Abstract
Aim
Mucins comprise a family of high-molecular-weight glycoproteins. MUC4, a large transmembrane mucin, has recently emerged as a novel marker for diagnosis, prognosis and therapy in several malignancies. However, its role in skin pathologies remains unknown. The aim of this study was to analyse the expression of MUC4 in cutaneous pathologies by immunohistochemistry for potential diagnostic, prognostic and therapeutic applications.
Methods
A total of 330 tissue spots representing the normal skin, and benign and malignant cutaneous diseases, were analysed after staining with the monoclonal antibody to human MUC4 (clone 8G7).
Results
While the normal epidermis showed a negative to weak-positive expression of MUC4, its expression was significantly upregulated in squamous cell carcinomas (SCCs) where the intensity of staining correlated negatively with tumour grade and positively with age. A moderately strong MUC4 expression was also noted in 2/20 cancer adjacent normal skin and 2/21 chronically inflamed skin tissues, while 10/19 cases of vulval condyloma acuminate, 3/12 of vulval hyperplasia and 2 cases of verruca vulgaris also showed strong MUC4 positivity. Malignant melanoma, basal cell carcinoma and cutaneous cysts were negative.
Conclusion
The results indicate that MUC4 expression is aberrantly upregulated in cutaneous SCCs, vulval condylomas and verruca vulgaris. Further, it appears that MUC4 expression in the skin may be modulated by chronic inflammation and the presence of an adjacent cutaneous malignancy in certain cases. These observations suggest a novel role for MUC4 mucin in the pathogenesis of cutaneous SCC and a possible application as a diagnostic and/or prognostic marker in cutaneous pathologies.
INTRODUCTION
The skin is the largest organ in the human body and subserves several vital functions. Skin diseases are not just a cosmetic problem, but have been shown to have an impact on the quality of life of the affected individuals.1
Mucins are high-molecular-weight glycoproteins expressed predominantly by epithelial cells. In recent years, their role has been better defined, revealing key roles in cell signalling and immune response. Human mucin genes are functionally classified into two major categories: secreted (MUC2, MUC5AC, MUC5B, MUC6-MUC8 and MUC19) and membrane bound (MUC1, MUC3, MUC4, MUC12, MUC13, MUC15, MUC17 and MUC20).2
MUC4 is a membrane-bound mucin encoded by a gene on chromosome 3q29. The largest and most distinct feature of MUC4 is its extracellular tandem repeat (TR) domain, which is comprised of a stretch of 16 amino acids repeated in tandem for up to 500 times.3 We have previously developed and characterised a monoclonal antibody (clone 8G7) raised against the TR region of MUC4.4 Employing this antibody, we and others have demonstrated an aberrant overexpression of MUC4 in cholangiocarcinoma,5 6 and pancreatic,7 8 cervical,9 gastric,10 ovarian,11 12 lung13 and prostate cancer.14 However, the status of MUC4 expression in cutaneous pathologies remains unknown.
In the present study, we examined the expression of MUC4 in the normal skin, benign skin diseases and cutaneous malignancies by immunohistochemistry. The significance of the study lies in the potential use of MUC4 as a novel diagnostic or prognostic marker and therapeutic target in malignant and potentially malignant disorders.
MATERIALS AND METHODS
Tissue samples and antibodies
Tissue microarrays made from formalin-fixed, paraffin-embedded tissues were purchased from US Biomax (Rockville, Maryland, USA) comprising skin disease progression (catalogue no. SK2081), cutaneous squamous cell carcinoma (catalogue no. SK802) and malignant melanoma (catalogue no. ME481). The cores in SK2081 were in duplicate, while in SK802 and ME481, there were no over-lapping cases.
The MUC4 monoclonal antibody (clone 8G7) that recognises an epitope on the highly repetitive TR region of human MUC4 was developed by us and described previously.4
Immunohistochemistry
The technique for immunostaining has been described previously.7 Briefly, after baking at 56°C overnight, the tissues were dewaxed in xylene, followed by rehydration through graded ethanol. Endogenous peroxidase activity was quenched by incubating the slides with 0.3% hydrogen peroxide in methanol for 30 min. Antigen retrieval performed in 0.01 M pre-heated citrate buffer (pH 6.0, 95°C) in a microwave oven for 15 min. Non-specific reactivity with the antibody was blocked by incubating the slides with 2.5 U horse serum (ImmPRESS Universal antibody kit; Vector Laboratories, Burlingame, California, USA) for 2 h followed by addition of the primary antibody (0.67 μg/ml of the MUC4 monoclonal antibody 8G7 in PBS). After overnight incubation at 4°C, the slides were washed with PBS and incubated with anti-mouse secondary antibody (ImmPRESS Universal antibody kit; Vector Laboratories) for 30 min. Colour was developed by adding 3,3′-diaminobenzidine solution (DAB substrate kit; Vector Laboratories). The slides were counter-stained with Gill’s haematoxylin (Vector Laboratories) and dehydrated in graded ethanol. The slides were mounted with a drop of Permount permanent mounting medium (Fisher Scientific, Pittsburgh, Pennsylvania, USA).
