection between low-risk type persistence and HPV 16/18 persistence (HR= 1.12 95% CI .79–1.57). Persistence of high-risk non HPV 16/18 had a slightly higher risk of a new infection compared to persistence of HPV 16/18 (HR = 1.38 95% CI 1.04–1.81). No differences were found for 3rd infection between HPV 16/18 persistence and either persistence of high-risk non HPV 16/18 or low-risk HPV types.
Multivariate analysis for risk of 2nd and 3rd event with new HPV types
Because the reported and the documented STI histories are correlated, having them in the same model would likely result in collinearity issues. Consequently, we created 2 models. In the first model using laboratory documented STIs, significant associations for acquisition of a new HPV type included persistent HPV infection from 1st visit with HPV, African-American race (vs white), mixed race (vs white), number of new partners in past 8 months, recent anal sex, diagnosis of bacterial vaginosis at the previous visit, and diagnosis of an STI (inclusive of N. gonorrhoeae, C. trachomatis and T. vaginalis) at the previous visit. Increasing age and having greater than 10 PMNs/hpf at the incident visit were protective. Multivariate associations are summarized in Table 3.
Table 3.
Multivariate analysis for risk of newly acquired HPV infection at 2nd and 3rd new type events
| Model 11,4, 5 | 2nd Event | 3rd Event | ||
|---|---|---|---|---|
| Hazard Ratio (95% confidence interval) | P | Hazard Ratio (95% confidence interval) | p | |
| Persistent HPV from prior event | 4.51 (3.78 – 5.37) | <0.0001 | 3.37 (2.69 – 4.23) | <0.0001 |
| Age (per year) | 0.95 (0.91 – 0.98) | 0.005 | 0.91 (0.86 – 0.96) | 0.0004 |
| African-American (vs White) | 1.65 (1.30 – 2.08) | <0.0001 | 1.22 (0.90 – 1.66) | 0.19 |
| Mixed race (vs. White) | 1.82 (1.18 – 2.81) | 0.007 | 1.22 (0.72 – 2.06) | 0.46 |
| Recent anal sex | 1.28 (1.00 – 1.64) | 0.05 | 1.50 (1.09 – 2.06) | <0.01 |
| Number of new partners in past 8 months (per partner) | 1.10 (1.05 – 1.15) | <0.0001 | 1.09 (1.05 – 1.14) | <0.0001 |
| Laboratory diagnosis of Bacterial Vaginosis at previous visit | 1.60 (1.07 – 2.39) | 0.03 | 0.99 (0.63 – 1.56) | 0.95 |
| Laboratory diagnosis of an STI2 at previous visit | 1.47 (1.00 – 2.17) | 0.05 | 2.10 (1.30 – 3.39) | 0.003 |
| Greater than 10 PMN/hpf at current visit (vs ≤ 10 PMNs) | 0.39 (0.19 – 0.79) | 0.009 | 1.04 (0.51 – 2.13) | 0.92 |
| Model 23,4,5 | H.R. (95% C.I.) | P | H.R. (95% C.I.) | p |
| Persistent HPV from prior event | 4.51 (3.78 – 5.38) | <0.001 | 3.45 (2.75 – 4.32) | <0.0001 |
| Age (per year) | 0.94 (0.90 – 0.98) | 0.002 | 0.91 (0.86 – 0.96) | 0.0004 |
| African-American (vs White) | 1.70 (1.34 – 2.15) | <0.0001 | 1.25 (0.93 – 1.69) | 0.14 |
| Mixed race (vs. White) | 1.85 (1.20 – 2.86) | 0.005 | 1.19 (0.70 – 2.01) | 0.51 |
| Recent anal sex | 1.28 (1.00 – 1.65) | 0.05 | 1.47 (1.06 – 2.03) | 0.02 |
| Number of new partners in past 8 months (per partner) | 1.10 (1.00 – 1.15) | <0.0001 | 1.10 (1.05 – 1.14) | <0.0001 |
| Recent reported STI2 | 1.42 (1.00 – 2.04) | 0.05 | 1.83 (1.12 – 2.94) | 0.02 |
| Recent reported HSV | 2.43 (1.58 – 3.73) | <0.0001 | 1.50 (.88 – 2.57) | 0.14 |
Model 1 includes laboratory documented STI history only.
