Abstract
The microcirculation is not merely a passive conduit for red cell transport, nutrient and gas exchange, but is instead a dynamic participant contributing to the multiple processes involved in the maintenance of metabolic homeostasis and optimal end-organ function. The microcirculation’s angioarchitechture and surface properties influence conduit function and flow dynamics over a wide spectrum of conditions, accommodating many different mechanical, pathological or organ-specific responses. The endothelium itself plays a critical role as the interface between tissues and blood components, participating in the regulation of coagulation, inflammation, vascular tone, and permeability. The complex nitric oxide pathways affect vasomotor tone and influence vascular conduit caliber and distribution density, alter thrombotic propensity, and modify adhesion molecule expression. Nitric oxide pathways also interact with red blood cells and free hemoglobin moieties in normal and pathological conditions. Red blood cells themselves may affect flow dynamics. Altered rheology and compromised NO bioavailability from medical storage or disease states impede microcirculatory flow and adversely modulate vasodilation. The integration of the microcirculation as a system with respect to flow modulation is delicately balanced, and can be readily disrupted in disease states such as sepsis. This review will provide a comprehensive description of these varied and intricate functions of the microvasculature.
Introduction
The microcirculation is not just a passive conduit for red blood cell transport, oxygen delivery, and carbon dioxide removal. Rather, it comprises a highly complex system that is dynamically regulated and is a critical participant in multiple biological processes involved in vascular homeostasis. In a variety of pathological states, this integrated system is significantly perturbed in clinically relevant ways. We will summarize the microcirculation from the perspective of its critical conduit function: its angioarchitecture and endothelial surface, the influences from nitric oxide pathways, its effects related to red cells and/or plasma hemoglobin, and its integration as a system with respect to flow modulation. Microvascular perturbations in illustrative clinical conditions will be noted. Some of these have specific implications for transfusion medicine.
Global hemodynamics and measures such as the mixed venous oxygen saturation and lactic acid levels are useful in clinical patient management, but they may not accurately reflect clinically relevant metabolic perturbations occurring at the cellular and tissue levels (1,2). Indeed, local microcirculatory dynamics are important for regulating tissue oxygenation and minimizing organ dysfunction (3).
Operational Requirements
Achievement of these essential end-points demands certain operational requirements of the microcirculation. Its angioarchitecture must retain some degree of functional integrity despite a wide variety of conditions ranging from traction forces along extensor surfaces to compression and compaction forces within contracting muscles. In addition, perfusion must be reduced to the splanchnic and superficial integumentary beds while preserving flow to vital organs and muscles during fight-or-flight stress. Tissue-specific responses that are appropriate to variable stimuli are also required. For instance, in the pulmonary vascular bed, capillaries must limit their perfusion to nonventilated alveoli in order to minimize intrapulmonary shunting and venous admixture, a process termed hypoxic pulmonary vasoconstriction. A diametrically opposite response, hypoxic vasodilatation, is required in the systemic circulation so that local hypoxia stimulates vasodilation to maintain blood flow and sustain cellular and tissue metabolic homeostasis.
The microcirculation meets these operation requirements via a number of well-defined strategies.
Architecture and Function
The microcirculatory system includes the precapillary arterioles, capillaries, and postcapillary venules that allow for tissue oxygen delivery. These vessels are generally defined as those less than 100 µm in diameter, although feeder arterioles may be as large as 150 µm. As the arteriole proceeds from proximal to distal, its diameter gradually decreases and its muscular layer becomes more sparse and discontinuous in a helical arrangement that overlies a continuous basement membrane and endothelial layer (4).
The arteriolar level is primarily the site of autoregulation whereby steady forward flow is maintained despite a wide range of systemic perfusion pressures (3,5). Sympathetic input, nitric oxide and carbon monoxide, endothelin-1, and other factors, contribute to flow regulation in the arterioles (6). Resistance introduced at this level of the circuit allows for a dampening effect and helps to preclude pressure-related damage to the distal, more fragile, capillary bed (7). This dissipation of pressure is also facilitated by the huge increase in vascular cross-sectional surface area presented by capillary branching. Furthermore, vessel recruitment (with opening and perfusion of previously closed capillaries) allows for continued flow without a rise in resistance (4).
Ranvier (1876) and Krough (1919) described a regular spatial distribution of capillaries intercalated in a parallel manner along muscle fibers (8,9). Krough also modeled oxygen diffusion from a capillary to a surrounding cylinder of tissue. This concept of diffusion limitation is still believed to be accurate, and it places strict constraints on how far tissue cells can be from an oxygen source, predicating a necessary underlying capillary density. He clearly showed that metabolically active tissues have a higher structural capillary density, thereby establishing the physiological necessity of preserving microvascular function. (9,10)
Krough also noted that gentle massage or electrical stimulation of muscle resulted in capillary recruitment and increased blood flow, presciently inferring that this was likely a dynamic, and not merely a passive, process (11). The fraction of nonperfused vessels constitutes a perfusion volume reserve that can be called upon under conditions of high metabolic demand. Given that prolonged vessel closure and nonperfusion can contribute to structural loss (12), variable capillary opening must constitute a normal state of affairs. Moreover, it appears that this reserve can be optimized; for example, trained athletes possess a coronary flow reserve twice that of healthy young individuals (7). Orthogonal polarization spectral imaging and sidestream dark field microscopy have demonstrated mixing of flow from adjacent capillary networks (3,13). In addition, oxygen diffusion can also take place at the precapillary arteriole and postcapillary venule level. These two factors, dynamic regulation of perfused volume and some dispersion of oxygen diffusion, combined with the spatial and temporal heterogeneity in capillary perfusion, allow for homogenous distribution of oxygen in the tissues (14,15).
The capillaries drain into a thin walled, high capacitance, low-pressure venules. The venule system may contain as much as 75% of total blood volume and act as a preload reservoir for cardiac output (6). The venous muscular layer gradually becomes more pronounced as it moves closer to the central circulation (4). Thereby, active venoconstriction due to adrenergic stimulation via neural and humoral pathways can allow for an increase in mean circulatory filling pressure, preload augmentation, and improved cardiac output. This must occur in concert with an increase in arterial resistance in order to maintain forward steady flow, prevent engorgement of postcapillary capacitance vessels, and limit fluid extravasation from the capillary bed. (16)
Thus, dynamic mechanical and metabolic modulation of the vascular conduit is a necessity for vascular homeostasis.
