Abstract
Objective:
To investigate age, gender, and left-right differences in cutaneous electrical perceptual threshold (EPT) testing in an able-bodied, Australian sample.
Study Design:
Prospective experimental.
Setting:
Hospital-based spinal cord injuries unit.
Methods:
Cutaneous electrical stimulation of the 28 dermatomes at ASIA sensory key points (C2-S4/S5) was performed on 29 female and 16 male healthy volunteers aged 21 to 76 years. Mean EPTs for each dermatome were compared (repeated measures ANOVA) for left-right, gender-related, and age-related (</>50 years of age) differences.
Results:
There was no group difference between sides (repeated measures ANOVA, P = 0.934). Women across all ages had lower group mean EPTs than men (P < 0.0001). Women younger than age 50 years had lower mean EPTs than those older than age 50 years (P = 0.008). There was no group difference between younger and older men (P = 0.371). Analysis of individual dermatomes revealed no significant differences in thoracic dermatomes between genders or age groups, contrary to the limb dermatomes.
Conclusion:
There were gender differences in EPT values across all ages. Women had higher EPTs as they advanced in age, but this was less clear in men. There was considerable somatotopic variability in EPTs, especially in the lower limbs. If EPT testing is to be applied to detect subclinical changes within a dermatome, establishment of age- and gender-specific somatotopic normograms is a prerequisite.
Keywords: Electrical perceptual thresholds, gender, age; Dermatomes; Quantitative sensory testing; Cutaneous electrical stimulation; Demographic differences
INTRODUCTION
Recent years have seen an escalation in experimental interventions proposed to improve functional outcomes after spinal cord injury (SCI). There is a growing imperative to develop appropriate and sensitive outcome measures for assessing any intervention's efficacy (1). The Clinical Guidelines Panel of the International Campaign for Cures of Spinal Cord Injury Paralysis has recognized that quantitative sensory testing (QST) can be a valuable adjunct to evaluating sensory dysfunction as it correlates to somatosensory-evoked potential recordings and ASIA sensory scores (2). However, QST has not been widely used due to lack of standards for testing, lack of normative data, and the lack of consensus and guidelines on how to interpret data from QST in the most general sense (3). Electrical perceptual threshold (EPT) testing has been acknowledged as a simpler QST method but still provides a quantitative map of the level and completeness of SCI, including the zone of partial preservation (4).
Previous reports using a variety of stimulation techniques suggest differences in sensory thresholds between genders related to differences in skinfold thickness and epidermal nerve fiber density (5–7). In addition, the majority of neurophysiologic variables demonstrate an age-dependent relationship (8,9). Gender and age have not been systematically assessed in the development of EPT testing by the technique described by Davey et al (10) for application to patients with SCI.
Somatotopic differences between dermatomes have been demonstrated, with higher EPTs and larger variation in the lumbar and sacral dermatomes (4,10,11). A normative template for EPT has been developed from combining data from 14 men and 16 women aged 20 to 55 years (4). However, S3–S5 dermatomes were not assessed, which are key to the ASIA classification. Gender differences in the study were assessed in a limited number of dermatomes.
Our aim was to investigate whether there are differences in EPT values in relation to side, gender, and age in able-bodied volunteers. To our knowledge, our study is the largest and only one investigating this relationship in all 28 ASIA sensory key points, including the clinically important S3–S5 dermatomes, using the technique described by Davey et al (10).
MATERIALS AND METHODS
Approval for the study was given by the Northern Sydney Health Human Research Ethics Committee in Sydney, Australia. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Participants
Posters were placed around common areas of our hospital over a period of 5 to 6 months requesting healthy volunteers of all ages and both genders. Patients were recruited in a stratified manner. Forty-five healthy volunteers (29 women, 16 men, aged 21–76 years) participated in the study after giving written informed consent. All denied history of neurologic, dermatologic, or systemic diseases that are commonly known to affect sensation. Volunteers were not paid for their participation.
Technique
Participants were tested lying supine in a quiet room thermostatically set at 22° to 26°C. After skin preparation with alcohol swabs, disposable, self-adhesive electrodes (3M Red Dot repositionable monitoring electrode, St. Paul, MN) were applied to the 56 ASIA sensory points bilaterally, with an inactive anode attached to each participant's lumbar quadrant ipsilateral to the tested side. When required, participants were shaved to improve electrode adhesion.