STI variables reflect C. trachomatis, N. gonorrhoeae, and T. vaginalis.
Model 2 uses questionnaire reported STI history only.
Adjusted for cohort and prevalent vs. incident HPV at 1 st detected visit.
Latino, Native American, and Asian Pacific Islander vs. white were not significant and not shown in the model.
Likelihood ratio statistic for model 1 = 325.77 (p < 0.0001) for 2 nd event and 190.04 (p < 0.0001) for 3 rd event.
Likelihood ratio statistic for model 2 = 311.69 (p < 0.0001) for 2 nd event and 187.23 (p < 0.0001) for 3 rd event
Although having a current STI was not significant in the univariate analysis, we ran the model with current STI and found it not significant in the multivariate as well. When we examined the multivariate model with all the individual significant STIs from Table 2, N. gonorrhoeae at the previous visit [HR = 2.48 (95% CI 1.07–5.74)] was significant but not C. trachomatis [HR =1.08 (95% CI .67–1.72)].
The second model using reported STI history had similar findings. The only difference was that reported history of bacterial vaginosis was not significant and the trend for weekly drug use was no longer seen.
The analysis showed similar risks for the 3rd event as the 2nd event except race, diagnosis of bacterial vaginosis and presence of PMNs at incident visit were not significant. The model with reported STI history showed similar findings.
DISCUSSION
This is the first study that we are aware that has looked at behavioral factors and laboratory based infection status as risks for repeated HPV detection events with new types. Not surprisingly, since HPV is a sexually transmitted infection, we found that significant risks were primarily those associated with sexual behavior. As in many incident studies, reporting a recent new partner was one of the strongest risks.(12) There was a 9% increase in risk for every partner reported in the past 8 months. Interestingly, abstinence was not protective compared to having no new partners underscoring the protection associated with monogamous relationships. On closer examination, the risk associated with a new partner was greater if the new partner was reported within the past 8 months rather than the past 4 months (data not shown). This risk found within the past 8 months was also shown by Winer and colleagues (3) suggesting that it may take up to 8 months before an infection is established and replication has reached a level adequate for detection. We also note that total number of sexual partners was not significant in either event underscoring the importance of recent exposure rather than past history.
As found in numerous prevalence studies, increasing age was protective. It is reported that as a woman ages, she has fewer current sexual partners. (20) However, the lower risk in older women has been found to be independent of the number of reported new partners suggesting that young age also reflects a biologic vulnerability. (13) One reason for this protection is the natural immunity women develop after clearing HPV infection. Hence, the older the woman, the more likely she has acquired and cleared infections and remains protected from repeat exposures. This was supported by the observation that the old cohort was less likely to have repeat infections than the new cohort. Since the old cohort had more number of lifetime sexual partners, pregnancies and STIs at baseline, they were also more likely to have had previous HPV exposures. On the other hand, the topography of the cervix in young versus older women may also explain this difference. Young women have a predominance of metaplastic tissue on the ectocervix, which may be more vulnerable than squamous epithelium.
The finding associated with race, specifically African Americans is not new to STI epidemiology. African Americans have higher rates of C. trachomatis, N. gonorrhoeae, syphylis, herpes simplex virus, and HIV. (21) These behaviors are not explained by individual sexual behaviors rather are thought to be a complex interaction of determinants of social health specifically access to health care and sexual networks. (22) We found African Americans in our study were an independent risk factor for the 2nd event underscoring the vulnerability of this group. The loss of race/ethnicity for the 3rd event may imply that access to health care did, in fact, influence risk early in the study. Since we offer reproductive health care to all of our subjects, we would have expected that the longer our subjects were in the study, the more likely they were all receiving similar health care regarding STI screening.
The role of anal sex certainly is interesting as well as plausible specifically in those who practice both vaginal and anal intercourse regularly. Several studies have shown that HPV infections of the anus are quite common in women with some studies showing higher rates of HPV in the anus than the cervix. (23–25) Many have suggested that the anus is an important reservoir for HPV since similar types can be detected in the anus and the cervix. (23) Most of our women did not practice anal sex without also engaging in vaginal intercourse during the same sexual encounter and condom use during anal sex was rarely practiced (data not shown).
