Abstract
Background:
Inadequate levels of vitamin D increase the risk of osteoporosis, a highly prevalent condition in patients with traumatic spinal cord injury (SCI). Reduced sunlight and dark skin further contribute to low vitamin D levels.
Objectives:
To compare serum 25-hydroxy vitamin D [vitamin D25(OH)] levels in acute and chronic SCI and to explore seasonal and ethnic differences among patients with acute and chronic SCI.
Patients/Methods:
Patients (N = 96) aged 19 to 55 years with C3-T10 motor complete SCI participated. Acute SCI was 2 to 6 months after injury, whereas chronic SCI was at least 1 year from injury. Serum vitamin D25(OH), calcium, and parathyroid hormone were drawn during summer or winter months. Vitamin D deficiency (<13 ng/mL), insufficiency (<20 ng/mL), and subtherapeutic (<32 ng/mL) levels were compared for all groups. A 3-way analysis of covariance was adopted to determine significant main effects of season, chronicity, and ethnicity. Interactions between season and chronicity, season and ethnicity, and chronicity and ethnicity were evaluated. Evaluation of a 3-way interaction among season, chronicity, and ethnicity was completed.
Results:
In summer, 65% of patients with acute SCI and 81% of patients with chronic SCI had subtherapeutic vitamin D levels, whereas in winter, 84% with acute SCI and 96% with chronic SCI had vitamin D25(OH) (<32ng/mL). Lower vitamin D25(OH) levels were observed in African Americans relative to whites. Significant main effects were noted for season (P = 0.017), chronicity (P = 0.003), and ethnicity (P < 0.001). However, interactions between 2 or more factors were not found.
Conclusions:
Vitamin D insufficiency and deficiency are found in the majority of patients with chronic SCI and in many with acute SCI. Initial screening for serum vitamin D25(OH) levels should be performed early in rehabilitation. Periodic monitoring in the chronic setting is highly recommended.
Keywords: Spinal cord injuries, acute, chronic; Nutritional deficiencies; Vitamin D deficiency; Bone loss; Hyperparathyroidism; Hypovitaminosis; Osteoporosis; Cholecalciferol; Ergocalciferol; Ethnicity
INTRODUCTION
Vitamin D deficiency and insufficiency are among the many factors contributing to the development of osteoporosis, a condition that universally affects patients with motor complete spinal cord injury (SCI) (1–3). National guidelines for practitioners have been developed for prevention of many secondary complications of SCI, including pressure ulcers, autonomic dysreflexia, neurogenic bowel and bladder, and deep vein thromboses. Despite the prevalence of immobilization osteoporosis in the SCI population, no national recommendations have been adopted in an effort to reduce bone loss.
The storage form of vitamin D, specifically 25-hydroxy vitamin D [vitamin D25(OH)], or calcifediol, is the functional indicator of vitamin D status in terms of nutrition (4). The term vitamin D deficiency refers to serum vitamin D25(OH) levels from 0 to 12 ng/mL, the range in which osteomalacia or rickets has been known to develop. However, Heaney (5) maintains that insufficiency of vitamin D occurs in the range of 13 to 32 ng/mL, values at which serum parathyroid hormone (PTH) continues to be upregulated and calcium absorption is compromised. The starting level of insufficiency for serum vitamin D25(OH) is a point of contention in the literature (5–9). The assignment of a consistent serum vitamin D25(OH) level across laboratories is lacking but typically ranges from 10 to 20 ng/mL (10). At levels >20 ng/mL, insufficiency is not recognized by many institutional laboratories, including the one used in this study (11); rather, these levels are considered in the normal range (10–12). Recently, an international consensus panel established that 20 ng/mL is the minimum therapeutic level of serum vitamin D25(OH) but fell short of recommending the higher level of 32 ng/mL (9).
Because the range between the perceived normal vitamin D25(OH) starting point of 20 ng/mL and the higher level of 32 ng/mL remains an issue of debate, some have termed all serum vitamin D25(OH) values <32 ng/mL as subtherapeutic (13). For this study, we considered serum vitamin D25(OH) values <32 ng/mL to be subtherapeutic, levels specifically <20 ng/mL insufficient, and those <12 ng/mL deficient. We have chosen to consider patients with vitamin D25(OH) levels <32 ng/mL to be in a high-risk category, given upregulation of PTH and impaired calcium absorption beyond the established minimum therapeutic level of 20 ng/mL (6).
Using definitions similar to Heaney's (7), Parfitt (14) asserts that deficient as well as insufficient levels of vitamin D carry increased risk of osteoporosis due to suboptimal calcium absorption. Thus, screening of high-risk groups for both deficient and insufficient levels has been recommended (7,15,16), so that treatment can be instituted prior to onset of osteoporosis. Early intervention is more essential in high-risk populations, such as individuals who are institutionalized or predominantly remain indoors. Among those at greatest risk of low vitamin D levels are persons lacking weight-bearing activity, as in the case of complete SCI and the elderly (16–18), and among individuals requiring various anticonvulsant medications (19).
The prevalence of low bone mineral density (BMD) in association with SCI-related immobility is well established (20–24). The onset of this bone loss occurs shortly after injury. Although Garland and colleagues (20) observed a 24% decline in the BMD of the distal femur just 4 months from original SCI and a 34% loss by 16 months after injury, Bauman (24) found that further reduction in BMD occurs far beyond 16 months after injury. Additional bone loss in the chronic phase of injury has been attributed to reduced osteoblastic function and is adversely influenced by inadequate levels of vitamin D.
In addition to genetic factors, weight-bearing activity, and calcium intake, inadequate stores of vitamin D can contribute to the onset of osteoporosis. Because weight-bearing activity is greatly limited in patients with motor complete SCI, efforts to monitor nutritional factors associated with low bone mineral density deserve greater emphasis. Although many measures of surveillance involve routine screening and straightforward treatment, all too often nutritional factors and comprehensive bone health are of secondary importance in the clinical setting. In the acute period, urgent medical issues of sepsis or respiratory status are usually the focus of attention, whereas neuropathic pain, spasticity, and bowel and bladder care may become the priorities during inpatient rehabilitation and in the outpatient setting. Protocols regarding routine screening and treatment for various ranges of vitamin D deficiency and insufficiency need to be instituted in the acute and chronic setting to assist clinicians in maximizing long-term outcomes in patients with SCI.
