Abstract
Eastern equine encephalomyelitis (EEE) was diagnosed in 19 horses and a flock of emus in the province of Quebec in fall 2008. The EEE virus caused unusual gross lesions in the central nervous system of one horse. This disease is not usually present in Quebec and the relation between the outbreak and favorable environmental conditions that summer are discussed.
Résumé
Éclosion d’encéphalomyélite équine de l’Est (ÉÉE) au Québec à l’automne de 2008. Une éclosion d’encéphalomyélite équine de l’Est (ÉÉE) s’est produite chez 19 chevaux et dans un troupeau d’émeus au Québec à l’automne de 2008. Un cheval présentait une lésion macroscopique anormale dans le système nerveux central. Les causes de cette éclosion, en rapport avec les conditions environnementales favorables, sont discutées.
(Traduit par les auteurs)
Eastern equine encephalomyelitis (EEE) is an arthropod-borne viral encephalitis caused by an Alphavirus, which is endemic in North America, especially in the United States Atlantic coastal states. This disease is of particular importance in horses and humans, in whom it is frequently lethal (1). Birds generally act as reservoir hosts but some bird species (such as ratites) are highly susceptible and infection is associated with a high mortality (2,3). The main EEE transmission cycle is between birds and mosquitos, Culiseta melanura being the most important in maintaining the enzootic transmission cycle. Mosquitos that feed on both birds and mammals (bridge vectors), such as Aedes, Culex, and Coquilletidia genera, can become infected with the EEE virus and are responsible for transmitting the disease to mammals (4). A rise in these insect populations is expected due to climate changes in eastern Canada and a parallel rise in the number of EEE cases could follow (5). In Canada, EEE is only reported sporadically, generally in the southern part of Ontario where the last outbreak (12 cases) occurred in 2003 (6). After 5 y of relative calm, numerous cases (8) were again diagnosed there in 2008 (7). In Quebec, this disease is rare and was reported only in 1972 and again, in a single horse, in 1999 (8). We describe herein the emergence of this disease in fall 2008 in Quebec in several horses and a flock of emus.
Case description
On September 8, 2008, an 8-year-old female Quarter horse was presented to the Equine Hospital of the Centre Hospitalier Universitaire Vétérinaire de l’Université de Montréal for weakness. The mare was alert and healthy the day before the onset of clinical signs. On the morning of September 8, the mare was found weak, with its head down and tilted to the left. A few hours later, the mare became recumbent and was referred to our hospital. On arrival, the mare was ambulatory but extremely weak and severely ataxic. She was tachycardic (52 beats/min) and tachypneic (28 breaths/min). Her rectal temperature was 37.1°C. Other aspects of the physical examination were unremarkable. A neurological examination revealed dorsal strabismus of the left eye. The direct and indirect pupillary light reflexes were bilaterally normal. The menace response was absent on both sides and the lingual tone was weak. Additional diagnostic tests included a complete blood (cell) count (CBC), serum biochemistry, abdominal ultrasonography, and urine analysis, all of which revealed no abnormalities.
Based on the central neurological signs in the mare, a primary neurological disease was considered; however, cervical vertebral and skull radiographs revealed no abnormalities. An atlanto-occipital cerebrospinal fluid (CSF) tap under general anesthesia did not show any abnormalities on gross examination of the CSF. Total protein concentration was within normal limits [0.86 g/L; reference range: 0.05 to 1 g/L (9)]. Cytological examination of the fluid showed a strong neutrophilic pleiocytosis [618 × 106 leukocytes/L; reference range: 0 to 6 × 106 leukocytes/L (9)] without evidence of microorganisms.
Differential diagnoses included viral (EEE, West Nile virus, rabies), bacterial (Streptococcus equi, Rhodococcus equi) or verminous (Halicephalobus sp.) encephalomyelitis, bacterial meningitis, or toxicosis (fumonisin B1). The mare became extremely agitated during the next hours despite supportive treatment and did not respond to sedative medication. Due to the deterioration in her condition, the owners opted for euthanasia.
