Abstract
The objective of this study was to examine the association of urinary phytoestrogens with the risk of postmenopausal breast cancer. Participants in the Multiethnic Cohort Study included 36,458 postmenopausal women who provided blood or urine specimens. A nested case-control study of breast cancer with biospecimens was created in which cases diagnosed after specimen collection were matched to two controls. Two hundred fifty-one women with breast cancer and 462 controls had urine available for analysis of urinary phytoestrogens. Odds ratios (ORs) and 95% confidence intervals (CIs) were obtained using conditional logistic regression. A non-monotonic inverse trend (P = 0.04) in breast cancer risk was associated with increasing urinary excretion of genistein (OR 25th to 75th percentile, 0.88; 95% CI, 0.78 to 0.99) and total isoflavones (OR 25th to 75th percentile, 0.80; 95% CI, 0.65 to 0.99). A significant reduction in breast cancer risk in Japanese-American women was associated with the highest compared with the lowest quartile excretion of urinary daidzein (OR, 0.41; 95% CI, 0.19 to 0.89; P for trend, 0.005). The risk of breast cancer was reduced among white women with the highest compared to the lowest quartile excretion of equol (OR, 0.27; 95% CI, 0.08 to 0.95), although the trend in risk was not significant (P = 0.07). Our results provide some support to the hypothesis that a diet rich in isoflavones from soy products reduces the risk of postmenopausal breast cancer, particularly in populations with comparatively high excretion of phytoestrogens.
Keywords: breast cancer, prospective cohort study, phytoestrogens, urinary excretion, multiethnic
INTRODUCTION
Phytochemicals with weak estrogenic properties in mammalian systems termed ‘phytoestrogens’ are naturally occurring, non-steroidal plant compounds with a variety of biological activities that may influence the risk of breast cancer (1). Phytoestrogens of primary concern to human health include the isoflavones genistein, daidzein and its metabolite, equol, that are principally found in soy products; and the lignans, enterolactone and enterodiol, that are produced by intestinal bacteria from the metabolism of oilseeds, seaweed, cereals, legumes, vegetables, and fruits (2–5). Phytoestrogens are hypothesized to reduce the levels of biologically available estrogen through the stimulation of sex hormone binding globulin production or competitive binding of estrogen receptor (ER)-α and –β (6). Phytoestrogens might also decrease circulating estrogen concentrations through the inhibition of aromatase and downregulation of 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase type 1, or prevent breast cancer through other mechanisms (7–9).
Scientific interest in the relation of phytoestrogens to the risk of breast cancer is premised, in part, on the observation that Asian women who migrate to the United States substantially increase their risk of breast cancer over several generations, suggesting that adoption of a Western lifestyle, including dietary change, influences the incidence of breast malignancy (10,11). Breast cancer rates in Japan and other Asian countries have been traditionally much lower than in the United States and Europe, although this difference in incidence has been narrowing in recent years (12). Lignans occur in much higher concentrations in general American and European diets than do isoflavones because of a comparatively low intake of soy products (6).
Epidemiological studies of dietary isoflavone and lignan consumption with the risk of breast cancer have been combined recently in two meta-analyses (10,13). Trock et al. (10) reported a modest, but statistically significant, 14% reduction in breast cancer risk among women with a high soy intake compared to women with a low soy intake. In a second meta-analysis separating the dietary studies conducted in Asia or among Asian-Americans from the dietary studies conducted in Western populations, Wu et al. (13) found a statistically significant reduction in the risk of breast cancer among Asian women reporting a ‘high’ soy intake compared to women reporting a ‘low’ soy intake, but a similar finding was not observed in non-Asian women. These results suggest that the beneficial effects of soy against breast cancer may only occur at relatively high levels of consumption.
Fewer epidemiological studies of the association of dietary enterolactone and breast cancer have been conducted, and these have been limited to Western populations. Of the three case-control studies reviewed by Boccardo et al. (14), two reported an inverse association of dietary lignans with premenopausal breast cancer, but no association with breast cancer risk among postmenopausal women. By contrast, results from a recent French prospective study suggest that women with high dietary intakes of total plant lignans have a statistically significant reduced risk of ER-positive / progesterone receptor (PR)-positive postmenopausal breast cancer (15), but not premenopausal breast cancer (16).
Prospective investigations of the association of blood or urine levels of phytoestrogens with breast cancer risk have been inconclusive (17–25). The objective of our study was to examine the association of the major urinary phytoestrogens daidzein, genistein, equol, and enterolactone with the risk of postmenopausal breast cancer in a multiethnic population with a wide range of dietary intakes, including Japanese-Americans with relatively high intake of soy products.
MATERIALS AND METHODS
Study Population and Data Collection
We conducted a nested case-control study of breast cancer within the Multiethnic Cohort Study, established in Hawaii and Los Angeles, California, from 1993 to 1996 (26). More than 215,000 adults aged 45 to 75 entered the cohort by completing a detailed questionnaire on diet and lifestyle factors. Five racial-ethnic groups were targeted for inclusion in the cohort: African-Americans, Native Hawaiians, Japanese-Americans, Latinos, and whites. A prospective biospecimen repository was developed, largely between 2001 and 2006, when cohort members who agreed to participate provided a blood and urine specimen, and completed a short questionnaire. Biospecimens were prospectively collected from 36,458 postmenopausal women, 32,243 of whom provided a urine sample. An overnight (Hawaii) or a first morning (Los Angeles) urine specimen was preferentially collected; spot urine was requested otherwise.
