Abstract
Distinct mechanisms are believed to regulate growth of the liver during fetal development and after injury in adults because the former relies on progenitors while the latter generally involves replication of mature hepatocytes. However, chronic liver injury in adults increases production of Hedgehog (Hh) ligands, developmental morphogens that control progenitor cell fate and orchestrate various aspects of tissue construction during embryogenesis. This raises the possibility that similar Hh-dependent mechanisms might also regulate adult liver regeneration. The present analysis of murine liver regeneration after 70% partial hepatectomy (PH), an established model of adult liver regeneration, demonstrated that PH induced production of Hh ligands and activated Hh signaling in liver cells. Treatment with a specific Hh signaling inhibitor interfered with several key components of normal liver regeneration, significantly inhibiting progenitor responses, matrix remodeling, proliferation of hepatocytes and ductular cells, and restoration of liver mass. These global inhibitory effects on liver regeneration dramatically reduced survival after PH.
Conclusion
Mechanisms that mediate liver organogenesis, such as Hh pathway activation, are retained and promote reconstruction of adult livers after injury.
Keywords: developmental morphogens, adult hepatocyte proliferation, progenitors, ductular cells, fibrogenesis
Healthy adult livers have enormous regenerative capacity. This permits recovery of normal tissue-specific functions and mass within weeks of 70% partial hepatectomy (PH) in humans. Liver regeneration proceeds more rapidly in rodents, which accomplish liver reconstruction within 7–10 days after PH(1). Thus, rodents are often used as experimental models to investigate regenerative mechanisms. Such work has consistently demonstrated that striking increases in hepatocyte DNA synthesis occur within the initial 48h post-PH, followed by smaller (but highly significant) increases in hepatocyte mitoses and eventual recovery of liver mass, leading to consensus that liver regeneration after PH relies largely upon increased replication of mature hepatocytes(2–5). Nevertheless, changes in expression of progenitor markers, such as alpha-fetoprotein (AFP) and Fn14, have long been acknowledged to occur during regeneration(6–9). Severe inhibition of liver regeneration after toxic liver injury was recently reported to occur in mice with targeted disruption of Fn14, a member of the tumor necrosis factor receptor superfamily that promotes the growth of bipotent hepatic progenitors (i.e., oval cells)(10). These findings suggest that liver progenitors may have a larger role in regenerating adult livers post-PH, and perhaps following other types of acute injury, than previously appreciated.
Because mature hepatocyte replication is inhibited in many types of chronic liver injury, it is generally believed that progenitor populations contribute to regeneration of chronically injured livers. However, the mechanisms that mobilize progenitor cells, and that control their fate in damaged livers, are poorly understood (11–13). Recent studies have demonstrated that Hedgehog (Hh), a fetal morphogenic signaling pathway, becomes activated in many types of chronic liver injury(14). Hh ligands generally promote the growth and viability of progenitor-type cells(15–17), and have been shown to function as viability factors for human and rodent liver progenitors, including oval cells. During embryogenesis and cancer metastasis, Hh-pathway activation tends to preferentially expand stromal cell populations by retaining the primitive, migratory phenotype of existing mesenchymal cells and promoting epithelial-to-mesenchymal transitions (EMT) in certain types of immature epithelial cells(18–21). A similar process occurs when the Hh-pathway becomes activated during chronic liver injury because Hh ligands function as growth factors for myofibroblastic liver cells(15, 22), stimulate quiescent hepatic stellate cells to acquire a more myofibroblastic phenotype(23). and induce immature ductular cells to undergo EMT(13). As a result, Hh pathway activation promotes fibrogenic repair responses during chronic liver injury. Our group has also suggested that Hh pathway activation enhances the outgrowth of liver progenitors that help to replace mature hepatocytes that die, but the latter remains unproven.
The present study evaluates the hypothesis that Hh pathway activation occurs after PH and plays a role in regulating liver regeneration after a surgical insult which causes massive acute loss of mature hepatocytes. Our findings demonstrate the kinetics of Hh pathway activation after PH, identify the types of Hh-responsive cells, and characterize the effects of Hh-pathway inhibition on the regenerative process. The results support our hypothesis and identify Hedgehog as a major regulator of liver regeneration post-PH. This, in turn, suggests that common mechanisms regulate liver growth during organogenesis and when reconstruction of adult livers is necessitated by injury.
Materials and Methods
Animal experiments
B6:129Sv (in-house strain, Spain) and C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were maintained in respective animal facilities at the University of the Basque Country and Duke University. Animal care and surgical procedures were conducted in compliance with local Institutional guidelines, and those set forth in the “Guide for the Care and Use of Laboratory Animals” as published by the National Institute of Health.
To ascertain the kinetics of Hh-signaling during liver regeneration, 70% PH was performed on 8–10-week old female mice (n=102), according to the method of Higgins and Anderson(1). Mice were operated between 1400h and 1700h. The mice were sacrificed at 6h (n=6), 12h (n=6), 24h (n=6), 48h (n=12), 72h (n=12), 96h (n=12), 120h (n=12), 144h (n=12), 168h (n=12) and 216h (n=12) post-PH. Animals were administered BrdU i.p. (50 µg/g body weight) 2h before sacrifice. Animals were weighed before PH and at the time of sacrifice; resected quiescent liver (used as 0h comparisons) and regenerating liver remnants were weighed and then formalin-fixed or snap frozen in liquid nitrogen.
To determine if inhibiting the Hh-pathway altered liver regeneration, PH was performed in an additional 100 mice (10–13-week old males) that were injected i.p. with vehicle (olive oil) or cyclopamine (15 mg/kg/day, Toronto Research Chemicals, Toronto, Canada(12, 24)) 24h before PH and daily thereafter. Liver remnants and blood were harvested for subsequent analysis. In addition to evaluating Hh-signaling and BrdU incorporation, potential toxic actions of cyclopamine were assessed by examining liver histology and levels of serum aminotransferases, blood urea nitrogen (BUN) and glucose.
Hh signaling was also assessed in primary hepatocytes isolated from another 6 adult male mice 24h or 48h after sham surgery (n=2 mice) or PH (n=4 mice). Standard in situ liver perfusion and density gradient centrifugation techniques were used to isolate hepatocytes(18). Cells were processed immediately for immunocytochemistry or plated onto plastic dishes in 10% serum-supplemented DMEM (Sigma, St. Louis, MO) overnight. Medium containing non-adhered cells was removed the following morning, plates were washed with fresh medium, and adherent cells were harvested for analysis. To assess direct effects of Hh pathway inhibition on cell viability and proliferation, hepatocytes were similarly isolated from 4 additional mice 24h after sham surgery (n=2) or PH (n=2) and cultured with either vehicle or cyclopamine (5 µM) in the presence of BrdU for 24h.
Immunohistochemistry and BrdU Labeling
Formalin-fixed paraffin embedded (FFPE)-livers and hepatocyte cytospins were prepared as previously described(16). A detailed protocol and antibodies used are listed in Supplemental Materials and Methods.
Quantitative Real-time Reverse Transcription-Polymerase Chain Reaction (QRT-PCR)
QRT-PCR were performed using established protocols(15); details in Supplemental Materials and Methods and Supplementary Table 1.
Western Blot
Proteins were isolated from whole liver tissue or primary hepatocytes. After quantification, equal amounts of protein were separated by SDS-PAGE and Western blot analysis was performed. Detailed list of antibodies is listed in Supplemental Materials and Methods.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism v5.02 for Windows (GraphPad Software, San Diego, CA). Results of gene expression are grouped into Pre-Replicative (0h-to-<36h post-PH), Replicative (36h-to-<100h post-PH), and Post-Replicative (>100h-to-<216h post-PH) sets, and compared to 0h (quiescent) samples. Statistical significance was determined using one-way ANOVA and Bonferroni’s Multiple Comparisons Test. Where mice were treated with either vehicle or cyclopamine, results were compared with respective vehicle- or cyclopamine-treated, 0h (quiescent) samples. Statistical significance was determined using unpaired, two-tailed Student’s t-test. Significance was accepted at the 5% level, *P<0.05, **P<0.01 or ***P<0.001.
