Abstract
Serum selenium (Se), vitamin E, and resting thyroid hormone concentrations were measured in 201 horses in Prince Edward Island (PEI). Selenium concentrations were either marginal (0.0053 to 0.1200 ppm) or deficient (< 0.0053 ppm) in 79% of horses based on current reference ranges for Se in serum. Aged and young adult pleasure horses had a higher prevalence of inadequate Se concentrations compared to racehorses and broodmares (82% and 97% versus 45% and 72%, respectively). Overall, 13% of horses had inadequate (< 200 μg/dL) serum vitamin E concentrations; most of these were young pleasure horses. No horses were hypothyroid and, contrary to findings in other species, there was a positive relationship between serum thyroxine and Se concentrations (P < 0.05). We conclude that Se deficiency is widespread in PEI horse populations, especially in pleasure horses, and vitamin E deficiency is more common in young pleasure horses. Micronutrient supplementation practices employed by PEI horse owners appear inadequate to ensure sufficiency.
Résumé
Le statut de sélénium et de vitamine E chez des chevaux à l’Île-du-Prince-Édouard. Les concentrations de sérum de sélénium (Se), de vitamine E et d’hormones thyroïdiennes au repos ont été mesurées chez 201 chevaux à l’Île-du-Prince-Édouard (Î.-P.-É.). Les concentrations de sélénium étaient soit négligeables (de 0,0053 à 0,1200 ppm) ou déficientes (< 0,0053 ppm) chez 79 % des chevaux en se fondant sur les fourchettes de référence actuelles pour le Se dans le sérum. Les chevaux de promenade âgés et jeunes adultes présentaient une prévalence supérieure de concentrations inadéquates de Se par rapport aux chevaux de course et aux juments d’élevage (82 % et 97 % contre 45 % et 72 %, respectivement). Au total, 13 % des chevaux présentaient des concentrations inadéquates (< 200 μg/dL) de sérum de vitamine E; la plupart de ces animaux étaient des jeunes chevaux de promenade. Aucun cheval n’était hypothyroïdien et, contrairement aux résultats chez d’autres espèces, il y avait une relation positive entre les concentrations de sérum de thyroxine et de Se (P < 0,05). Nous avons conclu que la carence de Se est généralisée au sein des populations de chevaux de l’Î.-P.-É., particulièrement chez les chevaux de promenade, et qu’une carence en vitamine E est plus commune chez les jeunes chevaux de promenade. Les pratiques de supplémentation employées par les propriétaires de chevaux de l’Î.-P.-É. semblent inadéquates pour assurer la suffisance.
(Traduit par Isabelle Vallières)
Introduction
Selenium (Se) and vitamin E are essential nutrients for livestock (1). The Maritime Provinces, including PEI, have low soil Se concentrations and animals consuming locally grown feeds are at risk of becoming deficient unless supplemented (2,3). Vitamin E is found in adequate amounts in most livestock forages at early stages of growth; however, the concentration of this vitamin declines as forages mature, and deteriorates over time when they are stored as hay or silage. Thus vitamin E intake in forage-fed livestock is likely to fluctuate seasonally, being lowest during the winter months when livestock are fed conserved forages only (4,5).
Horses in PEI tend to be fed locally grown forage and cereal grain which typically contain Se at a concentration between 0.004 and 0.043 ppm dry matter (DM) (6), whereas the Se requirement for horses has been estimated at between 0.1 ppm and 0.3 ppm DM (7–9). Supplementary Se is recommended for horses in deficient regions, and can be administered daily in feed as inorganic sodium selenate or selenite, or in an organic form such as Se yeast (10). Vitamin E, in the form of α-tocopherol, is often added to equine concentrate feeds to compensate for low vitamin E content of the stored forage components of the typical equine diet. Selenium-vitamin E injections can be used for short-term supplementation (11,12). However, the extent of use of these supplements in PEI is unknown, and the prevalence of Se and vitamin E deficiencies in Maritime equine populations has not been previously investigated. In a recent study of the Se status of dairy herds in PEI, 59% of the herds surveyed had either a marginal or deficient Se status, suggesting that Se deficiency of livestock may be more widespread in PEI than previously recognized (13).
