Abstract
Background
Air pollution is thought to exert health effects through oxidative stress, which causes damage to DNA and lipids.
Objective
We determined whether levels of oxidatively damaged DNA and lipid peroxidation products in cells or bodily fluids from humans are useful biomarkers of biologically effective dose in studies of the health effects of exposure to particulate matter (PM) from combustion processes.
Data sources
We identified publications that reported estimated associations between environmental exposure to PM and oxidative damage to DNA and lipids in PubMed and EMBASE. We also identified publications from reference lists and articles cited in the Web of Science.
Data extraction
For each study, we obtained information on the estimated effect size to calculate the standardized mean difference (unitless) and determined the potential for errors in exposure assessment and analysis of each of the biomarkers, for total and stratified formal meta-analyses.
Data synthesis
In the meta-analysis, the standardized mean differences (95% confidence interval) between exposed and unexposed subjects for oxidized DNA and lipids were 0.53 (0.29–0.76) and 0.73 (0.18–1.28) in blood and 0.52 (0.22–0.82) and 0.49 (0.01–0.97) in urine, respectively. The standardized mean difference for oxidized lipids was 0.64 (0.07–1.21) in the airways. Restricting analyses to studies unlikely to have substantial biomarker or exposure measurement error, studies likely to have biomarker and/or exposure error, or studies likely to have both sources of error resulted in standardized mean differences of 0.55 (0.19–0.90), 0.66 (0.37–0.95), and 0.65 (0.34–0.96), respectively.
Conclusions
Exposure to combustion particles is consistenly associated with oxidatively damaged DNA and lipids in humans, suggesting that it is possible to use these measurements as biomarkers of biologically effective dose.
Keywords: biomarker, DNA damage, lipid peroxidation products, oxidative stress, particulate matter
Exposure to particulate matter (PM) from combustion processes contributes substantially to cardiovascular and pulmonary ill health and premature mortality globally (Brook 2008; Salvi and Barnes 2009). PM represents highly complex mixtures with large variations in size, chemical composition, shape, surface, reactivity, and charge, in both time and space, due to variable sources, atmospheric chemical reactions, and meteorological conditions (Sioutas et al. 2005). Nevertheless, for exposure assessment for epidemiological association with health outcomes and regulation, PM is usually only considered as mass defined by size cutoff at 2.5 and 10 μm in aerodynamic diameter (PM2.5 and PM10, respectively). These measures are often little affected by ultrafine particles (UFPs) from, for example, diesel engine emission because of their low mass, although UFPs are thought to have important health effects due to their high alveolar deposition, small size (≤ 0.1 μm in aerodynamic diameter), large surface area, and potential to translocate (Delfino et al. 2005). Moreover, in epidemiological studies, exposure levels are often assigned crudely and groupwise according to air monitoring data and sources near the residence, because the modest individual risks require large numbers and long observation times to assess. Personal exposure can be assessed by portable monitors or carefully registered time–activity patterns in well-defined microenvironments of exposure in small numbers of subjects (Zou et al. 2009). However, the internal dose of PM also depends on breathing patterns and airway deposition, and cardiovascular and other systemic effects of PM require further translocation of PM constituents or signaling molecules or cells from the airways (Mills et al. 2009). Oxidative stress with inflammation is thought to be central in the mechanisms of action for both the pulmonary and extrapulmonary health effects of PM (Mills et al. 2009; Risom et al. 2005). Thus, biomarkers of oxidative stress should serve as proxy measures of the true internal exposure to PM to compare potential health impacts of different sources in both small controlled exposure settings and large population approaches. Oxidative modification of DNA and lipids are particularly relevant for cancer and cardiovascular disease where oxidative stress in the circulation is important (Mills et al 2009; Risom et al. 2005). Experimental studies in animals and cell cultures have consistently shown that combustion-related PM induces oxidative stress and DNA damage in relevant organs and cells (Møller et al. 2008a, 2010). In our experience the effects on biomarkers of oxidized DNA and lipids are observed within a lag period < 24 hr after the exposure to PM.
A number of studies of PM exposure in humans have applied biomarkers of oxidative damage to DNA and lipids in the blood compartment or in terms of products excreted in urine or exhaled breath condensate (EBC), as outlined in Table 1. The biomarkers of oxidatively damaged DNA include 8-oxo-7,8-dihydroguanine (8-oxoGua) or the corresponding deoxynucleoside 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) measured in DNA and urine, the exocyclic M1 adduct to guanine (M1dG), and lesions detected as sites in DNA sensitive to formamidopyrimidine DNA glycosylase (FPG) and endonuclease III (ENDOIII). The biomarkers of lipid peroxidation (LPO) products include conjugated dienes (CDs), lipid hydroperoxides, malondialdehyde (MDA), thiobarbituric acid–reactive substances (TBARS), and F2-isoprostanes measured in EBC, plasma, serum, or urine. However, a systematic approach is required to evaluate the validity of their use as biomarkers of biological effective dose in this context. We undertook a systematic review of the published studies to assess the extent and consistency of associations between exposure to combustion-related PM and the biomarkers of oxidative damage to DNA and lipids.
Table 1.
Summary of biomarkers of oxidatively damaged DNA, nucleobases, and LPO products used in studies of the effect of combustion particles.
| Biomolecule | Description | Assays |
|---|---|---|
| Oxidatively damaged DNA or nucleobases | ||
| ENDOIII/FPG | DNA base lesions detected by bacterial ENDOIII or FPG enzymes, representing mainly oxidized purine (including 8-oxodG) and pyrimidine lesions, respectively | Comet assay |
| 8-oxodG | Major oxidation product in nuclear DNA; detection of 8-oxodG in urine or plasma mainly originates from oxidation of deoxyguanosine triphosphate in the nucleotide pool | HPLC-ECD, LC-MS/MS, antibodies |
| 8-oxoGua | Major oxidation product in nuclear DNA; detection of 8-oxoGua in urine or plasma is likely to arise from cleavage of the oxidized base from DNA by repair enzymes (e.g., OGG1) | HPLC-ECD, LC-MS/MS, antibodies |
| M1dG | Exocyclic DNA damage formed by reactive carbonyl compounds released from oxidized lipids | LC-MS, antibodies |
| LPO products | ||
| CDs | Breakdown products of fatty acids considered to represent an early stage of the LPO process | Spectrophotometry |
| Lipid hydroperoxides | Reaction product between O2 and carbon radical in lipids | Spectrophotometry |
| MDA/TBARS | Breakdown carbonyl product of LPO; the reaction with thiobarbituric acid forms adducts that can be detected by spectrophotometry; prepurification of urine or plasma before the reaction with thiobarbituric acid can be considered as a specific measurement of LPO products, whereas the simple TBARS assay is highly unspecific | Spectrophotometry |
| F2-isoprostanes | Products that arise mainly from oxidation of arachidonic acid in phospholipids, often referred to as 8-iso-PGF2α or 15-F2t-isoprostanes | GC-MS, LC-MS/MS, antibodies |
Abbreviations: 8-oxodG, 8-oxo-7,8-dihydro-2′-deoxyguanosine; 8-oxoGua, 8-oxo-7,8-dihydroguanine; CDs, conjugated dienes; ECD, electrochemical detection; ENDOIII, endonuclease III; FPG, formamidopyrimidine DNA glycosylase; GC-MS, gas chromatography–mass spectrometry; HPLC, high-performance liquid chromatography; LC-MS, liquid chromatography–mass spectrometry; LC-MS/MS, liquid chromatography with tandem mass spectrometry; LPO, lipid peroxidation; M1dG, exocyclic M1 adduct to guanine; MDA, malondialdehyde; OGG1, 8-oxoguanine DNA glycosylase; TBARS, thiobarbituric acid–reactive substances. For descriptions and critical assessments of the assays as biomarkers, see Griffiths et al. (2002) and Halliwell and Whiteman (2004).
Materials and Methods
Studies included in meta-analysis
The publications were identified by searches in the PubMed, EMBASE, and Web of Science databases, as well as reference lists in the identified papers [see Supplemental Material for the search strategy (doi:10.1289/ehp.0901725)]. We included studies that investigated measures of effects of environmental air pollution exposure. This encompassed studies of subjects who had been exposed at work to environmental air pollution (e.g., policemen exposed to traffic exhaust). We excluded studies on occupational exposures to air pollution, such as metal smelting or incineration, because these are characterized by exposure to other air pollution components than those found in urban air. We searched for publications with reported data on LPO products and oxidatively damaged DNA by means of the biomarkers outlined in Table 1 in airways, the blood compartment, and urine. Isolated leukocytes, lymphocytes, or mononuclear blood cells are all referred to as white blood cells (WBCs). We used the term “oxidized nucleobases” for the urinary excretion of 8-oxodG and 8-oxoGua because they are not measured in DNA. Tables 2–5 summarize details of the included studies. The results from some of the studies have been reported in multiple publications; we discuss these as studies rather than as individual publications because they originated from the same investigation.
Table 2.