A triggering unit drove a stimulator (Digitimer DS7A, Digitimer Ltd, Letchworth Garden City, United Kingdom), which produced constant current square-wave electrical pulses (0.5 ms, width, 3 Hz) delivered via the electrode. The stimulus current was increased from zero at a constant rate of 0.24 mA per second by a custom-made motor until the volunteer verbally indicated first sensation at the ASIA sensory point (method of limits) (12). This current was recorded as the “ascending” EPT. The current was then increased further to slightly exceed the threshold and then decreased until the stimulus was no longer sensed. Each participant was familiarized with the process in a trial run, followed by repeating the process 3 times on each dermatome. If the difference between consecutive ascending recordings exceeded 0.5 mA, an additional trial was undertaken. The lowest ascending stimulus intensity of the 3 trials was recorded as the EPT. Sessions lasted between 2 and 3 hours, inclusive of short rest intervals.
Throughout testing, the volunteers were blind to the amplitude of the stimulus current. Testers were instructed not to look at the amplitude of the stimulus current but at the patient and to turn off the motor and record the EPT value as soon as the subject indicated a sensation, thus reducing tester bias. Our use of a constant current motor and self-adhesive electrodes reduced variability from manually increasing intensity and handheld thermodes, adding further precision to the quantification of sensory thresholds. The technique is described in more detail in our previous study (11). Although asking the person to verbally indicate sensation may introduce variability, this technique is thought to be more easily applicable to patients with tetraplegia than the use of a manual response.
Statistical Analysis
The average age of a person with SCI is 39.5 years, with a life expectancy of 7.3 to 34.1 years, depending on the level and severity of injury if injured at age 40 years (National Spinal Cord Injury Statistical Center, Birmingham, AL). There is acceleration of the aging process after SCI because of diminished physiologic reserves and increased demands on functioning body systems (13). We thus selected the age 50 years as a point for comparison against the SCI population instead of using 65 years, the age used in the able-bodied population for defining the geriatric age group. To calculate sample size required in each group to achieve 80% power as a comparison measure, we used sample size estimates per group for independent groups (unpaired t test), resulting in a size of 12 for each group. The software package SPSS 15.0 (SPSS, Chicago, IL) was used for all statistical comparisons. For each dermatome, the mean EPT value ± SD was determined from the total number of readings on both sides for the 45 volunteers.
The unpaired (2-tailed) t test was used to evaluate differences between the groups of mean age of men and women. This was used to assess for potential bias between the 2 sample populations. Repeated measure ANOVA was performed on sensory thresholds to identify group differences. The repeated measure was the dermatome and the test factor was the grouping (side specific, gender specific, or age specific). A 2-factor ANOVA was performed on mean threshold values for each dermatome to further analyze somatotopic variation of EPTs. All data were presented as mean ± SD, with P < 0.05 considered significant.
We have addressed within- and between-subject variability in various dermatomes in our previous paper (11), so this was not reassessed in this study.
RESULTS
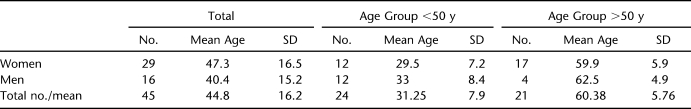
Of the 45 volunteers who participated in this study, 29 were women and 16 men (age range 21–76 years, mean age [SD] 47.3 years [16.5] and 40.4 years [15.2] years, respectively) (Table 1). There was no statistical group difference in mean age between genders (unpaired t test). There were 24 people younger and 21 older than age 50 years. All but 2 were right-hand dominant. For each dermatome, there were 90 EPT recordings from both left and right sides of the 45 volunteers.
Table 1.
Demographics of the Volunteer Population
No complications resulted from the procedure. One volunteer developed a transient contact dermatitis from the application of the self-adhesive electrodes, which resolved spontaneously within 48 hours.
Somatotopic Variability
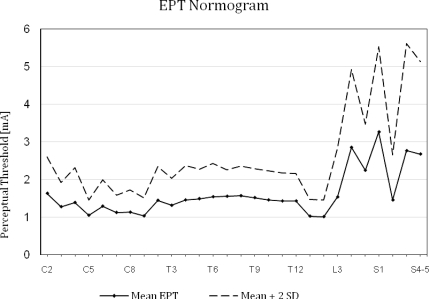
For each dermatome, the mean EPT value ± SD was determined from the total number of readings on both sides. Figure 1 shows the EPT normogram illustrating the variability in EPT means and their SDs for each dermatome. The smallest SD was 0.2 mA at C5 dermatome, and the largest was 1.43 mA at the S3 dermatome. In general, there was more variability in the lumbosacral dermatomes than upper limb and thoracic dermatomes, consistent with the findings of previous papers, which have reported on somatotopic variability (4,11).