Selenium and vitamin E are essential components of several major metabolic pathways and have complementary roles as antioxidants. A deficiency of Se and/or vitamin E diminishes protection against cellular oxidative stress, making the cell membrane more susceptible to disruption by free radicals resulting from cellular metabolism (14–16). The most common clinical manifestations of decreased anti-oxidant activity associated with vitamin E and/or Se deficiency are muscular and neuromuscular diseases, such as white muscle disease (WMD), equine motor neuron disease (EMND), and equine degenerative myeloen-cephalopathy (EDM) (5,15,17,18). Deficiencies in Se and vitamin E have also been associated with significant decreases in immune response in horses when compared to horses that received supplementation (19,20).
Thyroid hormone metabolism is dependent on Se status: iodothyronine 5′-deiodinase, a Se-containing enzyme, is responsible for converting thyroxine (T4) to the more active 3′,5′,3′-triodo-L-thyronine (T3) (21). Calves and lambs grazing on Se-deficient soils have increased peripheral concentrations of T4 and decreased T3 when compared to Se-supplemented animals due to alteration in the activity of type I iodothyronine 5′-deiodinase (22,23). Such alterations in thyroid hormone concentrations may be responsible for the poor growth, ill thrift, and lowered performance commonly noted in Se-deficient herds. To our knowledge, the relationship between Se status and resting thyroid hormone concentrations in horses has not been previously reported.
The objectives of this study were to 1) estimate the prevalence of Se and vitamin E deficiencies in representative functional populations of horses in Prince Edward Island, 2) examine the relationship between the Se status and resting thyroid hormone concentrations in these populations, and 3) determine if certain feeding and supplementation practices are associated with Se and vitamin E status.
Materials and methods
Experimental animals
Information brochures were sent to owners and trainers in PEI to elicit interest in one of several studies, described below. Owners or trainers approached the investigators and volunteered 1 or more horses as study subjects. To be eligible, all horses were required to be healthy, and (except for broodmares and foals) to be under the care of the same individual for at least 3 mo prior to enrolment, to ensure accurate recall of supplementation history. In total, 201 horses (151 adults and 50 foals) were enrolled. The horses were derived from 5 functional populations of interest. Groups 1 and 2 comprised 34 aged and 31 young adult pleasure horses, aged ≥ 20 and 4 to 12 y, respectively. The pleasure horse groups comprised 51 light horses, 10 ponies, and 4 draft horses, and had been recruited for another study on age-related changes in immune response to vaccination (24). The pleasure horses were recruited and sampled over a period of 2 y (2004 to 2006). Group 3 comprised 36 Standardbred racehorses in training at the Charlottetown Driving Park between the ages of 2 and 8 y, recruited specifically for this study and sampled on a single day (July 20, 2004). Groups 4 and 5 comprised 50 Standardbred mixed-age broodmares and their healthy foals (< 72 h of age), respectively, recruited for a study on passive transfer of immunoglobulin (25).
Sample collection and laboratory analysis
The pleasure horse groups were sampled for serum Se and vitamin E concentration at the start of the study and again 6 mo later, whereas all other horses were sampled just once. Blood samples were collected via jugular venipuncture into evacuated tubes. The blood was allowed to clot, then it was centrifuged, and serum was decanted into cryovials for storage at −80°C until assays were performed. Vitamin E was assayed using modified high performance liquid chromatography (HPLC) with fluorescence detection (26). Selenium analysis was performed using a method of additions calibration using a palladium-magnesium nitrate matrix modifier on an atomic absorption spectrometer (27–29). Both analyses were carried out following standard procedures at the Toxicology and Analytical Services Laboratory at the Atlantic Veterinary College, Charlottetown, Prince Edward Island. Serum T4 and T3 chemiluminence enzyme immunoassays were performed at Diagnostic Services, Atlantic Veterinary College (Immulite Total T4 and T3; Diagnostic Products Corporation, Los Angeles, California, USA).