Summary of controlled exposure studies on exposure to air pollution PM from combustion processes.
| Biomarker | Subjects (n) | Sex, age, smoking | Exposure assessmenta | Potential measurement error | Findings | Study |
|---|---|---|---|---|---|---|
| 8-oxodG (ELISA) F2-isoprostanes (LC-MS/MS) |
Subjects with metabolic syndrome exposed to diesel exhaust or FA for 2 hr (10) | MF 18–49 years NS |
PM2.5, 4.8 and 205 μg/m3 NO, 38.6 and 1,516 ppb NO2, 15.5 and 25.5 ppb |
Biomarker (8-oxodG) | No difference in urinary excretion | Allen et al. 2009b |
| 8-iso-PGF2 (ELISA) 8-oxodG, 8-oxoGua (HPLC/GC-MS) FPG sites (comet) MDA (HPLC-FD) |
Healthy subjects exposed to wood smoke in a chamber for 4 hr (13) | MF 20–56 years NS |
PM2.5, 261 and 14–27 μg/m3 UFPs, 137,500 and 5,950 particles/cm3 NO2, 10 and 8.5 ppb |
Biomarker (8-iso-PGF2 ) | Increased urinary excretion of 8-iso-PGF and MDA levels in EBC; unaltered FPG sites (WBCs) and 8-oxodG and 8-oxoGua (urine) | Barregard et al. 2006, 2008; Danielsen et al. 2008c |
| FPG sites (comet) | Healthy subjects exposed in a chamber for 24 hr (29) | MF 20–40 years NS |
Personal UFPs, 6,169–15,362 particles/cm3 (non-FA), and 91–542 particles/cm3 (FA) NOx, 25.3 ppb (non-FA), 28.3 ppb (FA), 11.6 ppb (back ground), and 59.5 ppb (busy street) O3, 12.1 ppb (non-FA), 4.3 ppb (FA), 30.1 ppb (back ground), and 19.5 ppb (busy street) |
No | Decreased levels in WBCs by exposure to FA | Bräuner et al. 2007 |
| 8-iso-PGF2 (ELISA) | Elderly subjects exposed in the homes (41) | MF 60–75 years NS |
Personal UFPs, 10,016 particles/cm3 (non-FA) and 3,206 particles/cm3 (FA) PM2.5, 12.6 (non-FA) and 4.7 μg/m3 (FA) NO2, 20.0 (non-FA) and 20.0 μg/m3 (FA) |
Biomarker | Unaltered urinary excretion | Bräuner et al. 2008 |
| 8-Isoprostane (ELISA) | Subjects with stable coronary heart disease (12) and controls (12) exposed to CAPs for 2 hr | M 54 ± 2 and 59 ± 2 years NS |
UFPs, 99,400 and zero particles/cm3 NOx, 7.2 and 6.3 ppb SO2, 0.13 and 0.13 ppb O3, 5.0 and 6.0 ppb |
Biomarker | Increased in EBC by CAPs exposure | Mills et al. 2008 |
| MDA (HPLC) | Subjects exercising in location with low and high traffic intensity (12) | M 21± 2 years NS |
Personal UFPs, 252,290 and 7,382 particles/cm3 | No | Increased after exercise at location with high exposure | Rundell et al. 2008d |
| 8-oxoGua (HPLC-ECD) | Healthy subjects exposed to traffic exhaust at a street intersection for 4 hr (3) | M 22–25 years NS |
None (49,000 cars/12 hr) | Exposure | Increased urinary excretion during the first 12 hr and 24 hr after exposure; normalized levels at 36 and 48 hr after the exposure | Suzuki et al. 1995e |
| FPG sites (comet) | Subjects bicycling in Copenhagen (15) | MF 25 ± 3 years NS |
Personal UFPs (32,400 and 13,400 particles/cm3) PM10, 23.5 μg/m3 (street) and 16.9 μg/m3 (background) NO2, 32.1 and 24.2 μg/m3 (street) and 11.3 μg/m3 (background) |
No | Increased after cycling in the traffic compared with cycling in the laboratory | Vinzents et al. 2005 |
Abbreviations: ECD, electrochemical detection; ELISA, enzyme-linked immunosorbent assay; FA, filtered air; FD, fluorescence detection; GC-MS, gas chromatography–mass spectrometry; LC-MS, liquid chromatography–mass spectrometry; LC-MS/MS, liquid chromatography with tandem mass spectrometry; M, male; MF, male and female; NO, nitric oxide; NS, nonsmoker; iso-PGF2, 8-iso-PGF2, 8-iso-prostaglandin F2; SO2, sulfur dioxide.
The values represent exposure assessment in the high-exposure and low-exposure group, respectively, unless stated otherwise by specific footnotes.
We calculated the mean and SD from the mean difference and 95% CI assuming no missing data in the pair analysis.
We calculated the net difference in MDA from preexposure values and baseline-adjusted the data according to the level of MDA in the group of subjects exposed to filtered air. The SD was calculated from 90% CI.
We used the mean level of MDA from the exercises at the locations with low and high PM concentration.
The data correspond to the mean of the whole exposure period (0–48 hr).
Table 5.
Summary of cross-sectional studies on exposure to air pollution PM from combustion processes in different areas.
| Biomarker | Subjects (n) | Sex, age, smoking | Exposure assessmenta | Potential measurement error | Findings | Study |
|---|---|---|---|---|---|---|
| FPG sites (comet) | Taxi-motor drivers, people living/working near busy roads and rural controls (135) | M 34 ± 10 years NS |
Ambient (stationary) sampling of UFPs (201,691 and 6,961 particles/cm3) (midday 1-hr concentration in a busy street intersection and town square in a rural village, respectively) and urinary excretion of S-PMA | Exposure | Association between S-PMA excretion and FPG sites in WBCs | Avogbe et al. 2005 |
| 8-oxodG (HPLC-ECD) | Children living in rural and urban area (75) | M 9–13 years NS |
Benzene (ambient monitoring and personal exposure) | Exposure | Increased in WBCs and urine | Buthbumrung et al. 2008 |
| 8-oxodG (immunohistochemistry) | Children living in a low-polluted area and Mexico City (98) | MF 6–13 years NR |
O3 (stationary monitoring data) | Biomarker exposure | Higher level in nasal biopsies from children in Mexico City compared with children in the low polluted area | Calderón-Garcidueñas et al. 1999 |
| 8-iso-PGF (ELISA) | Subjects living in areas of high and low pollution (120) | MF 18–22 years NS |
PM10, 42.3 (25.7–67.9) and 25.6 (17.8–28.6) ppb NO2, 39.7 (8.3–49.9) and 21.6 (11.4–29.6 ) ppb O3, 42.9 (28.5–65.3) and 26.9 (17.6–33.5) ppb (stationary monitoring stations with subsequent modeling) |
Biomarker exposure | Highest level in plasma of subjects living in the most polluted area | Chen et al. 2007b |
| 8-oxodG (ELISA) | Subjects living in Flanders, Belgium (399) | MF 50–65 years S/NS |
1-HOP (urine) tt-MA (urine) |
Biomarker exposure | Association between exposure biomarkers (1-HOP and tt-MA) and 8-oxodG excretion in urine | De Coster et al. 2008c |
| TBARS (SPM) and CDs (SPM) | Medical doctors who lived in (24) or who recently moved to (21) Mexico City and controls (17) | NR 17–32 years NS |
O3, 152 and 29 ppb (stationary monitoring) | Biomarker exposure | No difference in serum level between subjects who had permanently or who had never lived in Mexico City; subjects who had recently (within one week) moved to Mexico City had elevated levels in serum | Hicks et al. 1996d |
| TBARS (SPM) | Subjects exposed to residential biomass smoke (28) and controls (15) | F 31–63 years NS |
None | Biomarker exposure | Highest level in serum of exposed subjects | Isik et al. 2005 |
| CDs MDA |
Children living in Isfahan, Iran (374) | MF 10–18 years NR |
PM10, 122 ± 34 μg/m3 NO2, 34 ± 13 ppbO3, 38 ± 12 ppb SO2, 36 ± 14 ppb (stationary monitoring) |
Biomarker exposure | Association between PM10 and CDs in plasma | Kelishadi et al. 2009e |
| TBARS (SPM) | Subjects living in rural (125) and urban (167) areas of Mexico | MF 34 ± 6 and 69 ± 8 years NS |
O3 (155 vs. 46 ppb) PM10 (122 vs. 104 μg/m3) Stationary monitoring station |
Biomarker exposure | Highest level in plasma of subjects living in Mexico City | Sanchez-Rodriguez et al. 2005f |
| 8-oxodG (HPLC-ECD) | Subjects living in a rural village (100) and two suburbs of Antwerp, Belgium (100) | MF 17.2 ± 0.8 years S/NS |
1-HOP (urine) tt-MA (urine) |
Exposure | Highest level in urine from exposed subjects; no correlations between exposure markers (1-HOP and tt-MA) and 8-oxodG excretion in urine | Staessen et al. 2001g |
| 8-oxodG (ELISA) | Children living in areas of low and high air pollution exposure (894) | MF 6–11 years NS |
PM2.5, 22.7 and 16.8 μg/m3 PM10, 30.0 and 20.4 μg/m3 Stationary monitoring station |
Biomarker exposure | Positive association between air pollution exposure and urinary excretion of 8-oxodG in the area with high air pollution (Teplice, Czech Republic); same association statistically nonsignificant in the area with low level of air pollution (Prachatice, Czech Republic) | Svecova et al. 2009 |
| ENDOIII/FPG sites (comet) 8-oxodG (HPLC-ECD) |
Subjects living in Copenhagen, Denmark (40) | MF 36.5 (27–46 years) S/NS |
Benzene (personal exposure and urinary S-PMA excretion) | Exposure | Positive association between urinary S-PMA excretion and 8-oxodG (WBCs); no associations with ENDOIII/FPG sites (WBCs) or urinary excretion of 8-oxodG | Sørensen et al. 2003ch |
| TBARS (SPM) | Children living in Pancevo (industrial area) and Kovacica (village) in Serbia (128) | NR 12–15 years NR |
None | Biomarker exposure | Highest level in plasma from exposed subjects | Vujovic et al. 2009i |
Abbreviations: CDs, conjugated dienes; ECD, electrochemical detection; ELISA, enzyme-linked immunosorbent assay; F, female; M, male; MF, male and female; NR, not reported; NS, nonsmoker; SPM, spectrophotometry; iso-PGF2, 8-iso-PGF2, 8-iso-prostaglandin F2; MDA, malondialdehyde; SO2, sulfur dioxide.