The Endothelium
The endothelial layer comprises a critical physical and functional interface between tissues and blood. It is involved in - and provides biological linkages between - the dynamic modulation of coagulation, inflammatory processes, and vasomotor tone. Its permeability barrier function regulates fluid and solute flux across the membrane. These biological functions normally optimize conduit function and integrity of the vascular space.
The endothelial lining differs in terms of morphology, permeability, and functional properties both within and between tissues. For example, endothelial cells of the blood-brain barrier have extensive tight junctions without fenestrations and minimal pinocytotic transport. By contrast, there are numerous fenestrations present in the endothelium of the kidney that assist in filtration (17, 18).
Independent of such dramatic structural heterogeneity is an important, yet minimally understood, heterogeneity of endothelial function. For example, adhesion molecule expression is variable within and between different tissue beds. Notably, the pulmonary microvascular bed exhibits a high constitutive expression of adhesion molecules, predominately at the venular level, which promotes heightened margination of leukocytes in the lungs. (17) This characteristic of the pulmonary vascular endothelium likely contributes to the fact that the lung is the most common organ to be targeted by harmful consequences of acquired systemic inflammatory states. Examples include the pathogenesis of transfusion-related acute lung injury (19) and sepsis (discussed below), as well as ischemia/reperfusion injury in which even a localized occlusion can cause systemic endothelial dysfunction (20).
Shear Responsiveness
Above and beyond the influence of biological mediators, applied shear stress also significantly affects endothelial gene regulation and surface phenotype and function. The dose-response is exceedingly complex and is different for various shear stress regimes. It is believed that execution of endothelial mechanosensitivity requires shear sensing, and then mechanotransduction into the cell, followed by transduction to a chemical signal. Various factors have been implicated in this, notably the glycocalyx (see below) for the first two steps, and possibly endothelial transcription factor Egr-1 in the latter step. (21,22) Importantly, the endothelial response to shear stress not only promotes nitric oxide generation but also is negatively modulated by nitric oxide (22) (see below). Multiple endothelial genes are shear stress responsive.
The Glycocalyx
Microvascular endothelial cells are covered by a thin (60–111 nm) layer of glycocalyx consisting of glycolipids, glycoproteins, and proteoglycans. This dense mat helps provide an unstirred layer where, for example, solute concentration is different from that of the bulk plasma. Overlying this is a further layer (estimated to be 300–1000 nm thick) created by branching extensions of glycocalyx components (e.g., heparin sulfate proteoglycans and hyaluronic acid) that has a gradient of fluid flow (increasing with distance from the membrane lipid bilayer) but which comprises a cell-free zone that excludes moving blood cells. This layer dampens fast shear stress fluctuations while allowing for mechanical force transduction to the underlying endothelial cell. (17)
Overall, the glycocalyx provides the endothelial cell with a negatively charged, anti-adhesive, ”lubricated” (i.e., smooth) surface that helps maintain an anti-coagulant barrier (17,23). It participates in permeability regulation, transduction of shear stress, and blood cell interaction with the endothelium. Notably, the glycocalyx is dynamic in composition (but over much longer time frames than, for example, chemical signal transduction); it can be degraded by cytokines and oxidized-LDL and altered by hyperglycemia. Such glycocalyx perturbations have been suggested to be an underlying feature of early diabetic microangiopathy and a cause of microalbuminuria.
It has been suggested that cultured endothelial cells exhibit minimal glycocalyx compared to the same cells in vivo (e.g., 29.4 nm versus 878 nm, respectively for human umbilical vein endothelial cells). (24) If true, this raises the looming question as to whether endothelial cell results observed in vitro are accurately informative regarding cell behavior in vivo; and additionally, challenges our understanding of their pathobiology.
The Endothelium as a Virtual Organ
Given that the endothelial layer covers a large surface area (perhaps 350m2 in an adult [23]), it functions as a distributed signaling network. Endothelial cells secrete soluble mediators and microparticles that can affect other vascular beds. For example, endothelial liberation of endothelin-1 can exert both local and long-range effects on the vascular wall. Endothelial-derived microparticles can express tissue factor (25), and they can have distal signaling effects. Paracrine and cell-to-cell signaling via gap junction signal transmission also contributes to flow modulation.
Adhesion Biology
The endothelium is a central player in the inflammatory response by means of its dynamically modulated display of surface adhesion molecules for blood cells such as granulocytes, monocytes, and erythrocytes. The inflammatory response involves a complex dance between leukocyte and endothelial cell, with each signaling to and altering the other. Leukocyte behavior includes tethering and rolling (or other means of capture), activation, firm adhesion, crawling and transmigration into the extravascular space. An extremely complex and diverse set of well-defined adhesion molecules underlie these biological events, and they can notably differ depending on organ (e.g., brain differs from hepatic sinusoids, and both differ from cremaster/mesenteric post-capillary venules), and are illustrative of endothelial heterogeneity. (26)
Nitric Oxide Pathways
eNOS/NO
Endothelial cells have a constitutive form of nitric oxide synthase (eNOS) which is the primary isozyme involved in the regulation of vascular tone via the production of the vasodilatory molecule nitric oxide (NO) (27). Nitric oxide is a highly diffusible, labile, and multiply reactive molecule; and it functions as a ubiquitous signaling molecule with numerous downstream effects. It acts in a paracrine manner to attenuate vasomotor tone through a second messenger system by activating soluble guanylate cyclase to produce cGMP that, in turn, alters intracellular calcium fluxes and protein kinase activity. (28) NO has antithrombogenic activity in that it inhibits both tissue factor expression and platelet aggregation, and it down regulates endothelial expression of certain adhesion molecules (e.g., VCAM) and thereby decreases leukocyte adhesion to the vascular endothelium. Administration of N-nitro L-arginine methyl ester (L-NAME) inhibits eNOS activity; and therefore, results in up-regulation of endothelial cell adhesion molecules such as ICAM-1 and PECAM-1. (29) Conversely, boosting eNOS activity (e.g., with arginine supplementation or with a NO donor) down-regulates expression of some of these molecules. Such manipulations of eNOS activity are accompanied by the expected changes in leukocyte interaction with endothelium. NO has also been shown to inhibit neointimal hyperplasia and minimize smooth muscle cell proliferation in the vessel wall (30,31,32).
