Abstract
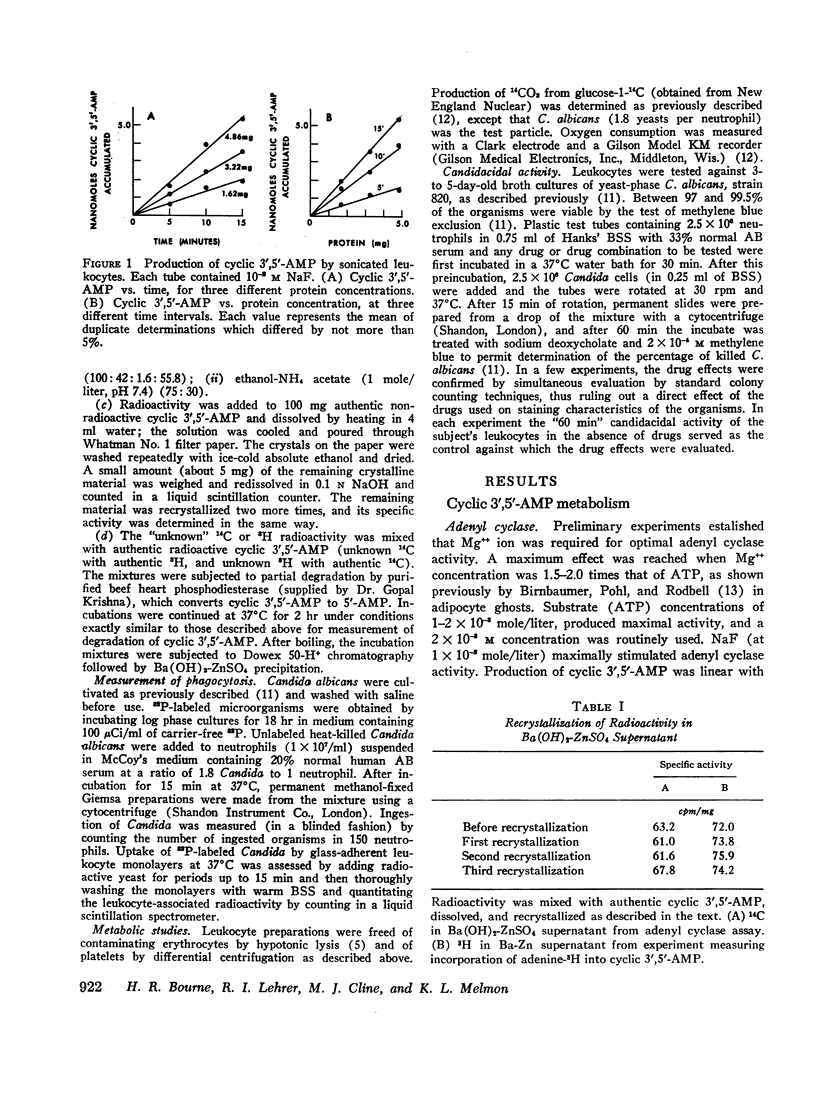
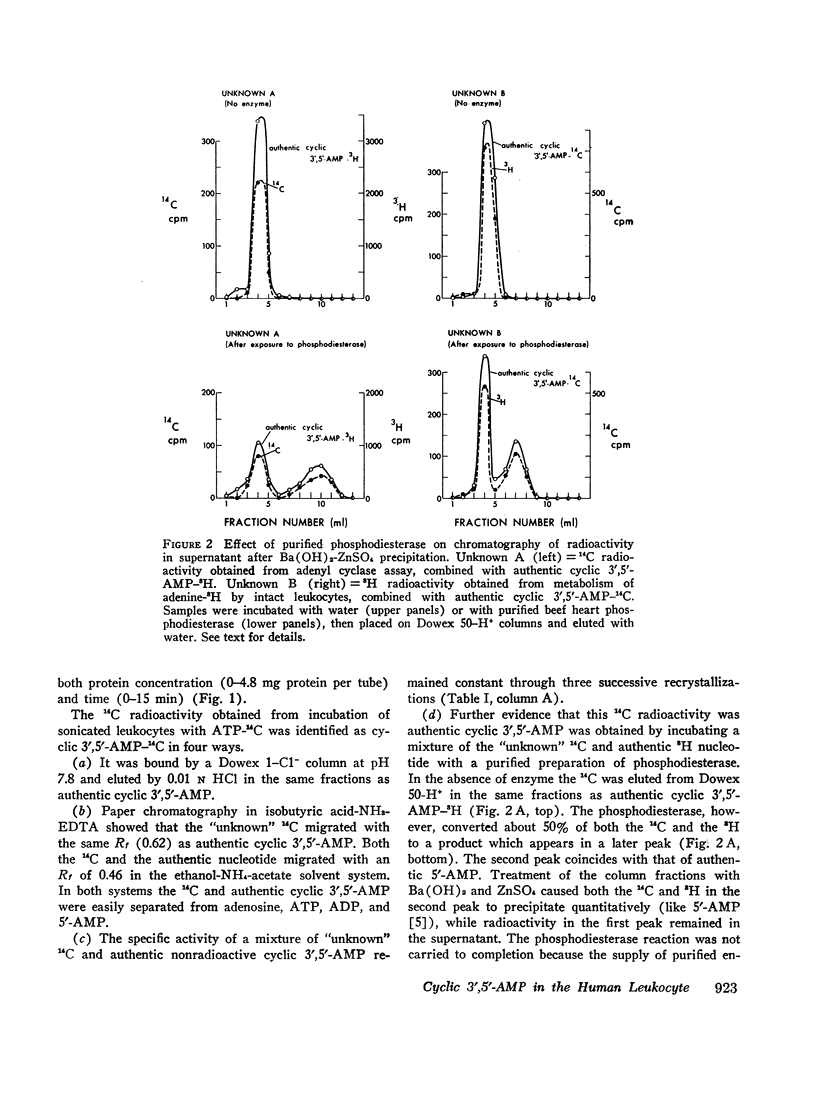
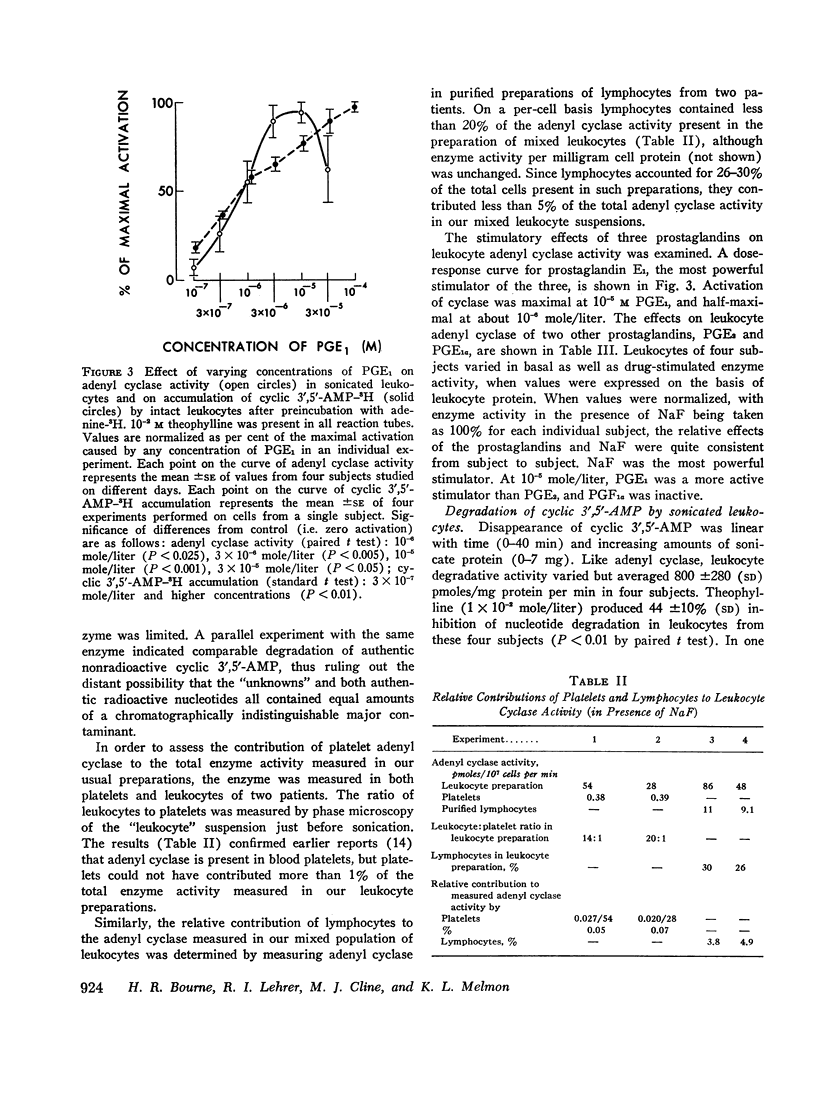
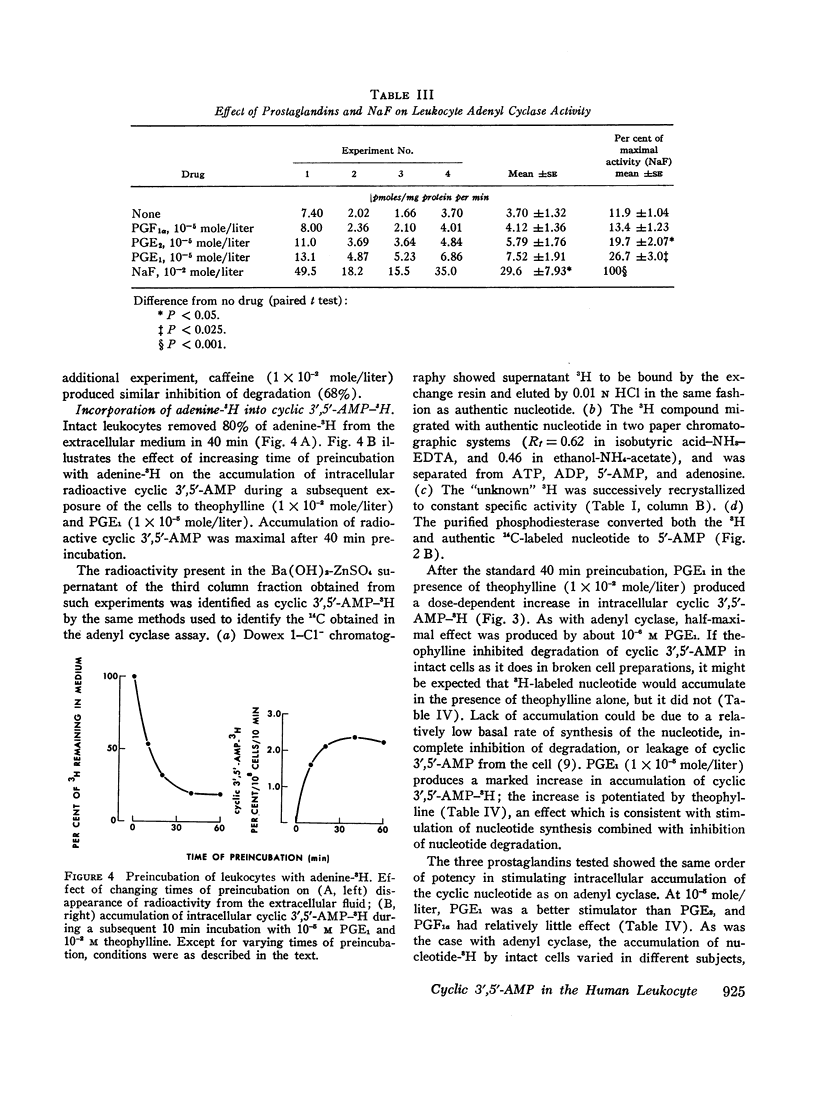
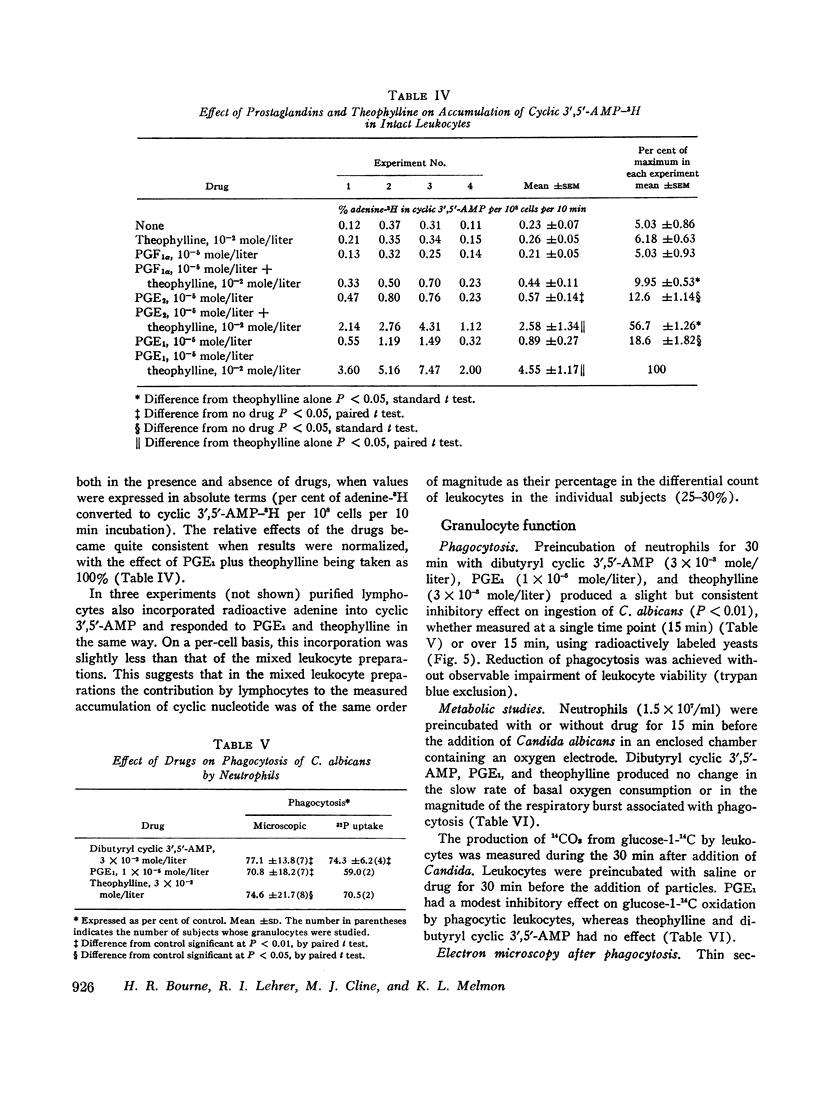
Prostaglandins E1 and E2 (PGE1 and PGE2) stimulate adenyl cyclase activity in broken cell preparations of normal human leukocytes, whereas prostaglandin F1a produces no effect. PGE1 and PGE2 also cause increased accumulation of cyclic 3′,5′-adenosine monophosphate-3H (3H-labeled AMP) in intact leukocytes which have been preincubated with adenine-3H in vitro. Theophylline inhibits leukocyte phosphodiesterase activity and potentiates the stimulatory effect of the prostaglandins on intracellular accumulation of cyclic 3′,5′-AMP-3H.
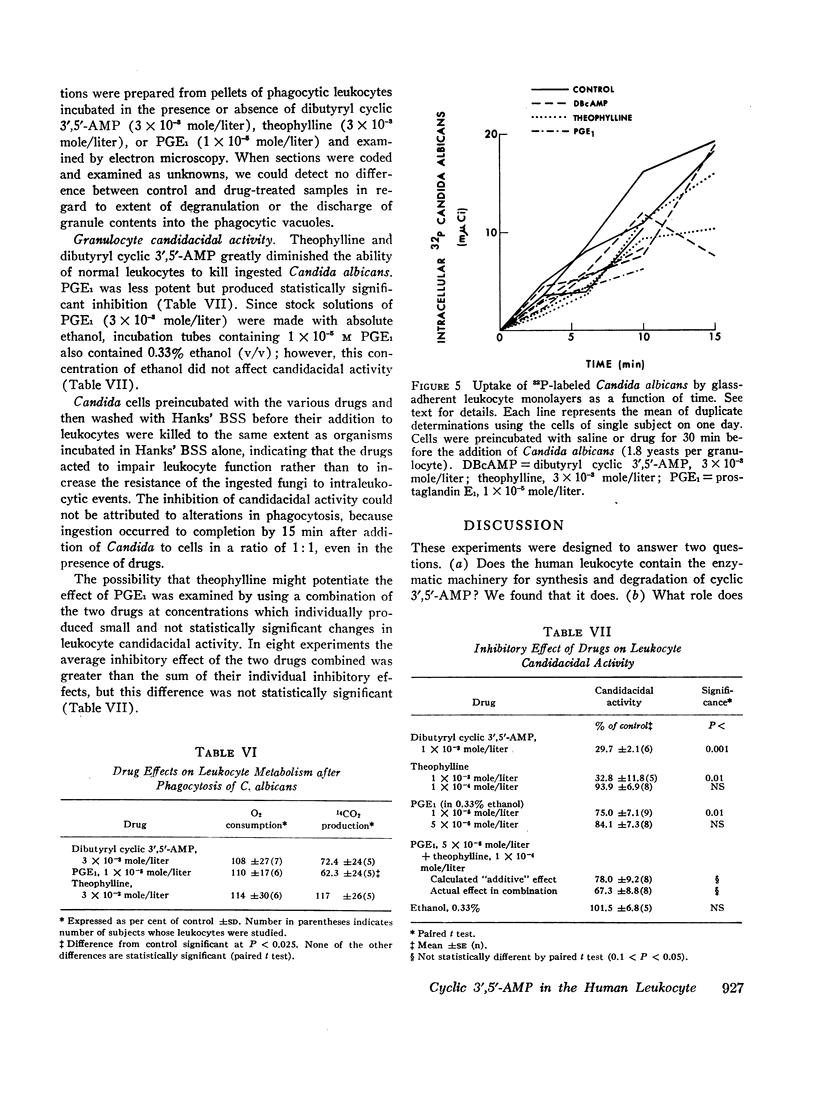