Results
Hedgehog pathway activation follows PH
Hepatic expression of mRNAs that encode Hh-pathway inhibitors (e.g., Hh interacting protein, Hip), Hh ligands (Indian Hh, Ihh, and Sonic Hh, Shh), the receptors that repress (Patched, Ptc) or promote (Smoothened, Smo) Hh signaling, and Hh-inducible transcription factors (Glioblastoma (Gli)1 and Gli2) were evaluated at several distinct time points after PH. For each gene, results were normalized to expression in the liver at the time of PH (0h) and graphed as a function of time to demonstrate how gene expression varied during the pre-replicative period, period of maximal liver cell replication, and post-replicative period (Fig 1A).
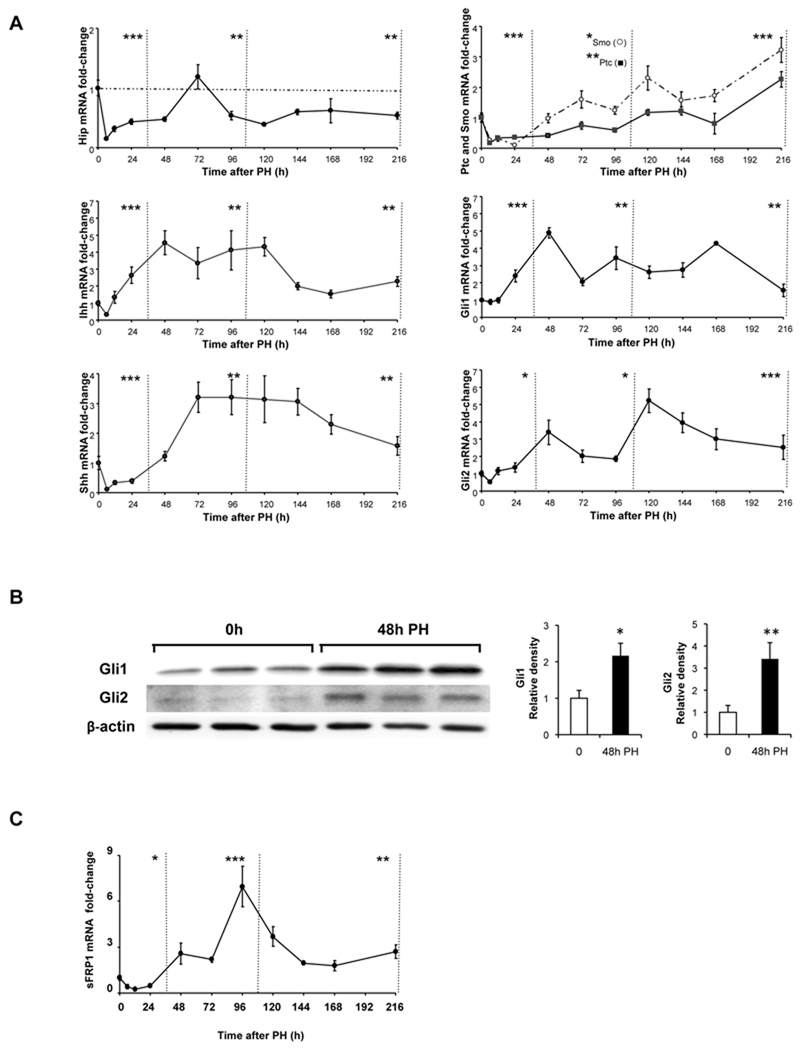
Figure 1. Activation of the Hedgehog pathway after partial hepatectomy (PH).
PH was performed on 102 mice; 6–12 mice/group were sacrificed at 6h, 12h, 24h, 48h, 72h, 96h, 120h, 144h, 168h and 216h post-PH. Quiescent (0h) livers and regenerated livers were harvested and total RNA from each mouse was examined in triplicate by qRT-PCR for (A) Hh pathway signaling components, Hip, Ihh, Shh, Ptc/Smo, Gli1, and Gli2 and (C) sFRP1, a Hh-target gene. Results are expressed as fold change relative to 0h liver tissues; mean ± SEM are graphed. Data are grouped into pre-replicative, replicative and post-replicative sets for statistical analysis using one-way ANOVA. (B) Representative Western blot analysis of Gli protein expression in 0h and post-PH livers of 3 randomly selected mice that were sacrificed at 48h post-PH. β-actin was used as a loading control; blots were densitized; Gli expression was normalized to β-actin expression in each sample; cumulative data were graphed. *P<0.05 or **P<0.01 or ***P<0.001 vs 0h.
PH abruptly reduced hepatic expression of Hip, and Hip mRNA levels generally remained below pre-PH values during the pre-replicative, replicative, and post-replicative periods after PH. Reduced Hip expression was accompanied by increased expression of Hh ligands. mRNA levels of Ihh began to increase during the pre-replicative period, remained at their highest values during the replicative period, then gradually declined. Expression of Shh did not increase until the middle-end of the replicative period but remained high throughout the post-replicative period post-PH.
The relative abundance of Ptc and Smo mRNAs changed after PH, such that expression of Smo (the signaling competent Hh co-receptor) was greater than that of Ptc (the inhibitory Hh receptor) throughout the replicative and post-replicative periods. Together with the reciprocal changes in mRNA expression of Hh ligand antagonists and Hh ligands, the predominance of Smo relative to Ptc suggested that Hh signaling would increase post-PH. Changes in expression of Gli1 and Gli2 support this concept. Levels of Gli1 began to increase in the pre-replicative period and remained at high levels until the end of the post-replicative period. Increases in Gli1 expression were followed by increases in mRNA levels of Gli2, a Gli-regulated gene(20). Gli2 expression began to increase during the replicative period, peaked somewhat later, and then remained high throughout the post-replicative period. Increased Gli1 and Gli2 mRNA levels were accompanied by increased levels of Gli1 and Gli2 proteins at 48h post-PH (the time point of maximal mRNA expression of these genes during the replicative period) (Fig 1B), and followed by increased mRNA expression of sFRP1, a Gli-regulated, Hh-target gene (Fig 1C)(25). Hence, PH led to dramatic increases in Hh signaling, particularly during the time intervals when liver cell replication and remodelling responses are known to occur in the regenerating liver tissues.