Case Ascertainment and Control Selection
Identification of incident, invasive postmenopausal breast cancer cases was accomplished through linkage to the population-based cancer registries covering Hawaii and California, which are members of the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute (27). Deaths were identified by linkage to death-certificate files in Hawaii and California, as well as to the National Death Index. During the follow-up period (median follow-up, 1.57 years; range, 0.01 to 7.69 years), 334 eligible breast cancer cases were identified. Approximately two controls for each case were randomly selected from the women who were alive and free of breast cancer at the age of the case’s diagnosis by matching for geographic location (Hawaii or California), race-ethnicity, birth year (±1 year), date of specimen collection (±6 months), time of specimen collection (±2 hours), fasting hours (0-<6, 6-<8, 8-<10, and ≥10 hrs), and hormone replacement therapy use (HRT, as current versus not current). HRT use and fasting status were used as matching criteria to ensure that matched sets would be available for the assessment of analytes requiring fasting status or non-HRT use. A total of 251 cases and 462 of matched controls had an overnight (173 cases, 338 controls) or first morning (78 cases, 124 controls) urine sample as required for phytoestrogen assessment. This included 251 matched sets, 40 with one control and 211 with two controls.
Laboratory Assays
Daidzein, genistein, equol, and enterolactone were analyzed by HPLC with electrospray ionization tandem mass spectrometry, similar to our earlier reports (28–30). Mass spectrometric analysis was performed by multiple reaction monitoring as previously described (29). Limits of quantitation (LOQ) were 1 nM for daidzein (all subjects > LOQ), 1 nM for genistein (54 subjects < LOQ), 2 nM for equol (360 subjects < LOQ), and 5 nM for enterolactone (12 subjects < LOQ). Between-batch coefficients of variation ranged from 4% to 18% for all analytes, while within-batch variation was less than or equal to half these levels. Excretion of phytoestrogens was corrected for creatinine as nmoles per mg creatinine.
Data Analyses
A preliminary examination of the data included comparisons of cases and controls with respect to several demographic characteristics and potential risk factors of interest. Phytoestrogen values were compared between cases and controls by the two-sided median test. The sum of daidzein and genistein was calculated as an estimate of main non-metabolized isoflavones and the sum of daidzein, genistein, and equol as an estimate of major total isoflavones. Partial correlations among the phytoestrogens, adjusted for the matching variables, were analyzed using the Spearman rank-order test.
We evaluated risks associated with different excretion of phytoestrogens by means of conditional logistic regression modeling case-control status (31). The excretion was defined as quantiles based on the distribution in the combined population of cases and controls. The cut points differed little from those based on the distribution of the entire data set, except for the highest quartile. We computed odds ratios (ORs) and 95 percent confidence intervals (CIs) by exponentiating the coefficients (and CIs) for the binary indicator variables representing the quartile excretion of urinary phytoestrogens. Matched sets were used as strata in the conditional logistic models, which accounted for the matching criteria: location, race-ethnicity, birth year, date and time of urine collection, HRT use, and fasting hours. We additionally included age at specimen collection and fasting hours as continuous variables to further adjust for differences within matched sets. We also considered other potential risk factors as adjustment variables, such as parity and family history, but these did not materially alter the fit of the models according to the likelihood ratio test. The Wald χ2 test was employed to evaluate the significance of the linear trend based on the median value for each quantile. Analyses were repeated for continuous log-transformed variables, and the resulting parameter estimates were utilized in computing the ORs for the difference between the 25th and 75th percentile of urinary phytoestrogens. Only Japanese-American and white women were considered in a race-ethnic-specific analysis because the number of cases was limited for the other subgroups. Tests for interaction between urinary phytoestrogen excretion and race-ethnicity (Japanese-American versus white) in relation to breast cancer risk were based on the Wald test of cross-product terms. Separate analyses were performed for strata by body mass index (BMI) category and smoking status, and for equol producers, identified by a ratio of equol to daidzein over 0.018 (32). These analyses used unconditional logistic regression, adjusted for the matching criteria variables, to maximize the number of subjects available. All tests were two sided, and P < 0.05 was considered statistically significant. Major analyses were repeated after omitting the 98 cases and matched controls with less than 1 year of follow-up, with little change in our findings, i.e., the direction of all associations remained the same, although there was some loss of statistical significance.
RESULTS
Few differences were found between breast cancer cases and their matched controls with regard to demographic or other background variables (Table 1). The mean age of cases and controls at urine collection was 67 years. Japanese-American women were the largest race-ethnic group represented, with smaller numbers of white, Latina, African-American, and Native Hawaiians. The mean BMI for cases and controls was similar, with 16% of cases and 15% of controls reporting a BMI ≥ 30, suggesting obesity. No significant differences between cases and controls in any of the reproductive variables were observed, likely because of the matching criteria. A family history of breast cancer was reported by 11% of cases and 13% of controls. A somewhat greater percentage of cases than controls had ever smoked tobacco, or were current alcohol drinkers.
Table 1.
Baseline characteristics of postmenopausal breast cancer cases and matched controls*, Multiethnic Cohort Study
| Cases | Controls | |
|---|---|---|
| (N=251) | (N=462) | |
| Mean age (years) at urine collection (± SD)† | 66.9 (±7.8) | 66.9 (±7.7) |
| Ethnicity†, n (%) | ||
| Japanese-American | 112 (44.6) | 216 (46.8) |
| African-American | 29 (11.6) | 45 (9.7) |
| Latino | 33 (13.2) | 54 (11.7) |
| White | 51 (20.3) | 96 (20.8) |
| Native Hawaiian | 26 (10.4) | 51 (11.0) |
| Years of education (± SD) | 14.0 (±2.9) | 14.0 (±2.8) |
| Body mass index (kg/m2) (± SD) | 25.6 (±5.1) | 25.2 (±5.6) |
| Age (years) at menarche (± SD) | 12.9 (±1.6) | 13.1 (±1.6) |
| Age (years) at menopause‡ (± SD) | 49.3 (±4.9) | 49.4 (±4.9) |
| Ever pregnant (%) | 89 | 90 |
| Age (years) at first child birth (± SD) | 23.9 (±4.6) | 23.7 (±4.5) |
| Ever used hormone replacement therapy† (%) | 32 | 32 |
| Family history of breast cancer (%) | 11 | 14 |
| Ever smoked tobacco (%) | 42 | 37 |
| Currently smoke tobacco (%) | 12 | 9 |
| Currently drink alcohol (%) | 40 | 35 |
Cases and controls matched on geographic area, ethnicity, birth year, date and time of specimen collection, fasting status and hormone replacement use.