All slides were observed under a Nikon light microscope, and photographs of representative areas were taken with the Q-capture Micropublisher 5.0 camera (Leeds Precision Instruments, Minneapolis, Minnesota, USA) using the Q-capture suite software package (QImaging, Surrey, Canada). The intensity of MUC4 expression was graded on a scale of 0–3 (0, no staining, 1+, weakly positive, 2+, moderately positive, 3+, strongly positive). The percentage of MUC4-positive cells was reported as focal (<25%), restricted (25–50%), or diffuse (>50%).
Statistical analysis
Data were analysed using Medcalc for Windows version 9.6.4.0 software. For the purpose of analysis, each spot was considered as an individual sample. Age and intensity of MUC4 expression were considered as continuous variables, while the type of cutaneous pathology, grade of SCC and gender of the patient were considered as categorical variables. Continuous variables were compared using Student’s two-tailed t test assuming unequal variance, while the categorical variables were compared using a two-way analysis of variance (ANOVA). The correlation between MUC4 expression and age or gender of the patient was examined by calculating the Spearman coefficient of rank correlation. A p value of <0.05 was considered significant.
RESULTS
A total of 330 tissue spots were examined for MUC4 expression. The histological types, patient age and results of MUC4 staining in the various groups are summarised in table 1.
Table 1.
Summary of MUC4 expression in cutaneous pathogenesis
| Pathology | Number | Age, mean±SE (years) |
Age range (years) |
MUC4 intensity, mean±SE |
Range | Number with MUC4 intensity ≥2, n (%) |
p-Value (vs normal skin) |
Correlation of MUC4 expression with age |
Correlation of MUC4 expression with gender |
|---|---|---|---|---|---|---|---|---|---|
| Normal skin | 32 | 35±2.7 | 1–75 | 0.7±0.1 | 0–1.5 | 0 (10) | 0.214 (p=0.18) | ||
| Cancer adjacent skin | 20 | 50±4.2 | 16–75 | 0.6±0.2 | 0–2 | 2 (10) | 0.21 | 0.03 (p=0.86) | |
| Benign diseases | |||||||||
| Hyperplasia (vulval skin) | 12 | 36±5 | 10–59 | 1.25±0.3 | 0–3 | 4 (12) | 0.08 | −0.22 (p=0.25) | NA |
| Cutaneous cyst | 2 | 57±0 | 57 | 0.5±0.5 | 0–1 | 0 (0) | 0.43 | – | |
| Chronic inflammation | 19 | 55±2 | 39–69 | 0.7±0.3 | 0–2.5 | 3 (16) | 0.15 | −0.31 (p=0.67) | 0.13 (p=58) |
| Papilloma | 10 | 62±3.8 | 40–73 | 0.5±0.2 | 0 | 0 (0) | 0.08 | NA | |
| HPV-associated diseases | |||||||||
| Condyloma acuminatum | 19 | 35±3 | 23–66 | 1.9±0.2 | 0–3 | 10 (53) | 0.0004 | 0.16 (p=0.38) | NA |
| Verruca vulgaris | 2 | 10 | – | 2±0 | – | 2 (100%) | <0.0001 | NA | |
| Malignant neoplasms | |||||||||
| Squamous cell carcinoma | 115 | 65±1.5 | 25–91 | ||||||
| Grade 1 | 62 | 64±2 | 32–86 | 1.2±0.2 | 0–3 | 4 (6) | 0.058 | 0.23 (p=0.10) | −0.13 (p=0.33) |
| Grade 2 | 42 | 66±2 | 25–91 | 1.0±0.2 | 0–3 | 2 (12) | 0.03 | 0.17 (p=0.76) | 0.03 (p=0.82) |
| Grade 3 | 11 | 62.5±5 | 45–84 | 0.6±0.4 | 0–2.5 | 1 (9) | 0.56 | 0.89 (p=0.03) | −0.66 (p=0.003) |
| Basal cell carcinoma | 30 | 65±1.8 | 48–82 | 0.3±0.1 | 0–1.5 | 0 (0) | 0.82 | NA | |
| Malignant melanoma | 69 | 54–1.7 | 16–88 | 0.5±0.1 | 0–1.5 | 0 (0) | 0.0006 | NA | |
HPV, human papillomavirus; NA, not applicable.