The following experiments demonstrate eNOS function, as well as its potential utility in translational vascular manipulation via gene therapy. Adenovirus mediated transfer of the eNOS gene directly into mouse lung resulted in a decrease in pulmonary vascular resistance with an attenuated vasoconstrictor response to endothelin-1, angiotensin-II, hypoxia, and bleomycin insult, as well as enhanced vasodilatory response to bradykinin and zaprinast. (33) Intravenous eNOS plasmid DNA delivery in the systemic hypertensive rat model resulted in a reduction in blood pressure (34). Ligation of the femoral artery of a rat to create an ischemic limb model followed by adenovirus/eNOS cDNA injection into the surrounding hindlimb musculature improved angiogenesis and limb reperfusion compared to sham injected control animals (35). In the injured carotid artery model in rabbits, eNOS gene transfer resulted in increased NO production, enhanced endothelium-dependent relaxation, and diminished contractile response to the vasoconstrictor phenylephrine. These effects were reversed by L-NAME administration. (36) Transgenic mice that over express eNOS also appear to have some cardioprotective resiliency against congestive heart failure and myocardial infarction (37,38).
An interesting and rational target disease for approach via manipulating eNOS activity is found in pulmonary arterial hypertension. Animal and human pathology studies of advanced pulmonary hypertension demonstrate reduced expression of eNOS (39–42). Given that pulmonary arterial hypertension also manifests a pathophysiology of vasoconstriction, endothelial cell dysfunction, intimal fibrosis, medial thickening, and vascular plexiform lesion formation, eNOS gene therapy is an attractive tool and a subject of translational research for this disorder. (31,43–45) We have recently utilized a novel delivery vehicle for in vivo expression of eNOS, namely engineered blood outgrowth endothelial cells; injection of which ameliorated pulmonary hypertension in a rat model of this disease (Somani and Hebbel, unpublished observations, 2009; submitted for publication, 2010).
Nitrate-Nitrite-NO Pathway
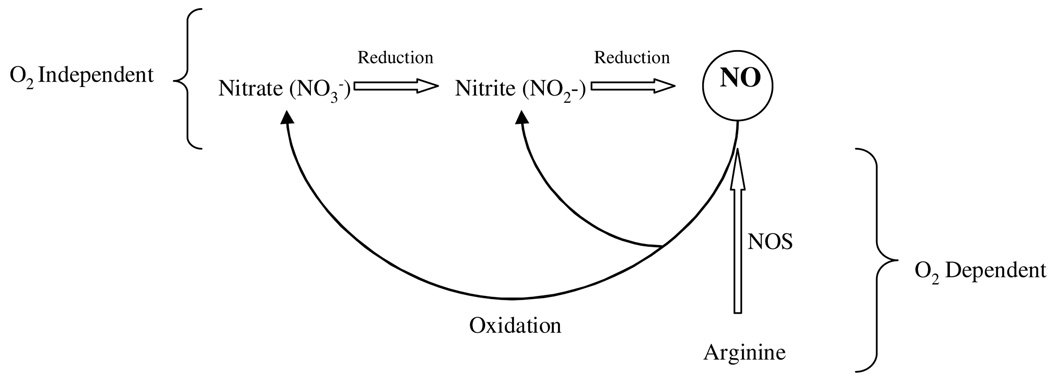
There is increasing evidence that nitrate and nitrite may serve as metabolic storage pools for NO and thus complement the NOS pathway (46). This is summarized in the flow diagram (Figure 1, adapted from discussions in reference 47).
Figure 1.
NO generation from oxygen independent and dependent pathways.
Since mammals lack nitrate reductase enzymes, a symbiotic relationship with commensal bacteria is required to reduce dietary nitrate to nitrite (48,49). Notably, dietary nitrate and nitrite have been shown to limit ischemia reperfusion injury in animal models (50). Of historical interest, medieval Chinese physicians used nitrate from gunpowder to optimize qi (or harmonious flow) in a patient ”… struck with evil, acute heart pains, and cold in the hands and the feet…” (47). Remarkably, in his 1919 paper, Krough alludes to the utility of nitrite to dilate the microcirculation (9).
Nitrite is a relatively stable anion in blood and tissues and may be reduced to NO by a number of pathways. Non-enzymatic disproportionation of nitrite in an acidic environment (pH 6.5) results in NO generation. (46,51) The endothelial enzymes xanthine oxidoreductase and eNOS also convert nitrite to NO under acidic or hypoxic conditions (52). Specifically, electron donation from xanthine, nicotinamide adenine dinucleotide or aldehydes reduce nitrite at the molybdenum site of xanthine oxidoreductase under anaerobic conditions. Moreover, this reaction is accelerated with a decrease in pH. (53). Under hypoxic conditions eNOS functionally switches from arginine metabolism to that of nitrite reduction to produce NO (53). These mechanisms potentially allow for NO mediated vasodilation to counteract conditions of compromised oxygen delivery and/or flow. (51). Under non-stressed standard physiological conditions, nitrite reduction is primarily carried out by deoxyhemoglobin and deoxymyoglobin. The clinical application of manipulating the nitrate-nitrite-NO pathway has been suggested for treatment of high blood pressure, stroke, myocardial infarction, gastric ulcer prophylaxis, and pulmonary hypertension.
Hemoglobin functions as an allosterically regulated nitrite reductase with both hypoxia and pH sensor properties. The deoxygenated form of hemoglobin binds nitrite readily and reduces it to NO. In addition, proton donation for nitrite reduction is favored at lower pH. (51,54,55). This may be the basis of enhanced NO generation along a decreasing oxygen and pH gradient allowing for hypoxic vasodilation at the microvascular level, and consequently, the availability of oxygen at the mitochondrial level. Cosby and co-workers demonstrated that brachial artery nitrite infusions resulted in increased blood flow in the forearm, even if NOS itself was inhibited. This increase in blood flow was associated with NO generation as well as the formation of iron-nitrosylated hemoglobin (NO bound to the heme group), and to a lesser extent S-nitroso-hemoglobin (NO bound to the cysteine-93 residue of the beta chain) (55). Other investigators have suggested that S-nitroso-hemoglobin is the predominant component of RBC mediated vasorelaxation and is unaffected by eNOS inhibition (56–58). S-nitrosylation of hemoglobin is proposed to occur in the pulmonary vascular bed under conditions of high oxygen tension (perhaps with NO transfer from the iron-heme moiety to the beta-93 cysteine residue) while NO release occurs during arterial to venous transit in parallel with a decreasing oxygen gradient (57,59).
