Abstract
The present study examined whether manipulation of the early life experience of rat pups might alter the later ability of an interoceptive challenge to recruit central neural circuits that receive visceral sensory signals and generate stress responses. For this purpose, litters were exposed to daily maternal separation for either 15 min (MS-15) or 180 min (MS-180) from postnatal days (P)1 to P10. Pups in control litters were raised under standard conditions (i.e., no separations). Similar to previous reports in adult rats, adolescent rats (P35–45) with a developmental history of MS-15 displayed less anxiety-like behavior on the elevated plus maze compared to control and MS-180 rats. As young adults (P50–60), rats were anesthetized and perfused with fixative 90 min after viscerosensory stimulation via lithium chloride (LiCl, 0.15M, 1% BW, i.p.) or saline control. In all three rearing groups, Fos activation within brainstem and forebrain regions of interest was significantly enhanced after LiCl vs. saline. MS-15 rats tended to display fewer LiCl-activated neurons in most brain regions compared with rats in the other two rearing groups. This trend reached significance within the dorsal bed nucleus of the stria terminalis. The ability of MS-15 to alter limbic forebrain activation in rats after an interoceptive challenge may contribute to the effect of early life experience to modulate physiological and behavioral stress responses more generally.
Introduction
In the late 1950s, Seymour Levine and colleagues reported that laboratory rats with an early developmental history of repeated brief maternal separation tended to show truncated corticosterone responses to stress when tested as adults [1,2]. In a now standard experimental model [3–6], separation of rat pups from their dams for brief periods (e.g., 10–15 min/day) during the first 1–2 weeks postnatal increases the active maternal care received by the pups (e.g., licking and grooming; [7,8]), and yields adult rats with lower levels of anxiety-like behavior on many laboratory tasks, accompanied by reduced synthesis and stress-induced release of ACTH, CRH, and corticosterone [9–11]. By contrast, longer periods of maternal separation (e.g., 180 min/day) during the same postnatal period reduce the cumulative active maternal care received by pups [8], and yields adult rats with higher levels of anxiety-like behavior accompanied by increased synthesis and release of stress hormones (reviewed in [12–18]). The utility of these paradigms to alter life-long stress responsiveness and emotionality has been validated in laboratories around the world, and they provide an opportunity to test hypotheses about developmental alterations of central visceral/emotional circuits and how circuit alterations contribute to alterations in behavioral and physiological responses.
Though many studies have documented the behavioral and neuroendocrine consequences of manipulating early dam/pup interactions, only limited data exist to inform opinions of how such manipulations might impact the development and functional organization of neural circuits that underlie individual responses to stressful and emotive events. The standard maternal separation paradigms are performed in rats during a potentially sensitive postnatal period of development, during which central visceral sensory and motor circuits undergo significant anatomical and functional maturation (reviewed in [19–21]). For example, we recently reported that brainstem and forebrain Fos responses to an acute interoceptive stressor (i.e., lithium chloride, LiCl) are decidedly immature in neonatal rats, undergoing significant maturation during the first few weeks of postnatal life [21]. Manipulating early life experiences through maternal separation will necessarily impact the amount and quality of sensory stimulation experienced by the developing pups, including altered visceral sensory stimulation [22]. We predict that this will affect the ongoing development and later functional organization of central circuits that process interoceptive signals, which in turn will influence lifelong neural, behavioral, and physiological responses to stressful events.
In the present study, a variation of the standard “Plotsky and Meaney” model [3–6] was used to manipulate the early life experience of pups from postnatal day (P)1 through P10, inclusive. We selected this model of altered early life experience because this time period overlaps with major events in the structural and functional maturation of the visceral circuits we are studying (reviewed in [19]). This is, therefore, a period of potential plasticity, and a potential sensitive period of development for central visceral circuits in rats. After weaning, adolescent (P35–45) rats exposed to different early life experiences were assessed for anxiety-like behavior in the elevated plus maze (EPMZ) to confirm the expected behavioral effects. When rats reached young adulthood (P50–60), they were treated acutely with LiCl in order to document potential rearing group effects on central neural Fos activation within stress-responsive brainstem, hypothalamic, and limbic forebrain components of the central visceral neuraxis.
Materials and Methods
Animals
All procedures were conducted under the oversight of the University of Pittsburgh Institutional Animal Care and Use Committee, in accordance with NIH standards for the care and use of animals in experimental research. As with previous studies in our laboratory [20], multiparous pregnant female Sprague-Dawley rats (Harlan, Indianapolis, IN) entered our animal care facility in late gestation (between days 13 and 16). The facility is temperature controlled (20–22 deg C), and has a light:dark cycle of 12:12h, with lights on at 0700h. Pelleted rat chow (Purina 5001) and drinking water was available ad libitum. Pregnant dams were individually housed in standard plastic caging filled with soft wood chip bedding. Cages were checked daily to accurately date parturition, designated postnatal day (P)0. Litters were culled to 10 pups on P0 or P1. Both female and male pups were used.
Early life experience (maternal separation)
Litters were randomly assigned to one of three developmental treatment groups. Control litters were animal facility reared (AFR; N=30 pups from 3 litters; 13 males and 17 females), with a weekly transfer of dam and pups together to a clean tub cage, but no other outside interventions. Pups in MS-15 litters received 15 min of daily separation from their dam from P1 to P10 (N=20 pups from 2 litters; 9 males and 11 females). For this purpose, the dam was removed from the home cage between 0900 and 1030h each day. Pups were then transferred en masse together with littermates and some home cage bedding to a smaller Nalgene tub, which was placed into an incubator (Animal Care Products; 33° C, 50–60% humidity) in an adjacent room. Following removal of pups, the dam was returned to the home cage, and pups were returned 15 min later. Pups in MS-180 litters were similarly removed from their home cage and transferred to the incubator from P1 to P10, but were held there together with littermates for 180 min each day (N=20 pups from 2 litters; 10 males and 10 females).
Litters remained undisturbed after P10, except for weekly transfer of dam and pups together to a clean cage. On P21, pups were weaned and housed with 1–3 same-sex littermates until they reached 150 g, after which all rats were pair-housed with a same sex littermate.
Behavioral Testing
All rats were tested on the elevated plus maze (EPMZ; reviewed in [23]) between P35 and P45 during the middle of the light cycle. Rats were placed on the maze in random order, and their behavior was video recorded for 5 min. The EPMZ was cleaned with a mild odor-neutralizing cleanser and allowed to dry between rats. Video records were scored for time spent by each rat in each segment of the maze (open arms, closed arms, and center), and for the number of entries into each segment. A rat was judged to have entered a segment when its head and both forepaws initially occupied it.
Treatment-Induced Fos Activation
A subset of male and female EPMZ-tested rats (N=36) was randomly chosen for perfusion after LiCl or control saline injection when they reached 50–60 days of age. Rats were weighed the day before perfusion but were not otherwise acclimated to handling or i.p. injections, as potential developmental group differences in central Fos responses to control treatment (i.p. saline) were of interest. Using a 28 gauge needle, rats were injected i.p. with a 1% BW volume of either aqueous 0.15M NaCl [Abbot, North Chicago, IL; n=4 MS-15 (2 male, 2 female), n=4 MS-180 (2 male, 2 female), n=8 AFR (5 male, 3 female)], or aqueous 0.15M LiCl [Sigma; n=6 MS-15 (4 male, 2 female), n=8 MS-180 (5 male, 3 female), n=6 AFR (2 male, 4 female)]. NaCl and LiCl solutions were syringe-filtered (0.45 µm) and equilibrated in a water bath to 37° C before injection. After injection, each rat was tail marked as to treatment using indelible ink, returned to its home cage, and left undisturbed for 90 min prior to anesthesia and perfusion (described below). This post-treatment survival time is similar to our previous Fos studies examining the effects of LiCl in neonatal and adult rats [21,24], and is based on evidence that nuclear Fos protein immunolabeling peaks approximately 1 hr after significant neural activation and persists for at least 2 hrs [25].