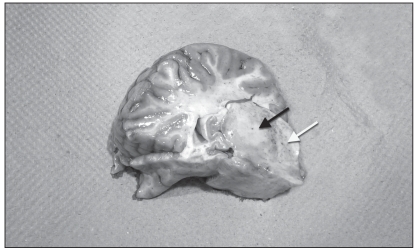
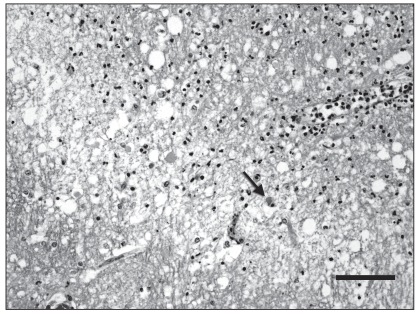
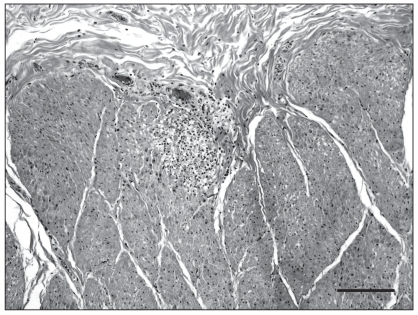
A complete necropsy was performed. On gross examination, there was diffuse flattening of cerebral gyri, suggesting cerebral edema. On cut section, a 2-cm, well-delineated, yellowish and soft focus was present in the left thalamus, surrounded by numerous microhemorrhages (Figure 1). Examination of the brain with a UV lamp showed mild fluorescence in this thalamic area and multifocally throughout the cerebral cortex. Multiple small hemorrhages were scattered all along the spinal cord, especially in the dorsal horns of the grey matter. No significant findings, except for diffuse and moderate pulmonary edema, were present in other organs. Microscopic examination of the brain revealed scattered but extensive neuronal necrosis with satellitosis, gliosis, and spongiosis, more pronounced in the cerebral cortex and thalamus, and marked, perivascular to diffuse, predominantly neutrophilic inflammation with congestion and perivascular hemorrhage. There was also a mild and multifocal infiltration of the leptomeninges by lymphocytes and neutrophils. The yellow focus observed grossly in the thalamus was a sharply circumscribed zone with necrotic neurons, marked vacuolation of the neuropil and severe neutrophilic infiltration (Figure 2). Lesions similar to those present in the brain were found in all segments of the spinal cord, mainly in the grey matter but also multifocally in the white matter. Another interesting finding was the presence of small foci of myodegeneration in the external muscularis of the small intestine, with mild infiltration by lymphocytes and macrophages (Figure 3). Cerebral and visceral tissues were positive for EEE by the immunoperoxidase test (Penn State University) and culture (NCFAD, Winnipeg). The brain was negative by immunofluorescent antibody (IFA) test for rabies and bacteriology yielded no significant finding in samples of cerebral cortex, cerebellum and spinal cord.
Figure 1.
Transverse section of left thalamocortex (index horse). There is a fairly well-delineated, 2-cm, pale focus in the thalamus (black arrow). Note also numerous microhemorrhages in the surrounding neuroparenchyma (white arrow).
Figure 2.
Microphotograph of the left thalamus (index horse). This is the junction between the necrotic zone (up right) and surrounding thalamus (down left). There is intense vacuolization of the neuropil and infiltration by numerous neutrophils in the necrotic area. Note a necrotic neuron (arrow). Hematoxylin-eosin-saffron (HES). Bar = 50 μm.
Figure 3.
Microphotograph of the jejunum (index horse). There is focal myodegeneration in the external muscularis, with mild infiltration by lymphocytes and macrophages. HES. Bar = 100 μm.