Matching criteria.
Defined as age at natural or surgical menopause.
Abbreviations: SD, standard deviation.
Urinary excretion of each of the phytoestrogens was generally lower among breast cancer cases than among controls, although we found few significant differences in median excretion for any of the race-ethnic groups (Table 2). Substantial race-ethnic variation was observed in the mean excretion of daidzein and genistein, with the highest excretion among Japanese-American controls and the lowest excretion among African-American controls. Mean urinary genistein concentrations were nearly three-fold greater among Japanese-American controls than among white controls, the next highest group, and more than seventeen-fold greater than among African-American controls. The highest excretion of urinary enterolactone was found among white controls, with lower excretion among the other race-ethnic groups.
Table 2.
Urinary excretion of phytoestrogens (nmoles/mg creatinine) among postmenopausal breast cancer cases and matched controls * by race-ethnic group, Multiethnic Cohort Study
| All subjects |
Japanese-American |
White |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | ||||
| Phytoestrogen | (N=251) | (N=462) | P-value† | (N=112) | (N=216) | P-value† | (N=51) | (N=96) | P-value† |
| Daidzein | |||||||||
| mean (± SD) | 2.50(± 6.02) | 3.86(± 8.25) | 3.36(± 7.69) | 5.60(± 9.34) | 2.24(± 5.07) | 2.86(± 7.77) | |||
| median (IQR) | 0.54(0.18–2.05) | 0.66(0.18–3.04) | 0.40 | 1.21(0.32–2.52) | 1.46(0.43–6.75) | 0.35 | 0.54(0.13–2.12) | 0.31(0.10–1.36) | 0.20 |
| Genistein | |||||||||
| mean (± SD) | 0.85(± 3.03) | 1.35(± 3.51) | 1.42(± 4.34) | 2.27(± 4.47) | 0.52(± 1.28) | 0.84(± 2.99) | |||
| median (IQR) | 0.10(0.02–0.55) | 0.10(0.02–0.73) | 0.84 | 0.22(0.06–1.05) | 0.34(0.07–1.95) | 0.35 | 0.06(0.01–0.35) | 0.04(0.01–0.32) | 0.20 |
| Equol | |||||||||
| mean (± SD) | 0.18(± 0.90) | 0.37(± 1.73) | 0.27(± 0.89) | 0.49(± 2.04) | 0.04(± 0.20) | 0.57(± 2.10) | |||
| median (IQR) | 0.004(0.001–0.014) | 0.004(0.001–0.012) | 0.72 | 0.003(0.001–0.017) | 0.003(0.001–0.009) | 0.49 | 0.006(0.001–0.019) | 0.007(0.002–0.023) | 0.42 |
| Daidzein + genistein | |||||||||
| mean (± SD) | 3.35(± 8.72) | 5.21(± 11.00) | 4.78(± 11.69) | 7.87(± 13.00) | 2.76(± 6.23) | 3.71(± 9.98) | |||
| median (IQR) | 0.71(0.21–2.61) | 0.78(0.22–3.97) | 0.72 | 1.44(0.39–3.31) | 1.92(0.56–8.89) | 0.64 | 0.93(0.17–2.52) | 0.33(0.11–1.65) | 0.20 |
| Daidzein+genistein+equol | |||||||||
| mean (± SD) | 3.53(± 8.88) | 5.58(± 11.41) | 5.05(± 11.83) | 8.35(± 13.31) | 2.81(± 6.25) | 4.27(± 11.00) | |||
| median (IQR) | 0.76(0.21–2.71) | 0.81(0.24–4.52) | 0.84 | 1.81(0.39–3.58) | 2.17(0.56–10.74) | 0.64 | 0.93(0.17–2.64) | 0.34(0.13–2.38) | 0.20 |
| Enterolactone | |||||||||
| mean (± SD) | 2.48(± 3.51) | 3.63(± 8.40) | 2.24(± 3.32) | 3.17(± 6.01) | 4.07(± 4.85) | 6.92(± 15.11) | |||
| median (IQR) | 1.32(0.50–2.73) | 1.42(0.33–3.71) | 0.50 | 1.00(0.33–2.55) | 1.13(0.15–3.62) | 0.25 | 2.36(1.15–5.56) | 2.85(1.00–5.56) | 0.42 |
| African-American |
Latino |
Native Hawaiian |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | ||||