Statistical analysis
Descriptive statistics are presented in tabular form classified according to currently accepted clinical reference ranges for serum Se (30), vitamin E (31), T4, and T3 concentrations (32). General linear models were used to evaluate factors influencing serum Se, vitamin E, T4, and T3 concentrations (Minitab 14; Minitab, State College, Pennsylvania, USA). The serum Se, vitamin E, and T4 concentrations were square root-transformed before analysis to meet model assumptions. The following categorical predictors were included in the initial model: population group (younger pleasure horses, older pleasure horses, racehorses, broodmares), breed (pony, light horse, draft), gender, and season of sampling (fall: August–October; winter: November–January; spring: February–April, and summer: May–July). Selenium and vitamin E values were included in the initial models for vitamin E and Se, respectively, and both were included in the initial models for T4 and T3. To avoid any intermediate effects of these predictors on the comparison of population groups, the predictors were centered by subtracting the respective population group means from each value. All 2-factor interactions were assessed. Predictors significantly associated with the dependent variable (P ≤ 0.05) were retained in the model. Effects of significant categorical predictors were presented as least square means with pairwise comparisons adjusted by the Bonferroni method. For the pleasure horses only, the paired data for serum Se and vitamin E were analyzed using the paired t-test.
Pearson’s correlation was used to describe the relationship between foal and dam serum Se, vitamin E, T4, and T3. Data from foals were not included in the linear models because the distribution and range of the values of the dependent variables obtained for foals made comparisons with the adult groups inappropriate.
Data on feeding and micronutrient supplementation practices, based on responses to a standardized interview of the owner or trainer conducted by the principal investigator, were summarized and assessed for their effects on Se and vitamin E status using linear models as described above, with population group included to control for confounding.
Results
Selenium
Serum Se concentrations were inadequate (≤ 0.14 ppm) in 79% of horses, based on currently accepted reference ranges (Table 1). Among the adult horses, aged and young adult pleasure horses had a higher prevalence of inadequate Se concentrations when compared to racehorses and broodmares (82% and 97% versus 45% and 72%, respectively). Foals also had a high prevalence of inadequate serum Se concentrations (96%), considerably higher than that of the broodmares from which they were derived (72%).
Table 1.
Mean ± standard deviation (s), and proportion of the population falling into currently accepted clinical reference ranges, for serum Se, vitamin E, T4, and T3 in PEI horses
| Young pleasure horses (n = 31) | Aged pleasure horses (n = 34) | Racehorses (n = 36) | Broodmares (n = 50) | Foals (n = 50) | Overall (n = 201) | |
|---|---|---|---|---|---|---|
| Se concentrations | ||||||
| Mean ± s | 0.061 ± 0.031 | 0.080 ± 0.051 | 0.14 ± 0.032 | 0.11 ± 0.031 | 0.059 ± 0.053 | |
| % Deficient (0.008–0.0053 ppm) | 36 | 41 | 0 | 6 | 60a | 29 |
| % Marginal (0.0053–0.120 ppm) | 61 | 41 | 45 | 66 | 36 | 50 |
| % Adequate (0.140–0.250 ppm) | 3 | 18 | 55 | 28 | 4 | 21 |
| Vitamin E concentrations | ||||||
| Mean ± s | 4473.0 ± 2327.0 | 7454.2 ± 3563.2 | 8686.1 ± 2779.4 | 7973.8 ± 2166.9 | 9465.6 ± 3637.8 | |
| % Deficient (< 3480 μmol/L) | 39 | 9 | 0 | 0 | 0 | 8 |
| % Marginal (3480–4643 μmol/L) | 16 | 15 | 3 | 2 | 0 | 6 |
| % Adequate (4644–23 220 μmol/L) | 45 | 76 | 97 | 98 | 100 | 86 |
| T4 concentrations | ||||||
| Mean ± s | 29.34 ± 9.730 | 24.46 ± 119.8 | 10.18 ± 5.039 | 26.11 ± 47.27 | 281.9 ± 78.6 | |
| % Inadequate (< 15 nmol/L) | 6 | 12 | 78 | 58 | 2 | 32 |
| % Adequate (15–51 nmol/L) (foal: 279–464 nmol/L) | 94 | 88 | 22 | 42 | 98 | 68 |
| T3 concentrations | ||||||
| Mean ± s | 1.26 ± 0.420 | 1.29 ± 5.04 | 1.29 ± 0.673 | 1.76 ± 2.47 | 12.89 ± 3.714 | |
| % Inadequate (< 0.4 nmol/L) | 0 | 0 | 0 | 0 | 0 | 0 |
| % Adequate (0.4–1.7 nmol/L) (foal: 10–19 nmol/L) | 100 | 100 | 100 | 100 | 100 | 100 |
Se-deficient foals were born to 27 of the 36 Se-deficient/marginal broodmares, while Se-deficient foals were born to only 3 of the 14 Se-adequate mares.