Perfusion and Tissue Collection
Perfusion and tissue collection have been described [21]. Briefly, rats were anesthetized with sodium pentobarbital (Nembutal, 10 mg/kg BW i.p., Abbot, North Chicago, IL) and transcardially perfused using aqueous 0.15M NaCl for the first minute followed by 4% paraformaldehyde in 0.1M phosphate buffer solution (pH 7.4) for 10 min. Fixed brains were removed from the skull, blocked, frozen, and sectioned coronally (35 µm) on a freezing-stage microtome. Sections from the upper cervical spinal cord through the rostral corpus callosum were collected serially in six adjacent sets and stored in cyropreservant solution at −20° C prior to processing for immunohistochemistry. One set of tissue sections per animal (i.e., representing brain regions sampled at 210 µm intervals) was used for analysis of Fos expression, as detailed below.
Immunohistochemistry
Immunohistochemical procedures have been described [21]. Briefly, sections from each rat were processed for dual immunoperoxidase visualization of nuclear Fos protein and cytoplasmic dopamine beta hydroxylase (DbH), the latter to identify noradrenergic neurons and fibers. DbH immunolabeling was used as a guide to help standardize the anatomical regions and subnuclei subjected to quantitative analyses of Fos expression across rats.
Fos protein was localized using a rabbit antiserum (kindly provided by Dr. Philip Larsen, Denmark, 1:50K). The specificity of this antiserum for Fos protein in rats has been reported [26]. Tissue sections were processed as previously described [21] and reacted with nickel-intensified diaminobenzidine (DAB) to produce a blue-black nuclear peroxidase label for Fos protein.
DbH was subsequently localized in Fos-reacted tissue sections using a mouse monoclonal antibody (1:30K; Millipore) as previously described [21]. Sections were reacted using plain DAB to produce a brown cytoplasmic peroxidase label. Double-labeled tissue sections were then rinsed and mounted, dehydrated and defatted, and placed under coverslips.
Quantification and Data Analysis
Cell counting in double-labeled tissue sections was performed as previously described [21] to document the extent of treatment-related Fos immunoreactivity in the area postrema (AP), nucleus of the solitary tract (NTS), parabrachial nucleus (PBN), central nucleus of the amygdala (CeA), paraventricular nucleus of the hypothalamus (PVN), and bed nucleus of the stria terminalis (BNST). Anatomical landmarks were defined with reference to the atlas of Paxinos and Watson [27], with sampling regions further standardized across animals based on the subregional distribution of DbH immunolabeling. Fos positive neurons were defined by the appearance of blue/black nuclei, or blue/black punctae distributed throughout the nucleus, independent of intensity. DbH positive cell bodies were defined by a brown stained cytoplasm with a clearly defined nucleus that was either Fos-positive or negative.
Each area of interest was sampled bilaterally in sections spaced by 210 µm. The AP was sampled along its full rostrocaudal extent in approximately 4 sections per rat. NTS subregional analyses were conducted for DbH-positive, Fos-positive, and double-labeled neurons caudal to the level of the AP, at the level of the AP, and rostral to the AP (up to the level at which the dorsal vagal complex moves laterally away from the wall of the 4th ventricle), in a total of approximately 10 sections per rat. Counting of Fos-positive cells within the PBN was restricted to its external lateral subdivision, in approximately 2–3 sections per rat. Fos-positive cell counts within the PVN were performed on 2–3 sections per rat, restricted to the medial parvicellular subdivision, immediately caudal to the optic chiasm and medial to the lateral magnocellular subdivision. Fos-positive CeA neurons were counted in the same 2–3 sections per rat in which PVN counts were performed. Fos-positive BNST neurons were counted within 2–3 sections per rat that were centered on the rostro-caudal level at which the anterior commissure (AC) crosses the midline. Counts in the BNST were divided into regions dorsal to the AC (dorsal BNST) and ventral to the AC (ventral BNST). For each brain region, the total number of neurons counted in each rat was divided by the number of sections in which counts were made to derive the average number of immunopositive cells per section.
Statistical Analysis
Anxiety-like behaviors were measured as the ratio of time spent in the open versus closed arms of the EPMZ during the 5 min exposure period, and as the number of entries into each arm of the maze. These behavioral data from the EPMZ were analyzed in two stages. The assumption of normally distributed data was evaluated by visual inspection of probability plots of all data subdivided by rearing group and/or injection type. The assumption of homogeneity of variance was evaluated using Levene's test. Within each rearing group, data were compared across litters using a one factor (litter) ANOVA to look for large pre-existing differences between litters. As no litters within any treatment group came close to being significantly different (i.e., p >0.10; see Results), data from all litters within each group were combined for later analyses.
Behavioral data were analyzed using the general linear model (Systat, version 9). The effects of three factors (rearing group, sex, and litter) were included in the model. To isolate and control for small, non-significant differences between litters, the factor for litter was nested within rearing group. Results were considered statistically significant when p<0.05.
Cell count data are presented as group means ± S.E.M., except as noted. The assumption of normally distributed data was evaluated by visual inspection of probability plots of all data subdivided by rearing group and/or injection type. The assumption of homogeneity of variance was evaluated using Levene's test. Cell count data were then analyzed in two stages. Within each rearing group, data first were compared across litters using a one factor (litter) ANOVA. As no litters within treatment came close to significance (i.e., p >0.10; see Results), all litters were included in later analyses.
Cell count data were then analyzed to determine the effects of rearing, i.p. injection, sex, and litter on Fos labeling, including activation of DbH-positive neurons in the brainstem, using the general linear model. As above, the effect for litter was nested within rearing group. Interaction effects among injection, rearing, and sex were also examined. Results were considered statistically significant when p<0.05. If there were interactions among the various factors considered in this study (i.e., when p was greater than or equal to 0.25), the interaction terms were dropped from the model, and the data were reanalyzed to get a better estimate of the true effects of i.p. injection, rearing group, sex, and litter (28).
Because the general linear model tests only the null hypothesis and not hypotheses of biological interest, statistically significant effects of rearing group on treatment-induced Fos activation were further investigated using linear contrast analysis [28,29]. We evaluated the hypothesis that the MS-15 group would have the lowest Fos counts after LiCl treatment, while AFR rats would have intermediate counts, and MS-180 rats would have the highest counts. We predicted that this hypothesis would best explain data from forebrain regions (i.e., the CeA, BNST, and PVN) that receive viscerosensory signals relayed from the caudal medulla. Conversely, rearing group effects on LiCl-induced Fos activation were considered to be less likely within the more primary viscerosensory regions (i.e., AP, NTS, and PBN) whose circuitry appears to be more mature at birth compared to the later-developing projections from brainstem to hypothalamus and limbic forebrain [21,31–37].
For Fos analyses, effect sizes (LiCl versus saline injection) were computed in terms of Cohen's d [38,39], where d represents the difference between the means of two groups divided by the pooled standard deviation. Cohen’s d is thus a measure of the number of standard deviations of difference between the means of the two groups. Values range from 0.0 (i.e., no difference) to highs of 2.0 or greater (i.e., large differences). By convention, effect sizes of 0.8 and greater are considered “large”, while those of 0.2 and lower are considered “small” [38].
Results
Effect of early life experience on anxiety-like behavior in the EPMZ
There were no significant differences between litters within the AFR, MS-15, and MS-180 groups in terms of the ratio of time spent in the open vs. closed arms, or on the number of entries into the open or closed arms (statistics shown below). Therefore, data from all litters were pooled within each rearing group for final analysis with the general linear model.