Possibly counterbalancing the above process is the ability of hemoglobin to scavenge nitric oxide. Indeed, the ability of pathophysiologically elevated levels of plasma free hemoglobin (in disorders involving intravascular hemolysis) to do this has been supported (60). In particular it has been asserted that this accounts for the clinical NO biodeficiency of sickle cell anemia, and even is the underlying cause of pulmonary hypertension in hemolytic disorders. However, whether this process can actually have such a major impact on vascular homeostasis is, at best, uncertain. It may critically depend upon whether concomitant, co-morbid processes have adversely impacted the function of eNOS, for example. Nonetheless, it has been presented that, despite administration of an NO donor, vasodilation is impaired in sickle cell disease and possibly in other chronic hemolytic disorders and, in fact, correlates inversely with plasma free hemoglobin concentration (60).
At higher concentrations, nitric oxide may have negative effects in terms of activating inflammatory pathways, inducing DNA damage, and contributing to cell cycle arrest and death. These effects are probably attenuated by the buffering action of albumin and cysteine 93 on the β-hemoglobin chain (28). Depending on the level of inflammation, the enhanced generation of superoxide from activated leucocytes can provide a buffering or even a quenching effect by virtue of its ability to scavenge NO. (29)
Red Blood Cells
The red blood cell (RBC) is the means by which oxygen is carried from the gas exchange unit (the alveolus) to its final destination (the individual cell) for aerobic utilization. The RBC also participates in microcirculatory regulation in order to match oxygen delivery with oxygen demand. Experiments have shown that RBCs affect microcirculatory flow by sensing either low regional oxygen tension or low pH. In response, native RBCs release adenosine 5’-triphosphate (ATP). The intraluminal rise in ATP produces vasodilation, which then facilitates RBC flow and improved oxygen delivery. (61–63)
RBC concentration, shape and deformability affect blood viscosity and, in turn, influence flow dynamics. For example, anemia may be tolerated if sufficient compensatory cardiorespiratory mechanisms increase blood flow to meet metabolic demand. Progressive hemodilution decreases whole blood viscosity resulting in a finite increase in microvascular blood flow that can compensate for lower oxygen content. In moderate hemodilution, it is estimated that half of the increase of the microvascular flow results from a reduction of blood viscosity. (64) However, continued extreme hemodilution beyond this point results in vasoconstriction, decreased flow, deficient oxygen delivery, and oxygen debt.
Transfusion of stored RBCs is a common therapy, with over 14 million units administered to patients each year in the United States (65). Stored RBCs are typically administered to correct anemia or blood loss, the underlying assumption being that transfused RBCs will function sufficiently like native RBCs. Changes during RBC storage may impact subsequent RBC function in vivo. (66,67) During storage, glycolysis produces protons and lactate, leading to decreased levels of the major allosteric modifier of hemoglobin, 2,3-diphosphoglycerate (2,3-DPG), an effect that causes a decrease in hemoglobin P50, i.e., an increase in hemoglobin oxygen binding affinity which may compromise oxygen unloading (68). Although 2,3-DPG is repleted in vivo after transfusion, studies examining the effect on oxygen dynamics and microcirculatory regulation are conflicting (69–74).
Decreased red cell pH and ATP levels during storage are associated with an alteration of RBC shape from a biconcave disc to an echinocyte (75). This change is associated with a loss of deformability (75–78) that impedes flow through the microcirculation (79). Stored RBCs have also been shown to adhere to the endothelium, potentially occluding the microcirculation. (80–82)
In respect to NO, the intact red blood cell membrane and the unstirred layer overlying the erythrocyte and the endothelium, serve as diffusion barriers and limit NO scavenging by intra-erythrocytic hemoglobin. (23,51,83) However, RBC integrity undergoes graduated disruption during blood bank storage, releasing iron and free hemoglobin in increasing amounts with storage duration (84). It has been postulated that the free hemoglobin delivered with transfusion of stored RBCs may act as a NO scavenger, potentiate vasoconstriction, and disrupt NO-mediated vasodilation in a manner similar to pathologic hemolytic conditions (85). In conjunction with this, nitric oxide adducts diminish during storage, compromising the capacity of banked RBCs to mediate hypoxic vasodilation (86). It is very important that this be further studied, as its truth would imply that the sickle patient, for example, with pre-existing NO biodeficiency might display vascular instability in response to red cell transfusion.
Although RBC transfusion increases oxygen carrying capacity, many studies have been unable to demonstrate a concomitant increase in oxygen utilization. A recent review found that RBC transfusion generally increased oxygen content and delivery; however, actual oxygen consumption increased in only 3 of the 18 studies (87). Clinical studies comparing outcomes in patients who received RBCs stored for different periods of time have yielded conflicting results (88,89).
Sepsis
Sepsis, defined as the presence of infection with a systemic inflammatory response, is a prime example of the perturbations that occur at the microvascular level. This dysregulation results in inadequate cellular tissue perfusion with an activation of anaerobic metabolism pathways, contributing to tissue oxygen debt and acidosis. (1,90)
Animal models demonstrate that sepsis is associated with a significant decrease in functional capillary density, an increase in stopped flow capillaries, and an increase in the intercapillary distance (91). Similarily, septic patients have microvascular blood flow alternations consisting of a decrease in small vessel (<20 µm) density and an increase in non-perfused or intermittently perfused vessels. This underlying microvascular heterogeneity was more severe in nonsurvivors and persisted in those developing multiple organ failure (92,92). Furthermore, even with the application of early goal directed therapy, nonsurvivors tended to have continued derangements in microcirculatory indices compared to survivors (93). Even the use of norepinephrine to improve cardiac index and increase mean arterial pressure did not improve capillary skin blood flow, red blood cell velocity, or microvascular perfusion (94,95).