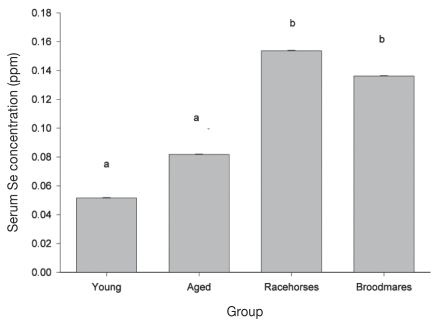
Mean serum Se concentrations differed by population group (P ≤ 0.001; Table 2). Racehorses and broodmares had significantly higher mean serum Se concentrations when compared to the young and aged pleasure horses (P < 0.05; Figure 1). There was a significant seasonal effect on mean serum Se concentration (P < 0.05; Table 2) such that horses tested in the winter months had significantly higher Se concentrations compared to those tested during the spring months.
Table 2.
Results of final linear models for serum Se, vitamin E, T4, and T3 concentrations in PEI horses
| Outcome | Effect | Category (n) | Estimatea | SEb | P-value |
|---|---|---|---|---|---|
| Se | Group | Young pleasure (31) | 0.227 | 0.019 | 0.001 |
| Aged pleasure (34) | 0.286 | 0.018 | |||
| Racehorses (36) | 0.392 | 0.020 | |||
| Broodmares (50) | 0.369 | 0.019 | |||
| Season | Winter (Nov, Dec, Jan) | 0.421 | 0.051 | 0.041 | |
| Spring (Feb, March, April) | 0.272 | 0.020 | |||
| Summer (May, June, July) | 0.308 | 0.010 | |||
| Fall (Aug, Sept, Oct) | 0.303 | 0.014 | |||
| Vitamin E | Group | Young pleasure (31) | 13.39 | 0.58 | 0.001 |
| Aged pleasure (34) | 17.42 | 0.56 | |||
| Racehorses (36) | 19.13 | 0.54 | |||
| Broodmares (50) | 18.37 | 0.46 | |||
| Se (Group) | Young pleasure | −1.95 | 19.45 | 0.010 | |
| Aged pleasure | 13.44 | 11.05 | |||
| Racehorses | 58.38 | 17.08 | |||
| Broodmares | −7.075 | 8.713 | |||
| T4 | Group | Young pleasure (31) | 5.333 | 0.171 | 0.001 |
| Aged pleasure (34) | 4.887 | 0.163 | |||
| Racehorses (36) | 3.101 | 0.158 | |||
| Broodmares (50) | 3.784 | 0.139 | |||
| Se | n/a | 3.830 | 1.781 | 0.033 | |
| T3 | Group | Young pleasure (31) | 1.258 | 0.095 | 0.784 |
| Aged pleasure (34) | 1.171 | 0.090 | |||
| Racehorses (36) | 1.287 | 0.088 | |||
| Broodmares (50) | 1.201 | 0.077 |
Regression coefficients for continuous predictors and least squares means for categorical predictors, computed at the mean Se in the group, when Se is present in the model. The estimates for Se, vitamin E and T4 refer to a square-root transformed scale.
SE — standard error.
Figure 1.
Comparison of the least square means (LSM) ± standard error of the mean (Sx) for serum Se concentrations between horse population groups on PEI. Means with different letters are significantly different (P < 0.05).