The values represent exposure assessment in the high-exposure and low-exposure group, respectively, unless stated otherwise by specific footnotes.
We used the median and interquartile range as surrogates for the mean and SD.
We used data from Antwerp, Belgium, and a rural area in the analysis because they had emissions of PAHs, and we estimated the SD from the 95% CI.
We calculated the mean level of LPO products from TBARS and CDs.
We used data based on the difference in interquartile range of the exposure (PM10) and assuming that the median concentration of exposure (122 μg/m3) corresponds to the mean level of MDA (0.7 μM) and CDs (2.5 μM). We calculated the SD from the mean coefficient of variation (11%) of the LPO products.
We pooled mean and SD from adult and elderly subjects.
We pooled data from Wilrijk and Hoboken, Belgium, for the analysis and calculated the SD from 95% CI.
We used the mean and SD in the groups of subjects being either higher or lower than the median urinary excretion of S-PMA.
We estimated the SD from 95% CI.
We stratified the studies into three broad categories: controlled exposures, panel studies, and cross-sectional studies. Studies with controlled exposure to air pollution PM are the most robust type of design as either crossover studies or parallel groups of subjects exposed to air pollution constituents or filtered air. In panel studies, samples are collected from the same individuals at different times of the year in order to exploit contrasts in exposure due to temporal changes. This study design minimizes the influence of interindividual variation because the subjects are their own controls. However, the design is vulnerable to confounding because other factors such as diet and sunlight show temporal (e.g., seasonal) variation, which can affect the value of the biomarker as shown, for instance, for DNA damage in WBCs detected by the comet assay (Møller and Loft 2006; Møller et al. 2002). In addition, the quality of the panel study depends on the exposure characterization; personal exposure characterization shows a closer association with biomarker levels than does exposure assessed from stationary monitoring stations (Sørensen et al. 2003a, 2003b, 2003c; Vinzents et al. 2005). Cross-sectional studies have a less controlled design than do the panel studies because the exposure gradient is obtained by collecting samples from subjects from different geographical areas or occupations. The cross-sectional studies can have optimal exposure characterization, but confounding can be a problem because individual factors such as lifestyle, including diet, may influence the biomarker and covary with the exposure. This problem typically arises, for instance, when policemen and office personal or subjects from rural and urban areas are being compared.
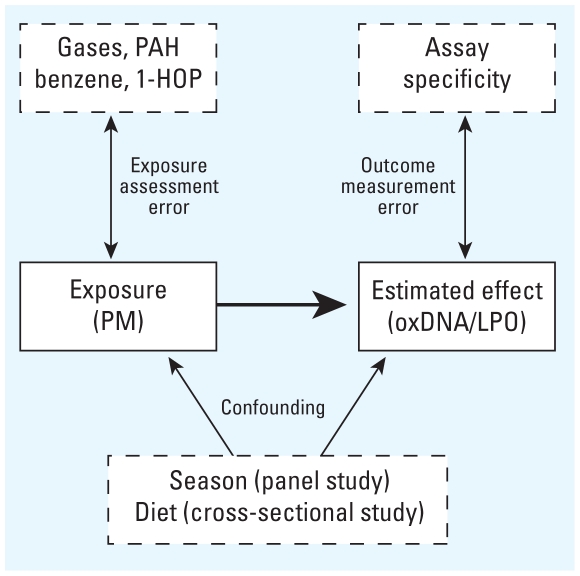
We critically analyzed the studies with special focus on suboptimal exposure assessment and the quality of biomarkers. We referred to these problems as potential measurement error in the exposure assessment and biomarkers because they can be regarded as systematic errors that may affect the validity. Figure 1 outlines the relationship between the measurement error in exposure assessment and biomarker. We should emphasize that specific studies included in our analysis may have other sources of bias, including selection bias and small numbers of observations, which can affect the estimated effect size in a particular study.
Figure 1.
Types of errors in studies of the effect of combustion air pollution. oxDNA, oxidatively damaged DNA.
Measurement error in exposure assessment
The primary exposure assessment in our analysis is the mass concentration of particles as PM2.5 or PM10 or the number concentration of UFPs. The exposure characterization encompasses data obtained from stationary monitor stations and personal monitors. We regarded personal exposure to PM as the optimal exposure assessment; for studies without PM measurements we regarded data on ambient gasses [nitrogen oxides (NOx), ozone (O3), or sulfur dioxide], polycyclic aromatic hydrocarbons (PAHs), and benzene as indirect estimates of PM exposure with greater potential for error. Similarly, the urinary excretion of metabolites of PAHs [e.g., 1-hydroxypyrene (1-HOP)] and benzene [S-phenylmercapturic acid (S-PMA) and trans,trans-muconic acid (tt-MA)] generated by their biotransformation are potentially biased estimates of the ambient concentration of PM, although they may be important biomarkers of internal dose of the parent compound. The measured PM showed the strongest association with the biomarkers of oxidized DNA and lipids in studies that measured the exposure as both PM and nitrogen dioxide (NO2), 1-HOP, S-PMA, and tt-MA (Avogbe et al. 2005; Bräuner et al. 2007; Sørensen et al. 2003a, 2003b). We expect that the measurement error will be nondifferential, which usually biases effect estimates toward the null because they tend to obscure contrasts between the exposed and unexposed and those with or without the outcome of interest.
Measurement error related to biomarkers
The potential for biomarker measurement error originates from unspecific measurements or analytic flaws due to poor assay conditions. For instance, suboptimal assay procedures used to detect oxidatively damaged DNA may cause spurious oxidation that increases the apparent level of DNA damage. The level of 8-oxodG in DNA from unexposed mammals is approximately 1 lesion/106 dG (deoxyguanosine); the European Standards Committee on Oxidative DNA Damage (2003a, 2003b; Gedik et al. 2005) recommended that publications that report levels of 8-oxodG above a threshold of 5 lesions/106 dG should be interpreted with caution. The comet assay detects DNA damage by migration of DNA in agarose gels, and the end points are usually reported as extent of migration, although they can be transformed to lesions per unaltered nucleotides by calibration with ionizing radiation (Forchhammer et al. 2008; Møller et al. 2004). The level of oxidatively damaged DNA measured by the comet assay in WBCs of humans is < 1 lesion/106 nucleotides (Møller 2006). Oxidatively damaged DNA, nucleobases, and LPO products can be measured by antibody-based methods, but artificially high background levels can occur because of unspecific binding of the antibodies to other biomolecules (Halliwell and Whiteman 2004; Møller et al. 2008b). The simple assay of TBARS and CDs has been seriously criticized and is not recommended for in vivo detection of LPO products, whereas improved methods using high-performance liquid chromatography (HPLC) purification steps are more reliable assays of TBARS (Halliwell and Whiteman 2004). We classified biomarkers with suboptimal biochemical analysis as follows: (1) simple spectrophotometric measurement of TBARS without a prepurification step; (2) simple assays for CDs and lipid hydroperoxides, (3) levels of 8-oxodG exceeding a threshold of 5 lesions/106 dG in the unexposed group, and (4) detection of oxidatively damaged DNA, nucleobases, or lipids by antibody-based methods without prepurification steps. We expect that the biomarker measurement error will result in reduced effect estimates for both nonspecific biomarkers and assays having low sensitivity or a high limit of detection.
Assessment of estimated effect size
The studies differ considerably in design, and the results are reported in ways and units that preclude direct comparison of the estimated effect size in the studies. Thus, we have estimated the effect of exposure on biomarkers as standardized mean differences with 95% confidence intervals (CIs) between exposed subjects and referents in a random effects meta-analysis by means of Review Manager (RevMan; version 5.0; Nordic Cochrane Centre, Cochrane Collaboration 2008, København Ø, Denmark). The standardized mean difference is the difference in means of groups divided by the pooled standard deviation (SD). It is a measurement of estimated effect, which can be used in a meta-analysis when all studies assess the same outcome (level of oxidized biomolecules in this analysis), but it is measured in a variety of ways with different scales. We assessed the estimated effect size in a random model meta-analysis because it incorporates heterogeneity among studies. The heterogeneity between studies was analyzed by tau squared, chi squared, and I2 tests; tests for subgroup differences were carried out using the chi-square test. We obtained means, SDs, and the number of subjects from the studies, or we calculated these values from data reported in the publications (see Tables 2–5). The variance was reported in different ways in the original publications; thus, our estimates of 95% CIs may be biased, but the central estimates (means) should not be. We calculated the mean and SD from regression analyses for studies that modeled continuous data and defined the exposure gradient as equal to either the interquartile range (Kelishadi et al. 2009; Liu et al. 2009a, 2009b) or a 10 μg/m3 increase in PM (Liu et al. 2007). The interquartile range in PM2.5 was in the same range as a 10-μg/m3 increase in PM2.5 in the studies carried out in Windsor, Ontario, Canada (Liu et al. 2007, 2009a, 2009b), whereas the interquartile range (66.5 μg/m3 measured as PM10) measured by Kelishadi et al. (2009) in Iran was substantially higher, which could reflect different sources of exposure or PM fraction. We calculated overall means and SD for studies that included more than one group of exposed subjects or investigated the same subjects under different exposure scenarios (Avogbe et al. 2005; De Coster et al. 2008; Mills et al. 2008; Novotna et al. 2007; Rundell et al. 2008; Singh et al. 2007; Staessen et al. 2001; Sørensen et al. 2003a, 2003b). We assumed that the interquartile ranges would be equal to the SDs found in studies that used nonparametric analyses and reported variation as ranges (Chen et al. 2007; Romieu et al. 2008; Sørensen et al. 2003a, 2003b).