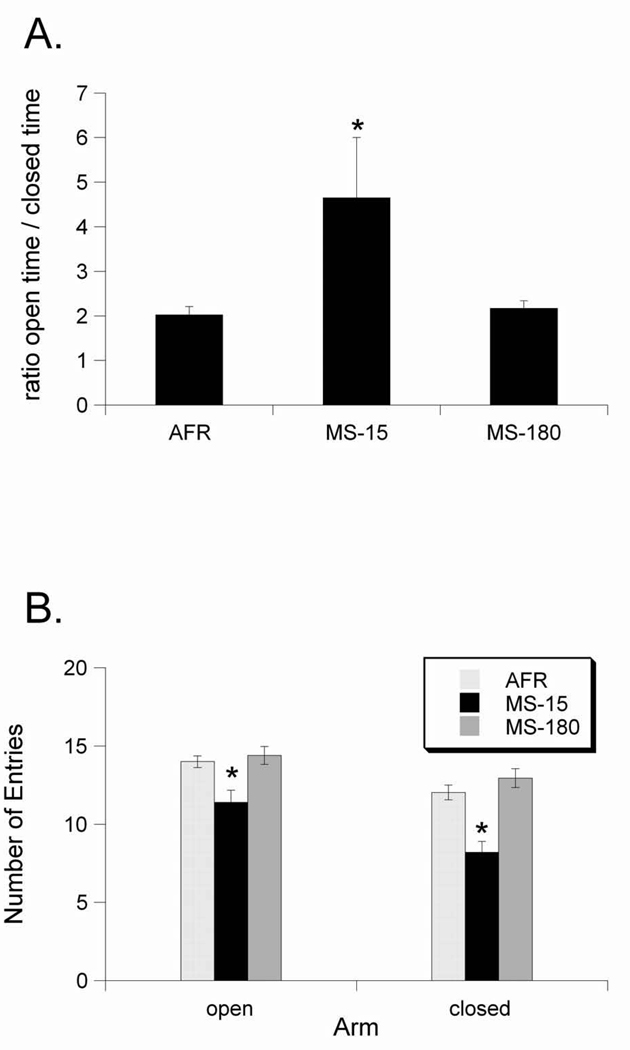
As expected, early life experience affected EPMZ behavior assessed in adolescent rats (P35–45), similar to previous reports in adult rats. Our analyses revealed that rats with a developmental history of MS-15 spent significantly more time in the open arms vs. the closed arms of the maze (i.e., higher open:closed arm time ratio) compared to rats from the AFR and MS-180 rearing groups (Fig. 1A), evidence for reduced anxiety-like behavior in the MS-15 group. Our mixed-model analysis confirmed a significant effect of rearing group (F2,59=4.754, p=0.01) but not sex (F1,59=0.258, p=0.61) on open:closed arm time ratios. Membership in a particular litter did not significantly affect open:closed arm time ratios (F4,59=2.133, p=0.08). No interaction terms approached significance (data not shown), so they were dropped from the model results presented here.
Figure 1.
Effects of early life experience on exploration of the elevated plus maze. A. Rats in the MS-15 group spent relatively more time in the open arm than the closed arm (i.e., higher open time/closed time ratio) compared to AFR and MS-180 rats. B. Rats in the MS-15 group had fewer entries into both the open and closed arms than rats in the AFR and MS-180 groups. Values are group means ± standard error. * p < 0.05.
Early life experience also affected the number of entries that rats made into the arms of the EPMZ (Fig. 1B). Overall, rats in the AFR and MS-180 groups made more entries into both the open and closed arms compared to rats in the MS-15 group. For entries into the open arm, the general linear model revealed a significant effect of rearing group (F2,62=7.947, p=0.001), but not sex (F1,62=0.784, p=0.37), with no significant contribution of litter (F4,62=0.940, p=0.44). For entries into the closed arm, ANOVA also revealed a significant effect of rearing group (F2,62=17.678, p<0.001) but not sex (F1,62=0.642, p=0.42). There was no significant contribution of litter to these effects (F4,62=1.89, p=0.12). Thus, compared to AFR and MS-180 rats, rats with a developmental history of MS-15 displayed significantly less anxiety-like behavior in the EPMZ when tested as adolescents. Conversely, there were no differences in EPMZ behavior between AFR and MS-180 rats. No interaction terms approached significance (data not shown), so they were dropped from the model results presented here.
Effect of early life experience on LiCl-induced Fos activation
There were no significant differences between litters within the AFR, MS-15, or MS-180 groups in terms of Fos responses to LiCl or saline injection in any of the brain regions in which Fos-positive cells were counted (statistics presented below). Therefore, data from all litters were pooled within each rearing group.
Nucleus of the Solitary Tract (NTS)
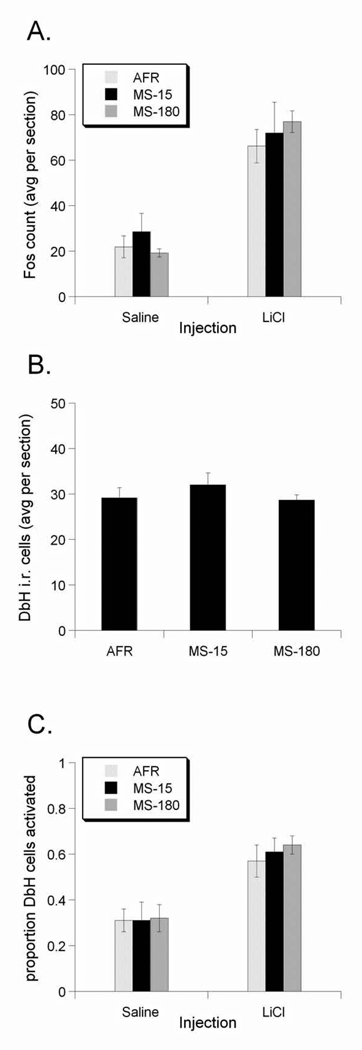
Injection of LiCl increased Fos expression within the NTS of rats from all rearing groups compared to Fos labeling after saline injection (F1,25=6.719, p=0.01; Figure 3A). Examples are depicted in Figure 2. Effect sizes (Cohen's d) show that, compared to Fos activation after control saline injection, the LiCl-induced increase in Fos expression was highest in the MS-180 group, intermediate in the AFR group, and lowest in the MS-15 group (Table 1). In other words, MS-15 appeared to attenuate the ability of LiCl treatment to activate Fos compared to the other two rearing groups. However, the general linear model revealed that the effect of rearing did not reach statistical significance (F2,25=0.326, p=0.725). Similarly, neither sex (F1,25=0.028, p=0.868) nor litter (F4,25=0.201, p=0.935) had a significant effect on LiCl-induced Fos expression. No interaction terms approached significance (data not shown), so they were dropped from the model results presented here.
Figure 3.
Effect of early life experience on LiCl-induced Fos expression within the NTS. A. LiCl Injection increased Fos counts in the NTS in all groups, irrespective of early life experience. B. Early life experience did not significantly alter the number of DbH-positive NTS neurons. C. The proportion of DbH-positive cells that expressed Fos in LiCl-treated rats was greater than in saline-treated rats in all three rearing groups, with no significant differences among groups. Values are group means ± standard error.