Hh pathway activation is accompanied by increased liver epithelial progenitors and fibrogenic repair after PH
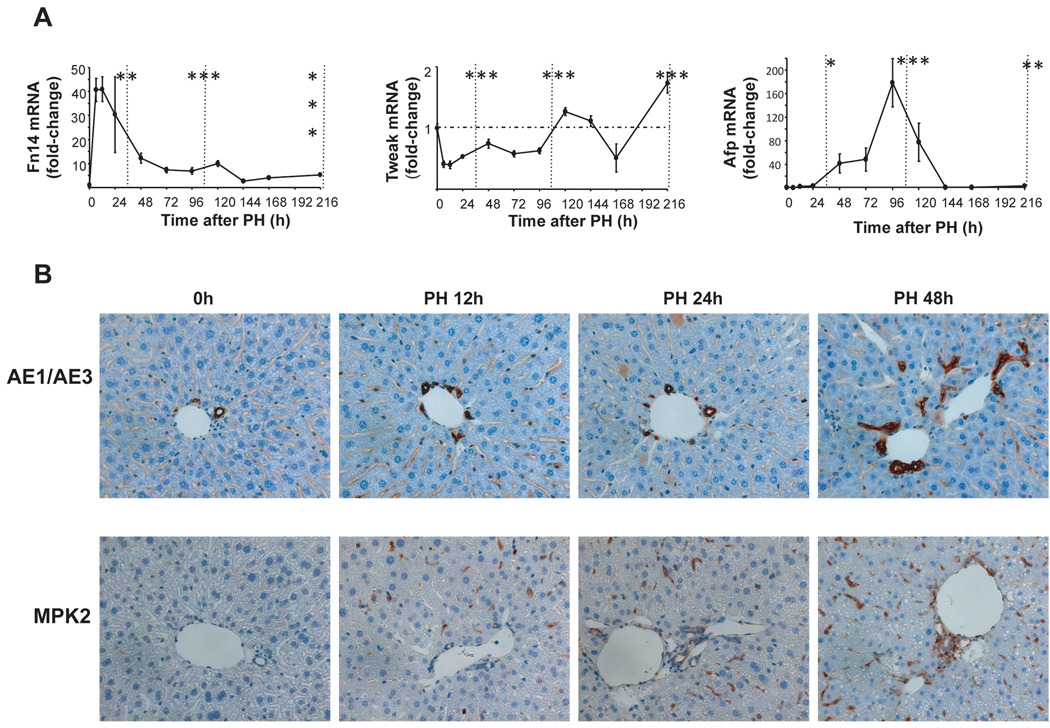
During chronic liver injury, Hh pathway activation promotes accumulation of liver epithelial progenitor cells and myofibroblasts, and stimulates fibrogenic repair. Hepatic expression of progenitor markers, such as AFP and Fn14, increase following PH(8, 9). We confirmed these observations (Fig 2). Fn14 increased 40-fold during the early pre-replicative period and remained at least 5-fold above basal values throughout the entire post-replicative period, although expression of the Fn14 ligand, TWEAK, remained relatively constant after PH. Early increases in Fn14 were followed by increases in AFP expression, which peaked sharply (at 160-fold above basal values) late in the replicative period (Fig 2A). Hepatic progenitor populations are known to be heterogeneous. Therefore, to verify that increased mRNA levels of Fn14 and AFP were actually accompanied by hepatic accumulation of progenitors, two other progenitor markers, AE1/AE3 and MPK (26, 27) were evaluated using immunohistochemistry. Results demonstrated that cells expressing these markers increased steadily in regenerating livers from 12h–48h post-PH (Fig 2B). These findings show that Hh pathway activation is associated with accumulation of liver progenitors before and during the replicative period following PH, as is known to occur when the pathway becomes activated during chronic liver injury(14).
Figure 2. Changes in markers of liver epithelial progenitors after PH.
Effects of PH on mRNA levels of progenitor markers were examined in all 102 mice described in Figure 1. (A) QRT-PCR analysis of Fn14, Tweak, Afp. Results are expressed as fold change relative to 0h liver tissues; mean ± SEM are graphed. Data are grouped into pre-replicative, replicative and post-replicative sets for statistical analysis. *P<0.05 or **P<0.01 or ***P<0.001 relative to time 0h (ANOVA). (B) Two other progenitor markers, AE1/AE3 and Mpk2, were evaluated using immunohistochemistry in 0h and post-PH livers from randomly selected mice that were sacrificed at either 12h, 24h or 48h post-PH (n=4 mice/time point). Representative sections are displayed. AE/AE3 or Mpk2-stained cells are brown.
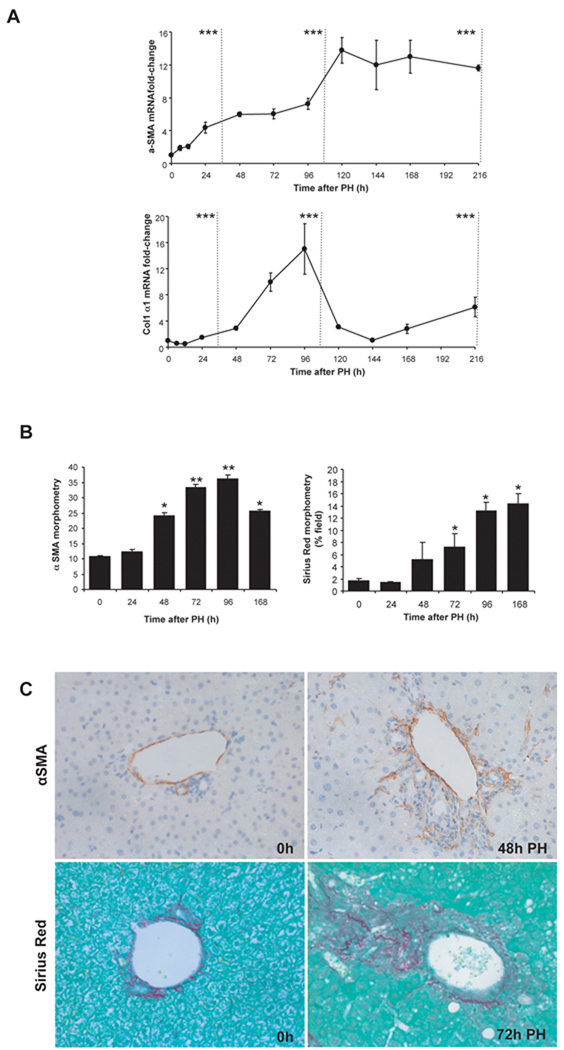
Accumulation of myofibroblastic cells and increased production of collagen matrix has also been demonstrated after PH(28–30). Our studies confirmed these findings (Fig 3). α-smooth muscle actin (α̃sma) mRNA levels increased steadily after PH, peaking at more than 12-fold above basal levels early during the post-replicative period and remaining in this range until the end of the study (216h post-PH). Collagen expression also increased significantly, with collagen1α1 mRNA peaking ~15-fold above basal values 96h post-PH (Fig 3A). Immunohistochemistry and morphometry confirmed hepatic accumulation of α̃SMA-immunoreactive cells and Sirius red fibrils in regenerating livers (Fig 3B,C). Therefore, Hh pathway activation post-PH is accompanied by progressive matrix accumulation, as is known to occur during fibrogenic repair of chronic liver injuries(31).
Figure 3. Changes in fibrogenesis after PH.
Effects of PH on mRNA levels of fibrogenic markers were examined in all 102 mice described in Figure 1. (A) QRT-PCR analysis of α-sma and collagen. Results are expressed as fold-change relative to 0h liver tissues; mean ± SEM are graphed. Data are grouped into pre-replicative, replicative and post-replicative sets for statistical analysis. *P<0.05 or **P<0.01 or ***P<0.001 relative to 0h (ANOVA). (B) Histochemistry was used to examine hepatic accumulation of α-sma(+) cells and collagen fibrils in 0h and post-PH livers from randomly-selected mice that were sacrificed at either 24h, 48h, 72h, or 168h post-PH (n=4 mice/time point). For α-SMA morphometry,15 randomly-selected, 40×fields/section were analysed by Metaview software; Sirius red staining was similarly assessed in 20× fields. Mean ± SEM results are graphed. *P<0.05 or **P<0.01 compared with vehicle-treated mice (Student’s t-test). (c) Representative sections demonstrating either α-SMA stained cells (brown) or Sirius-red stained collagen fibrils.