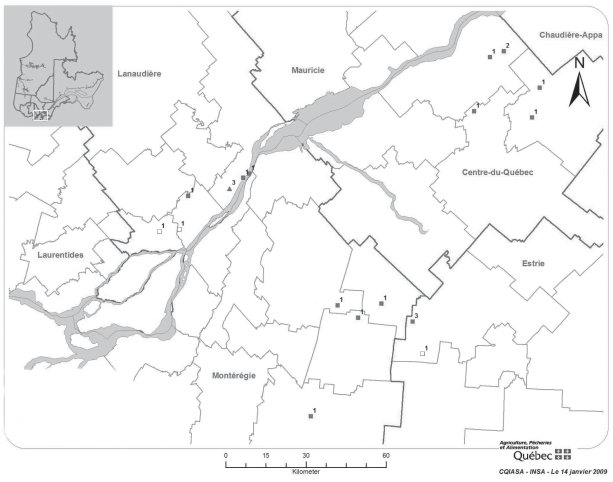
By October 14, 2008, EEE had been confirmed in a total of 19 horses from Quebec, by PCR and/or viral culture and/or immunohistochemistry (IHC) and/or serology (IgM capture and/or hemagglutination inhibition). Three horses were diagnosed by serology alone: these cases were classified, according to the OIE requirements for notification, as “presumptive” (Table 1). Two horses were diagnosed retrospectively by serology. Four regions were involved: Estrie, Montérégie, Centre-du-Québec, and Lanaudière (Figure 4). The average age of affected horses was 7 1/2 y, without sex or breed predilection. The main clinical signs in horses were ataxia, fever, and finally recumbency. In most cases, the clinical course was very short (24 to 48 h). All the affected horses were euthanized or died from the disease, except one diagnosed by serology, which survived with almost no sequela following symptomatic treatment. None of the horses had been vaccinated against EEE. No horse other than the index case had gross lesions.
Table 1.
Cases of eastern equine encephalomyelitis (EEE), Quebec, 2008, with results from diagnostic tests and OIE status for notification
| Case | Date of onset of clinical signs | Area | Viral isolation | PCR | IHC | Serology | Status (OIE) |
|---|---|---|---|---|---|---|---|
| Horse 1 | Sept. 1 2008 | Lanaudière | NP | NP | NP | + | P |
| Horse 2 | Sept. 6 2008 | Lanaudière | NP | NP | NP | + | P |
| Horse 3 | Sept. 8 2008 | Estrie | + | NP | + | NP | C |
| Horse 4 | Sept. 11 2008 | Estrie | + | NP | + | NP | C |
| Horse 5 | Sept. 17 2008 | Montérégie | + | + | + | NP | C |
| Horse 6 | Sept. 21 2008 | Estrie | NP | NP | + | NP | C |
| Emus (3) | Sept. 21 2008 | Lanaudière | + | + | NP | NP | C |
| Horse 7 | Sept. 22 2008 | Centre-du-Québec | − | + | + | + | C |
| Horse 8 | Sept. 24 2008 | Montérégie | + | + | + | + | C |
| Horse 9 | Sept. 30 2008 | Centre-du-Québec | NP | NP | + | + | C |
| Horse 10 | Sept. 30 2008 | Estrie | NP | NP | NP | + | P |
| Horse 11 | Oct. 1 2008 | Lanaudière | − | NP | + | NP | C |
| Horse 12 | Oct. 4 2008 | Lanaudière | + | NP | NP | NP | C |
| Horse 13 | Oct. 5 2008 | Centre-du-Québec | + | NP | + | NP | C |
| Horse 14 | Oct. 5 2008 | Lanaudière | NP | NP | + | − | C |
| Horse 15 | Oct. 7 2008 | Montérégie | NP | NP | + | NP | C |
| Horse 16 | Oct. 7 2008 | Centre-du-Québec | − | NP | + | + | C |
| Horse 17 | Oct. 11 2008 | Montérégie | NP | NP | + | NP | C |
| Horse 18 | Oct. 13 2008 | Centre-du-Québec | NP | NP | + | NP | C |
| Horse 19 | Oct. 14 2008 | Centre-du-Québec | NP | + | NP | NP | C |
+ — positive; − — negative; NP — not performed; P — presumptive; C — confirmed.