A given individual may require small or very large amounts of vitamin D to correct a nutritional deficit. The level of supplementation needed depends on several factors, including serum vitamin D25(OH) level at the onset of treatment, age and skin pigmentation, and probability of experiencing further bone loss based on lifestyle and geographic location (5,7,14,23–25). Sunlight can provide the requisite amounts of vitamin D for approximately 8 months of the year in the southern USA but for only 3 months of the year in the northern USA (26–28). In addition, dark-skinned individuals are less able to absorb sunlight due to skin pigmentation, thus increasing their risk of vitamin D deficiency, as noted in a number of reports (26,27). Finally, differences in vitamin D levels may be observed in acute vs chronic SCI. Identification of optimal time for intervention with vitamin supplementation, before a significant decline in serum vitamin D25(OH) levels, is pivotal in preventing bone loss and other medical complications.
The specific aims of this investigation were to examine seasonal and ethnic factors that could explain differences of vitamin D levels in patients with acute, motor complete SCI within 2 to 6 months of injury and in patients with chronic, motor complete SCI and to study differences between acute and chronic SCI in patients of similar ethnicity, keeping the season constant.
METHODS
Target Population
We chose to look at a number of different categories of individuals with traumatic, motor complete SCI for baseline levels of vitamin D deficiency/insufficiency. Participants were drawn from both the inpatient and the outpatient settings of a tertiary care center in the southern USA (Birmingham, Alabama). Patients with chronic SCI were defined as those whose SCI occurred at least 1 year from the date of participation. Baseline measurements were obtained during routine clinic visits. Our chronic cohort ranged from 1 to 25 years after injury.
Experimental Protocol and Patient Eligibility
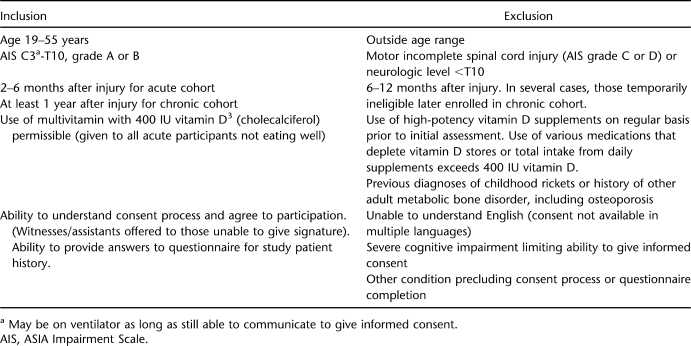
Patients with acute SCI were evaluated from 2 to 6 months after injury. In many ways, the latter group might be considered subacute. Based on the fact that serum vitamin D25(OH) levels indicate absorption over a 2- to 3-month period, examining levels any earlier than 8 weeks after injury might denote nutritional levels before the SCI. The choice of 8 weeks after injury as the earliest baseline measurement in acute SCI was based on research from Preece et al (29) and Dlugos et al (30). These authors separately examined serum vitamin D reduction in submarine sailors in settings of acute sunlight deprivation and found a 15 to 30 nmol/L decline over 2 months. Patients with known factors potentially causing vitamin D deficiency were ineligible for the study (see Table 1 for exclusion criteria).
Table 1.
Criteria for Participation
Because Bauman and colleagues had published a paper (1) on individuals >55 years of age, we chose to examine only people <56 years of age, because few data have previously been published on younger individuals. Our protocol involved chart review for confirmation of date of injury and ASIA injury level (31,32), as well as an ASIA examination at the time of consent to confirm continued status of motor complete injury. We limited our study group to people with motor complete SCI (ASIA impairment score A or B) with C3-T10 neurologic levels to ensure that subjects with cauda equina injuries would not be included arbitrarily. Each patient completed a questionnaire that included history of fractures unrelated to the original trauma; prior diagnosis of low bone mass; history of renal failure or liver disease (hepatitis or cirrhosis); medication use, specifically any seizure medications associated with vitamin D deficiency; dietary history, including lactose intolerance; and time spent outdoors if recruited in our summer groups.
Table 1 illustrates inclusion and exclusion criteria for the study. Although duration from injury differed in acute and chronic SCI, in all other respects, criteria for participation were the same. Those with acute injuries still in the hospital at the time of initial testing consumed a daily multivitamin containing 400 IU of vitamin D3 (cholecalciferol), part of the standard of care for inpatient hospitalization at our institution. These patients typically did not consume food sources with adequate vitamins and nutrients. Although use of a multivitamin in acute SCI does represent a difference between acute and chronic groups, the multivitamin substituted for nutrition from oral intake that patients with chronic SCI are likely to receive from food sources. Patients with acute SCI were ineligible if they were receiving either tube feeding or oral nutritional supplements that contained vitamin D in excess of 400 IU/day. Similarly, patients who were consuming a daily multivitamin containing 400 IU, as well as an oral supplement containing any amount of vitamin D, were ineligible, because the total vitamin D consumed would exceed 400 IU/day. No individuals with chronic SCI were excluded if they consumed a multivitamin daily, as long as it contained no more than 400 IU vitamin D. The few patients with chronic SCI who took a multivitamin also admitted to poor compliance with its use. No patients were on tube feedings or nutritional supplements that contained vitamin D at the time of consent.
Participation was limited to people with traumatic injuries as defined by inclusion in the NIDRR-funded (National Institute for Disability and Rehabilitation Research) SCI Model System of Care (31). Both genders and all ethnic groups were invited to participate; however, the only ethnic groups to enroll were whites and African Americans.
Patient Selection
Patients were selected from inpatient or outpatient settings of the SCI service from a rehabilitation hospital during the time intervals described below. During their hospitalization, inpatients are routinely informed about research studies for which they may be eligible, including the current study. Outpatients arriving at the spinal cord clinic are given a list of all studies conducted in the rehabilitation department that involve patients with SCI. Potential participants arriving for appointments were given verbal and written information about the current study, including basic eligibility requirements, travel commitment, duration of the study, benefits and risks of the study, and potential costs. If interested in learning more and sharing their personal health information, the potential study participant moved to the consent process.
Eligible patients were aged of 19 to 55 years with diagnoses of C3 to T10 motor complete traumatic SCIs. Potential patients in the winter cohort were recruited from outpatient clinic visits in Birmingham, Alabama, from January 1, 2005, through February 28, 2005; December 15, 2005, through February 28, 2006, and from December 15, 2006, through February 28, 2007. According to the National Weather Service (28), the above date ranges represent periods when sunlight levels are at their lowest points in the calendar year and extensive rays from the sun have been unavailable for at least the previous 60 days. The same age and neurologic levels of injury were included in summer patients as in winter ones described above.
Summer recruitment occurred from June 15 through October 15 in 2005, 2006, and 2007. The above time intervals were selected because Birmingham receives large amounts of sunlight from April through November. Summer participants were asked whether they spent a total (continuous or noncontinuous) of 15 minutes outdoors at least 5 days per week from the hours of 10 am to 3 pm. We also inquired whether patients wore sunscreen in that accumulated 15 minutes. Those with chronic and acute SCI underwent the same procedure for recruitment.