Microcirculatory perturbations in sepsis are multifactorial in nature and include: autoregulatory dysfunction, heterogeneous expression of iNOS, diminished red blood cell deformability, increased red blood cell aggregation, increased leukocyte activation, altered endothelial glycocalyx function, disordered coagulation with microthrombi formation, and capillary leakage. (90,96) Additionally, fast flow capillaries that deliver higher venular end-oxygen saturation contribute to intra-tissue shunting and compound dysregulated flow patterns. When combined with mitochondrial dysfunction, these factors contribute to the uncoupling of oxygen delivery and oxygen consumption.(96,97)
It is important to realize that these microcirculatory perturbations may not be adequately captured by global hemodynamic parameters (2), but still cause significant tissue hypoxia, organ dysfunction, and compromised patient prognosis. Moreover, the endothelium is the intersection between the biology of marked inflammation and disordered coagulation manifested by the septic state (98).
Systems Integration of Flow
In order to subserve a healthy organism, the microvasculature must be an integrated functional system with underlying control and feedback mechanisms at the arteriolar and capillary levels, and the red cell interface. As referenced earlier, the arteriolar level contributes to flow regulation by introducing resistance within the flow circuit. Resistance at this level is caused by myogenic contraction elicited from circumferential wall stress and smooth muscle elongation (5). This myogenic contraction is counterbalanced by a dilatory response induced by shear stress from increased blood flow (99). Given that arterioles supply a wider capillary network, dependence on feedback regulation at this level is likely to be imprecise and less anticipatory to local metabolic needs (3).
The capillary endothelium is a sensor and effector of sheer stress stimuli. Specifically, flow induced torque of glycocalyx anchoring molecules results in signal transduction to the endothelial cell with a compensatory upregulation of eNOS and increased NO production (17,100,101). Additionally, stimuli such as bradykinin, histamine, acetylcholine, and adenosine hyperpolarize endothelial cells and allow them to act as transducers of biochemical signals (102). The propagation of these transduced signals through gap junctions would allow the capillary endothelium to serve as a communicating medium and modulate upstream arteriolar diameter (102,103).
As alluded to earlier, hemoglobin and red blood cells can act as prime oxygen sensors and effect the release of nitric oxide or ATP respectively, allowing for intrinsic feedback to occur along a diminishing oxygen and/or decreasing pH gradient. The incorporation of these metabolic signals allows for a greater degree of specificity in modeling targeted blood flow (5).
Conclusion
Structure and function integration across a healthy microvascular bed is vital in maintaining tissue oxygenation and waste product removal, as is ongoing dynamic modulation of the ”conduit”. Nitric oxide formation from both eNOS and nitrite reduction pathways allows for targeted vasodilation to metabolically active tissue beds. The native RBC complements its primary role in oxygen delivery with an oxygen sensing capacity to modulate flow. Myogenic, sheer stress, and metabolic signals further allow for microvascular flow coordination in order to achieve metabolic homeostasis. Finally, the microvascular endothelium is an active - even critical - participant in both normal vascular homeostasis and in the pathobiology of various medical disorders. In particular, endothelial dysfunction can have widespread consequences. The clinical conditions of sepsis, transfusion-related acute lung injury, ischemia/reperfusion events, and chronic hemolytic disorders may all represent pathologic processes which reflect dysfunction of the microcirculation.
Acknowledgments
We apologize for not having been able to cite all relevant studies. We wish to acknowledge Jean Kulander and James Kiley for manuscript assistance. This work was support by the Vikings Children’s Fund and University Pediatrics Foundation Grant (to AS) and by the National Institutes of Health (PO1-HL55552 to RPH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We have no conflicts of interest to declare.
Contributor Information
Arif Somani, Pediatric Critical Care Medicine, University of Minnesota, soman007@umn.edu, 612-624-5649.
Marie E Steiner, Pediatric Hematology/Oncology and Pediatric Pulmonary/Critical Care, University of Minnesota, stein083@umn.edu, 612-626-2778.
Robert P. Hebbel, Director of Vascular Biology Center, Division of Hematology-Oncology-Transplantation, Department of Medicine, University of Minnesota hebbe001@umn.edu.
References
- 1.Shoemaker WC, Appel PL, Kram HB. Tissue oxygen dept as a determinant of lethal and nonlethal postoperative organ failure. Crit Care Med. 1988;16:1117–1120. doi: 10.1097/00003246-198811000-00007. [DOI] [PubMed] [Google Scholar]
- 2.De Backer D, Creteur J, Preiser JC, Dubois MC, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 3.Raat NJH, Ince C. Oxygenating the microcirculation: the perspective from blood transfusion and blood storage. Vox Sanguinis. 2007;93:12–18. doi: 10.1111/j.1423-0410.2007.00909.x. [DOI] [PubMed] [Google Scholar]
- 4.Reid L, Meyrick B. Microcirculation: Definition and organization at tissue level. NYAS. 1982;384:3–20. doi: 10.1111/j.1749-6632.1982.tb21357.x. [DOI] [PubMed] [Google Scholar]
- 5.Secomb TW. Theoretical models for regulation of blood flow. Microcirculation. 2008;15:765–775. doi: 10.1080/10739680802350112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.den Uil CA, Klijn E, Lagrand WK, et al. The microcirculation in health and critical disease. Prog Cardio Dis. 2008;51:161–170. doi: 10.1016/j.pcad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Levy BI, Schiffrin EL, Mourad JJ, et al. Impaired tissue perfusion: A pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118:968–976. doi: 10.1161/CIRCULATIONAHA.107.763730. [DOI] [PubMed] [Google Scholar]
- 8.Ranvier LA. Traite tech. d’Histologie, fas. 1876;4:511. [Google Scholar]