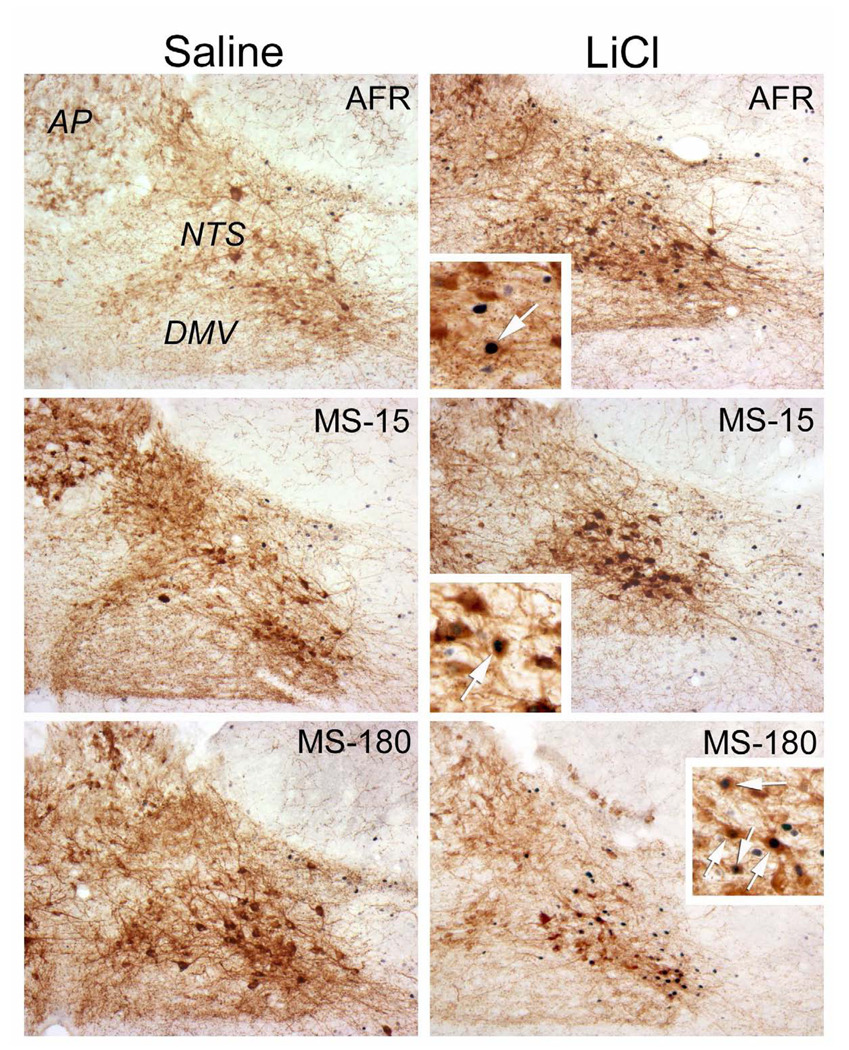
Figure 2.
Color images of saline- and LiCl-induced Fos expression (blue-black nuclear label) within the NTS. Sections are double-labeled for DbH (brown) to identify noradrenergic neurons. Rats injected with saline (left column) display fewer Fos-positive neurons compared to Fos expression in rats injected with LiCl (right column). There was no statistically significant effect of early life experience on the degree of Fos expression (see Figure 3). Insets provide higher-magnification views of Fos-positive noradrenergic neurons (arrows) in rats after LiCl treatment.
Table 1.
Effect of early life experience on LiCl- or saline-induced Fos within viscerosensory brain regions. LiCl induced robust activation of neurons in all depicted brain regions in all rearing groups, except in the ventral BNST of MS-15 rats. All data are group means, ± (SEM). Effect sizes are given as LiCl versus Saline injection, in terms of Cohen's d, where d represents the difference between the means of two groups divided by the pooled standard deviation. Values range from 0.0 (i.e., no difference) to highs of 2.0 or greater (i.e., large differences). By convention, effect sizes of 0.8 and greater are considered “large”, while those of 0.2 and lower are considered “small” [37]. In general, Fos responses to LiCl were lowest in MS-15 rats and highest in MS-180 rats, as judged by effect sizes.
| AFR | MS-15 | MS-180 | ||
|---|---|---|---|---|
| NTS | Saline | 21.93 (4.82) |
28.63 (8.00) |
19.24 (1.73) |
| LiCl | 66.18 (7.41) |
65.73 (13.42) |
76.99 (4.78) |
|
| effect size | 2.76 | 1.44 | 5.85 | |
| AP | Saline | 13.17 (3.78) |
11.79 (6.30) |
7.29 (1.07) |
| LiCl | 37.52 (10.72) |
27.89 (4.98) |
34.91 (3.78) |
|
| effect size | 1.23 | 1.35 | 3.58 | |
| PBN | Saline | 9.98 (2.06) |
19.55 (8.52) |
18.32 (3.63) |
| LiCl | 148.30 (29.15) |
125.11 (35.62) |
176.86 (15.14) |
|
| effect size | 2.73 | 1.83 | 5.16 | |
| CeA | Saline | 19.20 (2.75) |
61.64 (32.70) |
30.80 (7.60) |
| LiCl | 303.03 (51.28) |
306.66 (77.98) |
385.09 (27.00) |
|
| effect size | 3.19 | 1.86 | 6.43 | |
| BNSTd | Saline | 23.05 (4.98) |
28.42 (11.64) |
28.50 (5.42) |
| LiCl | 139.17 (26.95) |
98.32 (36.73) |
178.98 (17.80) |
|
| effect size | 2.44 | 1.28 | 4.13 | |
| BNSTv | Saline | 30.40 (5.22) |
76.08 (31.61) |
30.51 (9.04) |
| LiCl | 52.92 (18.10) |
35.12 (6.13) |
49.43 (5.03) |
|
| effect size | 0.69 | −0.90 | 1.16 | |
| PVNmp | Saline | 37.99 (11.45) |
71.63 (33.43) |
21.21 (2.46) |
| LiCl | 235.25 (49.75) |
171.50 (45.51) |
265.29 (40.28) |
|
| effect size | 2.22 | 1.16 | 3.03 |
Activation of neurons at each of three rostro-caudal levels of the NTS appeared to be parallel to the effects found in the NTS as a whole. Subregional differences based on early life experience were not predicted in advance, so further statistical analyses are not presented here. Table 2 presents a summary of effect sizes at each rostrocaudal level.
Table 2.
The overall pattern of Fos responses to LiCl were parallel at three rostrocaudal levels of the visceral NTS. LiCl induced the largest activation in MS-180 rats, intermediate levels of activation in AFR rats, and the lowest levels of activation were seen in the MS-15 rats. All data are group means, ± (SEM). Effect sizes are given as LiCl versus Saline injection, in terms of Cohen's d.
| AFR | MS-15 | MS-180 | ||
|---|---|---|---|---|
|
caudal NTS |
saline | 11.39 (2.59) |
15.08 (4.30) |
10.31 (1.80) |
| licl | 76.87 (17.22) |
60.25 (15.83) |
95.75 (7.96) |
|
| effect size | 2.16 | 1.61 | 5.30 | |
|
NTS at the level of the AP |
saline | 26.73 (7.57) |
49.17 (23.26) |
30.27 (4.64) |
| licl | 78.25 (12.86) |
97.98 (21.23) |
85.32 (7.32) |
|
| effect size | 1.91 | 0.99 | 3.43 | |
|
rostral NTS |
saline | 26.01 (4.45) |
33.67 (6.76) |
19.10 (2.13) |
| licl | 37.80 (4.02) |
37.51 (2.27) |
38.87 (3.90) |
|
| effect size | 1.04 | 0.37 | 2.37 |
Noradrenergic NTS activation
Neither rearing group (F1,27=0.48, p=0.624), nor sex (F1,27=0.08, p=0.776), nor litter (F4,27=0.94, p=0.454) had a statistically significant effect on the number of DbH-positive cells in the NTS (Fig. 3B).
Injection of LiCl increased the proportion of DbH-positive NTS neurons activated to express Fos compared to activation after saline treatment in all groups (F1,26=33.20, p<0.001; Figure 3C). There were no significant effects of rearing group (F2,26=0.25, p=0.778), sex (F1,26=0.43, p=0.517), or litter (F4,26=0.94, p=0.453) on LiCl-induced Fos activation in DbH positive NTS neurons. However, effect sizes (Table 3) indicated that LiCl activated more noradrenergic NTS neurons in MS-180 rats (Cohen's d = 2.83 vs saline) than in AFR (d = 1.59) or MS-15 rats (d = 1.94).
Table 3.