The ability of human granulocytes in vitro to kill Candida albicans was consistently inhibited by PGE1 and theophylline. This effect was reproduced by dibutyryl cyclic 3′,5′-AMP, a lipid-soluble analogue of the endogenous nucleotide. The inhibition of candidacidal activity could not be accounted for by drug effects on phagocytosis, oxygen consumption, or hexose monophosphate shunt activity. These results are consistent with the hypothesis that increased intracellular concentrations of cyclic 3′,5′-AMP impair the granulocyte's ability to kill C. albicans, but the precise mechanism of inhibition has not yet been defined.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Pohl S. L., Rodbell M. Adenyl cyclase in fat cells. 1. Properties and the effects of adrenocorticotropin and fluoride. J Biol Chem. 1969 Jul 10;244(13):3468–3476. [PubMed] [Google Scholar]
- FALLON H. J., FREI E., 3rd, DAVIDSON J. D., TRIER J. S., BURK D. Leukocyte preparations from human blood: evaluation of their morphologic and metabolic state. J Lab Clin Med. 1962 May;59:779–791. [PubMed] [Google Scholar]
- Humes J. L., Rounbehler M., Kuehl F. A., Jr A new assay for measuring adenyl cyclase activity in intact cells. Anal Biochem. 1969 Nov;32(2):210–217. doi: 10.1016/0003-2697(69)90077-3. [DOI] [PubMed] [Google Scholar]
- JOHNSON T. M., GARVIN J. E. Separation of lymphocytes in human blood by means of glass wool column. Proc Soc Exp Biol Med. 1959 Nov;102:333–335. doi: 10.3181/00379727-102-25237. [DOI] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- Kuo J. F., De Renzo E. C. A comparison of the effects of lipolytic and