- 9.Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol (Lond) 1919;52:409–415. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krogh A. The rate of diffusion of gases through animal tissues, with some remarks on the coefficient of invasion. J Physiol (Lond) 1919;52:391–408. doi: 10.1113/jphysiol.1919.sp001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krogh A. The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol (Lond) 1919;52:457–474. doi: 10.1113/jphysiol.1919.sp001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prewitt RL, Dowell R. Development of microvascular rarefaction in the spontaneously hypertensive rat. Am J Physiol. 1982;243:H243–H251. doi: 10.1152/ajpheart.1982.243.2.H243. [DOI] [PubMed] [Google Scholar]
- 13.De Backer D, Hollenberg S, Boerma C, et al. How to evaluate the microcirculation: report of a round table conference. Critical Care. 2007;11:R101–R109. doi: 10.1186/cc6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wieringa PA, Stassen HG, Van Kan JJ, Spaan JA. Oxygen diffusion in a network model of the myocardial microcirculation. Int J Microcirc Clin Exp. 1993;13:137–169. [PubMed] [Google Scholar]
- 15.Elbers WG, Ince C. Bench-to-bedside review: Mechanisms of critical illness-classifying microcirculatory flow abnormalities in distributive shock. Critical Care. 2006;10:221–228. doi: 10.1186/cc4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters J, Mack GW, Lister G. The importance of the peripheral circulation in critical illness. Intensive Care Med. 2001;27:1146–1458. doi: 10.1007/s001340101034. [DOI] [PubMed] [Google Scholar]
- 17.Moncada S, Higgs A, editors. The Vascular Endothelium. Berlin: Springer; 2006. [Google Scholar]
- 18.Aird WC, editor. Endothelial Biomedicine. New York: Cambridge University Press; 2007. [Google Scholar]
- 19.Kokkini G. Transfusion-Related acute lung injury. Transfusion Alt in Trans Med. 2002;4:58–61. [Google Scholar]
- 20.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathology. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Houston P, Dickson MC, Ludbrook V, et al. Fluid shear stress induction of the tissue factor promoter in vitro and in vivo is mediated by Egr-1. Arterioscler Thromb Vase Biol. 1999;19:281–289. doi: 10.1161/01.atv.19.2.281. [DOI] [PubMed] [Google Scholar]
- 22.Chiu JJ, Wung BS, Hsieh HJ, Lo LW, Wang DL. Nitric oxide regulates shear stress-induced early growth reponse-1: Expression via the extracellular signal-regulated kinase pathway in endothelial cells. Circulation Research. 1999;85:238–246. doi: 10.1161/01.res.85.3.238. [DOI] [PubMed] [Google Scholar]
- 23.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 24.Chappell D, Jacob M, Paul O, et al. The glycocalyx of the human umbilical vein endothelial cells. An impressive structure ex vivo but not in culture. Circulation Research. 2009;104:1313–1317. doi: 10.1161/CIRCRESAHA.108.187831. [DOI] [PubMed] [Google Scholar]
- 25.Shet AS, Aras O, Gupta K, et al. Sickle blood contains tissue factor positive microparticles from endothelial cells and monocytes. Blood. 2003;102:2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 26.Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: An in vivo perspective. J Immunology. 2008:6439–6446. doi: 10.4049/jimmunol.180.10.6439. [DOI] [PubMed] [Google Scholar]
- 27.Michelakis E. The role of the NO axis and its therapeutic implications in pulmonary arterial hypertension. Heart Failure Reviews. 2003;8:5–21. doi: 10.1023/a:1022150819223. [DOI] [PubMed] [Google Scholar]
- 28.Hollenberg SM, Cinel I. Bench-to-bedside review: nitric oxide in critical illness. Critical Care. 2009;13:218–226. doi: 10.1186/cc7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scalia R, Lefer AM. In vivo regulation of PECAM-1 activity during acute endothelial dysfunction in the rat mesenteric microvasculature. J. Leukoc. Biol. 1998;64:163–169. doi: 10.1002/jlb.64.2.163. [DOI] [PubMed] [Google Scholar]
- 30.von der Leyen HE, Gibbons GH, Morishita R, et al. Gene therapy inhibiting neointimal vascular lesion: In vivo transfer of endothelial cell nitric oxide synthase gene. Proc. Natl. Acad. Sci. USA. 1995;92:1137–1141. doi: 10.1073/pnas.92.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong D, Melo LG, Mangi AA, et al. Enhanced inhibition of neointimal hyperplasia by genetically engineered endothelial progenitor cells. Circulation. 2004;109:1769–1775. doi: 10.1161/01.CIR.0000121732.85572.6F. [DOI] [PubMed] [Google Scholar]
- 32.Galie N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovascular Research. 2004;61:227–237. doi: 10.1016/j.cardiores.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 33.Champion HC, Bivalacqua TJ, D’Souza FM, et al. Gene transfer of endothelial nitric oxide synthase to the lung of a mouse in vivo. Circ. Res. 1999;84:1422–1432. doi: 10.1161/01.res.84.12.1422. [DOI] [PubMed] [Google Scholar]
- 34.Lin K, Chao L, Chao J. Prolonged reduction of high blood pressure with human nitric oxide synthase gene delivery. Hypertension. 1997;30:307. doi: 10.1161/01.hyp.30.3.307. [DOI] [PubMed] [Google Scholar]
- 35.Smith RS, Jr, Lin K, Agata J, et al. Human endothelial nitric oxide synthase gene delivery promotes angiogenesis in a rat model of hindlimb ischemia. Arterioscler Thromb Vase Biol. 2002;22:1279–1285. doi: 10.1161/01.atv.0000026613.18742.67. [DOI] [PubMed] [Google Scholar]
- 36.Kullo IJ, Mozes G, Schwartz RS, et al. Enhanced endothelium-dependent relaxations after gene transfer of recombinant endothelial nitric oxide synthase to rabbit carotid arteries. Hypertension. 1997;30:314. doi: 10.1161/01.hyp.30.3.314. [DOI] [PubMed] [Google Scholar]
- 37.Jones SP, Greer JJ, van Haperen R, et al. Endothelial nitric oxide synthase overexpression attenuates congestive heart failure in mice. PNAS. 2003;100(8):4891–4896. doi: 10.1073/pnas.0837428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones SP, Greer JJ, Kakkar AK, et al. Endothelial nitric oxide synthase overexpression attenuates myocardial repurfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H276–H282. doi: 10.1152/ajpheart.00129.2003. [DOI] [PubMed] [Google Scholar]