Consent Process
The study protocol was approved by the Human Subjects Committee of the Institutional Review Board at the University of Alabama at Birmingham. Consent was obtained by the principal investigator, coinvestigator, or 1 of 2 Institutional Review Board–approved study nurses involved only in this aspect of the study consent process. All patients involved in the study provided informed, written consent. In cases in which a patient was unable to sign his or her name due to physical impairment, verbal consent was obtained in the presence of the principal or coinvestigator, the person's power of attorney who provided the signature, and a witness who was a hospital or clinic staff member.
Laboratory Testing
All patients had levels of serum vitamin D25(OH), PTH, and serum calcium drawn within 24 to 48 hours of signing the consent. Serum vitamin D25(OH) was measured using a radioimmunoassay (DiaSorin Inc, Stillwater, MN). All patients were notified of their results by letter. If found to have laboratory values strongly indicative of need for treatment, that patient also received a phone call from the principal investigator or her staff. In such instances, patients were advised to begin vitamin D supplementation. All were offered treatment through the SCI clinic, but in severe deficiency, patients were referred to metabolic bone centers for additional testing. Alternatively, they could choose to have laboratory results sent to their primary care physicians for treatment at alternative locations. Undergoing treatment was not a requirement for participation in this trial.
Data Extraction Protocol
Our protocol involved chart review for confirmation of date of injury and ASIA level, as well as an ASIA examination at the time of consent to confirm continued status of motor complete injury. We limited our study group to individuals aged 19 to 55 years with motor complete SCI and neurologic levels C3-T10. Information from the patient questionnaire was reviewed and verified to the extent possible. Topics of interest concerned history of fractures unrelated to the original trauma; prior diagnosis of low bone mass; history of renal failure or liver disease (hepatitis or cirrhosis); medication use, specifically any seizure medications associated with vitamin D deficiency; dietary history, including lactose intolerance; and time spent outdoors if recruited in our summer groups. This information was needed to limit bias in the final sample and assist with identifying potential patients with medical conditions excluding them from participation.
Statistical Analyses
Statistical analysis and graphical presentation were performed using SPSS Version 10 (Chicago, IL). To investigate the effects of season, ethnicity, and chronicity of injury on vitamin D levels, a 2 × 2 × 2 factorial analysis of covariance (ANCOVA) was utilized. Age was applied as a covariate. This analytical approach allowed not only for investigation of main effects of these factors but also for the possible interaction between these factors on vitamin D level outcome. An alpha level of P = 0.05 was adopted for all analyses. The same procedure described above using a 2 × 2 × 2 factorial ANCOVA was chosen to explore PTH levels. Detailed statistical testing on serum calcium levels was not performed. Calcium levels were obtained to rule out other conditions that potentially could have affected a participant's eligibility for the study.
RESULTS
Recruitment
Although people of all ethnic groups were invited to participate, the predominant ethnic group in Birmingham, Alabama, is African American. We were unsuccessful in recruiting test participants of other ethnic groups, perhaps because our consent was available only in English.
Subject Attrition
After consent, we determined that 3 of 10 patients in our chronic cohort needed to be withdrawn for use of medications that could significantly elevate or lower serum vitamin D status. All 3 were white women. Due to electrical power failure during a major hurricane in August 2005, 16 frozen samples were damaged by defrosting. Patients affected were notified and offered repeat testing, but only 4 were able to return to the testing site for reevaluation the following week. Two patients were reassessed in late winter and put in the winter cohort instead of the summer one. They did not start taking vitamin D in the interim. The remaining 10 were withdrawn, of which 7 were African American men and 3 were white (2 men, 1 woman). No patients with acute SCI were withdrawn.
Description of the Final Sample
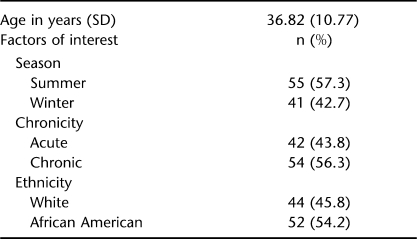
Demographic characteristics for the 96 patients who ultimately participated are shown in Table 2. Sixty-seven (69.8%) were male, 41 (42.7%) had tetraplegia, and 55 (57.3%) had paraplegia. Our overall results show exceptionally high numbers of SCI individuals with inadequate levels of vitamin D regardless of season, chronicity, or ethnicity. As predicted, higher rates of deficiency, as well as insufficiency, were observed during winter in chronic and in African American patients.
Table 2.
Group Characteristics
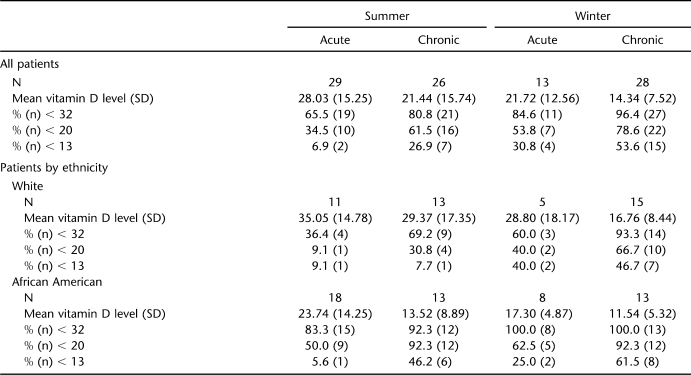
Relative percentages of patients according to subgroup are shown in Table 2. Percentages of subtherapeutic (<32 ng/mL) (13), insufficient (<20 ng/mL), and deficient (<13 ng/mL) levels are given in consecutive rows to emphasize the relative severities of low levels of vitamin D (4–7,10). Because calcium absorption is reduced at levels of vitamin D previously considered therapeutic (4,5,14), all patients with values of serum D25(OH) <32 ng/mL are at risk for bone loss. White participants with acute SCI during summer were the least affected group, but even in this category, >65% had inadequate stores of vitamin D. If we were to accept a minimum serum D25(OH) value of 20 ng/mL, which is considered the lower limit of normal at our institution's clinical laboratory, 35% to 78% of our patients would still fall short of therapeutic. Although we utilized the DiaSorin radioimmunoassay in determining our values, the reference values above are a reflection of published guidelines based on a variety of assays. Heaney (4–7) has advocated concern for levels <32 ng/mL based on all available tests at the time those reports were published, but multiple authors caution the reader on the variability of assays (7,12), as well as interlaboratory variation using the same assay (12,33). The ranges for deficiency, insufficiency, and subtherapeutic levels represent our best estimate based on available published guidelines, understanding the inherent limitations in measurement.