Effect of early life experience on anatomy and function of viscerosensory circuits in the NTS. All data are group means, ± (SEM). Effect sizes for DbH population are given as the difference between each treatment group and the AFR rats, in terms of Cohen's d. Effect sizes for proportion of DbH cells activated are given as LiCl versus Saline injection, in terms of Cohen's d.
| AFR | MS-15 | MS-180 | ||
|---|---|---|---|---|
|
DbH Population |
Count | 29.18 (2.22) |
32.03 (2.62) |
28.66 (1.16) |
|
effect size |
-- | 0.34 | −0.07 | |
|
Proportion of DbH cells activated |
saline | 0.31 (0.05) |
0.31 (0.08) |
0.32 (0.06) |
| licl | 0.57 (0.07) |
0.61 (0.06) |
0.64 (0.04) |
|
|
effect size |
1.59 | 1.94 | 2.83 |
Activation of DbH-positive neurons by LiCl appeared uniform across rostrocaudal levels of the visceral NTS. Subregional differences based on early life experience were not predicted in advance, and further statistical analyses are not presented here. Table 3 presents a summary of effect sizes across the three rostrocaudal levels examined.
Area Postrema (AP)
Compared to saline injection, LiCl increased AP Fos counts in all three rearing groups (F1,25=22.08, p<0.001; Table 1; Fig. 4A). Effect sizes (Cohen's d; Table 1) show that the LiCl-induced increase in Fos expression was highest in the MS-180 group and comparable between the AFR and MS-15 groups. However, the general linear model revealed that the effect of rearing did not reach statistical significance (F2,25=0.369, p=0.69). Similarly, neither sex (F1,25=1.075, p=0.31) nor litter (F4,25=1.76, p=0.16) had a significant effect on LiCl-induced Fos expression. No interaction terms approached significance (data not shown), so they were dropped from the model results presented here.
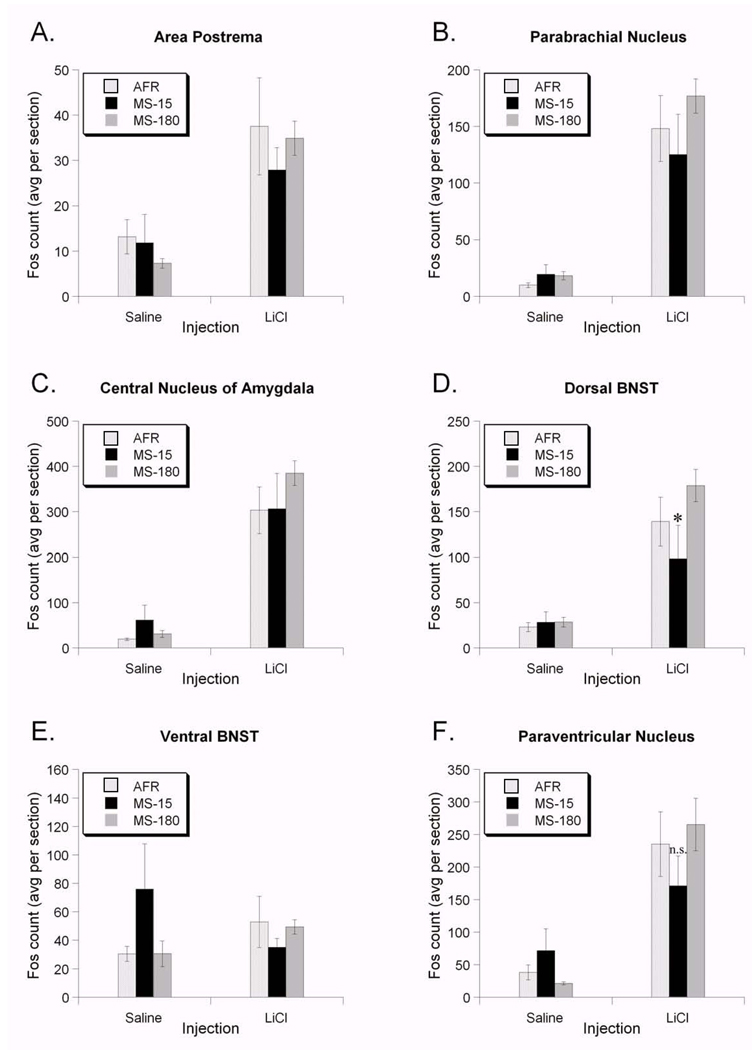
Figure 4.
Effect of early life experience on LiCl-induced Fos expression in several interoceptive brain regions. There was no statistically significant effect of early life experience on the degree of Fos expression within the AP (A), PBN (B), or CeA (C) after saline or LiCl injection. D. Early life experience significantly altered Fos responses to LiCl in the dorsal BNST. Rats in the MS-15 group had significantly lower Fos responses to LiCl than rats in the AFR and MS-180 groups. E. In the ventral BNST, Fos was not increased by LiCl injection. Rats in the MS-15 group had higher levels of Fos in response to saline injection compared to rats in the other two groups, although this did not reach statistical significance. There was no statistically significant effect of early life experience on the degree of Fos expression within the medial parvocellular PVN (F) after saline or LiCl treatment. All values are group means ± standard error. * p < 0.05.
Parabrachial Nucleus (PBN)
Compared to saline injection, LiCl increased Fos expression within the external lateral PBN in all three rearing groups (F1,25=57.4, p<0.001; Figure 4B). Effect sizes (Cohen's d; Table 1) show that the LiCl-induced increase in Fos expression was highest in the MS-180 group, intermediate in the AFR group, and lowest in the MS-15 group. However, the general linear model revealed that the effect of rearing did not reach statistical significance (F2,25=1.00, p=0.38). Similarly, neither sex (F1,25=1.34, p=0.25) nor litter (F3,25=1.17, p=0.34) had a significant effect on LiCl-induced Fos expression within the PBN. No interaction terms approached significance (data not shown), so they were dropped from the model results presented here.
Central Nucleus of the Amygdala (CeA)
Compared to saline injection, LiCl increased Fos expression in the CeA in all three rearing groups (F1,25=77.82, p<0.001; Figure 4C). Effect sizes (Cohen's d; Table 1) show that the LiCl-induced increase in Fos expression was highest in the MS-180 group, intermediate in the AFR group, and lowest in the MS-15 group. However, the general linear model revealed that the effect of rearing did not reach statistical significance (F2,25=1.73, p=0.19). Similarly, neither sex (F1,25=0.566, p=0.459) nor litter (F4,25=2.25, p=0.09) had a significant effect on LiCl-induced Fos expression. No interaction terms approached significance (data not shown), so they were dropped from the model results presented here.
Bed Nucleus of the Stria Terminalis (BNST)
Dorsal BNST
Compared to saline injection, LiCl increased Fos expression in the dorsal BNST in all three rearing groups (F1,19=56.43, p<0.001; Figure 4D; Figure 5). The general linear model revealed that rearing group alone did not affect Fos expression (F2,19=3.72, p=0.43). However, as we predicted, there was a significant interaction between early life experience and the magnitude of the LiCl-induced increase in Fos expression (F1,19=3.661, p=0.04).
Figure 5.