| Phytoestrogen | (N=29) | (N=45) | P-value† | (N=33) | (N=54) | P-value† | (N=26) | (N=51) | P-value† |
| Daidzein | |||||||||
| mean (± SD) | 0.60(± 1.53) | 1.12(± 2.53) | 1.05(± 3.19) | 1.56(± 4.27) | 3.22(± 4.67) | 3.19(± 9.20) | |||
| median (IQR) | 0.10(0.05–0.36) | 0.37(0.06–1.26) | 0.24 | 0.26(0.10–0.62) | 0.38(0.13–1.06) | 0.56 | 1.20(0.27–3.64) | 0.34(0.11–1.82) | 0.13 |
| Genistein | |||||||||
| mean (± SD) | 0.07(± 0.16) | 0.13(± 0.22) | 0.21(± 0.71) | 0.50(± 1.68) | 0.73(± 1.10) | 0.43(± 0.88) | |||
| median (IQR) | 0.01(0.00–0.04) | 0.03(0.01–0.16) | 0.03 | 0.04(0.01–0.10) | 0.05(0.01–0.15) | 0.56 | 0.19(0.04–1.12) | 0.07(0.03–0.29) | 0.13 |
| Equol | |||||||||
| mean (± SD) | 0.01(± 0.01) | 0.17(± 1.00) | 0.01(± 0.04) | 0.02(± 0.05) | 0.49(± 2.07) | 0.08(± 0.44) | |||
| median (IQR) | 0.003(0.001–0.014) | 0.005(0.002–0.013) | 0.24 | 0.004(0.002–0.008) | 0.005(0.002–0.011) | 0.15 | 0.005(0.002–0.011) | 0.004(0.001–0.011) | 0.58 |
| Daidzein + genistein | |||||||||
| mean (± SD) | 0.68(± 1.66) | 1.25(± 2.70) | 1.26(± 3.88) | 2.06(± 5.53) | 3.96(± 5.39) | 3.62(± 9.90) | |||
| median (IQR) | 0.10(0.06–0.38) | 0.44(0.08–1.58) | 0.10 | 0.37(0.13–0.73) | 0.47(0.16–1.20) | 0.56 | 1.50(0.31–5.75) | 0.41(0.17–2.54) | 0.05 |
| Daidzein+genistein+equol | |||||||||
| mean (± SD) | 0.69(± 1.66) | 1.42(± 3.02) | 1.28(± 3.88) | 2.07(± 5.53) | 4.45(± 6.28) | 3.69(± 9.92) | |||
| median (IQR) | 0.11(0.07–0.39) | 0.45(0.12–1.64) | 0.10 | 0.37(0.14–0.73) | 0.48(0.16–1.20) | 0.56 | 1.50(0.32–5.78) | 0.45(0.18–2.54) | 0.05 |
| Enterolactone | |||||||||
| mean (± SD) | 1.82(± 2.07) | 1.47(± 1.34) | 2.13(± 3.16) | 2.81(± 3.98) | 1.57(± 1.64) | 2.18(± 3.78) | |||
| median (IQR) | 1.35(0.64–2.22) | 1.27(0.36–1.90) | 0.81 | 1.21(0.54–2.06) | 1.84(0.91–3.32) | 0.15 | 0.91(0.50–2.17) | 0.77(0.15–2.42) | 0.94 |
Cases and controls matched on geographic area, ethnicity, birth year, date and time of specimen collection, fasting status and hormone replacement therapy use.
P-value for the difference between cases and controls based on the median test.
Abbreviations: SD, standard deviation; IQR, interquartile range.
Little two-way association in the urinary excretion of phytoestrogens was observed, with the exception of the excretion of daidzein and genistein that had a partial correlation of 0.89 (P < 0.0001), and the excretion of enterolactone and equol with a partial correlation of 0.27 (P < 0.0001).
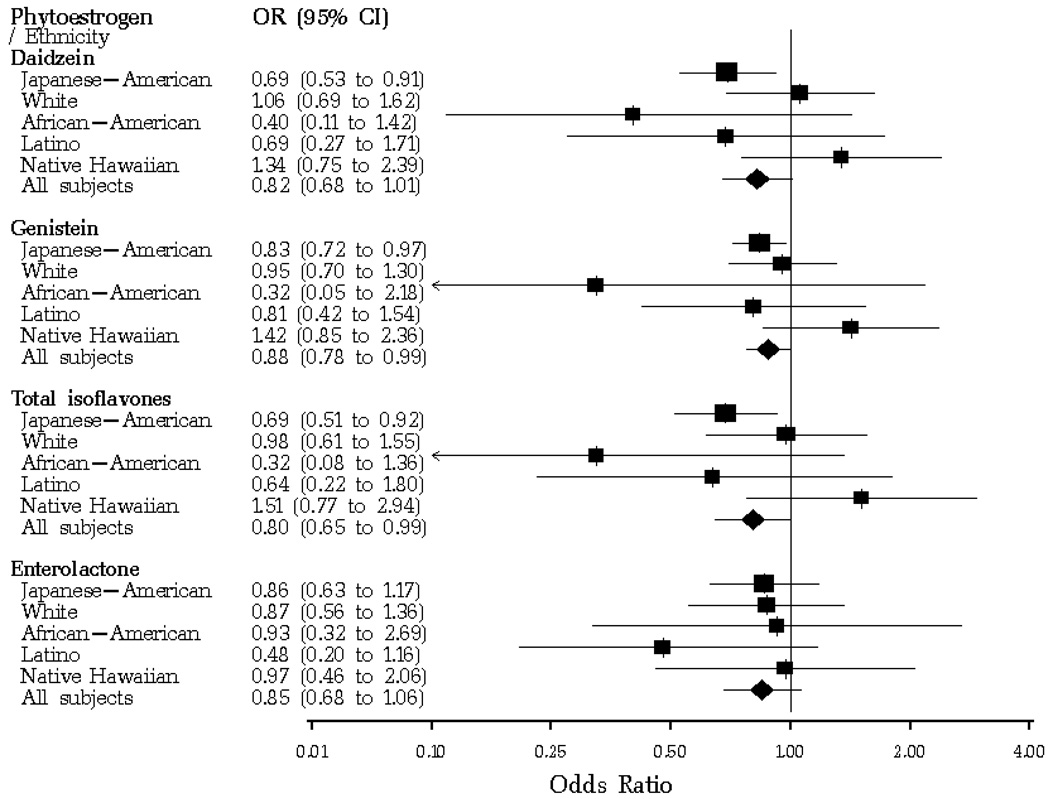
Breast cancer risk comparing women in the 75th to the 25th percentile of urinary phytoestrogen excretion is shown in Figure 1. Daidzein excretion was associated with a significantly reduced risk of breast cancer among Japanese-American women (OR, 0.69; 95% CI, 0.53 to 0.91), but not among women in other race-ethnic groups. A significantly reduced risk of breast cancer was also associated with genistein excretion and an index of total isoflavone excretion among Japanese-American women and all women combined. No relation of urinary equol or enterolactone to risk was found, although race- and ethnic-specific ORs for enterolactone excretion were all below one.