Figure 4.
Southern Quebec, St. Lawrence River crossing the map. Location and number of EEE positive cases. Black squares — confirmed equine cases. White squares — presumptive equine cases. Triangles — confirmed emu cases.
Microscopic lesions were similar in all horses with varying degrees of severity. Neutrophils were not evident in the brain of all horses, especially in cases with a longer clinical course. Muddy waters or wetlands along the St. Lawrence River or stagnant water in cranberry fields were found close to at least 3 farms, including a farm where 3 horses had died. Most owners reported a higher than average population of mosquitoes in August and September.
During the same period, an emu breeding farm in the Lanaudière region also reported heavy losses. Out of 35 birds, 16 died: they were mostly adults but some were juveniles. Most of the emu showed clinical signs compatible with EEE in this species, such as vomiting, bloody diarrhea, and dullness. Neurological signs were minimal and included ataxia in a few animals. A necropsy was performed on 3 birds. The main gross lesion was a severe necrotizing and hemorrhagic enteritis (Figure 5). Microscopic examination of tissues also revealed necrotic foci in the liver and spleen, as well as fibrinoid necrosis of splenic sheathed arterioles and small vessels in the intestinal lamina propria with subsequent necrosis and hemorrhages. The presence of EEE virus was confirmed by PCR and viral isolation in all 3 individuals. There was a variety of mammals and birds at this farm but no animals were sick except for emus. No human cases of EEE were diagnosed or suspected in Quebec during the outbreak.
Figure 5.
Segment of small intestine (emu). There is severe bowel distension by dark, unclotted blood and thickening of the intestinal wall.
An entomological survey was conducted in and around outbreak foci in the areas of Estrie and Lanaudière at the beginning of October. Due to the deterioration of weather conditions (freezing) between the first clinical cases and the period of insect trapping, only 118 mosquito specimens were captured. The most abundant group was Culex spp. Analyses by polymerase chain reaction (PCR) did not demonstrate the presence of EEE virus genetic material in the collected specimens.
Discussion
Eastern equine encephalitis is an uncommon disease in Canada and has been diagnosed only twice in Quebec during the last 40 y (8). So what happened in 2008 that created favorable conditions for the emergence of EEE in Quebec? First, this province is especially vulnerable to EEE clinical cases because, unlike Ontario and adjacent US states, its horse population is not routinely vaccinated against this virus and is thus sensitive to any increase in the mosquito viral load.
As the EEE prevalence is related to the presence and number of mosquito vectors, we can hypothesize that environmental conditions, especially rainfall, had been more favorable for mosquitoes in Quebec in 2008. In fact, several clinical cases occurred in farms close to swamps, muddy waters, or the St. Lawrence river marshes. Owners of sick animals also felt that there were more mosquitoes than normal in August and September; this assertion was impossible to confirm due to a rapid fall in temperature between the onset of clinical cases and the setting of insect traps. A link has been demonstrated between the occurrence of human EEE cases and the amount of precipitation in some regions of the US, especially autumn rainfall (August to October) of the year preceding the epidemic and summer rainfall (June to August) of the outbreak year (10). This could also have had an effect on the number of equine cases. When we compare the total precipitation in one of the most affected areas (Sherbrooke, Estrie) in June, July, and August 2008 (197, 206, and 154 mm, respectively — data were incomplete in the database for July and August, which indicates that these amounts were higher in reality), with previous years (86, 77, and 138 mm in 2007; 129, 60, and 297 mm in 2006), it seems there was more total rain in summer 2008 (557 mm) compared with the 2 preceding years (301 mm in 2007; 486 mm in 2006) (11). However, the amount of rainfall in the fall of 2007 (76 mm in September, 130 mm in October) was not abnormally high compared with the preceding years (56 and 163 mm in 2006; 102 and 208 mm in 2005) (11).