Our finding associated with STI acquisition was not surprising since STIs can induce inflammation resulting in breaks in the epithelial barrier allowing HPV direct access to basal epithelial cells. STIs also reflect partner risk (i.e. polygamy on part of one of the partners) and therefore is a marker for HPV exposure and not necessarily a biologic risk. Of interest, studies controlling for sexual behavior have not found STIs to be important. (26) We believe our close surveillance of STIs allowed a more thorough examination of risk compared to other studies. Of note, our laboratory documented and reported histories of infection gave similar results. Since we followed the women at close intervals, we likely enhanced their recall of STIs. Since the pooled STI variable appeared to reflect a stronger risk than any one single STI, it may imply the risk is primarily behavior-associated. On the other hand, inflammation in general, rather than the effects of a specific infectious agent, may be the risk. This latter hypothesis is supported by the significant association with a reported HSV event. In this case, reported history is likely more accurate than our lab documented variable since the brevity of HSV shedding in recurrences precluded us in documenting infection. In a previous publication, we found that HSV infection defined by serology was associated with HPV acquisition. (2) Unfortunately, serology was not available for this analysis. The lack of association with T. vaginalis alone was likely due to the small number of cases and insensitivity of the wet mount to diagnose T. vaginalis. Although the relationship with bacterial vaginosis with sexual activity is less well established, it has been associated the acquisition of other STIs such as HIV and HSV (27) but less so with HPV. (28, 29) The loss of association with the 3rd event may be due to the fact that we used a relatively imprecise measure of bacterial vaginosis. (30)
One of our findings not well explained was the protection seen with the presence of inflammation on wet mounts at the 2nd event. Since this association was no longer seen at the 3rd event, this finding may have been a product of a type I error and small sample size. On the other hand, the concomitant inflammation due to unknown factors may have resulted in the production of inflammatory cytokines which were protective of acquisition. (18, 31) The loss of association with the 3rd event may have been due to the imprecise measurement of PMNs associated with wet mounts.
One of the most striking findings in the study was the role of persistence and risk of acquisition. Of those with HPV persistence, almost three-quarters had a new type detected within one year compared to only one-third who showed clearance of initial infection. Persistence and detection of multiple types are interconnected. However, if a woman is unable to clear her infection rapidly and continues to acquire infections, she is more likely at any cross-sectional point to have multiple HPV types detected. A few studies have shown that detection of multiple HPV types is a risk for persistence and development of CIN. (32, 33) Some have also shown that having multiple types is a risk for acquiring additional types. (11, 34) Our data emphasize that the detection of multiple types is not a risk for acquiring a second infection similar to a study by Plummer et al. (35) Rather we believe the detection of multiple types likely reflects immune dysregulation in a woman. Immunocompromised women frequently have slower clearance rates resulting in the detection of multiple types at any one cross sectional point. (36) Only one other study examined persistence as a risk for subsequent infections for comparison. Rousseau et al (11), who followed women over a short period of time (18 months) and defined persistence over two consecutive visits, found no association. We found no difference between HP 16/18, other oncogenic or non-oncogenic underscoring the risk is associated with the women’s immune dysfunction and not the HPV type. However, we strongly doubt that these infections reflect reactivation since strong associations were observed with sexual risks.
Few studies are available for comparison. Our findings were similar to Ho et al (37) who showed that 70% of college women had a second infection with a new HPV type within 24 months with the majority occurring within 6 months and that risks included having a new sexual partner and being non-white. Interestingly, oral contraceptives were protective, a finding we reported in a previous study. (2) The study did not examine STIs. In an older group of women (mean of 33 years of age) who had a total of 4 visits, Rouseau et al (11) found similar results in that of women with HPV16 or 18, approximately 35% acquired another infection by 12 months whereas 25% of those with high-risk non HPV 16/18 or low-risk, had another infection. An important limitation in our study and similar studies is that infection events are usually only documented in broad intervals. In particular, inclusion of prevalent infections as incident events in analyses can lead to biased estimates of the distribution of times to subsequent events. Our estimates allowing for doubly interval censored event times suggests that prevalent infections may bias the 2nd event calculations. The observed slight shift to the right at 1 year (i.e. longer time to reinfection) would be expected since the assumption made by the analysis was that the prevalent infections may have occurred as early as the first day of sexual debut. The greater number of infections within the first 6 months in the study by Ho et al (37) may be a reflection of the greater number of prevalent infections in that study than ours. However, the bias seen in our study seems to lessen with time and corresponding bias in relative estimates of the effect of fixed covariates appears minimal.