Figure 1.
Differences in breast cancer risk between the 75th and the 25th percentile of urinary phytoestrogen excretion, by race-ethnic group, Multiethnic Cohort Study.*
Footnote: *Based on the logistic model for continuous log-transformed variables. The area of the solid squares represents the relative size of race-ethnic groups in the study. This figure was produced using a forest plot macro for SAS software, by Foster et al.33
In comparing cases and controls by quartile groupings in phytoestrogen values, no significant associations of individual phytoestrogens with the risk of breast cancer were found, although a non-monotonic inverse trend (P = 0.04) in risk associated with increasing urinary excretion of an index of total isoflavones was observed (Table 3). A borderline significant interaction (P = 0.05) was found between race-ethnicity (using two categories) and urinary daidzein excretion, modeled as a continuous variable, on the risk of breast cancer. Among Japanese-American women, but not among white women, a statistically significant reduction in breast cancer risk was associated with the highest compared with the lowest quartile of excretion of urinary daidzein (OR, 0.41; 95% CI, 0.19 to 0.89; P for trend, 0.005). By contrast, the risk of breast cancer was reduced among white women with the highest quartile of compared to the lowest urinary excretion of equol, although the trend in risk was not statistically significant. A similar inverse association was not observed among Japanese-American women. Adjustment for potential confounders of the relation between urinary phytoestrogens and breast cancer risk, including BMI, alcohol use, parity, and family history of breast cancer did not materially change the results from the crude model.
Table 3.
Risk of postmenopausal breast cancer associated with urinary phytoestrogen levels (nmoles/mg creatinine) by race-ethnicity, Multiethnic Cohort Study*
| All subjects |
Japanese-American |
White |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phytoestrogen/ | Cases | Controls | Odds Ratio† | P for | Cases | Controls | Odds Ratio† | P for | Cases | Controls | Odds Ratio† | P for |
| Quartile (range) | (%) | (%) | (95% CI) | trend | (%) | (%) | (95% CI) | trend | (%) | (%) | (95% CI) | trend |
| Daidzein | ||||||||||||
| Q1 (0–0.183) | 63 (25) | 115 (25) | 1 | 18 (16) | 24 (11) | 1 | 16 (31) | 38 (40) | 1 | |||
| Q2 (0.184–0.617) | 67 (27) | 111 (24) | 1.12 (0.73–1.71) | 27 (24) | 47 (22) | 0.78 (0.36–1.69) | 10 (20) | 24 (25) | 0.80 (0.31–2.07) | |||
| Q3 (0.618–2.535) | 70 (28) | 109 (24) | 1.18 (0.76–1.82) | 39 (35) | 57 (26) | 0.95 (0.45–2.03) | 15 (29) | 17 (18) | 2.07 (0.81–5.30) | |||
| Q4 (2.536– ) | 51 (20) | 127 (27) | 0.76 (0.47–1.21) | 0.07 | 28 (25) | 88 (41) | 0.41 (0.19–0.89) | 0.005 | 10 (20) | 17 (18) | 1.22 (0.46–3.22) | 0.63 |
| Genistein | ||||||||||||
| Q1 (0–0.022) | 66 (26) | 112 (24) | 1 | 16 (14) | 26 (12) | 1 | 16 (31) | 38 (40) | 1 | |||
| Q2 (0.023–0.101) | 60 (24) | 118 (26) | 0.87 (0.54–1.40) | 22 (20) | 48 (22) | 0.74 (0.32–1.74) | 13 (25) | 23 (24) | 1.16 (0.42–3.24) | |||
| Q3 (0.102–0.646) | 70 (28) | 109 (24) | 1.10 (0.70–1.71) | 38 (34) | 50 (23) | 1.24 (0.58–2.66) | 14 (27) | 18 (19) | 1.94 (0.73–5.18) | |||
| Q4 (0.647– ) | 55 (22) | 123 (27) | 0.79 (0.49–1.28) | 0.29 | 36 (32) | 92 (43) | 0.62 (0.29–1.32) | 0.08 | 8 (16) | 17 (18) | 0.98 (0.35–2.73) | 0.79 |
| Equol | ||||||||||||
| Q1 (0–0.001) | 65 (26) | 113 (24) | 1 | 37 (33) | 61 (28) | 1 | 13 (25) | 17 (18) | 1 | |||
| Q2 (0.002–0.004) | 63 (25) | 116 (25) | 0.95 (0.61–1.46) | 24 (21) | 67 (31) | 0.61 (0.33–1.14) | 10 (20) | 14 (15) | 0.75 (0.24–2.34) | |||
| Q3 (0.005–0.013) | 58 (23) | 119 (26) | 0.84 (0.53–1.34) | 20 (18) | 48 (22) | 0.70 (0.35–1.40) | 13 (25) | 27 (28) | 0.46 (0.15–1.37) | |||
| Q4 (0.014– ) | 65 (26) | 114 (25) | 0.99 (0.62–1.56) | 0.80 | 31 (28) | 40 (19) | 1.32 (0.70–2.49) | 0.06 | 15 (29) | 38 (40) | 0.27 (0.08–0.95) | 0.07 |
| Daidzein + Genistein | ||||||||||||
| Q1 (0–0.218) | 66 (26) | 112 (24) | 1 | 15 (13) | 23 (11) | 1 | 17 (33) | 37 (39) | 1 | |||
| Q2 (0.219–0.754) | 61 (24) | 117 (25) | 0.89 (0.57–1.40) | 27 (24) | 51 (24) | 0.85 (0.38–1.92) | 8 (16) | 25 (26) | 0.55 (0.20–1.53) | |||
| Q3 (0.755–3.216) | 73 (29) | 106 (23) | 1.19 (0.77–1.84) | 41 (37) | 52 (24) | 1.32 (0.59–2.95) | 16 (31) | 16 (17) | 2.41 (0.88–6.59) | |||
| Q4 (3.217– ) | 51 (20) | 127 (27) | 0.70 (0.44–1.13) | 0.08 | 29 (26) | 90 (42) | 0.51 (0.23–1.13) | 0.008 | 10 (20) | 18 (19) | 0.97 (0.37–2.54) | 0.72 |