Regardless of ethnicity, individuals with acute injuries had higher mean serum vitamin D25(OH) levels. This may be due to residual stores of vitamin D prior to the injury when dietary intake was favorable and outdoor activities were frequent. In addition, higher rates of vitamin D deficiency and insufficiency were observed in African Americans vs whites in both summer and winter months. We believe this finding may be due to baseline vitamin D levels in the immediate months preceding the SCI. Although both groups may have declined in the 2 to 3 months after injury, the prior level of vitamin D25(OH) stores in whites may have been significantly greater. Whites are more likely to have benefited from absorption of solar rays in the immediate months preceding injury, particularly in patients evaluated in September or early October. Although both groups were hospitalized in the immediate 8 to 12 weeks preceding enrollment, whites may have had higher serum vitamin D25 levels relative to African Americans.
Among patients with chronic SCI, 96.4% had serum vitamin D25(OH) levels <32 ng/mL during winter. Remarkably, the data show that during summer months, 81% of patients with chronic SCI and 65% with acute SCI do not receive adequate amounts of vitamin D. Therefore, a large number of people with SCI living in the southern USA still have inadequate levels of vitamin D, despite the strong presence of summer sunlight in this region.
Mean vitamin D levels across group factors are shown in Table 3. Our study group included a broad range of ages younger than 56 years. There were no significant differences between the mean ages of individuals with acute vs chronic SCI. Although the effects of age on vitamin D levels were controlled for in the analyses, our findings indicate that age had no effect on vitamin D status [F(1,87) = 0.487, not significant]. In interpreting these findings, we emphasize that the oldest patients were age 55 years; the majority of literature gives ages 70 years and older as a risk factor for hypovitaminosis D.
Table 3.
Mean Vitamin D Levels Across Group Factors
For vitamin D levels, primary results from the 3-way ANCOVA indicated a significant main effect for season, such that higher levels of vitamin D were found among summer patients vs winter ones [F(1,87) = 5.87, P = 0.017]. Levels of vitamin D were found to be higher among patients with acute injuries vs those with chronic injuries, as a significant main effect also emerged for this factor [F(1,87) = 9.64, P = 0.003]. The strongest main effect emerged for ethnicity, with whites showing higher levels of vitamin D than African Americans [F(1,87) = 16.51, P < 0.001]. There was no significant interaction between season and chronicity [F(1,87) = 0.016, not significant] or season and ethnicity [F(1,87) = 0.853, not significant]. Similarly, there was no significant interaction between chronicity and ethnicity [F(1,87) = 0.019, not significant]. Testing for a 3-way interaction between the factors did not reach significance [F(1,87) = 1.058, not significant].
For PTH levels, primary results from the 3-way ANCOVA indicated a significant main effect only for chronicity [F(1,87) = 0.531, P = 0.025], with higher PTH levels observed in chronic patients relative to acute ones. No main effect for season [F (1,87) = 0.602, not significant] or for ethnicity [F(1,87), = 1.33, not significant] was identified. No significant interaction was noted between season and ethnicity, between season and chronicity, or between chronicity and ethnicity. Finally, a 3-way interaction among season, chronicity, and ethnicity did not reach significance.
Selected patients did demonstrate secondary hyperparathyroidism, although our mean values for all groups fall in the normal range. Eight patients with chronic SCI but only 1 with acute SCI demonstrated markedly elevated PTH values from 71 to 169 pg/mL. All of the individuals exhibiting secondary hyperparathyroidism had severely depleted levels of vitamin D25(OH), averaging just 8 ng/mL (range = 2.5–16 ng/mL). We wish to emphasize that the significant main effect of chronicity for PTH values may have been partially influenced by the 8 above noted outliers.
Chart review as part of our screening process revealed some salient points of discussion. One African American man had previously received nutritional analysis while in the intensive care unit and had some preliminary laboratory tests at just 2 weeks after injury. At that time, he demonstrated serum vitamin D25(OH) of 27 ng/mL and PTH 9 pg/mL, slightly below the desired minimum values for both parameters (4,5). He was started on a multivitamin containing 200 IU of vitamin D, with an additional 200 IU of vitamin D in tube feeds that he received in the unit for 8 more weeks. He then resumed a regular diet and arrived in rehabilitation approximately 13 weeks after injury, at which time he became eligible for our study. Remarkably, his vitamin D level at 14 weeks after injury, performed by the same laboratory that had analyzed the 2-week sample, was now just 9 ng/mL. Moreover, his PTH was 71 pg/mL. This case illustrates how quickly such patients can transition from a near therapeutic vitamin serum D25(OH) level to severe deficiency with secondary hyperparathyroidism.
Among the 8 patients with chronic SCI identified as outliers, chart review revealed direct avoidance of vitamin D and calcium products as contributing factors to development of secondary hyperparathyroidism. More than half of the patients reported receiving advice to drastically limit or entirely eliminate any such products from their diet to minimize risk of developing kidney stones. Only 1 of these patients listed a family history of kidney stones. Composition of the outliers was entirely male, and 7 of 9 were white. Five of 9 were among the summer cohort, with the balance in the winter group.
We did attempt to estimate whether patients received adequate amounts of sunlight during summer months. Regrettably, many could not remember this information with sufficient accuracy to permit widespread inclusion of this data. Other confounding variables arose after further investigation by authors. In addition to use of sunscreen, we learned that a substantial number of female participants blocked absorption of vitamin D outdoors by wearing sun protection factor makeup.
Because lactose intolerance is common in African Americans, we did inquire about this in our questionnaire. Only 1 patient in the study was lactose intolerant and had avoided milk since childhood. He was in the chronic winter group found to be severely deficient [vitamin D25(OH) of 6 ng/mL] at the time of his evaluation.
DISCUSSION
Factors for vitamin D deficiency include a combination of inadequate diet and reduced sunlight exposure. Many patients with SCI are under a false impression that consuming foods with calcium would lead to kidney stones. Although this belief has been disproved, the misconception continues to be problematic and has led many patients to deliberately restrict milk consumption and other products rich in vitamin D. Some patients we studied were under the impression that vitamin D tablets would also cause kidney stones. Many of this group were among those ultimately identified as having secondary hyperparathyroidism, due to severely low vitamin D25(OH) levels. Other patients had considered taking vitamin D but feared the pills would be costly and thus dismissed the idea. The actual cost of supplementation using vitamin D3 would vary by the patient's needs but in many cases could be purchased for less than $10 a month, especially now that single-dose tablets of 1,000 IU are available at most retail pharmacies in the USA, with some major chains also carrying 2,000-IU tablets.