It has been speculated that in the last epizootic around Lac Brome (Quebec) in 1972 high winds from the south could have played a role in bringing infected insects from Connecticut to Quebec and initiating the propagation of the disease in this area (12). Unlike the 1972 epizootic in which all cases were distributed in a small area and clearly linked to a preceding outbreak in Connecticut some days before, the cases in 2008 were sparsely located throughout the St. Lawrence river lowlands, and the incidence of EEE cases in northeastern USA in 2008 was not unusually high compared to preceding years (12,13). Thus, in the 2008 epidemic, the relationship between winds and onset of the disease is more difficult to establish; more meteorological values are needed to investigate this hypothesis.
The annual average temperature rose 0.5°C to 1.2°C between 1960 and 1993 in southern Quebec, thus leading the Ouranos team on climate change to conclude that there was a warming tendency in the province (14). Public health authorities fear that diseases not present in Quebec but endemic in the USA, such as EEE, could eventually become more prevalent as elevated temperatures favor the survival and establishment of vectors in the northern part of the continent (5,14). The phenomenon of emerging “exotic” diseases partially related to climate change has been experienced in Europe with the appearance of Bluetongue and Chikungunya viruses, but this phenomenon remains to be confirmed in North America (15).
Studies have demonstrated that the EEE virus can overwinter in isolated northeastern regions including upstate New York, which borders Quebec (16,17). Other outbreaks should be expected to happen here in the future. The process by which the virus maintains itself in a region is unknown, but doesn’t seem related to persistence in birds, as demonstrated by a serological survey (16).
Finally, the unusual gross lesions in the brain and spinal cord of the index horse herein could be misleading in the differential diagnosis as macroscopic lesions are generally absent in equine EEE cases (18).
In conclusion, as other clinical cases of EEE can be expected to occur in Quebec in the future, it is strongly recommended that Quebec veterinary practitioners include EEE in their regular vaccination schedule to protect horses and emus. Equine vaccines are used off-label to protect emus as no vaccine has been officially approved for this species in North America (19). Public health authorities must also be aware that human EEE cases may also occur. Veterinarians and pathologists should include EEE in their differential diagnosis in horses having rapid onset of neurological signs that rapidly progress to death, and in emus that die suddenly or after a quick clinical course of hemorrhagic gastroenteritis.
Acknowledgments
The authors thank Christian Back from GDG Environnement for answering numerous questions on mosquito populations in Quebec, and Marco Langlois for technical assistance with the photographs. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Eastern Equine Encephalitis Fact Sheet [homepage on the Internet] Centers for Disease Control and Prevention (CDC); [Last accessed July 12, 2010]. [updated 2008/05/28]. Available from http://www.cdc.gov/ncidod/dvbid/Arbor/eeefact.htm. [Google Scholar]
- 2.Veazey RS, Vice CC, Cho D-Y, Tully TN, Jr, Shane SM. Pathology of eastern equine encephalitis in emus (Dromaius novaehollandiae) Vet Pathol. 1994;31:109–111. doi: 10.1177/030098589403100115. [DOI] [PubMed] [Google Scholar]
- 3.Martin E, Ojkic D, Archambault M. Emus die from eastern equine encephalitis virus (EEEV) infection. AHL Newsletter. 2003;7:42. [Google Scholar]