Expression of MUC4 in normal and cancer adjacent skin tissue
No MUC4 expression was detected in the epidermis of 20/32 normal skin tissue spots. In the remaining spots, a weak staining involving the entire thickness of the epidermis was observed (mean intensity 0.7±0.1, figure 1A). Nine of 21 cancer adjacent normal skin tissues (NATs) also expressed MUC4, with 7/21 showing weak and 2/21 moderately strong staining. Of the latter two, the first showed MUC4 expression limited to the stratum corneum (figure 1C), while in the second, a diffuse MUC4 staining was noted throughout the entire thickness of the epidermis, with the strongest expression noted in the stratum spinosum (figure 1B).
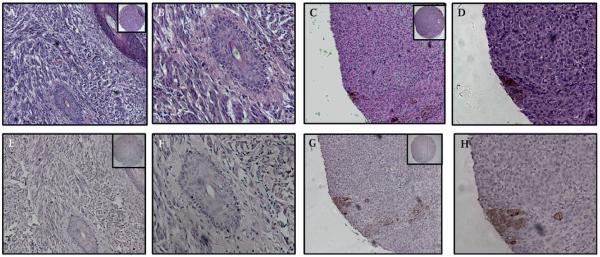
Figure 1.
Expression of MUC4 in normal epidermis, cancer adjacent normal skin and chronically inflamed skin tissue. (A) MUC4-negative normal skin epidermis. (B) Cancer adjacent normal skin tissue from the right elbow of a 40-year-old woman, showing MUC4 expression diffusely throughout the entire thickness of the epidermis, most prominently in the stratum spinosum. Note the melanin pigment granules present in cells of the stratum basalis. (C) Cancer adjacent normal skin tissue from the right hand of a 75-year-old woman. MUC4 expression is confined to the stratum corneum, with less intense staining in a few cells of the stratum granulosum. (D,E) Chronic inflammation of the left foot (D) and right thigh (E) with keratinisation of the squamous epithelium. MUC4 is expressed by the stratum corneum and stratum granulosum of the epidermis. MUC4 expression in all cases is in the cytoplasm and the membrane. (A–E) Magnification ×200 to demonstrate subcellular localisation of MUC4; insets, low magnification (×40).
Expression of MUC4 in benign skin diseases
In chronically inflamed skin tissues, MUC4 expression was either negative (10/19) or weakly positive (6/19), with three cases showing moderately strong expression (mean staining intensity 0.7±0.3, p=0.15). In two cases (a 52-year-old man with chronic inflammation of the left foot and a 50-year-old woman with inflammation of the right thigh), the stratum corneum and the stratum granulosum (but not the stratum lucidum) showed a diffuse and moderately strong MUC4 staining in the epidermal cells (figure 1D,E).
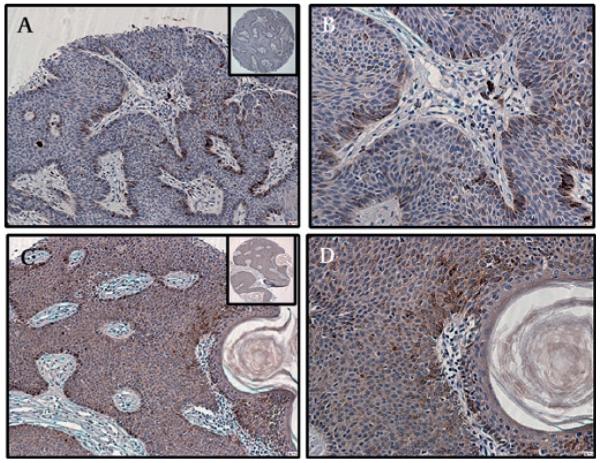
Ten of 19 cases of vulval condyloma acuminatum (VCA) showed strong to intense MUC4 staining (mean intensity 1.9±0.2, p=0.0004). Positive staining was localised specifically to the koilocytes and exhibited a cytoplasmic and a membrane staining pattern (figure 2E,F). A diffuse and strong MUC4 expression was also noted in 3/12 cases of hyperplasia of the vulval skin (figure 2C,D), while moderate MUC4 expression was observed in the epidermis of a verruca vulgaris lesion arising in the abdominal region of a 10-year-old girl (figure 2A,B). Cutaneous cysts were entirely negative for MUC4.