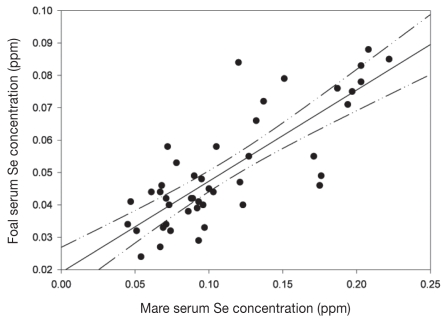
Moderate correlation was observed between foal and dam serum Se concentrations (r = 0.546; P ≤ 0.001). Five mares were considered outliers because their serum Se concentrations were very much higher than those of the other mares (0.203 to 0.232 ppm) suggesting they had been supplemented very recently; when these 5 foal/dam pairs were removed from the analysis, there was a high correlation between foal and dam Se concentrations (r = 0.805; P ≤ 0.001; Figure 2).
Figure 2.
The relationship between foal and dam serum Se concentration. The correlation line is shown with upper and lower 95% confidence limits (n = 45; Pearson’s correlation = 0.805; P < 0.001).
The aged and younger adult pleasure horses had follow-up serum samples taken 6 mo after the initial sampling (data not shown); there was no significant change in the concentrations between sampling times for either group.
Vitamin E
Only 13% of horses had inadequate serum vitamin E concentration; these horses were almost exclusively in the pleasure horse categories (Table 1) with young adult pleasure horses having the highest prevalence of inadequate serum vitamin E concentration (55%).
The aged pleasure horses, racehorses, and broodmares had significantly higher mean serum vitamin E concentrations compared to the young adult pleasure horses (P ≤ 0.001; Table 2; Figure 3). There was no significant difference in mean serum Se concentration between the aged pleasure horses, racehorses, and broodmares. There was a significant group × Se interaction (P < 0.05; Table 2) affecting vitamin E such that, in racehorses only, every 0.1 unit of increase in serum Se concentration was associated with a 5.8 unit increase in serum vitamin E concentration (Table 2).
Figure 3.
Comparison of the least square means (LSM) ± standard error of the mean (Sχ̄) for serum vitamin E concentrations between horse population groups on PEI. Means with different letters are significantly different (P < 0.05).
For the pleasure horse groups, there was no significant change in the prevalence of vitamin E deficiency at the 6-mo follow-up sampling when compared to the initial sampling (data not shown). There was no significant correlation between mares’ and their foals’ vitamin E concentrations (r = 0.232; P-value = 0.104).
Thyroid hormones
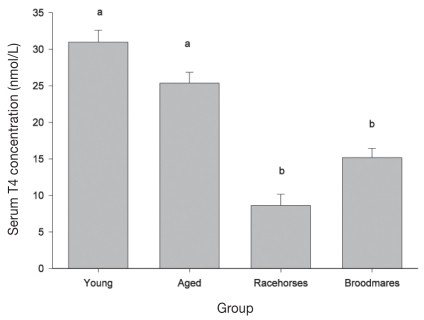
Based on accepted reference ranges for normal resting T4 and T3 concentrations in horses, all horses had serum T3 concentrations within the normal range. Thus none of the horses in this study were diagnosed as hypothyroid, defined as having resting concentrations of both T4 and T3 falling below the range considered normal. The highest prevalence of inadequate serum T4 concentrations was found in the racehorse group (78%) followed by broodmares (58%) (Table 1).
Three of the mares were outliers and were removed from the dataset used in the general linear model because of unusually high T4 concentrations (range: 92.8 to 291 nmol/L; normal adult range: 15 to 51nmol/L). Mean resting serum T4 concentrations differed between population groups (P ≤ 0.001). Mean T4 concentrations in racehorses were significantly lower than in broodmares, and mean T4 concentrations in both broodmares and racehorses were lower than in pleasure horses (Table 2; Figure 4). Overall, there was an effect of Se on T4 concentration such that every 0.1 unit of increase in serum Se concentration was associated with 3.8 units increase in T4 concentration. Mean resting T3 concentration did not differ between groups. There was a moderately significant negative mare-foal correlation for T3 concentrations (r = −0.442; P = 0.001).
Figure 4.