Results
Oxidative damage reported in controlled exposure studies
Table 2 summarizes studies on the association between air pollution exposure and oxidized DNA and lipids in controlled exposure studies. The number of subjects in these studies is rather low (3–41 subjects; mean ± SD, 18 ± 12), which may be because carrying out controlled exposure on a large number of subjects is demanding. A study of air pollution exposure to UFPs in persons bicycling for approximately 90 min in a laboratory or on traffic-intense streets reported that the level of FPG sites in WBCs was associated with the number concentration of UFPs (Vinzents et al. 2005). A subsequent investigation by the same group had a similar correlation between particulate fractions with median particle sizes of 23 nm and 57 nm (consistent with semivolatile organic compounds from diesel exhaust and carbonaceous soot emissions into the air of a busy street) and the level of FPG sites in WBCs (Bräuner et al. 2007). A subsequent study in elderly subjects showed a statistically non-significant decrease in urinary excretion of the F2-isoprostane 8-iso-prostaglandin-F2 after a period of home air filtration (Bräuner et al. 2008). In another study of elderly patients with coronary heart disease, Mills et al. (2008) reported that inhalation of concentrated air pollution particles (CAPs) was associated with increased concentration of 8-isoprostanes in EBC. This finding is in keeping with that found by Rundell et al. 2008) who observed that healthy young subjects had elevated level of MDA in EBC after intensive exercise at a location with high-traffic intensity compared with the same type of exercise at a location with less traffic.
Controlled exposure to wood smoke containing very high mass concentration of particles has been associated with increased levels of LPO products in serum, urine, and EBC (Barregard et al. 2006, 2008). However, Danielsen et al. (2008) observed no association between exposure and FPG sites in WBCs and suggested that this result may be due to increased DNA repair activity of oxidized nucleobases because urinary excretion of 8-oxoGua and WBC expression levels of the 8-oxoguanine DNA glycosylase (OGG1) base excision repair enzyme, which removes 8-oxoGua from DNA, were increased after exposure to wood smoke but not after exposure of the same subjects to clean air. Increased urinary excretion of 8-oxoGua was also observed in a study where subjects were exposed to exhaust on a traffic-intense street for 4 hr (Suzuki et al. 1995), but 2 hr of exposure to a high concentration of diesel exhaust was not associated with urinary excretion of 8-oxodG or F2-isoprostanes in subjects with metabolic syndrome (Allen et al. 2009).
Oxidative damage reported in panel studies
Table 3 summarizes studies on the association between air pollution exposure and oxidized DNA and lipids in panel studies. These studies involved multiple measurements over time, and the number of subjects in these studies was higher than the number of subjects in controlled exposure studies (2–182 subjects; mean ± SD, 62 ± 59). Several panel studies showed that concurrent air pollution exposure was associated with elevated levels of LPO products in EBC (Liu et al. 2009a; Romieu et al. 2008) and plasma (Liu et al. 2007; Medina-Navarro et al. 1997), as well as elevated levels of 8-oxodG in plasma (Chuang et al. 2007). Subjects without doctor-diagnosed cardiovascular diseases or who did not take medication for diabetes showed an association between outdoor levels of PM2.5 and TBARS in plasma, although the analysis of all subjects in the study only indicated statistically nonsignificant associations between personal exposure to PM2.5 and TBARS or 8-isoprostanes in plasma (Liu et al. 2009b). Personal exposure to PM2.5 was associated with increased levels of 8-oxodG in WBCs of students living in the center of Copenhagen, whereas the exposure was only associated with the MDA levels of women and not with the level of FPG sites in WBCs in any group (Sørensen et al. 2003a, 2003b). Interestingly, Sørensen et al. (2003a, 2003b) observed no correlation between 8-oxodG in WBCs and the background mass concentration of PM2.5 measured at stationary monitoring stations, suggesting that a relatively clean urban air may provide too little contrast in the long-range transported fractions of PM to be a reliable indicator of traffic-generated exposure. Moreover, they found significant association between the biomarkers and personal exposure to NO2 supporting the key role of PM. This is in keeping with observations from a controlled exposure study with constant NO2 exposure, which showed a strong effect of change in PM exposure on DNA oxidation (Bräuner et al. 2007).
Table 3.
Summary of panel studies on exposure to air pollution PM from combustion processes.
| Biomarker | Subjects (n) | Sex, age, smoking | Exposure assessmenta | Potential measurement error | Findings | Study |
|---|---|---|---|---|---|---|
| 8-oxodG (ELISA) | Students followed for 3 months (76) | MF 18–25 years NS |
PM2.5, 12.7–59.5 μg/m3 PM10, 22.2–87.2 μg/m3 SO2, 2.8–39.4 ppb NO2, 2.8–33.3 ppb O3, 22.5–48.3 ppb (stationary monitoring stations) |
Biomarker exposure | Positive association between 8-oxodG in plasma and SO2 and O3; no association with PM2.5, PM10, and NO2 | Chuang et al. 2007 |
| TBARS (FD) | Subjects with diabetes mellitus (25) followed for 7 weeks | MF 28–63 years NS |
Personal PM10, 25.5 (9.8–133) μg/m3 | Biomarker | Positive association between PM10 levels and TBARS in plasma | Liu et al. 2007b |
| TBARS (FD) 8-Isoprostanes (immunoassay) |
Asthmatics (182) followed for 4 weeks | MF 9–14 years NS |
PM2.5, 2.7–14.3 μg/m3 SO2, 1.3–13.8 ppb NO2, 12.3–27.0 ppb O3, 7.5–21.0 ppm Stationary monitoring |
Biomarker exposure | Positive association between TBARS in EBC and SO2, NO2 and PM2.5, but not with O3; the concentration of 8-isoprostanes in EBC was only associated with SO2 concentration | Liu et al. 2009ac |
| TBARS (SPM) 8-Isoprostanes (ELISA) |
Normal subjects living in Windsor, Ontario, Canada (29) followed for maximally 50 days | MF 64–96 years NS |
PM2.5, 6.3 (0.9–16.6) μg/m3 (personal exposure) and 15.3 (10.4–24.2) μg/m3 (outdoor) | Biomarker | No association with personal PM2.5 exposure and LPO products in plasma; an association with outdoor PM2.5 and TBARS in a subset of subjects without doctor-diagnosed cardiovascular disease or who did not take diabetic medication | Liu et al. 2009bd |
| TBARS (SPM) CDs (SPM) |
Medical doctors investigated 1 or 16 weeks after arrival in Mexico City (21) | NR 27–32 years NS |
O3, 141 ppb (no report of the O3 level in original residence) | Biomarker exposure | Increased TBARS in serum after the first week of the stay, normalized in samples obtained 16 weeks after | Medina-Navarro et al. 1997e |
| MDA (FD) | Asthmatics (107) followed for 2–16 weeks (average 8 weeks) | MF 10 ± 2 years NS |
PM2.5, 27.4 (4.2–89.5) μg/m3 NO2, 35.3 (13.9–73.5) ppb O3, 31.1 (9.8–60.7) ppb (stationary monitoring stations) |
Biomarker exposure | Positive association between ambient PM2.5 levels and MDA in EBC | Romieu et al. 2008f |
| 8-oxodG (HPLC-ECD) FPG sites (comet) MDA (HPLC) |
Students living in Copenhagen, Denmark (50) followed for 1 year | MF 20–33 years NS |
Personal PM2.5, 16.1 (10–24.5) μg/m3 PM2.5, 9.2 (5.3–14.8 μg/m3 (stationary monitoring stations) |
No | Correlation between personal exposure to PM2.5 and 8-oxodG in WBCs and MDA in plasma (only women); no correlation between PM2.5 and FPG sites in WBCs or 24-hr urinary excretion of 8-oxodG; no correlation between biomarkers and stationary (urban background) measurements of PM2.5 | Sørensen et al. 2003a, 2003bg |
| 8-oxodG (ELISA) | Security guards analyzed before and after a work shift (2) followed for 2 months | NR 18–20 years NS |
PM2.5, 243 (199–460) μg/m3 | Biomarker | Increased in urine after the work shift | Wei et al. 2009h |
Abbreviations: ECD, electrochemical detection; ELISA, enzyme-linked immunosorbent assay; FD, fluorescence detection; M, male; MF, male and female; NR, not reported; NS, nonsmoker; SO2, sulfur dioxide; SPM, spectrophotometry.
The values represent exposure assessment in the high-exposure and low-exposure group, respectively, unless stated otherwise by specific footnotes.
We calculated means and SD from the regression analysis in the study, based on 10-μg/m3 increase in PM10 and a coefficient of variation of 100%.
We calculated the mean level of LPO from TBARS and 8-isoprostanes, and the SD from the lower 95% CI assuming that it is similar to the 5th percentile and the coefficient of variation is the same in the exposed and reference group.
We calculated data from the regression model reported in the original publication. The data correspond to the difference in LPO products from the interquartile range in personal PM2.5 exposure. We calculated the SD from the coefficient of variation of data reported as 5th and 95th percentile, assuming that it is equivalent to 95% CI, and the mean level of LPO products from data of TBARS and 8-isoprostanes.
We calculated the mean level of LPO products from TBARS and CDs.