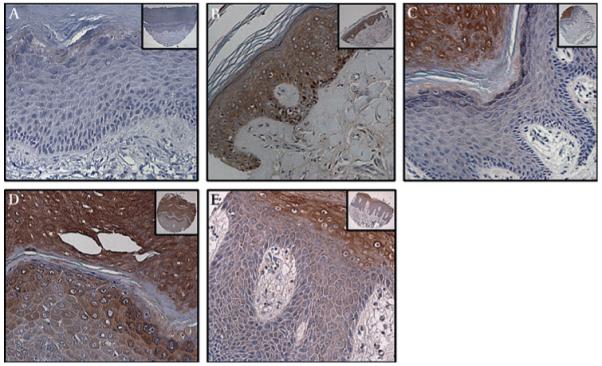
Figure 2.
Expression of MUC4 in verruca vulgaris, condyloma acuminatum and vulval hyperplasia. (A,B) MUC4 showed a restrictive expression (25–50% positive cells) with moderately strong staining (intensity 2+) in verruca vulgaris removed from the skin of the abdominal region of a 10-year-old girl. (C,D) Strong cytosolic and membrane staining for MUC4 was observed in a case of squamous hyperplasia of the vulval skin obtained from a 20-year-old woman. (E,F) Strong membrane and cytoplasmic MUC4 expression was also noted in the in condyloma acuminatum of the vulva (representative section from a 27-year-old woman), especially in the koilocytes. (A,C,E) Magnification ×100, and (B,D,F) magnification ×200, to demonstrate subcellular localisation of MUC4; insets, low magnification (×40).
Expression of MUC4 in cutaneous squamous cell carcinoma
MUC4 expression in grade 1 and 2 (but not grade 3) tumours was significantly increased compared with the normal epidermis (p=0.06 and 0.03, respectively). Further, while the mean intensity was not significantly different between the different grades of squamous cell carcinoma (SCC) (p=0.31, two-way ANOVA), a trend towards decreasing MUC4 expression with increasing grade of SCC was noted (figure 3). There was a weak but significant positive correlation between the age of the patient and MUC4 expression (Spearman’s rank correlation 0.2, 95% confidence interval 0.01 to 0.37, p 0.03). Patients with grade 1 SCC who showed moderate-to-strong MUC4 expression were significantly older than those with weak or no expression (83±2 years versus 63±6 years, respectively, p=0.009). No such difference was noted in grade 2 SCCs (68±2 years in moderate-to-strongly positive versus 65.5±3 years in weak-to-negative tumours, p=0.55). Due to the relatively small sample size of grade 3 SCCs, a similar comparison was not performed. MUC4 was expressed in the cytosol and plasma membrane of the positively staining tumour cells. To determine the correlation between MUC4 expression and gender of the patient, the cases were coded either as 1 (male) or 0 (female) and the rank order correlation coefficient was determined. There was no correlation between MUC4 expression and gender of the patient in well and moderately differentiated SCC. However, a significant negative correlation (r2=−0.66, p=0.003) was observed between MUC4 expression and gender of the patient in poorly differentiated SCC (suggesting that females had a significantly higher MUC4 expression than males).
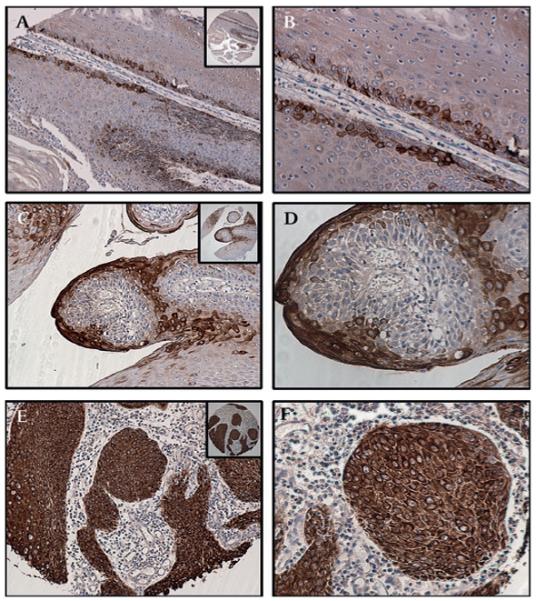
Figure 3.
Expression of MUC4 in cutaneous squamous cell carcinoma (SCC) by grade of tumour. (A) Well-differentiated SCC showing strong MUC4 expression in the malignant cells, and particularly in the keratin pearls (B). (C) MUC4 expression is equally intense but with fewer positive cells in moderately differentiated SCC. (D) Keratin pearls are evident and show strong MUC4 expression, similar to that seen in well-differentiated SCC. (E) Poorly differentiated SCC showing moderate-to-weak MUC4 expression, together with an absence of keratinisation. (F) A high-power view shows groups of malignant cells with mostly weak MUC4 expression in the cytoplasm and membrane, and few cells with moderate MUC4 positivity. (A,C,E) Magnification ×100, and (B,D,F) magnification ×200, to demonstrate subcellular localisation of MUC4; insets, low magnification (×40).