Hepatocytes and bile ductular cells express Hh-target genes after PH
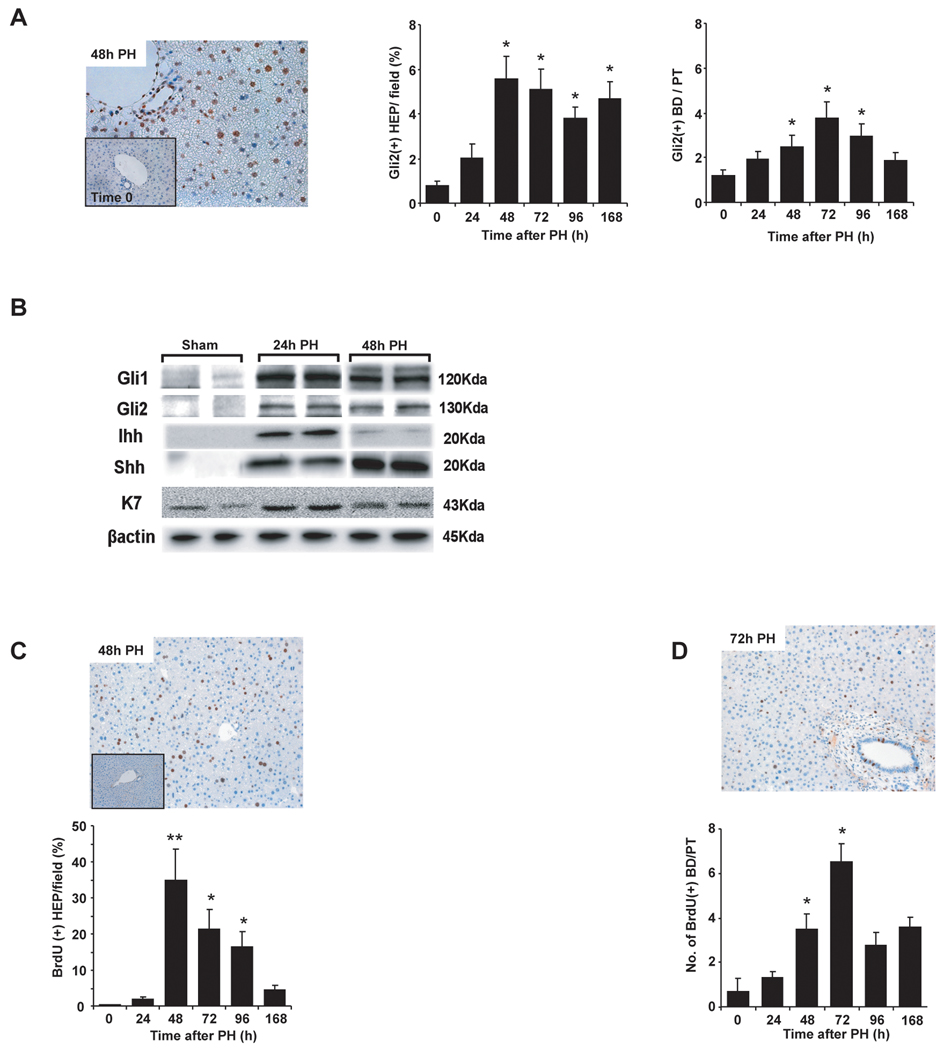
In healthy adult livers, mature hepatocytes generally do not express Hh-target genes, such as Gli2, although Gli2 can be demonstrated in occasional ductular cells in bile ducts and Canals of Hering(14). Thus, it was important to determine if these cell types became Hh-responsive when Hh pathway activity increased after PH. Immunohistochemistry demonstrated Gli2-staining in both hepatocytic and ductular cells in regenerating livers (Fig 4A). Numbers of Gli2(+) hepatocytes began to increase in the pre-replicative period, peaked at 48h post-PH, and remained at high levels throughout the post-replicative period. The number of Gli2(+) ductular cells increased significantly post-PH, but peak accumulation occurred a bit later (i.e., 72h post-PH).
Figure 4. Hepatocytes and bile-ductular cells are Hh-responsive and proliferative after partial hepatectomy.
(A) Cells with Gli2(+) nuclei were counted in sections from livers of randomly-selected mice at 0h and different time points ranging from 24h–168 h after PH (n=4 mice/time point). Gli2 staining in representative liver sections at 0h and 48h post-PH (Magnification 400×); mean ± SEM percentage of Gli2(+) hepatocytes and number of Gli2(+) ductular cells per portal tract are graphed. *P<0.05 vs 0h (Student’s t-test). (B) Additional mice were subjected to sham surgery (n=2) or PH (n=4) and hepatocytes were harvested 24h or 48h later; expression of Hh-regulated transcription factors (Gli1, Gli2), Hh ligands (Shh, Ihh) and K7 (a progenitor marker) was assessed in each isolate using Western blot analysis. β-actin was used as a loading control. (c) Representative photomicrographs demonstrating hepatocytes and ductular cells with BrdU nuclear staining 48h and 72h post-PH. Small insert demonstrates the absence of BrdU staining in 0h liver. To quantify BrdU staining, 15 randomly chosen, 20× fields/section were evaluated. Tissues from all mice were used for analyses. Mean ± SEM are graphed. *P<0.05 or **P<0.01 vs 0h (Student’s t-test).
In order to verify the unanticipated discovery that hepatocytes express Hh-target genes after PH, 6 additional mice were subjected to sham surgery (n=2 mice) or PH (n=4 mice) and primary hepatocytes were isolated 24h and 48h later. Cellular expression of Hh-target genes was then assessed. Western blot analysis demonstrated that primary hepatocytes from 24h and 48h post-PH livers expressed much higher levels of Gli1 and Gli2 proteins than sham-operated mice (Fig 4B). Immunocytochemistry showed that 100% of the analyzed cells expressed albumin, validating the purity of the preparation (Suppl Fig 1A). Some (<10%) of these albumin-expressing cells also expressed Gli1 or Gli2 (Suppl Fig 1B). Interestingly, a subset of the hepatocytic cells from the regenerating livers (but none of the cells from sham-operated mice) stained positively for AFP and these AFP(+) cells generally co-expressed the Hh-target gene, Gli2 (Suppl Fig 1C). Immunoblot analysis confirmed that hepatocyte populations from regenerating livers were enriched with cells that expressed keratin (K)7, a marker of immature hepatocytes (Fig 4B). Hepatocytic cells from regenerating livers also expressed Ihh and Shh ligands (Fig 4B), and immunostaining of 48h cytospins from regenerating (but not sham-operated) livers co-localized expression of Shh and albumin (Suppl Fig 1D). Thus, the aggregate data provide conclusive evidence that hepatocytic cells expressing progenitor markers and Hh ligands and/or target genes increase as the liver regenerates post-PH.
Proliferative activity parallels enrichment of liver epithelial cell populations with Hh-responsive cells
Hh ligands are known to promote the proliferation of various progenitors. Therefore, it was important to determine if the proliferative activity of hepatocytic and/or ductular cells increased as these populations became enriched with Hh-responsive cells. Mice received a single injection of BrdU 2h before sacrifice in order to label cells that were engaged in DNA synthesis. The numbers of hepatocytes and ductular cells with BrdU nuclear staining increased significantly after PH (Fig 4C,D). As with Gli2-staining (Fig 4A), BrdU nuclear staining peaked first in hepatocytes, and then in ductular cells. PH also increased nuclear accumulation of Ki67, another S phase marker, in both cell populations (Suppl Fig 2A,B). Thus, increased proliferative activity in hepatocyte and ductular cell populations closely paralleled their enrichment with Hh-responsive cells.