We calculated data (means) by regression analysis assuming that the SD is the same as the interquartile range.
We assumed that the SD and interquartile is the same value for the analysis of 8-oxodG and calculated the mean level of ENDOIII/FPG sites.
The study encompassed samples from two subjects analyzed on 29 working days.
Oxidative damage reported in cross-sectional studies
The design of the cross-sectional studies can be grouped into two main categories. The first category is characterized by studies that achieved the exposure contrast by studying subjects in occupations with different ambient air pollution levels (Table 4). The other category is characterized by studies of subjects, often with comparable occupations or ages, from geographical areas with different ambient air pollution levels (Table 5). The cross-sectional studies have generally included more subjects than the controlled exposure studies and panel studies. The number of subjects in the cross-sectional studies on different occupations has been in the range of 31–356 subjects (mean ± SD = 109 ± 95 subjects), whereas the studies that have contrasted exposure in different geographical areas have used even higher number of subjects (43–894 subjects; mean ± SD = 222 ± 234).
Table 4.
Summary of cross-sectional studies on exposure to air pollution PM from combustion processes in humans in different occupations.
| Biomarker | Subjects (n) | Sex, age, smoking | Exposure assessmenta | Potential measurement error | Findings | Study |
|---|---|---|---|---|---|---|
| 8-oxodG (HPLC-ECD) MDA (HPLC) |
Bus drivers (107) | MF 27–60 years NS |
1-HOP (urine) | Exposure | Bus drivers in the city center had higher levels of urinary 8-oxodG excretion than did bus drivers from the rural/suburban area; no clear differences between urinary excretions on working days and on days off observed; unaltered MDA in plasma between bus drivers in the city center and rural/suburban area | Autrup et al. 1999, Loft et al. 1999b |
| Lipid hydroperoxides (SPM) | Traffic officers and controls (32) | M 38–52 years S/NS |
None | Biomarker exposure | No difference between exposed and controls | Bonina et al. 2008c |
| FPG sites (comet) | Airport personnel (41) and controls (31) | NR 43 ± 9 years S/NS |
Stationary sampling of PAHs Urinary 1-HOP (urine) |
Exposure | Higher level in WBCs of exposed subjects | Cavallo et al. 2006d |
| 8-oxodG (ELISA) | Taxi drivers (95) and controls (75) | M 40 ± 4 and 44 ± 7 years S/NS |
1-HOP (urine) | Biomarker exposure | Highest level in urine of exposed subjects | Chuang et al. 2003 |
| 8-oxodG (HPLC-ECD) | Taxi-motor drivers and rural controls (41) | M 36 ± 5 years NS |
Ambient (stationary) concentration of PAHs and benzene Urinary excretion of S-PMA and 1-HOP |
Biomarker exposure | Highest level in WBCs of exposed subjects (high background level of 8-oxodG, 11.1 lesions/106 dG) | Ayi Fanou et al. 2006 |
| 8-oxodG (ELISA) | Highway toll workers and controls (74) | F 26 ± 6 and 27 ± 5 years S/NS |
Traffic intensity and urinary 1-HOP glucuronide excretion | Biomarker exposure | Highest level in urine of exposed subjects | Lai et al. 2005 |
| ENDOIII/FPG sites (comet) | Policemen from Prague, Czech Republic (65) | M 31 and 35 years (median) NS |
PM2.5 (stationary monitoring data, 33 ± 40 and 15 ± 9 μg/m3) PAHs (personal exposure, 8.5 ± 9 and 3.0 ± 3.4 ng/m3) |
Exposure | Highest level in WBCs of exposed subjects; positive correlation between PAH exposure and DNA damage in samples collected in January | Novotna et al. 2007e |
| FPG sites (comet) | Subjects exposed to traffic (44) and controls (27) | MF 35–64 years S/NS |
None | Exposure | Statistically nonsignificant higher level in WBCs of exposed subjects | Palli et al. 2009 |
| 8-oxodG (ELISA) 15-F2t-isoprostanes (immunoassay) |
Bus drivers (50) and controls (50) | M 50 ± 10 and 51 ± 11 years NS |
PM2.5 and PM10 (stationary monitoring station) and PAHs (personal exposure) PM2.5, 32.1 ± 8.1 and 20.9 ± 6.8 μg/m3 PM10, 38.6 ± 8.2 and 24.1 ± 6.5 μg/m3 |
Biomarker exposure | Highest levels in urine of exposed subjects | Rossner et al. 2007, 2008a, 2008bf |
| 8-oxodG (LC-MS/MS) M1dG (immunoslot blot) |
Policemen, bus drivers, and controls (356) | M 34.1 ± 9 years S/NS |
Concentration of PAHs in personal PM2.5 samples | Biomarker exposure | Policemen in Kosice, Slovakia, had higher levels of 8-oxodG in WBCs than did controls; no effect in policemen from Prague; 8-oxodG levels were very high (i.e., 53.6 lesions/106 nucleotides, corresponding to 244 lesions/106 dG); significantly higher levels of M1dG in exposed subjects in Sofia | Singh et al. 2007 |
Abbreviations: ECD, electrochemical detection; ELISA, enzyme-linked immunosorbent assay; F, female; LC-MS, liquid chromatography–mass spectrometry; LC-MS/MS, liquid chromatography with tandem mass spectrometry; M, male; MF, male and female; NR, not reported; NS, nonsmoker; S, smoker; SPM, spectrophotometry.
The values represent exposure assessment in the high-exposure and low-exposure group, respectively, unless stated otherwise by specific footnotes.
We used data from bus drivers on working days.
We pooled means and SD from smokers and nonsmokers of controls and traffic officers at the sampling before the intervention with phytochemicals (day 0).
The publication reports the mean level DNA damage without indication of the SD, whereas later studies by the same group showed a coefficient of variation of 40%.
The data represent the variation between sampling in January and September. The personal exposure to PAHs in the exposed and control group was 6.0 and 4.5 ng/m3, respectively.
The study had sampling of PM2.5 and PM10 by personal monitors, but the low amount of material precluded the assessment of individual exposure.
Using job titles as the basis for stratification of exposure, studies showed that subjects in occupations with high exposure to traffic emissions had higher levels of FPG sites (Avogbe et al. 2005; Cavallo et al. 2006; Palli et al. 2009) and 8-oxodG and M1dG (Ayi Fanou et al. 2006; Singh et al. 2007) in WBCs than did referents. However, the latter two studies reported levels of 8-oxodG that were above the threshold of 5 lesions/106 dG, suggesting the potential for spurious oxidation of the samples. Another study showed higher levels of FPG and ENDOIII sites in WBCs of traffic emission exposed policemen compared with other policemen working indoor during a month when air pollution was relatively high (i.e., January) but no association during a month with low air pollution exposure (i.e., September) (Novotna et al. 2007). In contrast, Bonina et al. (2008) found no difference in serum lipid hydroperoxides concentration when comparing traffic officers with healthy indoor workers; the primary purpose of their study appears to have been to compare lipid hydroperoxide levels between subjects that received phytochemicals and subjects that did not, and they evaluated associations with air pollution in a secondary analysis. Evidence of null associations in studies that evaluate air pollution as a secondary exposure suggests the possibly of a general trend toward publication bias favoring studies that report positive associations when air pollution is the primary exposure of interest, but the findings of the Bonina et al. (2008) study may also have been due to the use of a nonspecific spectro-photometric assay for the detection of LPO products, which can bias the estimated effect toward null. Studies using urinary biomarkers have also shown increased levels of 8-oxodG and 15-F2t-isoprostanes in subjects exposed to high concentrations of traffic-vehicle exhausts (Chuang et al. 2003; Lai et al. 2005; Rossner et al. 2008a, 2008b).
Cross-sectional studies of subjects living, working, or going to school in locations with different ambient air pollution levels encompass investigations of subjects with predefined age groups, such as children, adolescents, adults, or elderly. Studies of children living in areas with different levels of exposure have shown positive associations with 8-oxodG levels in nasal cells (Calderón-Garcidueñas et al. 1999), 8-oxodG in WBCs (Buthbumrung et al. 2008), LPO products in plasma (Kelishadi et al. 2009; Vujovic et al. 2009), and urinary excretion of 8-oxodG (Svecova et al. 2009). Studies of air pollution exposure in adults have provided more mixed results: benzene as a marker of urban air pollution exposure was associated with urinary excretion of S-PMA and 8-oxodG in WBCs, but not with ENDOIII and FPG sites in WBCs or urinary excretion of 8-oxodG (Sørensen et al. 2003c). Other studies of urinary excretion of 8-oxodG have shown positive associations (De Coster et al. 2008; Loft et al. 1999; Staessen et al. 2001; Wei et al. 2009), but studies of LPO products in plasma have indicated both positive associations with oxidative damage (Chen et al. 2007; Isik et al. 2005; Sanchez-Rodriguez et al. 2005) and no apparent effects (Autrup et al. 1999; Hicks et al. 1996).