Expression of MUC4 in basal cell papillomas and carcinoma
Basal cell papillomas showed either weak (6/10) or no MUC4 expression (4/10) (figure 4A,B). A similar pattern was observed in basal cell carcinomas (BCCs) where 8/30 cases showed a weak expression and the rest no MUC4 staining (figure 4C,D).
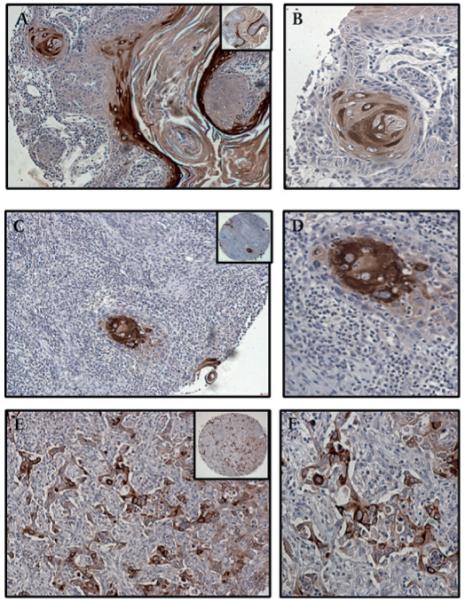
Figure 4.
Expression of MUC4 in basal cell papilloma and basal cell carcinoma. (A,B) MUC4 was not expressed by basal cell papillomas. Several pigmented cells are visible at the periphery of the papilloma. (C,D) Weak and diffuse cytoplasmic MUC4 staining of the tumour cells in a case of basal cell carcinoma arising in the back of a 70-year-old man. Pigmented cells containing melanin are also visible at the periphery of the tumour. (A,C) Magnification ×100, and (B,D) magnification ×200, to demonstrate subcellular localisation of MUC4; insets, low magnification (×40).
Expression of MUC4 in malignant melanoma
Of the 69 cases of malignant melanoma examined, 48% were negative for MUC4. The remaining (52%) cases showed only weak MUC4 expression, which was limited to those tumours wherein the malignant cells also contained prominent melanin granules (figure 5).
Figure 5.
Expression of MUC4 in malignant melanoma. Malignant melanoma without prominent melanin granules was uniformly negative for MUC4. Representative sections: (A,B) H&E; (E,F) MUC4 stained. Focal, weak MUC4 expression was noted in those melanomas where the tumour cells expressed melanin granules. Representative sections: (C,D) H&E to demonstrate melanin granules; (G,H) MUC4 stained. (A,C,E,G) Magnification ×100; (B,D,F,H) magnification ×200; insets, low magnification (×40).
DISCUSSION
Mucins are high-molecular-weight glycoproteins whose role as diagnostic markers and therapeutic targets is being increasingly recognised. MUC4, a membrane-bound mucin, is produced by several tissues, including the conjunctiva, cornea, tear film, lining epithelium of the respiratory tract (trachea to collecting ducts), inferior nasal turbinates, eustachian tubes, parotid and submandibular glands, and the endocervix.3
In this study, we examined the expression of MUC4 in healthy adult skin and compared it with that in benign and malignant skin diseases. MUC4 expression has recently emerged as a potential biomarker for the diagnosis and prognosis of several epithelial malignancies. In one study, MUC4 was strongly expressed in nearly 85% of primary SCCs of the upper aerodigestive tract (UADT). Further, MUC4 expression was also observed in 11/12 nodal metastases from these tumours and was associated with a lower rate of recurrence and significantly improved survival. It was also expressed diffusely throughout the dysplastic epithelium of the UADT, suggesting that MUC4 plays a role in the development and the progression of UADT malignancies.15 Other malignancies that have been shown to express high levels of MUC4 include salivary gland tumours, non-small-cell lung cancer, Barrett oesophagus and oesophageal carcinoma, cholangiocarcinoma and extrahepatic bile duct carcinoma, colon and pancreatic adenocarcinoma, and cervical squamous cell carcinoma and ovarian cancer.3 In contrast, MUC4 is downregulated in prostate and endometrial carcinoma. The diagnostic potential of MUC4 in cancer has been reviewed by us in a recent article.3
We observed that MUC4 expression in the normal skin was weak or absent. However, moderately strong MUC4 expression was noted in 2/21 NATs. MUC1, another membrane tethered mucin, has also been reported to be aberrantly expressed in the NATs associated with Bowen’s disease (SCC in situ) and cutaneous SCC.16 It is possible that MUC4 expression in the epidermis (of NATs) is regulated by the adjoining malignancy, possibly through signals from the tumour microenvironment. Unravelling the prognostic significance of MUC4 expression in NATs and its underlying pathobiology will provide a better insight into the role of MUC4 in cutaneous malignancies.