| Daidzein + Genistein + Equol | ||||||||||||
| Q1 (0–0.229) | 65 (26) | 114 (25) | 1 | 15 (13) | 25 (12) | 1 | 16 (31) | 37 (39) | 1 | |||
| Q2 (0.230–0.799) | 62 (25) | 115 (25) | 0.96 (0.61–1.51) | 27 (24) | 49 (23) | 0.95 (0.42–2.16) | 9 (18) | 24 (25) | 0.65 (0.24–1.79) | |||
| Q3 (0.800–3.683) | 75 (30) | 103 (22) | 1.30 (0.84–2.02) | 42 (38) | 50 (23) | 1.55 (0.70–3.45) | 17 (33) | 15 (16) | 2.93 (1.07–8.07) | |||
| Q4 (3.684– ) | 49 (20) | 130 (28) | 0.69 (0.43–1.10) | 0.04 | 28 (25) | 92 (43) | 0.53 (0.24–1.16) | 0.003 | 9 (18) | 20 (21) | 0.85 (0.32–2.25) | 0.82 |
| Enterolactone | ||||||||||||
| Q1 (0–0.378) | 56 (22) | 123 (27) | 1 | 33 (29) | 72 (33) | 1 | 4 (8) | 13 (14) | 1 | |||
| Q2 (0.379–1.373) | 73 (29) | 104 (23) | 1.45 (0.94–2.25) | 31 (28) | 46 (21) | 1.47 (0.77–2.79) | 12 (24) | 18 (19) | 2.22 (0.58–8.58) | |||
| Q3 (1.374–3.307) | 71 (28) | 108 (23) | 1.27 (0.82–1.97) | 28 (25) | 41 (19) | 1.36 (0.74–2.50) | 16 (31) | 23 (24) | 2.12 (0.56–8.09) | |||
| Q4 (3.308– ) | 51 (20) | 127 (27) | 0.83 (0.53–1.32) | 0.10 | 20 (18) | 57 (26) | 0.77 (0.41–1.45) | 0.20 | 19 (37) | 42 (44) | 1.26 (0.34–4.66) | 0.42 |
Cases and controls matched on geographic area, ethnicity, birth year, date and time of specimen collection, fasting status and hormone replacement therapy use.
Adjusted for age at blood draw and fasting hours prior to blood draw as continuous variables.
Abbreviations: CI, confidence interval.
Stratification by body mass index, tobacco smoking status, exogenous hormone use, and alcohol drinking had little influence on the relation of isoflavones or enterolactone with the risk of breast cancer (data not shown). No association of breast cancer with the excretion of equol was observed when we limited the analysis to the 97 cases and 161 controls who were equol producers (data not shown).
DISCUSSION
Results from this multiethnic cohort study add to the growing literature regarding the potential inverse association of phytoestrogen consumption with the risk of postmenopausal breast cancer. Excretion of genistein and an index of total isoflavones were significantly lower among women with breast cancer than among matched controls using continuous models, suggesting a modest relation between soy consumption and the risk of subsequent breast malignancy. The strongest dose-response relation was found for the inverse association of daidzein with the risk of breast cancer among Japanese-American women who had comparatively greater urinary concentrations of isoflavones than other race-ethnic groups, suggesting that the salutary effects of soy products in reducing postmenopausal breast cancer risk may require relatively high levels of intake. A suggestive relation between low equol excretion and breast cancer risk among white women should be interpreted with caution.
Only four prospective investigations of the association of baseline circulating or urinary isflavones with the subsequent risk of breast cancer have been conducted to our knowledge (17,22–25). In the first such study conducted within a Dutch population-based breast cancer screening program (17), no association of urinary genistein was found among 88 postmenopausal breast cancer cases compared to non-cancer controls. Phytoestrogen excretion was examined in spot urine specimens from 111 women and blood specimens from 92 women who later developed breast cancer within the European Prospective Investigation of Cancer and Nutrition-Norfolk study (22). Compared to controls, women with subsequent breast cancer had significantly higher levels of urinary and serum equol, and serum daidzein. However, a subsequent follow-up on this cohort with 237 breast cancer cases (194 postmenopausal) failed to confirm these associations (24). In general agreement with our results, an analysis performed within the Dutch Prospect cohort, including 87 pre- and peri-menopausal women and 296 postmenopausal women with breast cancer, showed a significant 32% reduction in risk among women in the highest compared with the lowest tertile of circulating genistein, but no association of breast cancer risk with circulating daidzein or equol (23). This finding is consistent with the results from the Japan Health Center prospective study of 59 premenopausal and 85 postmenopausal women which showed a significant 64% reduction in breast cancer risk associated with the highest versus the lowest quartile of plasma genistein, although no relation to plasma daidzein (25).