Combined effect estimates for markers in airways, blood, and urine
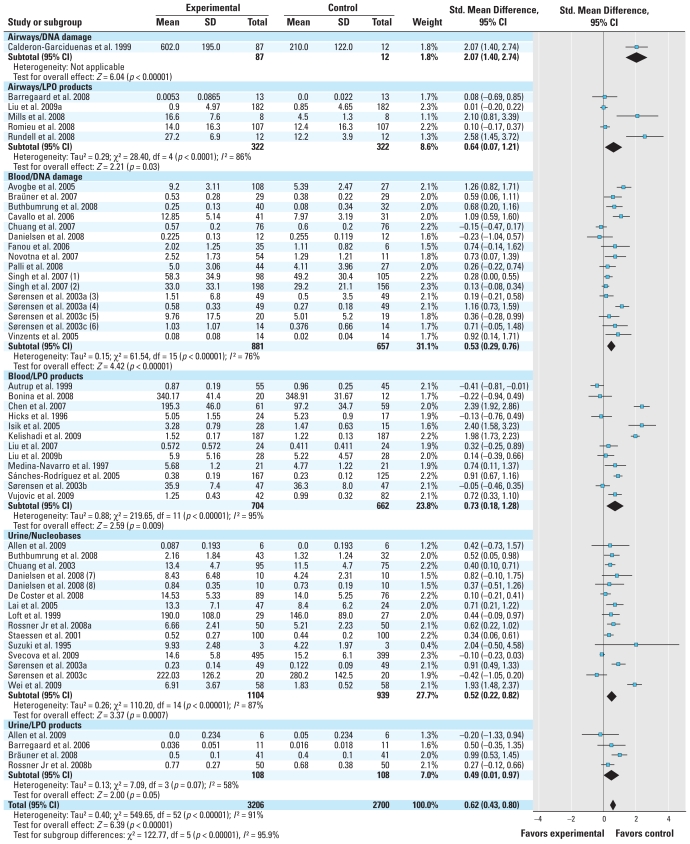
The qualitative assessment in the preceding sections indicates that most of the reports showed associations between air pollution exposure and oxidatively damaged DNA, nucleobases, and lipids. Most studies measured the biomarkers in surrogate tissue cells such as WBCs or noncellular bodily fluids such as plasma, urine, and EBC. The data on biomarkers of the airways mainly encompass measurements of LPO products in EBC, whereas only one study examined 8-oxodG in nasal cells (Calderón-Garcidueñas et al. 1999). Figure 2 shows study-specific and overall estimates of effect for exposure to PM on oxidatively damaged DNA, nucleobases, and lipids in the airways, blood, and urine. In general there is considerable heterogeneity between the studies and between subgroups; methodological diversity between studies may explain the heterogeneity. In addition, the categorization of the exposure does not take into account that exposure gradients most likely differ between the studies. It is not possible to calculate a standardized exposure unit (e.g., effect per 10-μg/m3 increase PM2.5) because the studies reported exposure measurements in different fractions of PM, or they contained no information about the concentration of PM. The overall standardized mean differences between exposed subjects and nonexposed referents for the oxidized DNA and for LPO products in the blood were 0.53 (95% CI, 0.29–0.76) and 0.73 (95% CI, 0.18–1.28), respectively. In the urine the estimated effect size by PM exposure versus nonexposed referents for oxidatively damaged DNA and nucleobases and for LPO products was 0.52 (95% CI, 0.22–0.82) and 0.49 (95% CI, 0.01–0.97), respectively. This suggests that exposure to PM is associated with comparable increases in oxidized DNA and lipids, although it should be emphasized that the heterogeneity between subgroups might mask real differences between the biomarkers. The effect on DNA damage in the airways is presently difficult to assess because there was only one study of oxidized DNA (Calderón-Garcidueñas et al. 1999). The estimated effect on LPO products in EBC was 0.64 (95% CI, 0.07–1.21), which is comparable to overall standardized mean differences in LPO products in the blood and urine with PM exposure. This finding suggests that LPO products in plasma and urine are suitable biomarkers of biologically effective PM dose reflecting oxidative stress in the airways.
Figure 2.
Forest plot of air pollution exposure on biomarkers of oxidized DNA, nucleobases, and lipids. Specific biomarkers in studies that have measured multiple assays of oxidized DNA and lipids are (1) 8-oxodG, (2) M1dG, (3) 8-oxodG, (4) ENDOIII/FPG sites, (5) ENDOIII/FPG sites, (6) 8-oxodG, (7) 8-oxodG, and (8) 8-oxoGua (the numbers in parentheses refer to references citations that are listed by first author/year only).
Combined estimates according to the potential for exposure or outcome measurement error
The focus of our analysis was on the estimated effect of exposure to PM on biomarkers of oxidized DNA, nucleobases, and lipids. A number of studies have not measured personal exposure to PM, which may be because of their focus on other air pollution constituents or lack of resources or because the study was too large to do personal exposure measurements. Ideally, all studies should have included personal measurements of PM, and the analysis should have included mutual adjustment for coexposures to other air pollutants. We assumed that PM is the most important contributor to oxidative stress among the air pollutants and that personal exposure can be estimated from the personal exposure to the other pollutants or ambient PM levels, although with potential bias. Of the studies identified in our meta-analysis, only Chen et al. (2007) has estimated personal exposure levels using mathematical modeling of data from stationary monitoring stations. We have categorized the studies according to whether or not they characterized exposure using personal versus ambient PM measurements. Based on the categorization of the studies according to the potential measurement error in Tables 2–5, the estimated effect size is 0.55 (95% CI, 0.19–0.90), 0.66 (95% CI, 0.37–0.95), and 0.65 (95% CI, 0.34–0.96) for studies categorized as having no potential measurement error, potential measurement error in either biomarker analysis or exposure assessment, and potential measurement error in both biomarker analysis or exposure assessment, respectively [see Supplemental Material for the Forest plots (doi:10.1289/ehp.0901725)]. The effect size is essentially identical in the three groups, which could be caused by opposite acting effects of potential measurement errors (regression toward null effect) and uncontrolled confounding factors (increased effect size) in panel and cross-sectional studies. The percentages of studies that did not use personal measurements to assess PM exposure were 8% (1 of 12), 36% (4 of 11), and 100% (29 of 29) in the studies categorized as controlled exposures, panel studies, and cross-sectional studies, respectively (χ2 = 36.7, p < 0.001). The use of error-prone biomarker assays was less likely in the controlled exposure studies (31%; 4 of 13) than in the panel studies (64%; 7 of 11) and cross-sectional studies (59%; 17 of 29), although no differences were found between the distributions (χ2 = 3.4, p > 0.05). Overall, the quality of the studies, in terms of the likelihood of exposure and outcome measurement error, appears highest in the controlled studies and lowest in the cross-sectional studies. Both of these measurement errors may bias the effect estimate toward null, whereas uncontrolled confounding factors in panel studies and cross-sectional studies most likely increased the estimated effect size. In addition, we emphasize that the studies that measured personal PM exposure and used more accurate biomarker assays mainly investigated the effect of air pollution particles in realistic urban air concentrations (Bräuner et al. 2007, 2008; Rundell et al. 2008; Sørensen et al. 2003a, 2003b; Vinzents et al. 2005), although one study used high concentrations of wood smoke particles (Barregard et al. 2006, 2008; Danielsen et al. 2008). Collectively, associations between PM exposure and biomarkers of oxidative stress estimated by studies likely to have more accurate exposure and outcome measurements cannot be explained by exposure to excessive concentrations of PM. Yet, the controlled exposure studies in our meta-analysis had few numbers of observations, which might reduce the precision with which effects have been estimated. In addition, controlled exposure studies may be more prone to selection bias due to random errors in selection and have limited generalizability because they are restricted to participants with specific characteristics.
Discussion
Our analysis shows that exposure to combustion particles is consistently associated with elevated levels of oxidatively damaged DNA and nucleobases and LPO products in human blood cells, plasma, urine, and EBC. The association is seen across studies with optimum designs, including controlled or personal exposure assessment and biomarkers with low potential measurement error due to indirect exposure assessment and/or use of biomarkers prone to artifacts. Still, we emphasize that the identified studies are inhomogeneous in design and quality of biomarkers, which weakens our conclusions about specific exposure–effect relationships for particulate air pollution. In addition, few numbers of subjects, especially in the controlled exposure studies, is a minor limitation that might affect the generalizability of our meta-analysis results.
Our critical analysis indicates that the range in exposure to realistic ambient concentrations of combustion particles is associated with a 50% increase in the level of oxidatively damaged DNA, nucleobases, and lipids, supporting the notion that they are suitable biomarkers of the biologically effective dose of PM. However, we caution against the use of suboptimal biomarkers; ideal biomarkers of oxidative damage should detect a major part of the total ongoing oxidative damage in vivo, have small assay variation, have smaller intraindividual than interindividual variation, not be confounded by diet, be stable on storage, and have the same level obtained in target and surrogate tissue (Halliwell and Whiteman 2004). They should also have predictive value of risk of disease, which can be firmly assessed only in prospective studies (Loft and Møller 2006). The biomarkers of urinary excretion of 8-oxodG and TBARS in plasma are among the few biomarkers that have been studied in prospective cohort studies; they have predictive value regarding development of lung cancer and cardiovascular diseases, respectively (Loft et al. 2006; Walter et al. 2004). Further development of oxidized DNA and LPO products as biomarkers of biological effective dose of air pollution exposure should focus on the most reliable and well-validated assays, including assays for the measurement of isoprostanes and techniques that consistently measure low background levels of oxidatively damaged DNA and nucleobases. The relevant biomarkers with low potential of measurement error are constantly developed to increase assay capacity toward high throughput, for instance, the comet assay and urinary excretion of 8-oxodG (Henriksen and Poulsen 2009; Stang and Witte 2009). This development will allow the use of these biomarkers of exposure to PM in large-scale population studies.
Footnotes
This work was supported by grants from the Danish Research Councils.
Supplemental Material is available online (doi:10.1289/ehp.0901725 via http://dx.doi.org/).