Non-melanoma skin cancer, comprising chiefly SCC and BCC, is the most common type of malignancy in the USA.17 Kathpalia et al, in a study to determine the cellular pathways involved in the development of cutaneous SCC, compared the global gene expression profile in well-differentiated SCCs from five Caucasians (age range 67–80 years) with lesion-free skin tissues from the same patients. They reported that MUC4 mRNA was upregulated nearly threefold in well-differentiated SCCs.18 In the present study, we observed a strong expression of MUC4 in a subset of well and moderately differentiated SCCs, while poorly differentiated tumours were weakly positive. This is similar to the pattern seen in other malignancies including salivary gland, and ovarian and endocervical adenocarcinoma,3 where MUC4 expression was observed to decrease with increasing grade of the tumour. The grade of tumour also has an impact on patient outcome in SCC, with poorly differentiated SCCs having a twofold greater risk of recurrence following treatment (compared with well-differentiated tumours).19 However, no further clinical information was available for the patients in our study, limiting the ability to assess the prognostic significance of MUC4 positivity.
Significantly, we noted that only about 14% of cutaneous SCCs showed moderate–strong positivity for MUC4 (intensity ≥2). Further, patients with grade 1 (but not grade 2) MUC4-positive SCCs were significantly older than grade-matched MUC4-negative SCC patients. This suggests that age-related factors such as years of sun exposure may play a role in inducing MUC4 in a subset of SCCs. Whether MUC4 expression is turned on in precursors of SCC-like actinic keratosis20 will be key to understanding the role of MUC4 in SCC.
An important agent that has been linked to SCCs, chiefly of the anogenital region, is the human papillomavirus (HPV). The HPV virus, a double-stranded non-enveloped DNA virus, is sexually transmitted and causes SCC and benign genital warts or condylomas.21 In the present study, we observed strong MUC4 staining in around 50% of the VCA lesions, and moderately strong staining in two cases of vulval verruca vulgaris. Further, while tissue from the chronically inflamed vulval skin was negative, two cases of simple hyperplasia of the vulva showed strong MUC4 expression. While nearly 90% of genital condylomas are caused by HPV-6 and HPV-11 (rarely progress to malignancy), rare cases are caused by HPV types 31, 33, 35, 39, 40, 42–45, 51–56 and 58 (moderate risk for malignancy) and types 16 and 18 (high risk for malignant transformation). An earlier study by our group revealed a progressive increase in MUC4 expression from the normal cervical epithelium through cervical intraepithelial neoplasia to cervical SCC.9 Taken together, the results of the two studies suggest, for what we believe is the first time, a novel association between HPV and MUC4. The E6 and E7 genes of HPV can produce oncoproteins that alter the regulation of cell growth, specifically by inactivation of the tumour suppressors p53 (by E6) and pRb (by E7). Although no direct association has been demonstrated between MUC4 and p53, the coordinated expression of the two proteins has been observed in pancreatic cancer.22 The study by Kathpalia et al also reported an upregulation of several p53-regulated genes.18 MUC4, through its interaction with the receptor tyrosine kinase ErbB2/HER-2, has been shown to promote survival and inhibit apoptosis in pancreatic cancer cells.23 24 Thus, MUC4 upregulation in VCA might constitute an early marker of transformation and could potentially be surrogate marker of HPV-associated transformation.
Mucins are not only players in malignancy, they also have a key role in the inflammatory process. For instance, an upregulation of Muc4 has been observed in a mouse model of acute colitis,25 while in colonic epithelial cells, MUC4 expression protects against Shigella infection.26 We observed two cases of chronic inflammation with a moderately strong MUC4 staining. In both cases, the stratum corneum was positive, and in one case, the stratum granulosum. Keratinocytes have been demonstrated to be capable of producing several pro-inflammatory cytokines, including interleukins 6 and 8, growth factors (GM-CSF), interferons, and tumour necrosis factors α and β,27 several of which also upregulate MUC4. While the relationship between keratinisation and MUC gene expression is not well studied, studies on the cornneal epithelium in rats has revealed that deficiency of vitamin A (required for normal keratinisation of epithelia) is associated with a decreased expression of asialo-glycoprotein (ASGP, rat Muc4) rat Muc5AC while the expression of rat Muc1 remained unaltered following a 20-week diet deficient in vitamin A.28 Understanding whether the production of MUC4 is a response to specific type of chronic stimulus or a general reaction to injury or irritation, and its relationship to the evolution and progression of the chronic inflammatory processes, will yield valuable information concerning the role of MUC4 in the cutaneous response to infection or injury.