In humans, sunlight is the primary source of vitamin D and typically provides up to 90% of our daily requirement (26,27). Ability of people to go outdoors and absorb vitamin D from the sun is limited by season, latitude, skin pigmentation, functional mobility, and tolerance to temperature changes. Patients with tetraplegia frequently depend on others to get them out of bed, and this assistance may not be available at desired times of peak sunlight. In addition, those with injures above T6 face thermoregulatory challenges and are highly sensitive to extreme heat. All patients with insensate skin are at risk of developing sunburn. Although use of sunscreen with a sun protection factor >8 will block harmful ultraviolet rays and prevent such burns, regrettably, cutaneous production of vitamin D3 is reduced by >95% (26–30). Exposure to sunlight for only 15 minutes between 10 am and 3 pm in spring, summer, and early fall is sufficient to obtain adequate vitamin D in persons with fair skin (34,35). Although this limited time will not result in sunburn, some with cervical or upper thoracic neurologic levels of SCI cannot tolerate even this short period due to altered temperature regulation.
Some patients with SCI are at lower risk of dysreflexia and may be able to tolerate the requisite 15 minutes outdoors to obtain adequate daily vitamin D during the summer. If they use the sun as their major vitamin D source, alternative plans should be made for winter months, unless their levels are at the higher end of the normal (32–100 ng/mL). We suggest that clinicians consider testing high-risk patients as the winter months approach. If patients do not otherwise receive supplementation during winter to compensate for the solar reduction of vitamin D, many are likely to experience a drop in their serum vitamin D25(OH) levels.
Reasons for lack of a combined effect among factors of interest (season, ethnicity, and chronicity) could be due to shared rather than independent contributions to serum levels of vitamin D. The underlying issue with absorption of sunlight in African Americans is interference with cutaneous absorption of vitamin D. Both dark skin and greater distance from the sun during winter hinder the body's ability to absorb vitamin D. The lack of an added effect from chronicity is less obvious. Potential of a floor effect may be one explanation. Because being African American had a more robust effect on serum vitamin D outcomes, addition of a second or third independent variable may add little to an already low serum vitamin D value.
The majority of research on vitamin D focuses on those with chronic SCI rather than on newly injured patients. Recent evidence suggests vitamin D levels are low among patients admitted to even a general rehabilitation unit (ie, nonSCI), and the problem is certainly magnified in those with SCI (36). Bauman et al (1) demonstrated the issue of vitamin D deficiency in a group of veterans living in New York. Among patients with paraplegia and tetraplegia with mean a age of 51 years and an average of 20 years after SCI, 32% were found to have vitamin D stores <16 ng/mL. Such deficiencies contribute to further bone loss, particularly for those with dark skin or whose location of residence contains many months of winter, such as the northern USA.
In severe forms of vitamin D deficiency, secondary hyperparathyroidism develops. Parathyroid hormone maintains serum calcium levels in a safe range, primarily by removing calcium from the bones and transferring it to the blood. When vitamin D stores are low, PTH is upregulated and its serum levels may be elevated as much as twofold (37). Only 1 patient with acute SCI in our study demonstrated secondary hyperparathyroidism, yet 14.5% of the patients with chronic SCI had elevated PTH. Chronically elevated PTH will lead to further bone loss in this high-risk group, a pivotal concern as the SCI population ages and life expectancies increase.
We suspect that the wide range of PTH values in both groups precluded identification of additional trends. Disparity in PTH values among patients with chronic SCI may be due to the wide range of years since injury in patients in the current study. According to our findings, we have 8 “outliers,” but do these outliers actually represent another high-risk group? A focused investigation of PTH values among a larger sample of chronically injured patients, stratified by years after injury, would aid in interpretation of results.
Age alone is an independent risk factor for vitamin D deficiency. Grados et al (38) studied nursing home patients and found that 53.6% had vitamin D25(OH) levels <12 ng/mL without any neurologic contributing factors. They ascribed their findings to age-associated reduced absorption of vitamins in the gastrointestinal tract, limited sunlight exposure, and low dietary intake of vitamin D.
A heightened risk of low vitamin D levels exists in patients who require anticonvulsant therapy. Vitamin D is rapidly hydroxylated by anticonvulsant medications, including phenytoin, phenobarbital, carbamazepine, and valproate (39–41). Hahn and colleagues (41) have shown that serum vitamin D25(OH) levels rapidly clear from circulation to become inactive forms in the presence of several different anticonvulsants. These medications are sometimes used to treat neuropathic pain in patients with SCI or to control seizures in patients with the dual diagnosis of SCI and traumatic brain injury.
New Recommendations for Therapeutic Ranges
A number of research groups (6,8,42) stress the need to advocate for considerably higher levels of vitamin D than traditionally recommended. At levels of serum vitamin D25(OH) from 13 to 32 ng/mL, PTH is not maximally suppressed and calcium absorption is decreased as much as 65% (6), effectively putting an individual at risk of additional fractures and other secondary conditions (8). In recognition of this fact, some laboratories have recently shifted their normal ranges for serum vitamin D levels upward. Nevertheless, the “normal” starting value of therapeutic serum vitamin D25(OH) levels remains 10 to 20 ng/mL in many institutions and commercial laboratories, despite the recent consensus statement by a panel of international experts (9).
Difficulties in establishing normal ranges of serum vitamin D are further complicated by variability in the assays utilized (12,33), as well as in precision in the performance of a given assay at a given laboratory. High-performance liquid chromatography is the gold standard used in the research setting but is expensive and rarely available at many institutions. Radioimmunoassay technology is the best performer commercially available in the USA at reasonable cost, but it too has limitations of accuracy and precision and depends on both the individual laboratory and the individual assay producer (12,33,43). Some of the earlier technologies using chemibioluminescence protein-binding assays gave falsely elevated readings but were widely used until recently (44). This presents a challenge in office settings and in many tertiary care centers, where samples are sent to outside laboratories and results are reported without details of assay and methodology employed. Because of assay variability and uncertainty, Cavalier et al (33) recommend ensuring a patient's vitamin D25(OH) level is at least 40 ng/mL if the clinician's goal is a level of 32 ng/mL.
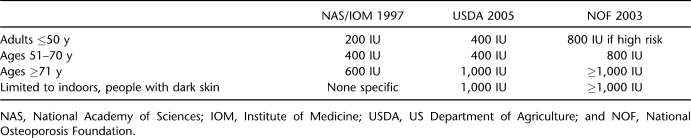
Not only are serum levels set too low by clinical laboratories, but recommended intakes to achieve desired serum vitamin D25(OH) status fall far short of what is needed (7,16). Until recently, only the elderly were recognized as needing an intake of oral vitamin D3 >400 IU/day. To address this concern, intakes for vitamin D developed by the Institute of Medicine in 1997 have been recently updated. The new recommendations appear in the Dietary Guidelines for Americans, produced in 2005 jointly by the US Department of Health and Human Services and US Department of Agriculture (45), with additional input from the US Department of Agriculture Advisory Committee (46). A summary of the old and new guidelines as well as those advised by the National Osteoporosis Foundation (47) appears in Table 4. For the first time, the recommended daily allowances now include a special category for populations at risk, regardless of age. The guidelines are applicable to persons with limited outdoor activity and those with dark skin. Individuals with SCI or other forms of immobility should also be considered high risk. Regrettably, many food cartons and dietary supplements continue to print outdated recommended daily allowances on their product labels.