- 39.Le Cras TD, Kim D, Markham NE, et al. Early abnormalities of pulmonary vascular development in the Fawn-Hooded rat raised at Denver’s altitude. Am J Physiol Lung Cell Mol Physiol. 2000;279:L283–L291. doi: 10.1152/ajplung.2000.279.2.L283. [DOI] [PubMed] [Google Scholar]
- 40.Gong F, Lin Y, Tang H, et al. VEGFmRNA and eNOSmRNA expression in immature rabbits with bleomycin-induced pulmonary hypertension. J Zhejiang Univ SCI. 2004;5(8):995–1000. doi: 10.1007/BF02947613. [DOI] [PubMed] [Google Scholar]
- 41.Giad A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 42.Tuder RM, Chacon M, Alger L, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol. 2001;195:367–374. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 43.Zhao YD, Courtman DW, Deng Y, et al. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells. Circ Res. 2005;96:442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 44.Campbell AI, Kuliszewski MA, Stewart DJ. Cell-based gene transfer to the pulmonary vasculature: endothelial nitric oxide synthase overexpresssion inhibits monocrotaline-induced pulmonary hypertension. Am J Respir Cell Mol Biol. 1999;21:567–575. doi: 10.1165/ajrcmb.21.5.3640. [DOI] [PubMed] [Google Scholar]
- 45.Somani A, Nair S, Milbauer LC, et al. Blood outgrowth endothelial cell based eNOS gene therapy prevents monocrotaline induced pulmonary hypertension. American Thoracic Society. 2009 abstract. [Google Scholar]
- 46.Gladwin MT, Raat NJH, Shiva S, et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection and vasodilation. Am J Physiol heart Circ Physiol. 2006;291:H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 47.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 48.Benjamin N, O'Driscoll F, Dougall H, et al. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 49.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raat NJH, Noguchi AC, Liu VB, et al. Dietary nitrate and nitrite modulate blood and organ nitrite and the cellular ischemic stress response. Free Rad Bio & Med. 2009;47:510–517. doi: 10.1016/j.freeradbiomed.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dejam A, Hunter CJ, Schechter AN, Gladwin MT. Emerging role of nitrite in human biology. Blood cells, Molecules, and Diseases. 2004;32:423–429. doi: 10.1016/j.bcmd.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Gautier C, van Faassen E, Mikula I, Martasek P, Slama-Schwok A. Endothelial nitric oxide synthase reduces nitrite anions to NO under anoxia. BBRC. 2006;341:816–821. doi: 10.1016/j.bbrc.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 53.van Faassen EE, Bahrami S, Fellisch M, et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schechter AN, Gladwin MT. Hemoglobin and the paracrine and endocrine functions of nitric oxide. N Engl J Med. 2003;348:1483–1485. doi: 10.1056/NEJMcibr023045. [DOI] [PubMed] [Google Scholar]
- 55.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Medicine. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 56.Diesen DL, Hess DT, Stamler JS. Hypoxic vasodilation by red blood cells: Evidence for an S-Nitrosothiol-based signal. Circ Res. 2008;103:545–553. doi: 10.1161/CIRCRESAHA.108.176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jia L, Bonaventura C, Bonaventura J, Stamler J. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 58.Datta B, Tufnell-Barrett T, Bleasdale RA, et al. Red blood cell nitric oxide as an endocrine vasoregulator: a potential role in congestive heart failure. Circulation. 2004;109:1339–1342. doi: 10.1161/01.CIR.0000124450.07016.1D. [DOI] [PubMed] [Google Scholar]
- 59.Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vase Biol. 2006;26:697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- 60.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Medicine. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 61.Ellsworth ML. The red blood cell as an oxygen sensor: what is the evidence? Acta Phys Scand. 2000;168:551–559. doi: 10.1046/j.1365-201x.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 62.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 63.McCullough WT, Collins DM, Ellsworth ML. Arteriolar responses to extracellular ATP in striated muscle. Am J Physiol. 1997;272:H1886–H1891. doi: 10.1152/ajpheart.1997.272.4.H1886. [DOI] [PubMed] [Google Scholar]
- 64.Cabrales P, Tsai AG. Plasma viscosity regulates systemic and microvascular perfusion during acute extreme anemic conditions. American Journal of Physiology. Heart and Circulatory. 2006;291(5):H2445–H2452. doi: 10.1152/ajpheart.00394.2006. [DOI] [PubMed] [Google Scholar]
- 65.US Department of Health and Human Services. The 2007 Nationwide Blood Collection and Utilization Survey Report. Washington, DC: DHHS; 2007. [Google Scholar]
- 66.Wagner GM, Chiu DT, Qju JH, Heath RH, Lubin BH. Spectrin oxidation correlates with membrane vesiculation in stored RBCs. Blood. 1987;69:1777–1781. [PubMed] [Google Scholar]
- 67.Izzo P, Manicone A, Spagnuolo A, Lauta VM, Di Pasquale A, Di Monte D. Erythrocytes stored in CPD SAG-mannitol: evaluation of their deformability. Clin Hemorheol Microcirc. 1999;21:335–339. [PubMed] [Google Scholar]
- 68.Hess JR, Greenwalt TG. Storage of red blood cells: new approaches. Transfus Med Rev. 2002;16:283–295. doi: 10.1053/tmrv.2002.35212. [DOI] [PubMed] [Google Scholar]
- 69.Arslan E, Sierko E, Waters JH, Siemionow M. Microcirculatory hemodynamics after acute blood loss followed by fresh and banked blood transfusion. Am J Surg. 2005;190:456–462. doi: 10.1016/j.amjsurg.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 70.Gonzalez AM, Yazici I, Kusza K, Siemionow M. Effects of fresh versus banked blood transfusions on microcirculatory hemodynamics and tissue oxygenation in the rat cremaster model. Surgery. 2007;141:630–639. doi: 10.1016/j.surg.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 71.Raat NJ, Verhoeven AJ, Mik EG, et al. The effect of storage time of human red cells on intestinal microcirculatory oxygenation in a rat isovolemic exchange model. Crit Care Med. 2005;33:39–45. doi: 10.1097/01.ccm.0000150655.75519.02. discussion 238-239. [DOI] [PubMed] [Google Scholar]