BCC and melanoma are not known to express mucin genes, except MUC15 in primary BCCs29 and focal MUC1 expression in basosquamous carcinoma.16 Both of these malignancies were largely negative for MUC4 in our study.
In summary, we present the first evidence demonstrating a differential expression of the MUC4 mucin in cutaneous diseases. We observed that while certain benign (chronic inflammation, verruca vulgaris), potentially malignant (VCA) and malignant (SCC) skin diseases express MUC4, its expression is lacking in others (BCC and malignant melanoma). Given the availability of a highly specific monoclonal antibody, our findings hold considerable clinical significance, as immunohistochemical analysis for MUC4 could be useful as a relatively simple technique to identify potentially malignant cutaneous lesions. Further, patients with MUC4+ lesions could be candidates for MUC4-antibody-based immunotherapy. Future studies will aim to extend the findings of this preliminary study to examine a possible correlation of MUC4 expression with clinicopathological variables, response to therapy and patient outcome.
Take-home messages.
-
▶
MUC4 is a high-molecular-weight glycoprotein whose expression is aberrantly upregulated in several malignant diseases, chiefly those arising from the gastrointestinal and respiratory tract.
-
▶
Our results show that while MUC4 expression is weak or entirely absent in the healthy epidermis, it is strongly upregulated in cutaneous squamous cell carcinomas (SCCs).
-
▶
MUC4 expression in SCCs correlates positively with grade of the tumour (well differentiated SCCs have the highest MUC4 expression).
-
▶
MUC4 is not expressed in melanomas or basal cell carcinomas.
-
▶
MUC4 expression is strongly upregulated in condyloma acuminatum, suggesting a potential link between human papillomavirus infection and MUC4 expression.
-
▶
MUC4 expression also appears to be modulated by chronic inflammation and presence of an adjacent area of malignancy in a small proportion of patients.
-
▶
Taken together, our results suggest that MUC4 might be a novel diagnostic or prognostic marker in skin cancer and could play a role in human-papillomavirus-induced cutaneous malignancies.
Acknowledgements
We thank Ms Kristi L Berger for editing the manuscript.
Funding The authors are supported by grants from the National Institutes of Health (RO1 CA78590, UO1 CA111294, RO1 CA131944, RO1 CA 133774 and P50 CA127297).
Footnotes
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Fleischer AB, Jr, Feldman SR, Rapp SR. Introduction. The magnitude of skin disease in the United States. Dermatol Clin. 2000;18:xv–xxi. doi: 10.1016/s0733-8635(05)70163-7. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966–81. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakraborty S, Jain M, Sasson AR, et al. MUC4 as a diagnostic marker in cancer. Expert Opin Med Diagn. 2008;2:891–910. doi: 10.1517/17530059.2.8.891. [DOI] [PubMed] [Google Scholar]
- 4.Moniaux N, Varshney GC, Chauhan SC, et al. Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans. J Histochem Cytochem. 2004;52:253–61. doi: 10.1177/002215540405200213. [DOI] [PubMed] [Google Scholar]
- 5.Shibahara H, Tamada S, Higashi M, et al. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology. 2004;39:220–9. doi: 10.1002/hep.20031. [DOI] [PubMed] [Google Scholar]
- 6.Tamada S, Shibahara H, Higashi M, et al. MUC4 is a novel prognostic factor of extrahepatic bile duct carcinoma. Clin Cancer Res. 2006;12:4257–64. doi: 10.1158/1078-0432.CCR-05-2814. [DOI] [PubMed] [Google Scholar]
- 7.Moniaux N, Chakraborty S, Yalniz M, et al. Early diagnosis of pancreatic cancer: neutrophil gelatinase-associated lipocalin as a marker of pancreatic intraepithelial neoplasia. Br J Cancer. 2008;98:1540–7. doi: 10.1038/sj.bjc.6604329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park HU, Kim JW, Kim GE, et al. Aberrant expression of MUC3 and MUC4 membrane-associated mucins and sialyl Le(x) antigen in pancreatic intraepithelial neoplasia. Pancreas. 2003;26:e48–54. doi: 10.1097/00006676-200304000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Munro EG, Jain M, Oliva E, et al. Upregulation of MUC4 in cervical squamous cell carcinoma: pathologic significance. Int J Gynecol Pathol. 2009;28:127–33. doi: 10.1097/PGP.0b013e318184f3e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senapati S, Chaturvedi P, Sharma P, et al. Deregulation of MUC4 in gastric adenocarcinoma: potential pathobiological implication in poorly differentiated non-signet ring cell type gastric cancer. Br J Cancer. 2008;99:949–56. doi: 10.1038/sj.bjc.6604632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chauhan SC, Singh AP, Ruiz F, et al. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125) Mod Pathol. 2006;19:1386–94. doi: 10.1038/modpathol.3800646. [DOI] [PubMed] [Google Scholar]