Figure 1.
Mean electrical perceptual threshold value + 2 SD for dermatomes C2-S4/S5.
Left-Right Difference
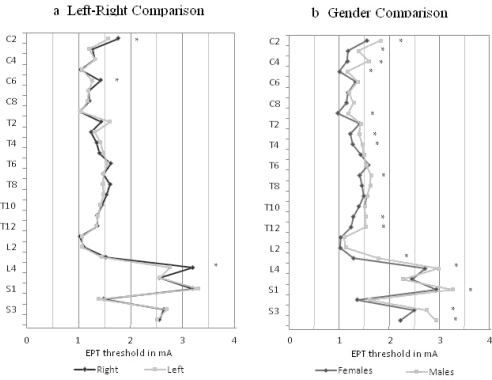
EPT values for left and right sides are shown in Figure 2a. Values for the right side ranged from 1.006 ± 0.198 (L1) to 3.31 ± 1.02 (S1) compared with values on the left of 1.002 ± 0.25 (L2) to 3.36 ± 1.37 (S1). Repeated measure ANOVA showed no group difference in EPT readings between the left and the right sides (P = 0.934). Two-factor ANOVA for each dermatome showed no significant difference for 25 of 28 dermatomes; however, there was a left-right difference (P < 0.05) in dermatomes C2, C6, and L4, with the right side showing consistently higher EPT values than the left.
Figure 2.
(a) Comparison of electrical perceptual threshold values for left and right sides for the total sample for all dermatomes. (b) Comparison of electrical perceptual threshold values for women and men for all dermatomes, * indicates significance with P < 0.05.
Gender Difference
For the total sample, women had lower group mean EPT values compared with men, (repeated measures ANOVA P < 0.0001) (Figure 2b). Statistically significant differences were found for 15 of the 28 dermatomes from cervical, thoracic, and lumbosacral regions. Variability in EPT values was similar in men and women (average SD for all dermatomes: men, 0.42; women, 0.38). For both genders, variability was high in all dermatomes below L3 and was lowest for C5.
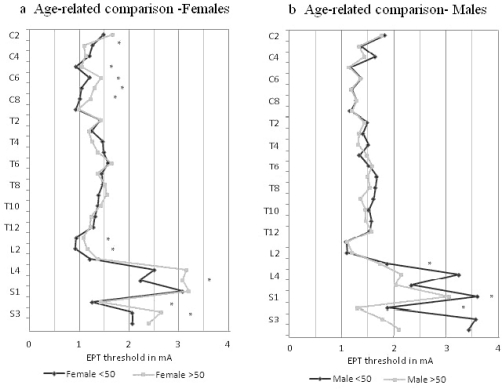
When each age group was analyzed separately, women younger than age 50 years had consistently lower EPTs than men younger than age 50 years, with statistical differences in 21 dermatomes (Figure 3a). In comparing women and men older than age 50 years (Figure 3b), women tended to have lower EPTs in the cervical and thoracic dermatomes, achieving statistical significance in only 6 dermatomes (C3, C4, T1, T3, T4, T11). In lumbosacral dermatomes, the trend appeared to reverse, with L4 and S3 EPT values significantly higher in women than in men.
Figure 3.
Comparison of electrical perceptual threshold values for gender differences in the (a) younger (<50 years of age) group and (b) older (>50 years of age) group.* indicates significance with P < 0.05.
Age Difference
There were 12 women younger than age 50 years and 17 older (Table 1). A group difference was noted between younger and older women (repeated measures ANOVA P = 0.008). Analysis of individual dermatomal differences in women (Figure 4a) showed the younger group had lower EPTs in 20 of 28 dermatomes, reaching statistical significance in 9 dermatomes (C5–C8, L1, L2, L5, S2, S3). In the C3 dermatome, younger women had a statistically significant higher EPT than did older women.
Figure 4.
Comparison of electrical perceptual threshold values for age-related differences for (a) women and (b) men. * indicates significance with P < 0.05.