Hh signaling promotes progenitor cell accumulation and fibrogenic liver repair after PH
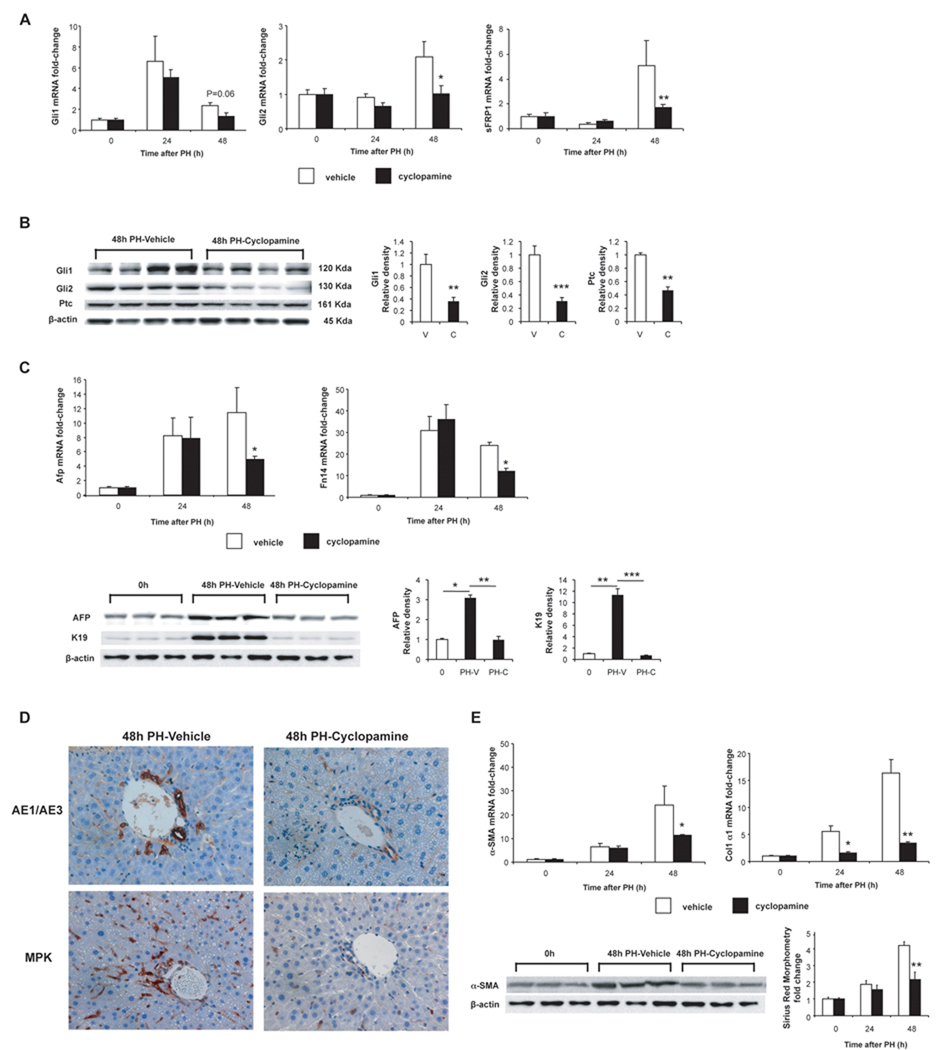
To determine how Hh-pathway activation impacts regenerative responses post-PH, mice were treated with cyclopamine, a specific Smo antagonist that abrogates Hh signal transduction(32) or vehicle (olive oil) before PH and at regular intervals (q24h) post-PH. As expected, cyclopamine did not prevent induction of Hh ligands (data not shown). However, it attenuated induction of Gli1 and Gli2 mRNAs (Fig 5A) and proteins (Fig 5B), and inhibited mRNA/protein expression of sFRP1 (Fig 5A) and Ptc (Fig 5B), two other Gli-regulated genes. Inhibiting Hh signaling also reduced mRNA and/or protein expression of various progenitor markers, such as AFP, Fn14, and keratin (K)19 following PH (Fig 5C), and prevented cells that expressed AE1/AE3 or Mpk (other progenitor markers) from accumulating in the liver (Fig 5D). In addition, it attenuated fibrogenic repair, as evidenced by decreased expression of α-sma and collagen mRNAs, α-SMA protein and picrosirius red staining (Fig 5E).
Figure 5. Inhibition of Hedgehog-signaling impairs post-PH induction of Hh signaling, liver progenitor cell accumulation, and fibrogenesis.
Mice were treated with cyclopamine, a Hh-inhibitor, or vehicle, before PH and at q24h post-PH; and followed for 12h (n=10), 24h (n=20), 48h (n=20) and 72h (n=50). BrdU was injected 2h before to sacrifice to assess proliferative activity. (A) Gli1, Gli2, and sFRP1 mRNA expression in all surviving vehicle (white bars) or Cyclopamine (solid bars) treated mice. Data graphed as mean ± SEM. (B) Western blot analysis of liver proteins obtained from the livers of randomly selected mice in cyclopamine- or vehicle-treatment groups at 48h post-PH. Each lane contains protein from an individual mouse. Blots were densitized; Hh-related gene expression was normalized to β-actin expression in each sample; Mean ± SEM data were graphed. *P<0.05 or **P<0.01 vs 0h (Student’s t-test). (C) QRT-PCR and Western blot analysis of progenitor markers in all mice in cyclopamine- or vehicle-treatment groups at 48h post-PH. Each lane of the Western blots contains protein pooled from 4–6 mice/treatment group. β-actin was used as a loading control. (D) 48h post-PH sections from 4 randomly-selected mice from each treatment group were also stained to demonstrate cells that expressed other progenitor markers, AE1/AE3 and Mpk2. Representative sections are shown. Cells staining for progenitors markers are brown. (E) Analysis of fibrogenic markers in all surviving mice from cyclopamine- or vehicle-treated mice at 48h. QRT-PCR analysis of α-sma and collagen1α1 mRNA expression; Western blot analysis of α-SMA protein expression; morphometric analysis of Sirius red staining.
Hh signaling promotes liver cell replication and is required for survival and liver regeneration after PH
Cyclopamine inhibition of Hh-regulated responses was associated with significantly reduced survival following PH. Increased mortality was evident as early as 48h post-PH and worsened over the subsequent 24h, such that only one of the 50 cyclopamine-treated mice survived to 3 days post-PH, compared to almost 90% of the 50 vehicle-treated controls (P=0.0001). Liver histology and serum aminotransferase values were no different in surviving cyclopamine-treated mice than controls at 24h and 48h post-PH, suggesting that cyclopamine was not directly hepatotoxic. This interpretation was validated by evidence that adding cyclopamine to cultures of regenerating hepatocytes from 24h and 48h post-PH mice abrogated Hh signaling, as evidenced by reduced expression of Gli1 protein and sFRP1 mRNA (both P<0.01 vs vehicle) but did not reduce cell viability. Because pancreatic beta cells and some renal cells are known to be Hh-responsive, levels of BUN and serum glucose were assessed. No differences were noted between cyclopamine- and vehicle-treated mice, suggesting that the increased cyclopamine-related mortality was not due to pancreatic or renal toxicity. Further study is needed to assure that cyclopamine did not exert other non-specific toxic actions that might have reduced post-PH survival.
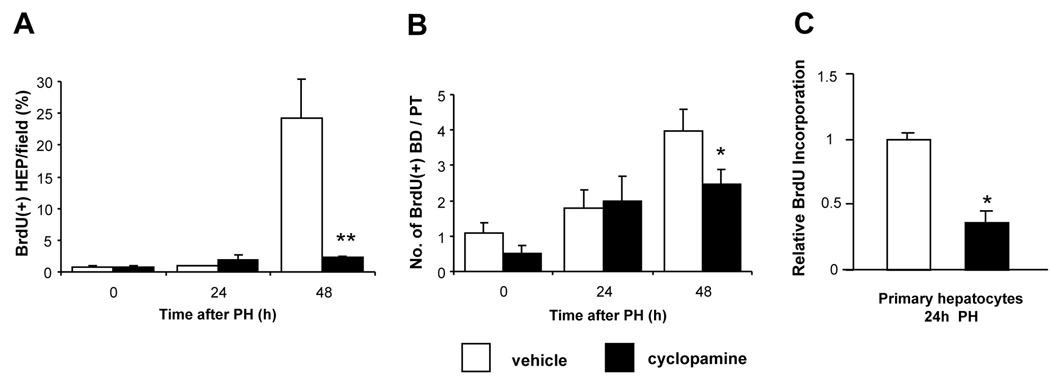
Notably, surviving cyclopamine-treated mice failed to recover liver weight (P=0.01). Hence, liver-to-body weight ratios in surviving cyclopamine-treated mice were lower than in vehicle-treated mice at 48h post-PH (P=0.03) (Table 1). The poor survival and restitution of liver mass in the cyclopamine-treated animals suggested that Hh-pathway inhibition impaired liver cell proliferation post-PH. Ki67-immunostaining and BrdU incorporation data supported this interpretation. At 48h post-PH, incorporation of BrdU was reduced by 90% in hepatocytes, and by ~40% in ductular cells of cyclopamine-treated mice compared to vehicle-treated controls (Fig 6A–B). Moreover, when primary hepatocyte cultures isolated from mice 24h post-PH were treated with cyclopamine in vitro for 24h, BrdU incorporation was inhibited by ~60% (P<0.01 vs tomatidine-treated controls) (Fig 6C). In contrast, cyclopamine had no effect on BrdU incorporation of hepatocyte cultures from sham-operated mice. Thus, cyclopamine specifically inhibited the proliferative activity of hepatocyte cultures that were enriched with Hh-responsive cells expressing progenitor markers (Fig 4B and Suppl Fig 1).