- 12.Ponnusamy MP, Singh AP, Jain M, et al. MUC4 activates HER2 signalling and enhances the motility of human ovarian cancer cells. Br J Cancer. 2008;99:520–6. doi: 10.1038/sj.bjc.6604517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsutsumida H, Goto M, Kitajima S, et al. MUC4 expression correlates with poor prognosis in small-sized lung adenocarcinoma. Lung Cancer. 2007;55:195–203. doi: 10.1016/j.lungcan.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Singh AP, Chauhan SC, Bafna S, et al. Aberrant expression of transmembrane mucins, MUC1 and MUC4, in human prostate carcinomas. Prostate. 2006;66:421–9. doi: 10.1002/pros.20372. [DOI] [PubMed] [Google Scholar]
- 15.Alos L, Lujan B, Castillo M, et al. Expression of membrane-bound mucins (MUC1 and MUC4) and secreted mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC7) in mucoepidermoid carcinomas of salivary glands. Am J Surg Pathol. 2005;29:806–13. doi: 10.1097/01.pas.0000155856.84553.c9. [DOI] [PubMed] [Google Scholar]
- 16.Heyderman E, Graham RM, Chapman DV, et al. Epithelial markers in primary skin cancer: an immunoperoxidase study of the distribution of epithelial membrane antigen (EMA) and carcinoembryonic antigen (CEA) in 65 primary skin carcinomas. Histopathology. 1984;8:423–34. doi: 10.1111/j.1365-2559.1984.tb02354.x. [DOI] [PubMed] [Google Scholar]
- 17.American Cancer Society . Cancer facts and figures 2009. Ameican Cancer Society; Atlanta: 2009. [Google Scholar]
- 18.Kathpalia VP, Mussak EN, Chow SS, et al. Genome-wide transcriptional profiling in human squamous cell carcinoma of the skin identifies unique tumor-associated signatures. J Dermatol. 2006;33:309–18. doi: 10.1111/j.1346-8138.2006.00075.x. [DOI] [PubMed] [Google Scholar]
- 19.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975–83. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 20.Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42:4–7. doi: 10.1067/mjd.2000.103342. [DOI] [PubMed] [Google Scholar]
- 21.Ganguly N, Parihar SP. Human papillomavirus E6 and E7 oncoproteins as risk factors for tumorigenesis. J Biosci. 2009;34:113–23. doi: 10.1007/s12038-009-0013-7. [DOI] [PubMed] [Google Scholar]
- 22.Bhardwaj A, Marsh WL, Jr, Nash JW, et al. Double immunohistochemical staining with MUC4/p53 is useful in the distinction of pancreatic adenocarcinoma from chronic pancreatitis: a tissue microarray-based study. Arch Pathol Lab Med. 2007;131:556–62. doi: 10.5858/2007-131-556-DISWPI. [DOI] [PubMed] [Google Scholar]
- 23.Chaturvedi P, Singh AP, Moniaux N, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5:309–20. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 24.Chaturvedi P, Singh AP, Chakraborty S, et al. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68:2065–70. doi: 10.1158/0008-5472.CAN-07-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoebler C, Gaudier E, De CP, et al. MUC genes are differently expressed during onset and maintenance of inflammation in dextran sodium sulfate-treated mice. Dig Dis Sci. 2006;51:381–9. doi: 10.1007/s10620-006-3142-y. [DOI] [PubMed] [Google Scholar]
- 26.Nutten S, Sansonetti P, Huet G, et al. Epithelial inflammation response induced by Shigella flexneri depends on mucin gene expression. Microbes Infect. 2002;4:1121–4. doi: 10.1016/s1286-4579(02)01636-2. [DOI] [PubMed] [Google Scholar]
- 27.Lever WF, Elder DE. Lever’s histopathology of the skin. 9th edn. Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- 28.Tei M, Spurr-Michaud SJ, Tisdale AS, et al. Vitamin A deficiency alters the expression of mucin genes by the rat ocular surface epithelium. Invest Ophthalmol Vis Sci. 2000;41:82–8. [PubMed] [Google Scholar]
- 29.Riker AI, Enkemann SA, Fodstad O, et al. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med Genomics. 2008;1:13. doi: 10.1186/1755-8794-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]