Table 4.
Revised Vitamin D Recommended Daily Allowances
Long-Term Benefits
Prevention of insufficiency fractures from minimal trauma is one of the primary objectives in recognizing and promptly treating low vitamin D levels in patients with SCI. Numbers of long bone fractures, subsequent to SCI and independent of fractures during initial trauma, vary by report and by subcategories of SCI (ie, complete vs incomplete). Estimates range from 8% to 34%, and fractures occur exclusively below the level of SCI (22,48). Patients with motor complete SCI are thought to have a fracture rate 10-fold greater than those with incomplete injuries (49). Even in the absence of identified fractures, osteoporosis and a high risk of potential fractures are nearly universal among patients with complete SCI (23,50). Acute osteoclastic upregulation immediately after injury is not responsive to supplementation of vitamin D alone, and much of the bone loss secondary to SCI occurs during the first 4 to 6 months. However, maintaining serum vitamin D25(OH) levels of at least 32 ng/mL during the acute and chronic phases of SCI should be strongly considered.
Despite this initial loss, supplementation of vitamin D is requisite yet insufficient to preserve remaining bone integrity in the years after SCI. At a time when patients with SCI are living longer, preservation of remaining bone structure is paramount for reduction of long-term morbidity and fracture prevention. As the Randomized Evaluation of Calcium or Vitamin D trial has demonstrated, need for oral supplementation with vitamin D increases with age, because older individuals are less able to process solar rays cutaneously (51). Thus, whatever small contribution vitamin D from limited solar exposure provides to patients with SCI, that portion will decline with advancing age. With the increasing focus on providing care for patients aging with SCI, taking important preventative measures in the early years after injury is essential.
Benefits Beyond Bone
The association between hypovitaminosis D and elevated risk of breast and colon cancers has been well established (52–54). What is less well publicized is the relationship between low vitamin D levels and muscle strength (independent of SCI and in the context of strength above the neurologic level of injury), musculoskeletal pain, chronic fatigue syndrome, and myositis. Mowe and colleagues (55) demonstrated the correlation of serum vitamin D25(OH) levels with arm muscle strength and with frequency of falls. In a randomized controlled study of 122 subjects, Bischoff et al (56) found improved grip strength as well as motor function in hip flexors and knee extensors in patients receiving vitamin D supplementation, relative to those not consuming the vitamins. The same investigation demonstrated a 49% reduction in falls among patients receiving vitamin D. In a cross-sectional investigation of 237 postmenopausal patients, Pfeifer et al (57) observed a significant correlation between serum vitamin D25(OH) levels with body sway and falls. Moreover, a correlation of vitamin D25(OH) levels was observed with trunk muscle strength and with activities of daily living and basic self-care.
Pain from muscle overuse has been frequently reported in patients with SCI, particularly in those who rely solely on their upper extremities for transfers and weight-bearing activities (58). Plotnikoff and Quigley (59) examined serum vitamin D25(OH) in those with persistent, nonspecific musculoskeletal pain unresponsive to traditional agents, such as nonsteroidal anti-inflammatory drugs. Their study found that 90% of patients had deficient serum D25(OH) levels, many of whom were <35 years of age or in groups in whom vitamin D deficiency is not traditionally observed. Consequently, the failure to identify hypovitaminosis D can potentially limit functional mobility in affected individuals. In a case series by Gloth and Tobin (60), patients with nonspecific and treatment-resistant musculoskeletal pain symptoms responded in <1 week to aggressive supplementation with vitamin D, after deficiency of serum stores was identified. Pain can be elicited from the bones themselves in hypovitaminosis D by simple palpation of anterior tibia, as illustrated by Holick (61).
Vitamin D Toxicity
Some practitioners may hesitate to place patients with SCI on vitamin D treatment due to fears regarding its potential toxicity. Vitamin D toxicity should be based on the serum concentration in the blood rather than a given level of oral intake (62,63). Symptoms of toxicity include an increase in urinary calcium and blood levels. As discussed in detail by Vieth et al (62,63), supplementation of 10,000 IU daily for several months was associated with toxicity in the 1987 Council Report of the American Medical Association. This assertion was based on a report published in 1948 in which actual intake studied was 150,000 to 600,000 IU and on a review article without a primary reference for the 10,000 IU limit. Other reports of toxic levels have been based on infants receiving 1,800 to 3,600 IU daily, not on adults.
Supplemental vitamin D can be given in 2 forms, ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). The latter form of the vitamin has several advantages: low expense, availability without a prescription, and possibly greater efficacy than vitamin D2 (64). Practitioners selecting this mode of treatment recommend a daily dose dependent on the degree of deficiency and typically prescribe oral tablets available in either 400-IU or 1,000-IU increments. Even the most conservative entities have endorsed doses of 2,000 IU daily as safe (18).
Study Limitations
This investigation is limited first by the relatively small number of subjects and by problems inherent in assays for vitamin D (12,33,44). A second limitation was the premise that patients were at the minimum therapeutic vitamin D levels at the time of their injuries in the acute cohort. Data from other centers at our institution that study vitamin D deficiency do show higher rates of hypovitaminosis D in the elderly, particularly among African Americans. However, a group of healthy age-matched controls for our patients was not available for comparison.
Limitations of recall bias should be noted for correlation of time spent outdoors with serum vitamin D levels. These data were obtained only for patients studied in the summer in order to better assess any outliers (particularly on the high end in case of a patient's spending multiple hours outside). Actual time spent outdoors required to absorb adequate vitamin D stores ranges from 3 to 20 minutes, depending on skin pigmentation (12). During this time, the face, hands, and arms would need to be exposed to sunlight to adequately absorb enough vitamin D (27). Few patients could remember whether they were wearing sleeved or sleeveless clothing. Consequently, we were unable to determine the influence of summer sun exposure with accuracy. Most patients were able to remember whether or not they used sunscreen, with an unusually high number reporting rare to no use of protective lotions. However, we determined that many women used makeup that contained protective agents proved to block ultraviolet rays. Recall bias also confounds dietary reporting. Of note, most patients admitted to avoiding milk products in favor of carbonated soda or more traditional southern beverages with high sugar content.