Table 1.
Mean Liver and Body weights of Cyclopamine vs. Vehicle-treated mice 12–48 hours post-PH
| Initial BW (g) | Final BW (g) | Final LW (g) | LW/BW | |
|---|---|---|---|---|
| 0h V | 26.3±0.3 | #1.05±0.03 | 4.0±0.1 | |
| 0h C | 25.8±0.6 | #1.03±0.04 | 4.0±0.1 | |
| 12h V | 27.4±0.8 | 26.2±0.4 | 0.64±0.09 | 2.4±0.3 |
| 12h C | 27.7±1.0 | 26.9±0.8 | 0.64±0.05 | 2.4±0.1 |
| 24h V | 25.5±0.6 | 23.5±0.6 | 0.64±0.03 | 2.7±0.1 |
| 24h C | 25.7±0.8 | 24.3±0.6 | 0.62±0.02 | 2.6±0.0 |
| 48h V | 26.5±0.4 | 23.1±0.6 | 0.75±0.03 | 3.3±0.1 |
| 48h C | 24.8±0.8 | 21.9±0.6 | 0.63±0.02* | 2.9±0.1* |
P<0.05; mean ± SEM.
V: vehicle
C: cyclopamine
BW: body weight; LW: liver weight
LW / BW: liver: body w LW/BW: liver to body weight ratio in g ×100
ns: non-significant
Estimated LW, considering that the resected LW is 70% of the original liver mass
Figure 6. Inhibition of Hedgehog-signaling impairs proliferation of hepatocytic and ductular cells after PH.
(A) Percentage BrdU(+) hepatocytes per field and (B) number of BrdU(+) ductular cells per portal tract in liver sections from all surviving cyclopamine- or vehicle-treated mice described in Fig. 5. *P<0.05, **P<0.01 vs comparable vehicle-treated control (Student’s t-test). (C) Direct effects of cyclopamine on proliferative activity of primary hepatocytes. Two additional pairs of mice were subjected to either sham surgery or PH; hepatocytes were isolated 24h later and cultured overnight in the presence of BrdU ± either tomatidine (an inactive cyclopamine analog) or cyclopamine (5 µM); for each treatment group BrdU incorporation was assessed in quadruplicate plates by ELISA. Mean ± SEM BrdU incorporation in cyclopamine-treated cultures was normalized to results in respective tomatidine-treated cultures. *P<0.05 vs comparable tomatidine-treated controls (Student’s t-test). Cyclopamine had no effect on BrdU incorporation in hepatocytes from sham-operated mice (data not shown).
Discussion
This study demonstrates that Hh pathway activation is critical for liver regeneration to occur after PH. The mechanisms mediating re-growth of the adult liver after a surgical insult that causes massive acute loss of mature hepatocytes have been investigated for decades(1). Several key growth regulators for this process have been identified, including hepatocyte mitogens, cytokines, pathogen-associated molecular pattern (PAMP) receptors, and intracellular factors involved in inflammatory and metabolic stress(33). Although a recent transcriptome analyses had documented that mRNA expression of certain Hh signaling components change after PH(34), information on the kinetics of Hh pathway activation and cellular targets of Hh signaling post-PH, as well as evidence that Hh pathway directly regulates liver regeneration after acute liver injury, are entirely novel. Additional research is now needed to clarify if and how Hh signaling interacts with other mechanisms that are known to modulate regenerative responses to PH.
The vast majority of the earlier work in regenerating livers post-PH had focused on mature hepatocyte replication, and attention was largely restricted to the time interval that immediately spans peak replicative activity in these cells (i.e., 0h–72h post-PH)(35–37). The current study encompassed a much longer time period (i.e., from 0h–216h post-PH) and monitored distinct components of the regenerative response. In addition to assessing hepatocyte proliferative activity, progenitor and stromal responses were analyzed concurrently, revealing a role for the Hh pathway in each of these activities. The aggregate results demonstrate that Hh signaling plays a pivotal role in integrating and coordinating various aspects of adult liver repair after acute injury. In retrospect, this discovery is not surprising because Hh pathway activation is well known to orchestrate tissue construction during fetal development(38, 39) and it provides a similar function during remodelling of chronically damaged livers(14). However, the new data in the PH model will undoubtedly spark controversy because liver regeneration post-PH is believed to be driven predominately by replication of mature hepatocytes(4, 33), while other types of tissue growth that rely on Hh signaling are known to involve progenitor populations(17, 39).
In this regard, it is important to emphasize that earlier studies of regenerating livers after PH have demonstrated increased expression of progenitor markers(7, 9), suggesting that immature liver cells may accumulate during this process. Feng et al., reported that mRNA levels of Fn14 increased dramatically within 2h–4h of PH and remained at high levels for the next 2–3 days, although they were unable to detect Fn14 mRNA by Northern blot analysis of primary hepatocytes or healthy adult livers pre-PH(9). The authors suggested that up-regulation of Fn14 expression in regenerating livers contributed to increased hepatocyte proliferative activity because they detected striking induction of Fn14 in many hepatoma cell lines and in human hepatocellular carcinoma samples. Subsequently, another group discovered that ductular type progenitors express Fn14. Moreover, they demonstrated that stimulating liver progenitors with the Fn14 ligand, TWEAK, increased cell proliferation, while knocking out Fn14 in mice virtually eliminated proliferation of bipotent hepatic progenitors (oval cells) in an in vivo model of oval cell-dependent liver regeneration. Thus, they concluded that Fn14 controlled regenerative activity of liver progenitors(10).
In the present study, qRT-PCR and Western blot analysis demonstrated that inhibiting Hh signaling with cyclopamine partially blocked induction of various progenitor markers (e.g., Fn14, AFP, K7, and K19) after PH. Immunohistochemistry showed that cyclopamine also prevented AE1/AE3-stained cells and Mpk(+) cells from accumulating in livers post-PH. Thus, several independent lines of evidence indicate that Hh pathway activation expands heterogeneous populations of hepatic progenitors following PH. Interestingly, blocking Hh signaling also inhibited proliferation of hepatocytes and ductular cells post-PH. In addition, post-PH recovery of liver mass and survival were negatively impacted by cyclopamine treatment. When considered in light of the aforementioned data about Fn14, these new results suggest that progenitor populations (which likely include Fn14(+) oval cells) give rise to some of the hepatocytes and ductular cells that proliferate to regenerate the liver post-PH. Moreover, Hh signaling appears to be necessary for these processes to occur. This is consistent with earlier evidence that Hh ligands function as viability factors for oval cells and other hepatic progenitors(17, 39).
The new data also demonstrate that the timing of progenitor accumulation after PH closely coincides with significant induction of mesenchymal markers, such as α-sma and collagen1αI. Others have reported that hepatic progenitor populations include multi-potent cells that co-express epithelial and mesenchymal markers(26, 40, 41). The latter is characteristic of cells that undergo EMT or mesenchymal-to-epithelial transition (MET)(42). During fetal development, organogenesis involves repetitive waves of EMT/MET(43). The Hh-pathway is known to promote EMT during development and cancer metastasis(20, 44, 45), and we recently reported that it stimulates adult liver ductular cells to undergo EMT(13). Interestingly, both hepatic epithelial progenitors and mesenchymal stem cells(10, 40, 46) express Fn14, raising the possibility that some Fn14(+) epithelial progenitors that help to regenerate the liver post-PH may be derived from hepatic stromal cells. This theory is particularly intriguing because PH is accompanied by dramatic expansion of myofibroblastic cells, as well as matrix remodelling which results in transient, but significant, accumulation of fibrous matrix(30). Hh pathway activation has been shown to promote expansion of mesenchymal-type progenitor cells in several tissues(17, 38) and mediates fibrogenic repair during chronic liver injury(14). The current study provides compelling evidence that Hh signaling is involved in expanding populations of myofibroblastic cells that contribute to hepatic matrix deposition after PH. Additional research is now justified to determine if Hh-regulated EMT/MET responses are involved in the post-PH matrix remodelling process and/or participate in repopulating the liver epithelial compartment after PH.