In summary, we found that new HPV infections occur repeatedly among young women with up to 28% to 48% having another infection within 12 months. Having recent new sexual partners and having a laboratory documented STI were the strongest sexual risks. We believe the influence of persistent HPV on repeat acquisition is reflective of immune dysfunction which is a risk for acquisition as well as the inability to clear the virus.
Acknowledgments
Financial Support: This work was supported by grant R37 CA051323 from the National Cancer Institute (NIH/NCI) and 7RT-0195 (Tobacco-Related Disease Research Program) from the University of California San Francisco and was carried out in part in the Pediatric Clinical Research Center, Clinical and Translational Science Institute at the University of California, San Francisco (NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131). Roche Molecular Diagnostics (Pleasanton, CA) provided supplies for HPV DNA detection.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest
References
- 1.Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Health. 2008;43:S5–25. S e1–41. doi: 10.1016/j.jadohealth.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Moscicki AB, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 3.Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645–54. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 4.Giuliano AR, Harris R, Sedjo RL, et al. Incidence, prevalence, and clearance of type-specific human papillomavirus infections: The Young Women’s Health Study. J Infect Dis. 2002;186:462–9. doi: 10.1086/341782. [DOI] [PubMed] [Google Scholar]
- 5.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural History of cervicovaginal papillomavirus infection in young women. NEJM. 1998;338:423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 6.Smith JS, Bosetti C, Munoz N, et al. Chlamydia trachomatis and invasive cervical cancer: a pooled analysis of the IARC multicentric case-control study. Int J Cancer. 2004;111:431–9. doi: 10.1002/ijc.20257. [DOI] [PubMed] [Google Scholar]
- 7.Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12:485–90. [PubMed] [Google Scholar]
- 8.Tamim H, Finan RR, Sharida HE, Rashid M, Almawi WY. Cervicovaginal coinfections with human papillomavirus and Chlamydia trachomatis. Diagn Microbiol Infect Dis. 2002;43:277–81. doi: 10.1016/s0732-8893(02)00403-0. [DOI] [PubMed] [Google Scholar]
- 9.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 10.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007;197:340–5. doi: 10.1016/j.ajog.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 11.Rousseau MC, Pereira JS, Prado JC, Villa LL, Rohan TE, Franco EL. Cervical coinfection with human papillomavirus (HPV) types as a predictor of acquisition and persistence of HPV infection. J Infect Dis. 2001;184:1508–17. doi: 10.1086/324579. [DOI] [PubMed] [Google Scholar]
- 12.Burchell A, Winer R, de Sanjose S, Franco E. Epidemiolgy and transmission dynamics of genital human papillomavirus infection. Vaccine monographs. 2006;24:52–62. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Munoz N, Mendez F, Posso H, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190:2077–87. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 14.Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000;92:464–74. doi: 10.1093/jnci/92.6.464. [DOI] [PubMed] [Google Scholar]
- 15.Farhat S, Nakagawa M, Moscicki AB. Cell-mediated immune responses to human papillomavirus 16 E6 and E7 antigens as measured by interferon gamma enzyme-linked immunospot in women with cleared or persistent human papillomavirus infection. Int J Gynecol Cancer. 2009;19:508–12. doi: 10.1111/IGC.0b013e3181a388c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277–84. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 17.Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364:1678–83. doi: 10.1016/S0140-6736(04)17354-6. [DOI] [PubMed] [Google Scholar]
- 18.Scott ME, Ma Y, Farhat S, Shiboski S, Moscicki AB. Covariates of cervical cytokine mRNA expression by real-time PCR in adolescents and young women: effects of Chlamydia trachomatis infection, hormonal contraception, and smoking. J Clin Immunol. 2006;26:222–32. doi: 10.1007/s10875-006-9010-x. [DOI] [PubMed] [Google Scholar]