There were 17 men younger than age 50 years and 4 older, with no group difference noted between them (repeated measures ANOVA P = 0.371). Analysis of individual dermatomal differences showed that younger men had statistically significant higher EPTs than older men did in 3 dermatomes in the lumbo-sacral (L3, S1, S2) region (Figure 4b).
No significant differences were found in EPT values in the thoracic region of either gender.
DISCUSSION
EPT testing is a promising adjunct to the ASIA examination for assessing sensory dysfunction. It is simple, requires minimal training, and has good inter- and intrarater reliability (11). It is thought to activate the largest, lowest threshold afferent fibers near the site of stimulation, such as those innervating Pacinian corpuscles and Merkel discs located in cutaneous and subcutaneous tissues, and appears to measure segmental posterior spinal cord function (11,14). Because it is a relatively new technique, its characteristics and clear reference ranges in the able-bodied population need to be further defined to enable clinical and research use. Previous studies have examined EPT values in several dermatomes, but to our knowledge, our study is the first to examine EPT in all dermatomes, including the clinically important dermatomes of S4 and S5 for ASIA determination.
Left-Right Difference
Previous studies using Davey's technique have found no left-right difference in EPT readings although this was tested in a limited number of dermatomes (4,10,11). Studies of quantitative sensory testing measures, such as thermal and pain thresholds, found symmetrical left-right results (15,16). Our results also found no overall group difference between sides. When individual dermatomes were analyzed, the right side had consistently higher EPTs than the left in dermatomes C2, C6, and L4 (P < 0.05). The significance of this is unclear and may relate to hand dominance (43 of 45 volunteers were right handed) or somatotopic variation. Nevertheless, left-right differences appear minimal, which argues for the use of a “mirror image normogram” for simplification.
Age and Gender Differences
It is well established that tactile sensitivity decreases with age, attributable to structural deformation, reduced density of sensory receptors and nerve fibers, and degraded information processing in the somatosensory cortex (8,17). This decline appears to be similar in men and women (18) and is steeper for vibration than other sensory modalities, such as touch (19). The literature for gender differences in somatosensory function is less clear. Some authors have found that women have lower sensory and pain thresholds than men, hypothesized to be due to method of stimulation (20), epidermal nerve fiber density (8), hair distribution and shaving (21), skin temperature, and skinfold thickness (6). Some have found no gender differences, arguing that age is the most significant factor in determining sensory thresholds, after adjusting for differences between genders in age, anthropometric parameters and height (17,18). There is also histologic evidence that gender, height, and body weight do not independently influence intraepidermal nerve fiber density (22).
Our study found that younger women have lower EPT values compared with men, with this difference becoming less pronounced with age. There was a group difference between women younger and older than 50 years of age, with individual dermatomal differences in women predominantly in the cervical and lumbosacral region. There was no significant group difference between younger and older men. Younger men had higher EPTs than older men in 3 dermatomes in the lumbosacral region, which may be due to unbalanced numbers in the 2 groups. It has been suggested that the aging effect on some nerve fibres is more significant in women than men (23), which may explain our findings of a significant difference in women but not in men.
Somatotopic Variability
Women have been found to have greater individual pain threshold variability compared with men (24), with variations in menstrual cycle debated as a possible cause (25). Greater female variability was not found in the present study, with SDs of EPT values being similar in men and women. Our previous paper (11) noted a large within-subject variation in dermatome S2, compared with 3 other dermatomes tested (C4, T1, L4). Although S2 also had high SDs in the present study, these were similarly large for all dermatomes below L4.
Interestingly, we found no significant differences in the thoracic dermatomes when comparing the 2 age groups in both genders. It has been postulated that age and height may not be directly associated with decline in vibrotactile sensitivity but could contribute through their effects on peripheral nerve function (18). Distal epidermal innervation decreases in a length-dependent manner with advancing age (22) and may explain why the thoracic dermatomes, being innervated by anatomically shorter peripheral nerves, did not exhibit a large change in EPT thresholds with age.
The rate of decline in sensation is reported to be diverse at different examined regions and between genders, being more prominent at the leg and dorsum of the foot and in men than women (16). Duke et al (26) found similar vibration thresholds with increasing age in the upper limbs between genders, but a statistical difference was observed in men vs women for both lower limbs from about the age of 60 years (26). This is in concordance with our findings, in which differences are most marked in the cervical and lumbosacral regions.