In summary, the current study provides novel evidence that a developmental morphogen that regulates progenitor and stromal cell fate (i.e., Hh) is required for optimal regeneration of the adult liver post-PH. This discovery complements growing evidence that Hh signaling guides repair of chronically injured livers, and is exciting because it suggests that common mechanisms mediate fetal liver development and repair of adult liver injury. Therefore, progress in delineating how Hh-responsive mechanisms regulate liver growth and development might help to unravel conserved mechanisms that control regeneration of injured livers in adults. Such knowledge has important implications for patients with various types of acute and chronic liver damage.
Supplementary Material
Six mice were subjected to sham surgery (n=2) or PH (n=4); primary hepatocytes were isolated 24h or 48h later and processed immediately for immunocytochemistry. (A) Representative cytospins from sham-operated (top panel) or partially hepatectomized (bottom panel) mice. For each, in phase image and results of albumin-staining are displayed. (Final magnification 200×). (B) Immunostaining for Gli1 (left panels) and Gli2 (right panels) in cytospins from regenerating livers 48h after PH. DAPI demonstrates nuclei of cells shown in the in-phase images. Merged views demonstrate co-localization (purple) Gli proteins in some cells. (Final magnification 400×). (C–D) 48h cytospins were further analyzed to better characterize the cellular targets (C) and sources (D) of Hh. Double-immunostaining for the Hh-target gene, Gli2, and α-fetoprotein (AFP), a marker of hepatocyte progenitors. DAPI demonstrates nuclei of cells shown in the in-phase image. The merged view confirms co-localization of nuclear Gli2 (purple) and AFP (D) Double-immunostaining for Shh ligand and albumin, a marker of hepatocytes. DAPI demonstrates nuclei of cells shown in the in-phase image. The merged view confirms co-localization (yellow) of Shh and albumin. (Final magnification 400×).
Ki67 nuclear staining in hepatocytes (A) and ductular cells (B) at different time points. To quantify Ki67 staining, 15 randomly chosen, 20× fields/section were evaluated. Tissues from all mice were used for analyses. *P<0.05 or **P<0.01 vs 0h (Student’s t-test).
Acknowledgements
Authors are grateful to Dr. Xiaoling Wang (Gastroenterology, Duke University) and to Dr. Gregory Michelotti (Anesthesiology, Duke University) for technical assistance, and Dr. Jiawen Huang (Gastroenterology, Duke University) for animal care assistance. The authors thank W.C. Stone (Gastroenterology, Duke University) for his administrative support to this work.
Financial Support
This work was supported by NIH grants RO1 DK077794 to AMD. Work done in the University of the Basque Country was supported by MEC (SAF2007/60211) and Gobierno Vasco (IT-325-07). BO was recipient of a Basque Government Mobility Grant (MV-2009-1-11).
List of Abbreviations
- AFP
alpha fetoprotein
- αSMA
α smooth muscle actin
- BD
bile ductular cells
- BrdU
5-bromo-2’-deoxyuridine
- EMT
epithelial-to-mesenchymal transition
- FFPE
formalin fixed paraffin embedded
- Gli
Glioblastoma
- Hh
Hedgehog
- HEP
Hepatocytic cells
- Hip
Hh interacting protein
- Ihh
Indian Hedgehog
- PH
partial hepatectomy
- PT
Portal tract
- Ptc
Patched
- sFRP1
secreted frizzled-related protein 1
- Shh
Sonic Hedgehog
- Smo
Smoothened
- QRT-PCR
Quantitative real-time polymerase chain reaction
Footnotes
Disclosures: Authors declare that they have no conflict of interests or financial interests.
Contributor Information
Begoña Ochoa, Email: begona.ochoa@ehu.es.
Wing-Kin Syn, Email: ws45@notes.duke.edu.
Igotz Delgado, Email: igotz.delgado@ehu.es.
Gamze F. Karaca, Email: fk14@notes.duke.edu.
Youngmi Jung, Email: youngmi.jung@duke.edu.
Jiangbo Wang, Email: wang0092@mc.duke.edu.
Ana M. Zubiaga, Email: ana.zubiaga@ehu.es.
Olatz Fresnedo, Email: olatz.fresnedo@ehu.es.
Alessia Omenetti, Email: alessia.omenetti@duke.edu.
Marzena Zdanowicz, Email: kzdanow@notes.duke.edu.
Steve S. Choi, Email: steve.choi@duke.edu.
Anna Mae Diehl, Email: annamae.diehl@duke.edu.
References
- 1.Higgins G, Anderson RM. Experimental pathology of the liver. Arch Pathol. 1931;(12):186–202. [Google Scholar]
- 2.Bucher NL, Swaffield MN. The Rate Of Incorporation Of Labeled Thymidine Into The Deoxyribonucleic Acid Of Regenerating Rat Liver In Relation To The Amount Of Liver Excised. Cancer Res. 1964;24:1611–1625. [PubMed] [Google Scholar]
- 3.Stocker E, Pfeifer U. [On the manner of proliferation of the liver parenchyma after partial hepatectomy. Autoradiography studies using 3H-thymidine] Naturwissenschaften. 1965;52(24):663. doi: 10.1007/BF00589634. [DOI] [PubMed] [Google Scholar]
- 4.Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137(2):466–481. doi: 10.1053/j.gastro.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39(6):1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 6.Chiu JF, Gabryelak T, Commers P, Massari R. The elevation of alpha-fetoprotein messenger RNA in regenerating rat liver. Biochem Biophys Res Commun. 1981;98(1):250–254. doi: 10.1016/0006-291x(81)91895-7. [DOI] [PubMed] [Google Scholar]
- 7.Petropoulos C, Andrews G, Tamaoki T, Fausto N. alpha-Fetoprotein and albumin mRNA levels in liver regeneration and carcinogenesis. J Biol Chem. 1983;258(8):4901–4906. [PubMed] [Google Scholar]
- 8.Bernuau D, Poliard A, Feldmann G. In situ cellular analysis of alpha-fetoprotein gene expression in regenerating rat liver after partial hepatectomy. Hepatology. 1988;8(5):997–1005. doi: 10.1002/hep.1840080504. [DOI] [PubMed] [Google Scholar]
- 9.Feng SL, Guo Y, Factor VM, Thorgeirsson SS, Bell DW, Testa JR, et al. The Fn14 immediate-early response gene is induced during liver regeneration and highly expressed in both human and murine hepatocellular carcinomas. Am J Pathol. 2000;156(4):1253–1261. doi: 10.1016/S0002-9440(10)64996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakubowski A, Ambrose C, Parr M, Lincecum JM, Wang MZ, Zheng TS, et al. TWEAK induces liver progenitor cell proliferation. J Clin Invest. 2005;115(9):2330–2340. doi: 10.1172/JCI23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung Y, Brown KD, Witek RP, Omenetti A, Yang L, Vandongen M, et al. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology. 2008;134(5):1532–1543. doi: 10.1053/j.gastro.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, et al. Hedgehog-Mediated Epithelial-to-Mesenchymal Transition and Fibrogenic Repair in Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118(10):3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omenetti A, Diehl AM. The adventures of sonic hedgehog in development and repair. II. Sonic hedgehog and liver development, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G595–G598. doi: 10.1152/ajpgi.00543.2007. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48(1):98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung Y, McCall SJ, Li YX, Diehl AM. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology. 2007;45(5):1091–1096. doi: 10.1002/hep.21660. [DOI] [PubMed] [Google Scholar]