Reference
- Allen J, Trenga CA, Peretz A, Sullivan JH, Carlsten CC, Kaufman JD. Effect of diesel exhaust inhalation on antioxidant and oxidative stress responses in adults with metabolic syndrome. Inhal Toxicol. 2009;21:1061–1067. doi: 10.3109/08958370902721424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autrup H, Daneshvar B, Dragsted LO, Gamborg M, Hansen ÅM, Loft S, et al. Biomarkers for exposure to ambient air pollution—comparison of carcinogen-DNA adduct levels with other exposure markers and markers for oxidative stress. Environ Health Perspect. 1999;107:233–238. doi: 10.1289/ehp.99107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avogbe PH, Ayi-Fanou L, Autrup H, Loft S, Fayomi B, Sanni A, et al. Ultrafine particulate matter and high-level benzene urban air pollution in relation to oxidative DNA damage. Carcinogenesis. 2005;26:613–620. doi: 10.1093/carcin/bgh353. [DOI] [PubMed] [Google Scholar]
- Ayi Fanou L, Mobio TA, Creppy EE, Fayomi B, Fustoni S, M⊘ller P, et al. Survey of air pollution in Cotonou, Benin—air monitoring and biomarkers. Sci Total Environ. 2006;358:85–96. doi: 10.1016/j.scitotenv.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Barregard L, Sällsten G, Andersson L, Almstrand AC, Gustafson P, Andersson M, et al. Experimental exposure to wood smoke: effects on airway inflammation and oxidative stress. Occup Environ Med. 2008;65:319–324. doi: 10.1136/oem.2006.032458. [DOI] [PubMed] [Google Scholar]
- Barregard L, Sällsten G, Gustafson P, Andersson L, Johansson L, Basu S, et al. Experimental exposure to wood-smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhal Toxicol. 2006;18:845–853. doi: 10.1080/08958370600685798. [DOI] [PubMed] [Google Scholar]
- Bonina FP, Puglia C, Frasca G, Cimino F, Trombetta D, Tringali G, et al. Protective effects of a standardised red orange extract on air pollution-induced oxidative damage in traffic police officers. Nat Prod Res. 2008;22:1544–1551. doi: 10.1080/14786410701740401. [DOI] [PubMed] [Google Scholar]
- Bräuner EV, Forchhammer L, M⊘ller P, Barregard L, Gunnarsen L, Afshari A, et al. Indoor particles affect vascular function in the aged: an air filtration-based intervention study. Am J Respir Crit Care Med. 2008;177:419–425. doi: 10.1164/rccm.200704-632OC. [DOI] [PubMed] [Google Scholar]
- Bräuner EV, Forchhammer L, M⊘ller P, Simonsen J, Glasius M, Wåhlin P, et al. Exposure to ultrafine particles from ambient air and oxidative stress-induced DNA damage. Environ Health Perspect. 2007;115:1177–1182. doi: 10.1289/ehp.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD. Cardiovascular effects of air pollution. Clin Sci. 2008;115:175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- Buthbumrung N, Mahidol C, Navasumrit P, Promvijit J, Hunsonti P, Autrup H, et al. Oxidative DNA damage and influence of genetic polymorphisms among urban and rural school-children exposed to benzene. Chem Biol Interact. 2008;172:185–194. doi: 10.1016/j.cbi.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Wen-Wang L, Zhang YJ, Rodriguez-Alcaraz A, Osnaya N, Villarreal-Calderón A, et al. 8-hydroxy-2’-deoxyguanosine, a major mutagenic oxidative DNA lesion, and DNA strand breaks in nasal respiratory epithelium of children exposed to urban pollution. Environ Health Perspect. 1999;107:469–474. doi: 10.1289/ehp.107-1566580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo D, Ursini CL, Carelli G, Iavicoli I, Ciervo A, Perniconi B, et al. Occupational exposure in airport personnel: characterization and evaluation of genotoxic and oxidative effects. Toxicology. 2006;223:26–35. doi: 10.1016/j.tox.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Chen C, Arjomandi M, Balmes J, Tager I, Holland N. Effects of chronic and acute ozone exposure on lipid peroxidation and antioxidant capacity in healthy young adults. Environ Health Perspect. 2007;115:1732–1737. doi: 10.1289/ehp.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CY, Lee CC, Chang YK, Sung FC. Oxidative DNA damage estimated by urinary 8-hydroxydeoxyguanosine: influence of taxi driving, smoking and areca chewing. Chemosphere. 2003;52:1163–1171. doi: 10.1016/S0045-6535(03)00307-2. [DOI] [PubMed] [Google Scholar]
- Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- Danielsen PH, Bräuner EV, Barregard L, Sällsten G, Wallin M, Olinski R, et al. Oxidatively damaged DNA and its repair after experimental exposure to wood smoke in healthy humans. Mutat Res. 2008;642:37–42. doi: 10.1016/j.mrfmmm.2008.04.001. [DOI] [PubMed] [Google Scholar]
- De Coster S, Koppen G, Bracke M, Schroijen C, Hond ED, Nelen V, et al. Pollutant effects on genotoxic parameters and tumor-associated protein levels in adults: a cross sectional study. Environ Health. 2008;7:26. doi: 10.1186/1476-069X-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ Health Perspect. 2005;113:934–946. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Standards Committee on Oxidative DNA Damage. Measurement of DNA oxidation in human cells by chromatographic and enzymic methods. Free Radic Biol Med. 2003a;34:1089–1099. doi: 10.1016/s0891-5849(03)00041-8. [DOI] [PubMed] [Google Scholar]
- European Standards Committee on Oxidative DNA Damage. Comparative analysis of baseline 8-oxo-7,8-dihydro-guanine in mammalian cell DNA, by different methods in different laboratories: an approach to consensus. Carcinogenesis. 2003b;23:2129–2133. doi: 10.1093/carcin/23.12.2129. [DOI] [PubMed] [Google Scholar]
- Forchhammer L, Bräuner EV, Folkmann JK, Danielsen PH, Nielsen C, Jensen A, et al. Variation in assessment of oxidatively damaged DNA in mononuclear blood cells by the comet assay with visual scoring. Mutagenesis. 2008;23:223–231. doi: 10.1093/mutage/gen006. [DOI] [PubMed] [Google Scholar]
- Gedik CM, Collins A European Standards Committee on Oxidative DNA Damage. Establishing the background level of base oxidation in human lymphocyte DNA: results of an interlaboratory validation study. FASEB J. 2005;19:82–84. doi: 10.1096/fj.04-1767fje. [DOI] [PubMed] [Google Scholar]
- Griffiths HR, M⊘ller L, Bartosz G, Bast A, Bertoni-Freddari C, Collins A, et al. Biomarkers. Mol Aspects Med. 2002;23:101–208. doi: 10.1016/s0098-2997(02)00017-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T, Hillestr⊘m PR, Poulsen HE, Weimann A. Automated method for the direct analysis of 8-oxo-guanosine and 8-oxo-2’-deoxyguanosine in human urine using ultra-performance liquid chromatography and tandem mass spectrometry. Free Radic Biol Med. 47:629–635. doi: 10.1016/j.freeradbiomed.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Hicks JJ, Medina-Navarro R, Guzman-Grenfell A, Wacher N, Lifshitz A. Possible effect of air pollutants (Mexico City) on superoxide dismutase activity and serum lipoperoxides in the human adult. Arch Med Res. 1996;27:145–149. [PubMed] [Google Scholar]
- Isik B, Isik RS, Akyildiz L, Topcu F. Does biomass exposure affect serum MDA levels in women? Inhal Toxicol. 2005;17:695–697. doi: 10.1080/08958370500189883. [DOI] [PubMed] [Google Scholar]
- Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis. 2009;203:311–319. doi: 10.1016/j.atherosclerosis.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Lai CH, Liou SH, Lin HC, Shih TS, Tsai PJ, Chen JS, et al. Exposure to traffic exhausts and oxidative DNA damage. Occup Environ Med. 2005;62:216–222. doi: 10.1136/oem.2004.015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Poon R, Chen L, Frescura AM, Montuschi P, Ciabattoni G, et al. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect. 2009a;117:668–674. doi: 10.1289/ehp11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Ruddy T, Dalipaj M, Poon R, Szyszkowicz M, You H, et al. Effects of indoor, outdoor, and personal exposure to particulate air pollution on cardiovascular physiology and systemic mediators in seniors. J Occup Environ Med. 2009b;51:1088–1098. doi: 10.1097/JOM.0b013e3181b35144. [DOI] [PubMed] [Google Scholar]
- Liu L, Ruddy TD, Dalipaj M, Szyszkowicz M, You H, Poon R, et al. Influence of personal exposure to particulate air pollution on cardiovascular physiology and biomarkers of inflammation and oxidative stress in subjects with diabetes. J Occup Environ Med. 2007;49:258–265. doi: 10.1097/JOM.0b013e31803220ef. [DOI] [PubMed] [Google Scholar]
- Loft S, M⊘ller P. Oxidative DNA damage and human cancer: need for cohort studies. Antioxid Redox Signal. 2006;8:1021–1031. doi: 10.1089/ars.2006.8.1021. [DOI] [PubMed] [Google Scholar]
- Loft S, Poulsen HE, Vistisen K, Knudsen LE. Increased urinary excretion of 8-oxo-2’-deoxyguanosine, a biomarker of oxidative DNA damage, in urban bus drivers. Mutat Res. 1999;441:11–19. doi: 10.1016/s1383-5718(99)00034-0. [DOI] [PubMed] [Google Scholar]