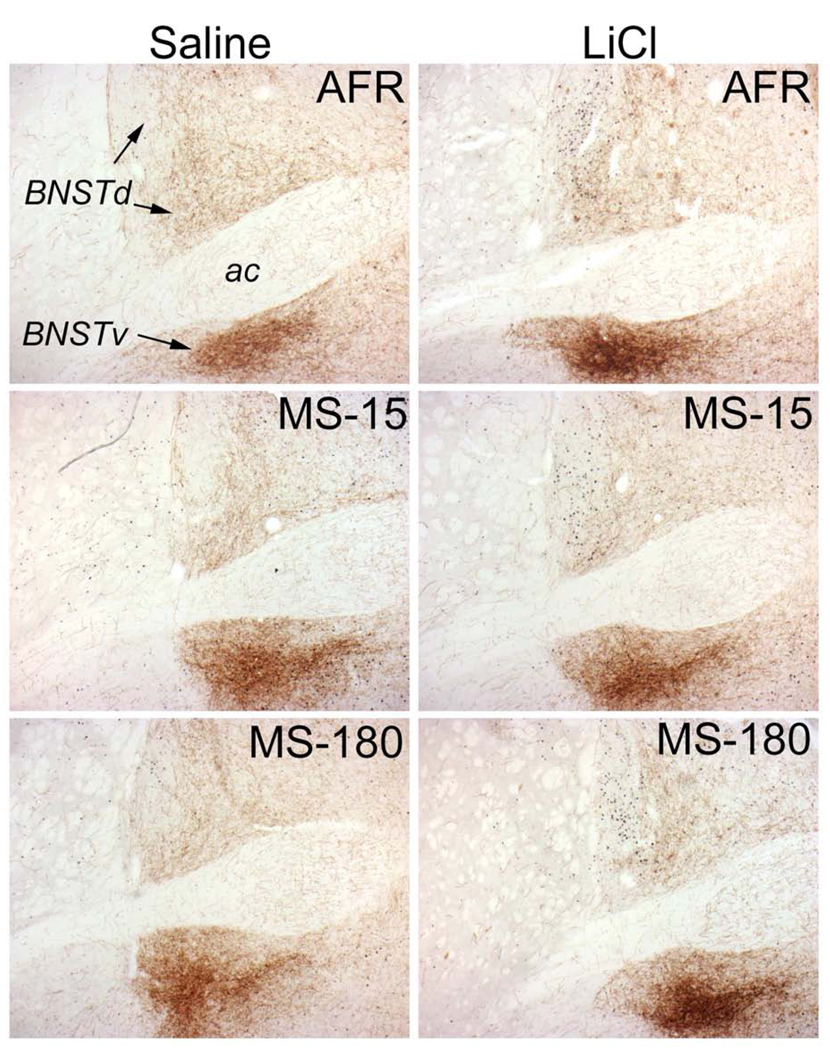
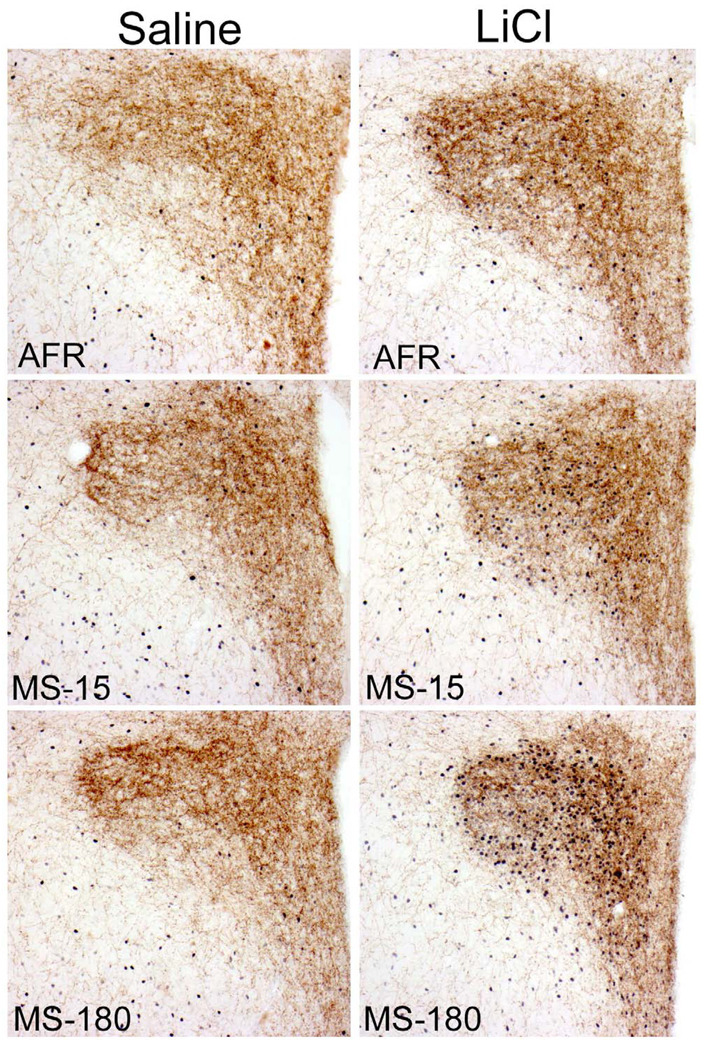
Color images of saline- and LiCl-induced Fos expression within the BNST. Tissue sections were double-labeled for cFos and DbH to identify activated neurons within noradrenergic terminal fields. Rats injected with saline (left column) display fewer Fos-positive neurons compared to rats injected with LiCl (right column). There was a statistically significant effect of early life experience on the degree of Fos expression within the dorsal BNST (see Figure 4D). Rats in the MS-15 group (center) had lower Fos responses to LiCl compared to rats in the AFR (top) and MS-180 (bottom) groups.
There were statistically significant differences based on sex (F1,19=6.01, p=0.02), and the sex*injection interaction was also significant (F1,19=6.77, p=0.017). However, there was no significant rearing*sex interaction (F1,19=1.26, p=0.30), nor any effect of litter (F1,19=1.10, p=0.38). Sex effects were not explored in depth owing to the small sample sizes. Linear contrast analysis (see Methods) demonstrated that these data are well fit by the hypothesis that the dorsal BNST in MS-180 rats has the highest Fos response to LiCl, the AFR group an intermediate response, and the MS-15 group the lowest response (Rcontrast=0.711, t30=5.540, p<0.001; see Fig. 4D).
Ventral BNST
Fos expression in the ventral BNST (Figure 4E; Figure 5) was less robust than in the dorsal BNST (Figure 4D; Figure 5). Effect sizes (Cohen's d; Table 1) show that the LiCl-induced increase in Fos expression was highest in the MS-180 group, intermediate in the AFR group, and lowest in the MS-15 group. Indeed, saline injected MS-15 rats had higher Fos counts than the LiCl-injected MS-15 rats, evidenced by the negative effect size (Table 1). The general linear model revealed that the effects of injection (F1,25=0.29, p=0.59) and rearing (F2,25=0.40, p=0.67) did not reach statistical significance. Similarly, neither sex (F1,25=1.15, p=0.29) nor litter (F4,25=1.26, p=0.31) had a significant effect on LiCl-induced Fos expression. No interaction terms approached significance (data not shown), so they were dropped from the model results presented here.
Paraventricular Nucleus of the Hypothalamus (PVN)
Compared to saline injection, LiCl increased PVN Fos expression in all three rearing groups (F1,25=27.19, p<0.001; Figure 4F; Figure 7). Effect sizes (Cohen's d; Table 1) show that the LiCl-induced increase in Fos expression was highest in the MS-180 group, intermediate in the AFR group, and smallest in the MS-15 group. However, the general linear model revealed that the effect of rearing did not reach statistical significance (F2,25=0.34, p=0.71). Similarly, neither sex (F1,25=0.83, p=0.36) nor litter (F1,25=0.13, p=0.96) had a significant effect on LiCl-induced Fos expression in the PVN. No interaction terms approached significance (data not shown), so they were dropped from the model results presented here.
Discussion
Early postnatal life events can strongly influence the functional development of central neural systems that mediate the expression of behavioral, emotional, autonomic, and endocrine responses to stress [19,22,40,41]. Although central visceral and emotional neural circuits are largely coextensive, only limited research has been directed towards understanding how these circuits are shaped by developmental events that profoundly impact later emotionality and stress responsiveness. Our experimental outcomes provide new evidence regarding the biological impact of early life experience on central circuits that respond to a prototypical interoceptive stressor (i.e., LiCl), and that are known to contribute to behavioral and physiological responses to this challenge.