- 17.Sicklick JK, Li YX, Melhem A, Schmelzer E, Zdanowicz M, Huang J, et al. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G859–G870. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- 18.Sicklick JK, Li YX, Jayaraman A, Kannangai R, Qi Y, Vivekanandan P, et al. Dysregulation of the Hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis. 2006;27(4):748–757. doi: 10.1093/carcin/bgi292. [DOI] [PubMed] [Google Scholar]
- 19.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15(6):740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Bailey JM, Singh PK, Hollingsworth MA. Cancer metastasis facilitated by developmental pathways: Sonic hedgehog, Notch, and bone morphogenic proteins. J Cell Biochem. 2007;102(4):829–839. doi: 10.1002/jcb.21509. [DOI] [PubMed] [Google Scholar]
- 21.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sicklick JK, Li YX, Choi SS, Qi Y, Chen W, Bustamante M, et al. Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab Invest. 2005;85(11):1368–1380. doi: 10.1038/labinvest.3700349. [DOI] [PubMed] [Google Scholar]
- 23.Choi SS, Omenetti A, Witek RP, Moylan CA, Syn WK, Jung Y, et al. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2009;297(6):G1093–G1106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipinski RJ, Hutson PR, Hannam PW, Nydza RJ, Washington IM, Moore RW, et al. Dose- and route-dependent teratogenicity, toxicity, and pharmacokinetic profiles of the hedgehog signaling antagonist cyclopamine in the mouse. Toxicol Sci. 2008;104(1):189–197. doi: 10.1093/toxsci/kfn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He J, Sheng T, Stelter AA, Li C, Zhang X, Sinha M, et al. Suppressing Wnt signaling by the hedgehog pathway through sFRP-1. J Biol Chem. 2006;281(47):35598–35602. doi: 10.1074/jbc.C600200200. [DOI] [PubMed] [Google Scholar]
- 26.Yovchev MI, Grozdanov PN, Joseph B, Gupta S, Dabeva MD. Novel hepatic progenitor cell surface markers in the adult rat liver. Hepatology. 2007;45(1):139–149. doi: 10.1002/hep.21448. [DOI] [PubMed] [Google Scholar]
- 27.Smith PG, Tee LB, Yeoh GC. Appearance of oval cells in the liver of rats after long-term exposure to ethanol. Hepatology. 1996;23(1):145–154. doi: 10.1002/hep.510230120. [DOI] [PubMed] [Google Scholar]
- 28.Grisham JW. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer Res. 1962;22:842–849. [PubMed] [Google Scholar]
- 29.Tanaka Y, Mak KM, Lieber CS. Immunohistochemical detection of proliferating lipocytes in regenerating rat liver. J Pathol. 1990;160(2):129–134. doi: 10.1002/path.1711600206. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto H, Murawaki Y, Kawasaki H. Hepatic collagen synthesis and degradation during liver regeneration after partial hepatectomy. Hepatology. 1995;21(1):155–161. [PubMed] [Google Scholar]
- 31.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134(6):1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16(21):2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43(2) Suppl 1:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 34.Katoh Y, Katoh M. Integrative genomic analyses on GLI2: mechanism of Hedgehog priming through basal GLI2 expression, and interaction map of stem cell signaling network with P53. Int J Oncol. 2008;33(4):881–886. doi: 10.3892/ijo_00000076. [DOI] [PubMed] [Google Scholar]
- 35.Factor VM, Jensen MR, Thorgeirsson SS. Coexpression of C-myc and transforming growth factor alfa in the liver promotes early replicative senescence and diminishes regenerative capacity after partial hepatectomy in transgenic mice. Hepatology. 1997;26(6):1434–1443. doi: 10.1002/hep.510260610. [DOI] [PubMed] [Google Scholar]
- 36.Arai M, Yokosuka O, Chiba T, Imazeki F, Kato M, Hashida J, et al. Gene expression profiling reveals the mechanism and pathophysiology of mouse liver regeneration. J Biol Chem. 2003;278(32):29813–29818. doi: 10.1074/jbc.M212648200. [DOI] [PubMed] [Google Scholar]
- 37.Horimoto M, Fulop P, Derdak Z, Wands JR, Baffy G. Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regeneration in mice. Hepatology. 2004;39(2):386–392. doi: 10.1002/hep.20047. [DOI] [PubMed] [Google Scholar]
- 38.Kolterud A, Grosse AS, Zacharias WJ, Walton KD, Kretovich KE, Madison BB, et al. Paracrine Hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology. 2009;137(2):618–628. doi: 10.1053/j.gastro.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirose Y, Itoh T, Miyajima A. Hedgehog signal activation coordinates proliferation and differentiation of fetal liver progenitor cells. Exp Cell Res. 2009;315(15):2648–2657. doi: 10.1016/j.yexcr.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 40.Yovchev MI, Grozdanov PN, Zhou H, Racherla H, Guha C, Dabeva MD. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology. 2008;47(2):636–647. doi: 10.1002/hep.22047. [DOI] [PubMed] [Google Scholar]
- 41.Dan YY, Riehle KJ, Lazaro C, Teoh N, Haque J, Campbell JS, et al. Isolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineages. Proc Natl Acad Sci U S A. 2006;103(26):9912–9917. doi: 10.1073/pnas.0603824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119(6):1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs. 2007;185(1–3):7–19. doi: 10.1159/000101298. [DOI] [PubMed] [Google Scholar]
- 44.Heisenberg CP, Solnica-Krezel L. Back and forth between cell fate specification and movement during vertebrate gastrulation. Curr Opin Genet Dev. 2008;18(4):311–316. doi: 10.1016/j.gde.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thiery JP, Chopin D. Epithelial cell plasticity in development and tumor progression. Cancer Metastasis Rev. 1999;18(1):31–42. doi: 10.1023/a:1006256219004. [DOI] [PubMed] [Google Scholar]
- 46.Girgenrath M, Weng S, Kostek CA, Browning B, Wang M, Brown SA, et al. TWEAK, via its receptor Fn14, is a novel regulator of mesenchymal progenitor cells and skeletal muscle regeneration. Embo J. 2006;25(24):5826–5839. doi: 10.1038/sj.emboj.7601441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Six mice were subjected to sham surgery (n=2) or PH (n=4); primary hepatocytes were isolated 24h or 48h later and processed immediately for immunocytochemistry. (A) Representative cytospins from sham-operated (top panel) or partially hepatectomized (bottom panel) mice. For each, in phase image and results of albumin-staining are displayed. (Final magnification 200×). (B) Immunostaining for Gli1 (left panels) and Gli2 (right panels) in cytospins from regenerating livers 48h after PH. DAPI demonstrates nuclei of cells shown in the in-phase images. Merged views demonstrate co-localization (purple) Gli proteins in some cells. (Final magnification 400×). (C–D) 48h cytospins were further analyzed to better characterize the cellular targets (C) and sources (D) of Hh. Double-immunostaining for the Hh-target gene, Gli2, and α-fetoprotein (AFP), a marker of hepatocyte progenitors. DAPI demonstrates nuclei of cells shown in the in-phase image. The merged view confirms co-localization of nuclear Gli2 (purple) and AFP (D) Double-immunostaining for Shh ligand and albumin, a marker of hepatocytes. DAPI demonstrates nuclei of cells shown in the in-phase image. The merged view confirms co-localization (yellow) of Shh and albumin. (Final magnification 400×).
Ki67 nuclear staining in hepatocytes (A) and ductular cells (B) at different time points. To quantify Ki67 staining, 15 randomly chosen, 20× fields/section were evaluated. Tissues from all mice were used for analyses. *P<0.05 or **P<0.01 vs 0h (Student’s t-test).