- Loft S, Svoboda P, Kasai H, Tjonneland A, Vogel U, M⊘ller P, et al. Prospective study of 8-oxo-7,8-dihydro-2’-deoxyguanosine excretion and the risk of lung cancer. Carcinogenesis. 2006;27:1245–1250. doi: 10.1093/carcin/bgi313. [DOI] [PubMed] [Google Scholar]
- Medina-Navarro R, Lifshitz A, Wacher N, Hicks JJ. Changes in human serum antioxidant capacity and peroxidation after four months of exposure to air pollutants. Arch Med Res. 1997;28:205–208. [PubMed] [Google Scholar]
- Mills NL, Donaldson K, Hadoke PW, Boon NA, MacNee W, Cassee FR, et al. Adverse cardiovascular effects of air pollution. Nat Clin Pract Cardiovasc Med. 2009;6:36–44. doi: 10.1038/ncpcardio1399. [DOI] [PubMed] [Google Scholar]
- Mills NL, Robinson SD, Fokkens PH, Leseman DL, Miller MR, Anderson D, et al. Exposure to concentrated ambient particles does not affect vascular function in patients with coronary heart disease. Environ Health Perspect. 2008;116:709–715. doi: 10.1289/ehp.11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M⊘ller P. Assessment of reference values for DNA damage detected by the comet assay in humans blood cell DNA. Mutat Res. 2006;612:84–104. doi: 10.1016/j.mrrev.2005.10.001. [DOI] [PubMed] [Google Scholar]
- M⊘ller P, Folkmann JK, Forchhammer L, Bräuner EV, Danielsen PH, Risom L, et al. Air pollution, oxidative damage to DNA, and carcinogenesis. Cancer Lett. 2008a;266:84–97. doi: 10.1016/j.canlet.2008.02.030. [DOI] [PubMed] [Google Scholar]
- M⊘ller P, Friis G, Christensen PH, Risom L, Plesner G, Kjærsgaard J, et al. Intra-laboratory comet assay sample scoring exercise for determination of formamidopyrimidine DNA glycosylase sites in human mononuclear blood cell DNA. Free Radic Res. 2004;38:1207–1214. doi: 10.1080/10715760400016824. [DOI] [PubMed] [Google Scholar]
- M⊘ller P, Jacobsen NR, Folkmann JK, Danielsen PH, Mikkelsen L, Hemmingsen JG, et al. Role of oxidative damage in toxicity of particulates. Free Radic Res. 2010;44:1–46. doi: 10.3109/10715760903300691. [DOI] [PubMed] [Google Scholar]
- M⊘ller P, Loft S. Dietary antioxidants and beneficial effect on oxidatively damaged DNA. Free Radic Biol Med. 2006;41:388–415. doi: 10.1016/j.freeradbiomed.2006.04.001. [DOI] [PubMed] [Google Scholar]
- M⊘ller P, Risom L, Lundby C, Mikkelsen L, Loft S. Hypoxia and oxidation levels of DNA and lipids in humans and animal experimental models. IUBMB Life. 2008b;60:707–723. doi: 10.1002/iub.109. [DOI] [PubMed] [Google Scholar]
- M⊘ller P, Wallin H, Holst E, Knudsen LE. Sunlight induced DNA damage in human mononuclear cells. FASEB J. 2002;16:45–53. doi: 10.1096/fj.01-0386com. [DOI] [PubMed] [Google Scholar]
- Novotna B, Topinka J, Solansky I, Chvatalova I, Lnenickova Z, Sram RJ. Impact of air pollution and genotype variability on DNA damage in Prague policemen. Toxicol Lett. 2007;172:37–47. doi: 10.1016/j.toxlet.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Palli D, Sera F, Giovannelli L, Masala G, Grechi D, Bendinelli B, et al. Environmental ozone exposure and oxidative DNA damage in adult residents of Florence, Italy. Environ Pollut. 2009;157:1521–1525. doi: 10.1016/j.envpol.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Risom L, M⊘ller P, Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res. 2005;592:119–137. doi: 10.1016/j.mrfmmm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Romieu I, Barraza-Villarreal A, Escamilla-Nunez C, Almstrand AC, Diaz-Sanchez D, Sly PD, et al. Exhaled breath malondialdehyde as a marker of effect of exposure to air pollution in children with asthma. J Allergy Clin Immunol. 2008;121:903–909. doi: 10.1016/j.jaci.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Rossner P, Jr, Svecova V, Milcova A, Lnenickova Z, Solansky I, Santella RM, et al. Oxidative and nitrosative stress markers in bus drivers. Mutat Res. 2007;617:23–32. doi: 10.1016/j.mrfmmm.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Rossner P, Jr, Svecova V, Milcova A, Lnenickova Z, Solansky I, Sram RJ. Seasonal variability of oxidative stress markers in city bus drivers. Part I. Oxidative damage to DNA. Mutat Res. 2008a;642:14–20. doi: 10.1016/j.mrfmmm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Rossner P, Jr, Svecova V, Milcova A, Lnenickova Z, Solansky I, Sram RJ. Seasonal variability of oxidative stress markers in city bus drivers. Part II. Oxidative damage to lipids and proteins. Mutat Res. 2008b;642:21–27. doi: 10.1016/j.mrfmmm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Rundell KW, Slee JB, Caviston R, Hollenbach AM. Decreased lung function after inhalation of ultrafine and fine particulate matter during exercise is related to decreased total nitrate in exhaled breath condensate. Inhal Toxicol. 2008;20:1–9. doi: 10.1080/08958370701758593. [DOI] [PubMed] [Google Scholar]
- Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in nonsmokers. Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- Sánchez-Rodríguez MA, Retana-Ugalde R, Ruiz-Ramos M, Munoz-Sanchez JL, Vargas-Guadarrama LA, Mendoza-Nunez VM. Efficient antioxidant capacity against lipid peroxide levels in healthy elderly of Mexico City. Environ Res. 2005;97:322–329. doi: 10.1016/j.envres.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Singh R, Kaur B, Kalina I, Popov TA, Georgieva T, Garte S, et al. Effects of environmental air pollution on endogenous oxidative DNA damage in humans. Mutat Res. 2007;620:71–82. doi: 10.1016/j.mrfmmm.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Sioutas C, Delfino RJ, Singh M. Exposure assessment for atmospheric ultrafine particles (UFPs) and implications in epidemiologic research. Environ Health Perspect. 2005;113:947–955. doi: 10.1289/ehp.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S⊘rensen M, Autrup H, Hertel O, Wallin H, Knudsen LE, Loft S. Personal exposure to PM2.5 and biomarkers of DNA damage. Cancer Epidemiol Biomarkers Prev. 2003a;12:191–196. [PubMed] [Google Scholar]
- S⊘rensen M, Daneshvar B, Hansen M, Dragsted LO, Hertel O, Knudsen L, et al. Personal PM2.5 exposure and markers of oxidative stress in blood. Environ Health Perspect. 2003b;111:161–166. doi: 10.1289/ehp.111-1241344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S⊘rensen M, Skov H, Autrup H, Hertel O, Loft S. Urban benzene exposure and oxidative DNA damage: influence of genetic polymorphisms in metabolic genes. Sci Total Environ. 2003c;309:69–80. doi: 10.1016/S0048-9697(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Nawrot T, Hond ED, Thijs L, Fagard R, Hoppenbrouwers K, et al. Renal function, cytogenetic measurements, and sexual development in adolescents in relation to environmental pollutants: a feasibility study of biomarkers. Lancet. 2001;357:1660–1669. doi: 10.1016/s0140-6736(00)04822-4. [DOI] [PubMed] [Google Scholar]
- Stang A, Witte I. Performance of the comet assay in a high-throughput version. Mutat Res. 2009;675:5–10. doi: 10.1016/j.mrgentox.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Inoue Y, Suzuki S. Changes in the urinary excretion level of 8-hydroxyguanine by exposure to reactive oxygen-generating substances. Free Radic Biol Med. 1995;18:431–436. doi: 10.1016/0891-5849(94)00152-a. [DOI] [PubMed] [Google Scholar]
- Svecova V, Rossner P, Jr, Dostal M, Topinka J, Solansky I, Sram RJ. Urinary 8-oxodeoxyguanosine levels in children exposed to air pollutants. Mutat Res. 2009;662:37–43. doi: 10.1016/j.mrfmmm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Vinzents P, M⊘ller P, S⊘rensen M, Knudsen LE, Hertel O, Jensen FP, et al. Personal exposure to ultrafine particles and oxidative DNA damage. Environ Health Perspect. 2005;113:1485–1490. doi: 10.1289/ehp.7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujovic A, Kotur-Stevuljevic J, Kornic D, Spasic S, Spasojevic-Kalimanovska V, Bogavac-Stanojevic N, et al. Oxidative stress and anti-oxidative defense in schoolchildren residing in a petrochemical industry environment. Indian Pediatr. 2009;47:233–239. doi: 10.1007/s13312-010-0045-y. [DOI] [PubMed] [Google Scholar]
- Walter MF, Jacob RF, Jeffers B, Ghadanfar MM, Preston GM, Buch J, et al. Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: a longitudinal analysis of the PREVENT study. J Am Coll Cardiol. 2004;44:1996–2002. doi: 10.1016/j.jacc.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Wei Y, Han IK, Shao M, Hu M, Zhang OJ, Tang X. PM2.5 constituents and oxidative DNA damage in humans. Environ Sci Technol. 2009;43:4757–4762. doi: 10.1021/es803337c. [DOI] [PubMed] [Google Scholar]
- Zou B, Wilson JG, Zhan FB, Zeng Y. An emission-weighted proximity model for air pollution exposure assessment. Sci Total Environ. 2009;407:4939–4945. doi: 10.1016/j.scitotenv.2009.05.014. [DOI] [PubMed] [Google Scholar]