The present study was the first prospective investigation of circulating or urinary enterolactone and the risk of breast cancer in a multiethnic population. The absence of an association of enterolactone with breast cancer incidence in our cohort is consistent with the majority of other published studies (17–24), although only a few investigators have distinguished pre- and post-menopausal women in their statistical analyses (20,21,23). While there is general consistency across studies in the null association of breast cancer risk with enterolactone, some subgroup analyses have been significant. Although no overall association with breast cancer incidence among 248 cases (112 postmenopausal) was found by quartile of plasma enterolactone within three cohort studies in northern Sweden (18), women in the lowest and highest 12.5 percentiles were at a statistically significant increased risk. An examination of 381 postmenopausal breast cancer cases nested within the Danish Diet, Cancer and Health Study showed no overall association of plasma enterolactone with risk, but a significant inverse association with risk was suggested comparing the 80 women with ERα-negative breast cancer to controls (19).
An advantage of our study design was the ability to examine the association of isoflavone excretion among women with a broad range of soy product and legume intake that contributes to the dietary markers selected for investigation. Indeed, Japanese-American women in our prospective cohort had substantially higher excretion of isoflavones than other participants, with more than a four-fold mean variation in total isoflavone excretion between ethnic groups. A further benefit of our study methodology is the greater concentration of phytoestrogens in the urine than serum which leads to lower quantitation limits. Urine also better reflects recent (24 to 48 hrs) dietary exposure because of the short half-life of the compounds, although excellent correlations between plasma levels and urine excretion of isoflavones and their metabolites was found in several studies that considered the appropriate timing of sample collection (34,35).
Future analyses of these data will include stratification by hormone receptor status which is presently missing for 54% of the cases who were identified through our rapid case ascertainment system. Our report was also limited by the small number of cases for several of the race-ethnic groups included in the analysis. The absence of an association of several established risk factors for postmenopausal breast cancer in this study, such as BMI and alcohol, might be explained by the close matching of cases and controls by age, race-ethnicity, and hormone replacement use or by the modest sample size. In interpreting our results, it is important to consider that a high intake of soy and lignans may be correlated with other lifestyle factors that reduce breast cancer risk, such as greater physical activity, reduced alcohol consumption, and lower BMI.
In conclusion, this prospective investigation provides evidence that the consumption of foods high in isoflavones may reduce the risk of postmenopausal breast cancer. The absence of an association of urinary enterolactone with breast cancer risk is consistent with the majority of other null studies. Because we were unable to examine long-term isoflavone exposure or the effects of increased soy product consumption among women at high genetic risk of breast cancer, much remains to be studied about the potential breast cancer risk reduction associated with the consumption of genistein and daidzein.
Acknowledgments
Research Support: This study was supported in part by the National Cancer Institute grants P01-CA-33619, P30-CA-71789, and R37-CA-54281, and by contracts N01-PC-5137 and N01-PC-35139 from the National Institutes of Health, Department of Health and Human Services.
Abbreviations
- CI
confidence interval
- BMI
body mass index
- ER
estrogen receptor
- HER2
herceptin-2
- OR
odds ratio
- SEER
Surveillance, Epidemiology, End-Results
- PR
progesterone receptor
REFERENCES
- 1.Peeters PH, Keinan-Boker L, van der Schouw YT, Grobbee DE. Phytoestrogens and breast cancer risk. Review of the epidemiological evidence. Breast Cancer Res Treat. 2003;77:171–183. doi: 10.1023/a:1021381101632. [DOI] [PubMed] [Google Scholar]
- 2.Murphy PA, Song T, Buseman G, et al. Isoflavones in retail and institutional soy foods. J Agric Food Chem. 1999;47:2697–2704. doi: 10.1021/jf981144o. [DOI] [PubMed] [Google Scholar]
- 3.Thompson LU, Robb P, Serraino M, Cheung F. Mammalian lignan production from various foods. Nutr Cancer. 1991;16:43–52. doi: 10.1080/01635589109514139. [DOI] [PubMed] [Google Scholar]
- 4.Valentín-Blasini L, Sadowski MA, Walden D, Caltabiano L, Needham LL, Barr DB. Urinary phytoestrogen concentrations in the U.S. population (1999–2000) J Expo Anal Environ Epidemiol. 2005;15:509–523. doi: 10.1038/sj.jea.7500429. [DOI] [PubMed] [Google Scholar]
- 5.Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med. 1998;217:335–339. doi: 10.3181/00379727-217-44241. [DOI] [PubMed] [Google Scholar]
- 6.Adlercreutz H. Phyto-oestrogens and cancer. Lancet Oncol. 2002;3:364–373. doi: 10.1016/s1470-2045(02)00777-5. [DOI] [PubMed] [Google Scholar]
- 7.Adlercreutz H. Phytoestrogens and breast cancer. J Steroid Biochem Mol Biol. 2002;83:113–118. doi: 10.1016/s0960-0760(02)00273-x. [DOI] [PubMed] [Google Scholar]
- 8.Mai Z, Blackburn GL, Zhou JR. Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells. Mol Carcinog. 2007;46:534–542. doi: 10.1002/mc.20300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson LU, Chen JM, Li T, Strasser-Weippl K, Goss PE. Dietary flaxseed alters tumor biological markers in postmenopausal breast cancer. Clin Cancer Res. 2005;11:3828–3835. doi: 10.1158/1078-0432.CCR-04-2326. [DOI] [PubMed] [Google Scholar]