- 19.Sun J. Statistical Analysis of Doubly Interval-censored Failure Time Data. In: Balakrishnan N, Rao CR, editors. Handbook of Statistics: Survival Analysis. 2004. pp. 105–22. [Google Scholar]
- 20.Mosher WD, Chandra A, Jones J. Sexual behavior and selected health measures: men and women 15–44 years of age, United States, 2002. Adv Data. 2005;362:1–55. [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) Sexually Transmitted Disease Surveillance, 2005. Atlanta, GA: US Department of Health and Human Services; 2006. [Google Scholar]
- 22.Parrish DD, Kent CK. Access to care issues for African American communities: implications for STD disparities. Sex Transm Dis. 2008;35:S19–22. doi: 10.1097/OLQ.0b013e31818f2ae1. [DOI] [PubMed] [Google Scholar]
- 23.Goodman MT, Shvetsov YB, McDuffie K, et al. Acquisition of anal human papillomavirus (HPV) infection in women: the Hawaii HPV Cohort study. J Infect Dis. 2008;197:957–66. doi: 10.1086/529207. [DOI] [PubMed] [Google Scholar]
- 24.Moscicki AB, Durako SJ, Houser J, et al. Human papillomavirus infection and abnormal cytology of the anus in HIV-infected and uninfected adolescents. AIDS. 2003;17:311–20. doi: 10.1097/00002030-200302140-00004. [DOI] [PubMed] [Google Scholar]
- 25.Palefsky JM, Holly EA, Ralston ML, Da Costa M, Greenblatt RM. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. J Infect Dis. 2001;183:383–91. doi: 10.1086/318071. [DOI] [PubMed] [Google Scholar]
- 26.Richardson H, Abrahamowicz M, Tellier PP, et al. Modifiable risk factors associated with clearence of type-specific cervical papillomavirus infections in a cohort of university students. Cancer Epidemiol Biomarkers Prev. 2005;14:1149–56. doi: 10.1158/1055-9965.EPI-04-0230. [DOI] [PubMed] [Google Scholar]
- 27.Allsworth JE, Lewis VA, Peipert JF. Viral sexually transmitted infections and bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Sex Transm Dis. 2008;35:791–6. doi: 10.1097/OLQ.0b013e3181788301. [DOI] [PubMed] [Google Scholar]
- 28.Boyle DC, Barton SE, Uthayakumar S, et al. Is bacterial vaginosis associated with cervical intraepithelial neoplasia? Int J Gynecol Cancer. 2003;13:159–63. doi: 10.1046/j.1525-1438.2003.13007.x. [DOI] [PubMed] [Google Scholar]
- 29.Watts DH, Fazzari M, Minkoff H, et al. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J Infect Dis. 2005;191:1129–39. doi: 10.1086/427777. [DOI] [PubMed] [Google Scholar]
- 30.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lieberman JA, Moscicki AB, Sumerel JL, Ma Y, Scott ME. Determination of cytokine protein levels in cervical mucus samples from young women by a multiplex immunoassay method and assessment of correlates. Clin Vaccine Immunol. 2008;15:49–54. doi: 10.1128/CVI.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trottier H, Mahmud S, Costa MC, et al. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2006;15:1274–80. doi: 10.1158/1055-9965.EPI-06-0129. [DOI] [PubMed] [Google Scholar]
- 33.Trottier H, Mahmud S, Prado JC, et al. Type-specific duration of human papillomavirus infection: implications for human papillomavirus screening and vaccination. J Infect Dis. 2008;197:1436–47. doi: 10.1086/587698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendez F, Munoz N, Posso H, et al. Cervical coinfection with human papillomavirus (HPV) types and possible implications for the prevention of cervical cancer by HPV vaccines. J Infect Dis. 2005;192:1158–65. doi: 10.1086/444391. [DOI] [PubMed] [Google Scholar]
- 35.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195:1582–9. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 36.Moscicki AB, Ellenberg JH, Farhat S, Xu J. Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type. J Infect Dis. 2004;190:37–45. doi: 10.1086/421467. [DOI] [PubMed] [Google Scholar]
- 37.Ho GY, Studentsov Y, Hall CB, et al. Risk factors for subsequent cervicovaginal human papillomavirus (HPV) infection and the protective role of antibodies to HPV-16 virus-like particles. J Infect Dis. 2002;186:737–42. doi: 10.1086/342972. [DOI] [PubMed] [Google Scholar]