antilipolytic agents on adenosine 3',5'-monophosphate levels in adipose cells as determined by prior labeling with adenine-8-14C. J Biol Chem. 1969 May 10;244(9):2252–2260. [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Margolis S. Histamine release in vitro: inhibition by catecholamines and methylxanthines. Science. 1968 Aug 30;161(3844):902–903. doi: 10.1126/science.161.3844.902. [DOI] [PubMed] [Google Scholar]
- May C. D., Levine B. B., Weissmann G. Effects of compounds which inhibit antigenic release of histamine and phagocytic release of lysosomal enzyme on glucose utilization by leukocytes in humans. Proc Soc Exp Biol Med. 1970 Mar;133(3):758–763. doi: 10.3181/00379727-133-34559. [DOI] [PubMed] [Google Scholar]
- Scott R. E. Effects of prostaglandins, epinephrine and NaF on human leukocyte, platelet and liver adenyl cyclase. Blood. 1970 Apr;35(4):514–516. [PubMed] [Google Scholar]
- Shimizu H., Daly J. W., Creveling C. R. A radioisotopic method for measuring the formation of adenosine 3',5'-cyclic monophosphate in incubated slices of brain. J Neurochem. 1969 Dec;16(12):1609–1619. doi: 10.1111/j.1471-4159.1969.tb10360.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMS H. E., FIELD J. B. Low leukocyte phosphorylase in hepatic phosphorylase-deficient glycogen storage disease. J Clin Invest. 1961 Oct;40:1841–1845. doi: 10.1172/JCI104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe S. M., Shulman N. R. Adenyl cyclase activity in human platelets. Biochem Biophys Res Commun. 1969 Apr 29;35(2):265–272. doi: 10.1016/0006-291x(69)90277-0. [DOI] [PubMed] [Google Scholar]