- 4.Morris CD. Eastern equine encephalitis. J Florida Mosquito Control Assoc. 1992;63:23–34. [PubMed] [Google Scholar]
- 5.Giguère M, Gosselin P. Maladies zoonotiques et à transmission vectorielle [monograph on the Internet] Québec, QC: Institut National de Santé Publique du Québec (INSPQ); c2006. [Last accessed July 12, 1020]. Available from: www.inspq.qc.ca/pdf/publications/519-ChangementsClimatiques_MaladiesZoonotiques.pdf. [Google Scholar]
- 6.Wright B, Kenney D. Equine viral encephalitis [monograph on the Internet] Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA); c2005. [Last accessed July 12, 2010]. Available from: http://www.omafra.gov.on.ca/english/livestock/horses/facts/info_equv.htm. [Google Scholar]
- 7.Carman S, McEwen B, DeLay J, Ojkic D. Equine viruses identified or isolated at the Animal Health Laboratory (AHL), 1998 to October 2008. AHL Newsletter. 2008;12:34. Available from: http://www.labservices.uoguelph.ca/labserv/units/ahl/news_archive.cfm. [Google Scholar]
- 8.Desjardins F, Vincent C, Major M. Avertissement vétérinaire no.21: Encéphalite équine de l’Est (EEE) [monograph on the Internet] Québec, QC: MAPAQ; c2000. [Last accessed 06/01/2009]. Request pdf from http://www.mapaq.gouv.qc.ca/Fr/Productions/santeanimale/raizo/advertissementszoosanitaires/archives. [Google Scholar]
- 9.Mayhew IG, Whitlock RH, Tasker JB. Equine cerebrospinal fluid: Reference values of normal horses. Am J Vet Res. 1977;38:1271–1274. [PubMed] [Google Scholar]
- 10.Letson GW, Bailey RE, Pearson J, Tsai TF. Eastern equine encephalitis (EEE): A description of the 1989 outbreak, recent epidemiologic trends, and the association of rainfall with EEE occurence. Am J Trop Med Hyg. 1993;49:677–685. doi: 10.4269/ajtmh.1993.49.677. [DOI] [PubMed] [Google Scholar]
- 11.National Climate Data and Information Archive [database on the Internet] Ottawa: Environment Canada; c2009. [Last accessed July 12, 2010]. Available from http://www.climate.weatheroffice.ec.gc.ca/climateData/canada_e.html?&. [Google Scholar]
- 12.Sellers RF. Eastern equine encephalitis in Quebec and Connecticut, 1972. Can J Vet Res. 1989;53:76–79. [PMC free article] [PubMed] [Google Scholar]
- 13.2008 Summary of Eastern Equine Encephalitis Cases in the United States [homepage on the Internet] USDA, APHIS; [Last accessed July 12, 2010]. [updated 2009/04/15]. Available from http://www.aphis.usda.gov/vs/nahss/equine/ee/2008_eastern_equine_encephalitis_final.pdf. [Google Scholar]
- 14.Bourque A, Simonet G. Quebec chapter — From Impacts to Adaptation: Canada in a Changing Climate 2007 [monograph on the Internet] Gouvernement du Canada; c2008. [Last accessed July 12, 2010]. Available from http://www.ouranos.ca/en/publications/about-climate-change.php. [Google Scholar]
- 15.Gould EA, Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103:109–121. doi: 10.1016/j.trstmh.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young DS, Kramer LD, Maffei JG, et al. Molecular epidemiology of eastern equine encephalitis virus, New York. Emerg Infect Dis. 2008;14:454–460. doi: 10.3201/eid1403.070816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong PM, Andreadis TG, Anderson JF, Stull JW, Mores CN. Tracking eastern equine encephalitis virus perpetuation in the northeastern United States by phylogenetic analysis. Am J Trop Med Hyg. 2008;79:291–296. [PubMed] [Google Scholar]
- 18.Maxie MG, Youssef S. Maxie MG, editor. Nervous system. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 2007;1:423–424. [Google Scholar]
- 19.Tengelsen LA, Bowen RA, Royals MA, Campbell GL, Komar N, Craven RB. Response to and efficacy of vaccination against eastern equine encephalomyelitis virus in emus. J Am Vet Med Assoc. 2001;218:1469–1473. doi: 10.2460/javma.2001.218.1469. [DOI] [PubMed] [Google Scholar]