CONCLUSIONS
Vitamin D deficiency/insufficiency is nearly universal among patients with chronic SCI but is also seen in large numbers within months of acute injuries. This condition exists regardless of season, ethnicity, or chronicity. Inadequate levels of vitamin D are seen more often in the winter for whites but exist year-round in African Americans. Maintaining adequate vitamin D levels reduces progression of osteoporosis and may minimize the risk for fractures. Lack of treatment for vitamin D deficiency can lead to additional health problems for patients with SCI, including secondary hyperparathyroidism, increased fall risk, muscle weakness, and secondary pain syndromes. Screening for serum vitamin D25(OH) and PTH levels should ideally be done at the beginning of rehabilitation. Levels should be repeated periodically during the first year after injury, because they can decline during this time as the recently injured patient adapts to a new lifestyle. Annual vitamin D surveillance should also be considered in chronic SCI. In many instances, PTH should also be measured annually.
Acknowledgments
We express our gratitude to Elizabeth Richardson, MS, and Kevin Spratt, PhD, for statistical support and to research assistants Ford Vox, MD, and John Miller, MS, for database management. We thank Linda K. Davis, RN, CRNP, Chi-Tso Huang, MD, and Amie B. Jackson, MD, for assisting with patient participation.
Footnotes
Funding for this project was made possible by the G. Heiner Sell Award of the American Spinal Injury Association, as well as through institutional support from the University of Alabama at Birmingham and the Rehabilitation Institute of Chicago.
References
- Bauman WA, Zhong YG, Schwartz E. Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism. 1995;44(12):1612–1616. doi: 10.1016/0026-0495(95)90083-7. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Pandian MR, Segal JL, Winer RL, Eltorai I, Brunnemann S. Vitamin D, parathormone, and calcitonin profiles in persons with long-standing spinal cord injury. Arch Phys Med Rehabil. 1994;75(7):766–769. [PubMed] [Google Scholar]
- Oleson CV, Chen D, Wuermser LA. Vitamin D Deficiency in Traumatic Spinal Cord Injury: Conference Proceedings of Contemporary Diagnosis and Treatment of Vitamin D–Related Disorder. American Society of Bone and Mineral Research; 2006. p. 41. [Google Scholar]
- Heaney RP, Abrams S, Dawson-Hughes B, et al. Peak bone mass. Osteoporosis Int. 2000;11(12):985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- Heaney RP. Lessons for nutritional science from vitamin D. Am J Clin Nutr. 1999;69(5):825–826. doi: 10.1093/ajcn/69.5.825. [DOI] [PubMed] [Google Scholar]
- Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22(2):142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr. 2004;80(6 suppl):1706S–1709S. doi: 10.1093/ajcn/80.6.1706S. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16(7):713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. Thirteenth workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103(3–5):204–205. doi: 10.1016/j.jsbmb.2006.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensmeyer GL, Binkley N, Drezner MK. New horizons for assessment of vitamin D status in man. In: Seibel MJ, Robins SP, Bilezikian JP, editors. Dynamics of Bone and Cartilage Metabolism. 2nd ed. San Diego, CA: Academic Press; 2006. pp. 513–527. [Google Scholar]
- University of Alabama at Birmingham. UAB Laboratory Handbook. Birmingham, AL: University of Alabama at Birmingham; 2005. Reference ranges for common laboratory tests. [Google Scholar]
- Binkley N, Krueger D, Cowgill CS, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004;89(7):3152–3157. doi: 10.1210/jc.2003-031979. [DOI] [PubMed] [Google Scholar]
- Boas SR, Hageman JR, Ho LT, Liveris M. Very high-dose ergocalciferol is effective for correcting vitamin D deficiency in children and young adults with cystic fibrosis. J Cystic Fibrosis. 2009;8(4):270–272. doi: 10.1016/j.jcf.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. Osteomalacia and related disorders. In: Avioli LV, Krane SM, editors. Metabolic Bone Disease and Clinically Related Disorders. 2nd ed. Philadelphia, PA: WB Saunders; 1990. pp. 329–396. [Google Scholar]
- Thomas MK, Lloyd-Jones DM, Thadhania RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- Vieth R. The role of vitamin D in the prevention of osteoporosis. Ann Med. 2005;37(4):278–285. doi: 10.1080/07853890510007313. [DOI] [PubMed] [Google Scholar]
- MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76(4):1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- Mulder JE, Kulak CA, Shane E. Secondary osteoporosis. In: Siebel MJ, Robins SP, Bilezikian JP, editors. Dynamics of Bone and Cartilage Metabolism. San Diego, CA: Academic Press; 2006. pp. 717–737. [Google Scholar]
- Garland DE, Stewart CA, Adkins RH, et al. Osteoporosis after spinal cord injury. J Orthop Res. 1992;10(3):371–378. doi: 10.1002/jor.1100100309. [DOI] [PubMed] [Google Scholar]
- Biering-Sorensen F, Bohr H, Schaadt O. Bone mineral content of the lumbar spine and lower extremities years after spinal cord lesion. Paraplegia. 1988;26(5):293–301. doi: 10.1038/sc.1988.44. [DOI] [PubMed] [Google Scholar]
- Ragnarsson KT, Sell GH. Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehabil. 1981;62(9):418–423. [PubMed] [Google Scholar]
- Demirel G, Yilmaz N, Paker N, Onel S. Osteoporosis after spinal cord injury. Spinal Cord. 1998;36(12):822–825. doi: 10.1038/sj.sc.3100704. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AN, Wang J, Pierson RN, Schwartz E. Continuous loss of bone during chronic immobilization: a monozygotic twin study. Osteoporos Int. 1999;10(2):123–127. doi: 10.1007/s001980050206. [DOI] [PubMed] [Google Scholar]
- Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998;67(6):1232–1236. doi: 10.1093/ajcn/67.6.1232. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Current Opin Endocrinol Diabetes. 2002;9(1):87–98. [Google Scholar]
- Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr. 1995;61(suppl 3):638S–645S. doi: 10.1093/ajcn/61.3.638S. [DOI] [PubMed] [Google Scholar]
- National Weather Service. Available at: www.srh.noaa.gov/bmx. Accessed April 10, 2005.