The summation of the existing evidence suggests a group difference between genders at a younger age. Although a group difference between genders is not found in the older population, some limb dermatomes may retain statistical difference. In trials promoting recovery, improvements may be limited to 1 to 2 dermatomes. If EPT is to be applied to detect subclinical deficits, we need gender- and age-specific normograms, including all dermatomes that draw on larger data sets with established means and SDs against which to compare. We calculated that a sample size of 12 for each group was required to achieve an 80% power in group comparison. We were able to recruit at least 12 people in all groups apart from men older than age 50 years. Hence, although we have obtained gender-specific normograms in this study for people younger than age 50 years for all dermatomes (including S4–S5), we were not able to establish age-specific normograms or draw definitive conclusions in older men. In addition to normograms, it will be important to ascertain when changes in EPT values are meaningful, if they remain above normal values. That is, the minimal physiologically important difference (the smallest difference in EPT value that is perceived as beneficial that would mandate a change in the patient's management) (27) in sensory function needs to be determined in future clinical trials. Further investigation into factors that can affect EPT testing is also required, such as clinical and demographic variables (eg, ethnicity).
CONCLUSION
EPT testing is a useful and simple adjunct in quantifying sensory dysfunction. Using this method, we found an overall group difference between genders. This difference was more marked in younger (<50 years of age) than older (>50 years of age) groups. EPT values tend to increase with age in women. Low numbers of volunteers in the older age group prevented us from drawing a conclusion in men. There was considerable somatotopic variation in EPT measurements, with larger variation in the lower limbs. This calls for establishment of age- and gender-specific somatotopic normograms.
Acknowledgments
We acknowledge Julia Lai Kwon, who helped conduct testing, sponsored by the University of Sydney Summer Scholarship Program. We thank Ms Bronwyn Loong for statistical advice, as well as the New South Wales Office of Science and Medical Research for funding the Program Grant and Exchange Fellowship.
Footnotes
This work was sponsored by the New South Wales Office of Science and Medical Research, Sydney, Australia.
REFERENCES
- Steeves J, Fawcett J, Tuszynski M. Report of International Clinical Trials Workshop on Spinal Cord Injury, February 20–21, 2004, Vancouver, Canada. Spinal Cord. 2004;42(10):591–597. doi: 10.1038/sj.sc.3101669. [DOI] [PubMed] [Google Scholar]
- Steeves JD, Lammertse D, Curt A, et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord. 2007;45(3):206–221. doi: 10.1038/sj.sc.3102008. [DOI] [PubMed] [Google Scholar]
- Backonja MM, Edwards RR, Sehgal N, et al. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain. 2009;25(7):641–647. doi: 10.1097/AJP.0b013e3181a68c7e. [DOI] [PubMed] [Google Scholar]
- Savic G, Frankel EM, Jamous MA, Ellaway PH, Davey NJ. Perceptual threshold to cutaneous electrical stimulation in patients with spinal cord injury. Spinal Cord. 2006;44(9):560–566. doi: 10.1038/sj.sc.3101921. [DOI] [PubMed] [Google Scholar]
- Leitgeb N, Schroettner J, Cech R. Electric current perception of the general population including children and the elderly. J Med Eng Technol. 2005;29(5):215–218. doi: 10.1080/03091900412331291705. [DOI] [PubMed] [Google Scholar]
- Maffiuletti NA, Herrero AJ, Jubeau M, Impellizzeri FM, Bizzini M. Differences in electrical stimulation thresholds between men and women. Ann Neurol. 2008;63(4):507–512. doi: 10.1002/ana.21346. [DOI] [PubMed] [Google Scholar]
- Irnich W, Batz L. The perception threshold for 50 Hz alternating voltage and current. Biomed Tech (Berl) 1989;34(9):207–209. doi: 10.1515/bmte.1989.34.9.207. [DOI] [PubMed] [Google Scholar]
- Gøransson LG, Mellgren SI, Lindal S, Omdal R. The effect of age and gender on epidermal nerve fiber density. Neurology. 2004;62(5):774–777. doi: 10.1212/01.wnl.0000113732.41127.8f. [DOI] [PubMed] [Google Scholar]