In agreement with results from previous studies in adolescent and mature rats exposed as neonates to repeated brief maternal separation [8,22,42], in the present study rats with a developmental history of MS-15 displayed less anxiety-like behavior on the EPMZ compared to rats in both the AFR and MS-180 rearing groups. Although the behavior of MS-180 rats on the EPMZ did not differ from that of AFR rats, this finding was not entirely unexpected. The ability of MS-15 to reduce later stress responsiveness and anxiety-like behavior seems to be more robust and reproducible across laboratories compared to the smaller and more variable effects of MS-180 to increase stress responsiveness and anxiety-like behavior [42–47]. Based on this evidence of early experience-induced alterations in later behavioral responsiveness to an exteroceptive stressor (i.e., the EPMZ), we predicted that the ability of an interoceptive stressor (i.e., LiCl) to activate central neural Fos expression would be blunted in MS-15 rats as compared to MS-180 rats and AFR controls. We also expected that potential between-group differences in LiCl Fos responsiveness would be most apparent within the hypothalamus and limbic forebrain, and less so within the caudal brainstem. The latter prediction was based on our earlier study demonstrating that brainstem Fos responses to LiCl mature earlier than hypothalamic and limbic forebrain Fos responses during the first few weeks of postnatal development [21], suggesting that brainstem regions may be less sensitive to potential modulatory effects of early life experience. However, at least a portion of the brainstem Fos response to LiCl likely occurs subsequent to recruitment of hypothalamic and limbic forebrain regions that project to the brainstem; thus, we also expected that any rearing group-related changes in forebrain Fos responses to LiCl might contribute to altered brainstem Fos responses.
Overall, the Fos activation data were consistent with our predictions (see Table 1 for summary data and effect sizes). Postnatal rearing group exerted nonsignificant effects on LiCl-induced Fos activation within most brain regions examined, except for the dorsal BNST, discussed below. However, Cohen’s d (a measure of effect size) revealed that LiCl exerted the largest effects to increase Fos expression across brain regions within MS-180 rats, intermediate effects in AFR control rats, and the smallest effects in MS-15 rats. This was true for LiCl-induced Fos activation within the NTS (including DbH-positive noradrenergic NTS neurons), PBN, CeA, and PVN. It also was true for Fos activation within the dorsal BNST, in which neural Fos responses to LiCl treatment were significantly attenuated in MS-15 rats compared to MS-180 rats and AFR controls.
The BNST is known to play a critical role in mediating anxiety-like behaviors (reviewed in [48,49]), and also participates importantly in stressor-induced activation of CRH-positive PVN neurons at the apex of the HPA axis (reviewed in [50]). In light of the dBNST Fos results, a subsequent reanalysis of the EPMZ data revealed substantial correlations between LiCl-induced Fos counts within dBNST and the ratio of time spent in the open vs closed arms of the maze by rats within the same rearing groups. In AFR rats, a modest but nonsignificant correlation between LiCl induced Fos and behavior was found (r=0.611, p=0.098), while MS-15 and MS-180 rats both showed large and significant correlations (r=0.976, p<0.005, and r=0.751, p=0.015, respectively). Thus, LiCl treatment may recruit central neural circuits that at least partially overlap circuits that mediate emotional and behavioral responses to EPMZ exposure. Ongoing studies are testing this prediction.
LiCl-induced Fos activation within the medial parvocellular PVN did not differ significantly between developmental treatment groups. Activation of the PVN, as assayed by stress-induced release of corticotropic releasing hormone, is substantially modified in response to manipulation of early life experience (see also [16,40,51]). Our data are broadly consistent with these findings, as PVN Fos activation in response to LiCl was lower (albeit non-significantly) in MS-15 rats compared to MS-180 rats and AFR controls (Figure 4F). The effect of developmental rearing may have been too small to generate statistical significance in samples of the size used in the present study. Alternatively, or in addition, our method of scoring Fos-positive cells by the mere presence of visible nuclear immunolabeling does not provide information regarding the strength of neuronal activation [26], which may have differed among rearing groups. It also is possible that the LiCl dose used in this study (i.e., 0.15M, 1% body weight) was high enough to create a ceiling effect, thereby obscuring any differential effects of rearing condition on Fos activation within the PVN or other brain regions that showed trends for differences that did not reach statistical significance. That seems unlikely, however, as this dose is half that typically used to study the effects of LiCl on central Fos activation (e.g., [24,52]). Moreover, early life experience did alter the pattern of Fos responses to LiCl in the dorsal BNST, evidence that large effects could be revealed within this data set. It remains to be determined whether different doses of LiCl or alternative interoceptive stressors affect the BNST, PVN, and other brain regions in different ways.
We are aware of one previous study that showed a difference in neural activation in the BNST as a result of manipulating early life experience in rat puts. Abraham and Kovacs [53] investigated Fos responses in the BNST of rats after brief maternal separations repeated daily from P0–P21, which reduced later Fos responses to restraint stress and ether exposure. Our own results with LiCl extend these findings by showing that viscerosensory stimuli have differential effects in AFR, MS-15, and MS-180 rats.
The effects of early experience on circuit development were previously probed by Card and coworkers [20], who showed that both MS-180 and MS-15 groups had fewer gastric pre-autonomic neurons overall in the BNST compared to AFR controls. In that study, there was no difference in gastric pre-autonomic neuron number between MS-180 and MS-15 rats. Our results, showing differential levels of dorsal BNST Fos activation in response to LiCl treatment across all three rearing groups, suggest that dorsal BNST responsiveness to an interoceptive challenge is altered, in addition to circuit anatomy. More recently, Banihashemi and Rinaman [54] demonstrated increased numbers of gastric pre-autonomic neurons in the PVN in adolescent rats with a developmental history of MS-15, further evidence that central visceral circuits can be altered by early postnatal experience.
Few studies have directly examined both the behavioral and functional neural consequences of altered early life experience. We have demonstrated that individual differences in neural responses to a stressful interoceptive challenge (i.e., LiCl injection) can be traced back to early life experiences that are known to shape the development and function of central visceral pathways that include the BNST, a region known for its role in behavioral responses to stressful and emotionally evocative events (48,49). Our new findings support the view that early life experience can shape the developmental trajectory of central visceral circuits, and suggest that altered central responses to interoceptive cues may contribute to the impact of early experience on later emotionality and stress responsiveness.
Figure 6.