- 10.Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98:459–471. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler RG, Hoover RN, Pike MC, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 12.Curado MP, Edwards B, Shin HR, et al., editors. Cancer Incidence in Five Continents, Vol. IX IARC Scientific Publications No. 160. Lyon: IARC; 2007. [Google Scholar]
- 13.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boccardo F, Puntoni M, Guglielmini P, Rubagotti A. Enterolactone as a risk factor for breast cancer: a review of the published evidence. Clin Chim Acta. 2006;365:58–67. doi: 10.1016/j.cca.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 15.Touillaud MS, Thiébaut AC, Fournier A, Niravong M, Boutron-Ruault MC, Clavel-Chapelon F. Dietary lignan intake and postmenopausal breast cancer risk by estrogen and progesterone receptor status. J Natl Cancer Inst. 2007;99:475–486. doi: 10.1093/jnci/djk096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Touillaud MS, Thiébaut AC, Niravong M, Boutron-Ruault MC, Clavel-Chapelon F. No association between dietary phytoestrogens and risk of premenopausal breast cancer in a French cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15:2574–2576. doi: 10.1158/1055-9965.EPI-06-0543. [DOI] [PubMed] [Google Scholar]
- 17.den Tonkelaar I, Keinan-Boker L, Veer PV, et al. Urinary phytoestrogens and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10:223–228. [PubMed] [Google Scholar]
- 18.Hultén K, Winkvist A, Lenner P, Johansson R, Adlercreutz H, Hallmans G. An incident case-referent study on plasma enterolactone and breast cancer risk. Eur J Nutr. 2002;41:168–176. doi: 10.1007/s00394-002-0373-3. [DOI] [PubMed] [Google Scholar]
- 19.Olsen A, Knudsen KE, Thomsen BL, et al. Plasma enterolactone and breast cancer incidence by estrogen receptor status. Cancer Epidemiol Biomarkers Prev. 2004;13:2084–2089. [PubMed] [Google Scholar]
- 20.Zeleniuch-Jacquotte A, Adlercreutz H, Shore RE, et al. Circulating enterolactone and risk of breast cancer: a prospective study in New York. Br J Cancer. 2004;91:99–105. doi: 10.1038/sj.bjc.6601893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilkkinen A, Virtamo J, Vartiainen E, et al. Serum enterolactone concentration is not associated with breast cancer risk in a nested case-control study. Int J Cancer. 2004;108:277–280. doi: 10.1002/ijc.11519. [DOI] [PubMed] [Google Scholar]
- 22.Grace PB, Taylor JI, Low YL, et al. Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European prospective investigation of cancer and nutrition-norfolk. Cancer Epidemiol Biomarkers Prev. 2004;13:698–708. [PubMed] [Google Scholar]
- 23.Verheus M, van Gils CH, Keinan-Boker L, Grace PB, Bingham SA, Peeters PH. Plasma phytoestrogens and subsequent breast cancer risk. J Clin Oncol. 2007;25:648–655. doi: 10.1200/JCO.2006.06.0244. [DOI] [PubMed] [Google Scholar]
- 24.Ward H, Chapelais G, Kuhnle GG, Luben R, Khaw KT, Bingham S. Breast cancer risk in relation to urinary and serum biomarkers of phytoestrogen exposure in the European Prospective into Cancer-Norfolk cohort study. Breast Cancer Res. 2008;10:R32. doi: 10.1186/bcr1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwasaki M, Inoue M, Otani T, et al. Plasma isoflavone level and subsequent risk of breast cancer among Japanese women: a nested case-control study from the Japan Public Health Center-based prospective study group. J Clin Oncol. 2008;26:1677–1683. doi: 10.1200/JCO.2007.13.9964. [DOI] [PubMed] [Google Scholar]
- 26.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ries LAG, Melbert D, Krapcho M, et al., editors. Bethesda, MD: National Cancer Institute; SEER Cancer Statistics Review, 1975–2005. 2008 http://seer.cancer.gov/csr/1975_2005/.
- 28.Franke AA, Custer LJ, Wilkens LR, et al. Liquid chromatographic-photodiode array mass spectrometric analysis of dietary phytoestrogens from human urine and blood. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:45–59. doi: 10.1016/s1570-0232(02)00216-7. [DOI] [PubMed] [Google Scholar]
- 29.Maskarinec G, Hebshi S, Custer L, Franke AA. The relation of soy intake and isoflavone levels in nipple aspirate fluid. Eur J Cancer Prev. 2008;17:67–70. doi: 10.1097/CEJ.0b013e3281108101. [DOI] [PubMed] [Google Scholar]
- 30.Franke AA, Custer LJ, Wang W, Shi CY. HPLC analysis of isoflavonoids and other phenolic agents from foods and from human fluids. Proc Soc Exp Biol Med. 1998;217:263–273. doi: 10.3181/00379727-217-44231. [DOI] [PubMed] [Google Scholar]
- 31.Breslow NE, Day NE. IARC Scientific Publication no. 32. Lyon, France: International Agency for Research on Cancer; 1980. Statistical methods in cancer research. Vol 1. The analysis of case-control studies. [PubMed] [Google Scholar]
- 32.Setchell KD, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr. 2006;8:2188–2193. doi: 10.1093/jn/136.8.2188. [DOI] [PubMed] [Google Scholar]
- 33.Foster G, Goldsmith CH. Problems commonly associated with forest plots addressed using high resolution graphics in SAS (Paper 139-31) San Francisco, CA: SAS Users Group International 31 Proceedings; 2006. [Google Scholar]
- 34.Franke AA, Yu MC, Maskarinec G, Fanti P, Zheng W, Custer LJ. Phytoestrogens in human biomatrices including breast milk. Biochem Soc Trans. 1999;27:308–318. doi: 10.1042/bst0270308. [DOI] [PubMed] [Google Scholar]
- 35.Franke AA, Halm BM, Ashburn LA. Isoflavones in children and adults consuming soy. Arch Biochem Biophys. 2008;476:161–170. doi: 10.1016/j.abb.2008.02.009. [DOI] [PubMed] [Google Scholar]