- 72.Cabrales P, Tsai AG, Intaglietta M. Modulation of perfusion and oxygenation by red blood cell oxygen affinity during acute anemia. Am J Respir Cell Mol Biol. 2008;38:354–361. doi: 10.1165/rcmb.2007-0292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.d'Almeida MS, Gray D, Martin C, Ellis CG, Chin-Yee IH. Effect of prophylactic transfusion of stored RBCs on oxygen reserve in response to acute isovolemic hemorrhage in a rodent model. Transfusion. 2001;41:950–956. doi: 10.1046/j.1537-2995.2001.41070950.x. [DOI] [PubMed] [Google Scholar]
- 74.Eichelbronner O, D'Almeida M, Sielenkamper A, Sibbald WJ, Chin-Yee IH. Increasing P(50) does not improve DO(2CRIT) or systemic VO(2) in severe anemia. Am J Physiol Heart Circ Physiol. 2002;283:H92–H101. doi: 10.1152/ajpheart.01066.2001. [DOI] [PubMed] [Google Scholar]
- 75.Berezina TL, Zaets SB, Morgan C, et al. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102:6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- 76.Wagner GM, Chiu DT, Qju JH, Heath RH, Lubin BH. Spectrin oxidation correlates with membrane vesiculation in stored RBCs. Blood. 1987;69:1777–1781. [PubMed] [Google Scholar]
- 77.Izzo P, Manicone A, Spagnuolo A, et al. Erythrocytes stored in CPD SAG-mannitol: evaluation of their deformability. Clin Hemorheol Microcirc. 1999;21:335–339. [PubMed] [Google Scholar]
- 78.Relevy H, Koshkaryev A, Manny N, Yedgar S, Barshtein G. Blood banking-induced alteration of red blood cell flow properties. Transfusion. 2008;48:136–146. doi: 10.1111/j.1537-2995.2007.01491.x. [DOI] [PubMed] [Google Scholar]
- 79.Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44:1626–1634. doi: 10.1111/j.0041-1132.2004.04128.x. [DOI] [PubMed] [Google Scholar]
- 80.Ho J, Sibbald WJ, Chin-Yee IH. Effects of storage on efficacy of red cell transfusion: when is it not safe? Crit Care Med. 2003;31:S687–S697. doi: 10.1097/01.CCM.0000099349.17094.A3. [DOI] [PubMed] [Google Scholar]
- 81.Eichelbronner O, Sibbald WJ, Chin-Yee IH. Intermittent flow increases endotoxin-induced adhesion of human erythrocytes to vascular endothelial cells. Intensive Care Med. 2003;29:709–714. doi: 10.1007/s00134-003-1698-y. [DOI] [PubMed] [Google Scholar]
- 82.Luk CS, Gray-Statchuk LA, Cepinkas G, Chin-Yee IH. WBC reduction reduces storage-associated RBC adhesion to human vascular endothelial cells under conditions of continuous flow in vitro. Transfusion. 2003;43:151–156. doi: 10.1046/j.1537-2995.2003.00310.x. [DOI] [PubMed] [Google Scholar]
- 83.Deonikar P, Kavdia M. Extracellular diffusion and permeability effects on NO-RBCs interactions using an experimental and theoretical model. Microvasc Research. 2010;79:447–455. doi: 10.1016/j.mvr.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aubuchon JP, Estep TN, Davey RJ. Effect of plasticizer di-2-ethylhexyl phthlatate on the survival of stored RBCs. Blood. 1988;71:448–452. [PubMed] [Google Scholar]
- 85.Gladwin MT, Kim-Shapiro DB. Storage lesion in banked blood due to hemolysis-dependent disruption of nitric oxide homeostasis. Curr Opin Hematol. 2009;16:515–523. doi: 10.1097/MOH.0b013e32833157f4. [DOI] [PubMed] [Google Scholar]
- 86.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. PNAS USA. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hebert PC, Van der Linden P, Biro G, Hu LQ. Physiologic aspects of anemia. Crit Care Clin. 2004;20:187–212. doi: 10.1016/j.ccc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 2009;96:93–103. doi: 10.1111/j.1423-0410.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- 89.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 90.Spronk P, Zandstra D, Ince C. Bench-to-bedside review: Sepsis is a disease of the microcirculation. Crit Care. 2004;8:462–468. doi: 10.1186/cc2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lam C, Tymi K, Martin C, Sibbald C. Microvascular perfusion is impaired in a rat model of normotensive sepsis. J. Clin. Invest. 1994;94:2077–2083. doi: 10.1172/JCI117562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sakr Y, Dubois ML, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32:1825–1831. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 93.Trzeciak S, Dellinger R, Parrillo J, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: Relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2006:1–11. doi: 10.1016/j.annemergmed.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 94.LeDoux D, Astiz M, Carpati C, Rackow E. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med. 2000;28:2729–2732. doi: 10.1097/00003246-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 95.Fries M, Ince C, Rossaint R, et al. Levosimendan but not norepinephrine improves microvascular oxygenation during experimental septic shock. Crit Care Med. 2008;36:1886–1891. doi: 10.1097/CCM.0b013e31817cede9. [DOI] [PubMed] [Google Scholar]
- 96.Ince C. The microcirculation is the motor of sepsis. Crit Care. 2005;9 supple 4:S13–S19. doi: 10.1186/cc3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ellis CG, Jagger J, Sharpe M. The microcirculation as a functional system. Critical Care. 2005;9 supp 4:S3–S8. doi: 10.1186/cc3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukocyte Biology. 2008;83:536–545. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 99.Pries AR, Secomb TW. Modeling structural adaptation of microcirculation. Microcirculation. 2008;15:753–764. doi: 10.1080/10739680802229076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qi J, Du J, Tang X, et al. The upregulation of endothelial nitric oxide synthase and urotensin-II is associated with pulmonary hypertension and vascular diseases in rats produced by aortocaval shunting. Heart Vessels. 2004;19:81–88. doi: 10.1007/s00380-003-0739-3. [DOI] [PubMed] [Google Scholar]
- 101.Li Y, Zheng J, Bird I, Magness R. Effects of pulsatile shear stress on signaling mechanisms controlling nitiric oxide production, endothelial nitric oxide synthase phosphorylation, and expression in ovine fetoplacental artery endothelial cells. Endothelium. 2005;12:21–39. doi: 10.1080/10623320590933743. [DOI] [PubMed] [Google Scholar]
- 102.Dietrich HH, Tyml K. Capillary as a communicating medium in the microvasculature. Microvascuclar Research. 1992;43:87–99. doi: 10.1016/0026-2862(92)90008-d. [DOI] [PubMed] [Google Scholar]
- 103.Koller A, Kaley G. Endothelial regulation of wall shear stress and blood flow in skeletal muscle microcirculation. Am J Physiol. 1991;260:H862–H868. doi: 10.1152/ajpheart.1991.260.3.H862. [DOI] [PubMed] [Google Scholar]