- Preece MA, Tomlinson S, Ribut CA, et al. Studies of vitamin D deficiency in man. Q J Med. 1975;44(176):575–589. [PubMed] [Google Scholar]
- Dlugos DJ, Perrotta PL, Horn WG. Effects of the submarine environment on renal-stone risk factors and vitamin D metabolism. Undersea Hyperbaric Med. 1995;22(2):145–152. [PubMed] [Google Scholar]
- 2006. University of Alabama at Birmingham Spinal Cord Injury Care System. Birmingham, AL;
- American Spinal Injury Association. International Standards for Neurological Classification of Spinal Cord Injury. Chicago, IL: ASIA; 2002. [Google Scholar]
- Cavalier E, Rozet E, Gadisseur R, et al. Measurement uncertainty of 25-OH vitamin D determination with different commercially available kits: impact on the clinical cut offs. Osteoporos Int. 2010;21(6):1047–1051. doi: 10.1007/s00198-009-1052-5. [DOI] [PubMed] [Google Scholar]
- Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(suppl 6):1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: importance in the prevention of cancers, type I diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- Kiebzak GM, Moore N, Margolis S, Hollis B, Kevorkian CG. Vitamin D status of patients admitted to a hospital rehabilitation unit: relationship to function and progress. Am J Phys Med Rehabil. 2007;86(6):435–445. doi: 10.1097/PHM.0b013e31805b7e20. [DOI] [PubMed] [Google Scholar]
- Pepe J, Romagnoli E, Nofroni I, et al. Vitamin D status as the major factor determining the circulating levels of parathyroid hormone: a study in normal subjects. Osteoporos Int. 2005;16(7):805–812. doi: 10.1007/s00198-004-1757-4. [DOI] [PubMed] [Google Scholar]
- Grados F, Brazier M, Kamel S, et al. Effects on bone mineral density of calcium and vitamin D supplementation in elderly women with vitamin D deficiency. Joint Bone Spine. 2003;70(3):203–208. doi: 10.1016/s1297-319x(03)00046-0. [DOI] [PubMed] [Google Scholar]
- Valimaki MJ, Tiihonen M, Laitinen K, et al. Bone mineral density measured by dual-energy x-ray absorptiometry and novel markers of bone formation and resorption in patients on antiepileptic drugs. J Bone Miner Res. 1994;9(5):631–637. doi: 10.1002/jbmr.5650090507. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick LA. Pathophysiology of bone loss in patients receiving anticonvulsant therapy. Epilepsy Behav. 2004;5(suppl 2):S3–S15. doi: 10.1016/j.yebeh.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Hahn TJ, Hendin BA, Scharp CR, Haddad JG. Effect of chronic anticonvulsant therapy on serum 25-hydroxycholecalciferol levels in adults. N Eng J Med. 1972;287(18):900–904. doi: 10.1056/NEJM197211022871803. [DOI] [PubMed] [Google Scholar]
- Vieth R, Kimball S, Hu A, Walfish PG. Randomized comparisons of the effects of the vitamin D3 adequate intake versus 100 mcg (4,000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J. 2004;3(Jul 19):8. doi: 10.1186/1475-2891-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips P, Chapuy MC, Dawson-Hughes B, Pols HA, Holick MF. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int. 1999;9(5):394–397. doi: 10.1007/s001980050162. [DOI] [PubMed] [Google Scholar]
- Glendenning P, Taranto M, Noble JM, et al. Current assays overestimate 25-hydroxyvitamin D3 and underestimate 25-hydroxyvitamin D2 compared with HPLC: need for assay-specific decision limits and metabolite-specific assays. Ann Clin Biochem. 2006;43(pt 1):23–30. doi: 10.1258/000456306775141650. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services and US Department of Agriculture. Dietary Guidelines for Americans. 6th ed. Washington, DC: US Government Printing Office; 2005. Available at: www.healthierus.gov/dietaryguidelines. Accessed December 18, 2006. [Google Scholar]
- US Department of Agriculture. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2005. August 2004. Available at: http://www.health.gov/DietaryGuidelines/dga2005/report/. Accessed December 18, 2006.
- National Osteoporosis Foundation. Physician's Guide to Prevention and Treatment of Osteoporosis. 2003. Available at: http://www.nof.org/professionals/Clinicians_Guide.htm. Accessed December 18, 2006.
- Vestergaard P, Krogh K, Rejnmark L, Mosekilde L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36(11):790–796. doi: 10.1038/sj.sc.3100648. [DOI] [PubMed] [Google Scholar]
- Nance PW, Schryvers O, Leslie W, Ludwig S, Krahn J, Uebelhart D. Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch Phys Med Rehabil. 1999;80(3):243–251. doi: 10.1016/s0003-9993(99)90133-8. [DOI] [PubMed] [Google Scholar]
- Uebelhart D, Demiaux-Domenech B, Roth M, Chantraine A. Bone metabolism in spinal cord injured individuals and in others who have prolonged immobilization: a review. Paraplegia. 1995;33(11):669–673. doi: 10.1038/sc.1995.140. [DOI] [PubMed] [Google Scholar]
- Hartman TJ, Albert PS, Snyder K, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomized evaluation of calcium or vitamin D, RECORD): A randomized placebo – controlled trial J Nutr 20051352252–259.15671222 [Google Scholar]
- Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16(2):83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- Robsahm TE, Tretli S, Dahlback A, Moan J. Vitamin D3 from sunlight may improve the prognosis of breast, colon, and prostate cancer (Norway) Cancer Causes Control. 2004;15(2):149–158. doi: 10.1023/B:CACO.0000019494.34403.09. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- Mowe M, Haug E, Bohmer T. Low serum calcidiol concentration in older adults with reduced muscular function. J Am Geriatr Soc. 1999;47(2):220–226. doi: 10.1111/j.1532-5415.1999.tb04581.x. [DOI] [PubMed] [Google Scholar]
- Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Begerow B, Minne HW, et al. Vitamin D status, trunk muscle strength, body sway, falls, and fractures among 237 postmenopausal women with osteoporosis. Exp Clin Endocrinol Diabetes. 2001;109(2):87–92. doi: 10.1055/s-2001-14831. [DOI] [PubMed] [Google Scholar]
- Ziambaras K, Dagogo-Jack S. Reversible muscle weakness in patients with vitamin D deficiency. West J Med. 1997;167(6):435–439. [PMC free article] [PubMed] [Google Scholar]
- Plotnikoff GA, Quigley BA. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78(12):1463–1470. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- Gloth FM, III, Tobin JD. Vitamin D deficiency in older people. J Am Geriatr Soc. 1995;43(7):822–828. doi: 10.1111/j.1532-5415.1995.tb07059.x. [DOI] [PubMed] [Google Scholar]
- Holick MF. The role of vitamin D for bone health and fracture prevention. Curr Osteoporos Rep. 2006;4(3):96–102. doi: 10.1007/s11914-996-0028-z. [DOI] [PubMed] [Google Scholar]
- Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001;73(2):288–294. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69(5):842–856. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- Trang HM, Cole DE, Rubin LA, Pierrator A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68(4):854–858. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]