- Verdú E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5(4):191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- Davey NJ, Nowicky AV, Zaman R. Somatotopy of perceptual threshold to cutaneous electrical stimulation in man. Exp Physiol. 2001;86(1):127–130. doi: 10.1113/eph8602086. [DOI] [PubMed] [Google Scholar]
- Leong GW, Gorrie CA, Ng K, Rutkowski S, Waite PME. Electrical perceptual threshold testing: validation study. J Spinal Cord Med. 2009;32(2):140–146. doi: 10.1080/10790268.2009.11760765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassioukov A, Wolfe DL, Hsieh JT, Hayes KC, Durham CE. Quantitative sensory testing in patients with incomplete spinal cord injury. Arch Phys Med Rehabil. 1999;80(10):1258–1263. doi: 10.1016/s0003-9993(99)90026-6. [DOI] [PubMed] [Google Scholar]
- Capoor J, Stein AB. Aging with spinal cord injury. Phys Med Rehabil Clin N Am. 2005;16(1):129–161. doi: 10.1016/j.pmr.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Kramer JL, Moss AJ, Taylor P, Curt A. Assessment of posterior spinal cord function with electrical perception threshold in spinal cord injury. J Neurotrauma. 2008;25(8):1019–1026. doi: 10.1089/neu.2007.0503. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Zimmerman IR, O'Brien PC. Introduction of automated systems to evaluate touch, pressure, vibration and thermal cutaneous sensation in man. Ann Neurol. 1978;4(6):502–510. doi: 10.1002/ana.410040605. [DOI] [PubMed] [Google Scholar]
- Meh D, Denislic M. Quantitative assessment of thermal and pain sensitivity. J Neurol Sci. 1994;127(1):164–169. doi: 10.1016/0022-510x(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Lin YH, Hsieh SC, Chao CC, Chang YC, Hsieh ST. Influence of aging on thermal and vibratory thresholds of quantitative sensory testing. J Peripher Nerv Sys. 2005;10(3):269–281. doi: 10.1111/j.1085-9489.2005.10305.x. [DOI] [PubMed] [Google Scholar]
- Deshpande N, Metter EJ, Ling S, Conwit R, Ferrucci L. Physiological correlates of age-related decline in vibrotactile sensitivity. Neurobiol Aging. 2008;29(5):765–773. doi: 10.1016/j.neurobiolaging.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands ML, Schwatz AV, Brown BW, Nevitt MC, Seeley DG, Kelsey JL. Relationship of neurological function and age in older women: the study of osteoporotic fractures. Neuroepidemiology. 1998;17(6):318–329. doi: 10.1159/000026186. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Rollman G. Sex differences in responsiveness to painful and non-painful stimuli are dependent upon the stimulation method. Pain. 1993;53(3):255–264. doi: 10.1016/0304-3959(93)90221-A. [DOI] [PubMed] [Google Scholar]
- Caissie R, Landry PE, Paquin R, Champigny MF, Berthod F. Quantitative method to evaluate the functionality of the trigeminal nerve. J Oral Maxillofac Surg. 2007;65(11):2254–2259. doi: 10.1016/j.joms.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Umapathi T, Tan WL, Tan NC, Chan YH. Determinants of epidermal nerve fiber density in normal individuals. Muscle Nerve. 2006;33(6):742–746. doi: 10.1002/mus.20528. [DOI] [PubMed] [Google Scholar]
- Takekuma K, Ando F, Niino N, Shimokata H. Age and gender differences in skin sensory threshold assessed by current perception in community-dwelling Japanese. J Epidemiol. 2000;10(1 suppl):S33–S38. doi: 10.2188/jea.10.1sup_33. [DOI] [PubMed] [Google Scholar]
- Lund I, Lundeberg T, Kowalski J, Svensson E. Gender differences in electrical pain threshold responses to transcutaneous electrical nerve stimulation (TENS) Neurosci Lett. 2005;375(2):75–80. doi: 10.1016/j.neulet.2004.10.068. [DOI] [PubMed] [Google Scholar]
- Oshima M, Ogawa R, Londyn D. Current perception threshold increases during pregnancy but does not change across menstrual cycle. J Nippon Med Sch. 2002;69(1):19–23. doi: 10.1272/jnms.69.19. [DOI] [PubMed] [Google Scholar]
- Duke J, McEvoy M, Sibbritt D, Guest M, Smith W, Attia J. Vibrotactile threshold measurement for detecting peripheral neuropathy: defining variability and a normal range for clinical and research use. Diabetologia. 2007;50(11):2305–2312. doi: 10.1007/s00125-007-0813-y. [DOI] [PubMed] [Google Scholar]
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]