Color images of saline and LiCl-induced Fos expression within the PVN. Tissue sections were double-labeled for cFos and DbH to identify activated neurons within noradrenergic terminal fields. Rats injected with saline (left column) display less Fos immunolabeling compared to rats injected with LiCl (right column). Rats in the MS-15 group (center) tended to have lower Fos responses to LiCl than rats in the AFR (top) and MS-180 (bottom) groups, though this effect did not reach statistical significance (see Fig. 4F for quantitative data).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supported by NIH grants T32 MH18273, 1F32MH075586, and MH59911
References
- 1.Levine S, Chevalier JA, Korchin SJ. The effects of early handling and shock on later avoidance behavior. Journal of Personality. 1956;24:475–493. doi: 10.1111/j.1467-6494.1956.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 2.Levine S. Infantile experience and resistance to stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- 3.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Molecular Brain Research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 4.Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiology and Behavior. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- 5.Huot RL, Gonzalez ME, Ladd CO, Thrivikraman KV, Plotsky PM. Foster litters prevent hypothalamic-pituitary-adrenal axis sensitization mediated by neonatal maternal separation. Psychoneuroendocrinology. 2004;29:279–289. doi: 10.1016/s0306-4530(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 6.Ladd CO, Huot RL, Thriikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. In: Mayer EA, Saper CB, editors. Progress in Brain Research. Vol. 122. 2000. pp. 81–103. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 8.Macri S, Mason GJ, et al. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring's HPA and fear responses in rats. European Journal of Neuroscience. 2004;20:1017–1024. doi: 10.1111/j.1460-9568.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- 9.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 10.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 11.Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ. Epigenetic programming of stress responses through variations in maternal care. Ann N Y Acad Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- 12.Denenberg VH. Commentary: Is maternal stimulation the mediator of the handling effect in infancy? Developmental Psychobiology. 1999;34:1–3. doi: 10.1002/(sici)1098-2302(199901)34:1<1::aid-dev2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Bhatnagar S, Shanks N, Plotsky PM, Meaney MJ. Hypothalamic-pituitary-adrenal responses in neonatally handled and nonhandled rats: differences in facilitatory and inhibitory neural pathways. In: McCarthy R, Agulara G, Sabba E, Stress R, editors. Molecular and Neurobiological Advances. New York: Gordon & Breach; 1996. pp. 1–24. [Google Scholar]
- 14.Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Developmental Neuroscience. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 16.Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- 17.Schwetz I, McRoberts JA, Coutinho SV, Bradesi S, Gale G, Fanselow M, Million M, Ohning G, Tache Y, Plotsky PM, Mayer EA. Corticotropin-releasing factor receptor 1 mediates acute and delayed stress-induced visceral hyperalgesia in maternally separated Long-Evans rats. American Journal of Physiology - Regulatory, Integrative, and Comparative Physiology. 2005;289:G704–G712. doi: 10.1152/ajpgi.00498.2004. [DOI] [PubMed] [Google Scholar]
- 18.Macrì S, Würbel H. Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm Behav. 2006;50:667–680. doi: 10.1016/j.yhbeh.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Rinaman L, Koehnle TJ. Development of Central Visceral Circuits. In: Blumberg M, Freeman J, Robinson S, editors. Handbook of Developmental Behavioral Neuroscience. Oxford University Press; 2010. [Google Scholar]
- 20.Card JP, Levitt P, Gluhovsky M, Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. J Neurosci. 2005;25:9102–9011. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koehnle TJ, Rinaman L. Progressive postnatal increases in Fos Immunoreactivity in the forebrain and brainstem of rats after viscerosensory stimulation with lithium chloride. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2007;292:R1212–R1223. doi: 10.1152/ajpregu.00666.2006. [DOI] [PubMed] [Google Scholar]
- 22.Weber BC, Manfredo HH, Rinaman LA. Potential gastrointestinal link between enhanced postnatal maternal care and reduced anxiety-like behavior in adolescent rats. Behav Neurosci. 2009;123:1178–1184. doi: 10.1037/a0017659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Rinaman L, Dzmura V. Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1495–R1503. doi: 10.1152/ajpregu.00393.2007. [DOI] [PubMed] [Google Scholar]
- 25.Spencer CM, Houpt TA. Dynamics of c-fos and ICER mRNA expression in rat forebrain following lithium chloride injection. Brain Res Mol Brain Res. 2001;93:113–126. doi: 10.1016/s0169-328x(01)00173-5. [DOI] [PubMed] [Google Scholar]
- 26.Rinaman L, Stricker EM, Hoffman GE, Verbalis JG. Central c-Fos expression in neonatal and adult rats after subcutaneous injection of hypertonic saline. Neurosci. 1997;79:1165–1175. doi: 10.1016/s0306-4522(97)00022-5. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The Rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 28.Bancroft TA. Analysis and inference for incompletely specified models involving the use of preliminary tests(s) of significance. Biometrica. 1964;20:427–442. [Google Scholar]
- 29.Rosenthal R, Rosnow RL, Rubin DB. Contrasts and effect sizes in behavioral research: a correlational approach. New York: Cambridge University Press; 2000. [Google Scholar]
- 30.Furr RM, Rosenthal R. Understanding Statistics. 1. Vol. 2. Lawrence Earlbaum; 2003. Evaluating Theories Efficiently: The Nuts and Bolts of Contrast Analysis; pp. 33–67. [Google Scholar]
- 31.Rinaman L, Levitt P. Establishment of vagal sensory-motor circuits during fetal development in rats. Journal of Neurobiology. 1993;24:641–659. doi: 10.1002/neu.480240509. [DOI] [PubMed] [Google Scholar]
- 32.Rinaman L, Hoffman GE, Stricker EM, Verbalis JG. Exogenous cholecystokinin activates cFos expression in medullary but not hypothalamic neurons in neonatal rats. Brain Res Dev Brain Res. 1994;77:140–145. doi: 10.1016/0165-3806(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 33.Rinaman L. Postnatal development of vagal-hypothalamic circuits in rats. Society for Neuroscience Abstracts. 1996;22 159.10. [Google Scholar]
- 34.Rinaman L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J Comp Neurol. 1998;399:101–109. doi: 10.1002/(sici)1096-9861(19980914)399:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Rinaman L, Levitt P, et al. Progressive postnatal assembly of limbic-autonomic circuits revealed by central transneuronal transport of pseudorabies virus. The Journal of Neuroscience. 2000;20:2731–2741. doi: 10.1523/JNEUROSCI.20-07-02731.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rinaman L. Postnatal development of catecholamine inputs to the paraventricular nucleus of the hypothalamus in rats. The Journal of Comparative Neurology. 2001;438:411–422. doi: 10.1002/cne.1324. [DOI] [PubMed] [Google Scholar]
- 37.Rinaman L. Postnatal development of hypothalamic inputs to the dorsal vagal complex in rats. Physiology and Behavior. 2003;9:65–70. doi: 10.1016/s0031-9384(03)00105-7. 7. [DOI] [PubMed] [Google Scholar]
- 38.Cohen J. Statistical Power Analysis for the Behavioral Sciences. NY: Academic Press; 1969. [Google Scholar]
- 39.Cohen J. The Earth is Round (p<.05) American Psychologist. 1994;49:997–1003. [Google Scholar]
- 40.Francis DD, Caldji C, et al. The role of corticotropin-releasing factor-norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biological Psychiatry. 1999;46:1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- 41.Gunnar MB. Quality of early care and buffering of neuroendocrine stress reactions: potential effects on the developing human brain. Preventive Medicine. 1998;27:208–211. doi: 10.1006/pmed.1998.0276. [DOI] [PubMed] [Google Scholar]
- 42.McIntosh J, Anisman H, Merali Z. Short- and long-periods of neonatal maternal separation differentially affect anxiety and feeding in adult rats: gender-dependent effects. Brain Res Dev Brain Res. 1999;113:97–106. doi: 10.1016/s0165-3806(99)00005-x. [DOI] [PubMed] [Google Scholar]
- 43.Roman E, Gustafsson L, Berg M, Nylander I. Behavioral profiles and stress-induced corticosteroid secretion in male Wistar rats subjected to short and prolonged periods of maternal separation. Horm Behav. 2006;50:736–747. doi: 10.1016/j.yhbeh.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Slotten HA, Kalinichev M, Hagan JJ, Marsden CA, Fone KC. Long-lasting changes in behavioural and neuroendocrine indices in the rat following neonatal maternal separation: gender-dependent effects. Brain Res. 1006;1097:123–132. doi: 10.1016/j.brainres.2006.04.066. [DOI] [PubMed] [Google Scholar]
- 45.Daniels WM, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 2004;19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- 46.Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Pharmacol Biochem Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 47.Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Reviews in the Neurosciences. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- 48.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 49.Davis M, Shi C. The Extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann. NY Acad. Sci. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- 50.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responseiveness. Front. Neuroeno. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Troakes C, Ingram CD. Anxiety behaviour of the male rat on the elevated plus maze: associated regional increase in c-fos mRNA expression and modulation by early maternal separation. Stress. 2008;2:1. doi: 10.1080/10253890802506391. [DOI] [PubMed] [Google Scholar]
- 52.Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. Am J Physiol. 1999;277:R1537–R1540. doi: 10.1152/ajpregu.1999.277.5.R1537. [DOI] [PubMed] [Google Scholar]
- 53.Abrahám IM, Kovács KJ. Postnatal handling alters the activation of stress-related neuronal circuitries. Eur J Neurosci. 2000;12:3003–3014. doi: 10.1046/j.1460-9568.2000.00176.x. [DOI] [PubMed] [Google Scholar]
- 54.Banihashemi L, Rinaman L. Repeated brief postnatal maternal separation enhances hypothalamic gastric autonomic circuits in juvenile rats. Neuroscience. 2010;165:265–277. doi: 10.1016/j.neuroscience.2009.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]