Comparison of the least square means (LSM) ± standard error of the mean (Sχ̄) for resting serum T4 concentrations between horse population groups on PEI. Means with different letters are significantly different (P < 0.05).
Feeding and supplementation data
The feeding and micronutrient supplementation history for the broodmares was unavailable. Interview data were obtained for all enrolled pleasure horses and racehorses. All horses were fed locally grown hay and all horses, with the exception of the racehorses, were allowed to graze on pasture during the grazing months. All racehorses were fed a commercially prepared pelleted complete feed or sweet feed which contained 50 to 55 ppm of Se on a dry matter basis, whereas only 52% of the young adult pleasure horses and 56% of the aged pleasure horses were fed this type of feed. Locally grown grains, such as corn and oats, were given to most racehorses (53%), and some young pleasure horses (7%), and aged pleasure horses (9%). The amount offered on a daily basis was not determined for any of these feeds.
Selenium supplementation in the form of either a fortified salt block or free choice mineral was made available to 45% of young adult pleasure horses, 50% of aged pleasure horses, and 22% of the racehorses. Vitamin E supplementation in the form of a top dress was given to 3% of young and aged pleasure horses, and 14% of racehorses.
Unconditional analysis of feeding and micronutrient supplementation practices suggested that feeding a commercial prepared feed was associated with a significantly higher mean serum Se concentration (P < 0.01) but when population group was included in the linear model, none of the feeding or supplementation practices were found to have a significant effect on mean Se or vitamin E concentration.
Discussion
Seventy-nine percent of the horses in this study had either deficient or marginal serum Se concentrations. This is in agreement with previous studies on the Se status of livestock in PEI (2,13) and confirms that horses grazing solely or primarily on forages grown in PEI are unlikely to receive sufficient dietary Se.
The dietary Se requirement for horses is estimated at between 0.1 ppm DM (7) and 0.3 ppm DM (8,9). Horses in PEI are fed locally grown forages containing low levels of Se, typically less than 0.05 ppm DM (2). Horses that received supplementary Se in this study were fed primarily an inorganic form of Se (selenite) which has been shown to be effective if fed appropriately (10,33). However, supplementation practices commonly employed in PEI appear to be inadequate to ensure sufficiency. Feeding practices were confounded by population group: all racehorses were fed commercially prepared feeds fortified with Se, whereas only about half of pleasure horses were fed such feed. Further, it was not possible to assess the amount of supplementary Se ingested, making it difficult to assess the effects of specific supplementation practices on serum Se concentration.
The incidence of broodmares with inadequate Se status was 72%. This was not significantly different from that of the racehorses. In general, broodmares may be more likely to be maintained on higher concentrate diets when compared to pleasure horses, to allow for their increased energy needs during pregnancy and lactation. It is also common practice by some broodmare owners to administer an injectable form of Se/vitamin E approximately 1 mo prior to foaling, even though this may give only a transient rise in concentrations (12,34). These management practices might account for the lower number of broodmares with inadequate Se concentrations.
Approximately 2/3 of the foals in this study had inadequate Se concentrations. This places these foals at higher risk for WMD and perhaps immune dysfunction. However, these results should be considered with caution because specific reference values for vitamin E and Se in neonatal foals have not been established. Based on animal health laboratory data from 1 US laboratory, it appears that neonatal reference values are likely to be considerably lower than those presented for adults in Table 1 (35). The Se concentration in the serum of the foal was strongly correlated to that of the mare, consistent with previous research (5,18,36). Mares with deficient serum Se concentrations are at risk of giving birth to a foal with WMD (18).
Selenium concentrations were affected by season: horses tested during the winter had significantly higher serum Se concentrations compared to horses tested in the spring. It may be that many horse owners offer commercial feeds, or simply increase the amount of feed offered, over the winter months to provide extra energy during the colder months.
Serum Se was tested in the adult and aged pleasure horses at the start of the study and again 6 mo later. The owners were informed of their horses’ serum Se status after the initial sampling; despite this, there was no significant improvement in serum Se concentrations between the 2 sampling times, suggesting that compliance may be an issue when offering advice on micronutrient deficiencies.
Routine analysis of locally grown forages and grains, and a better understanding of how to interpret feed labels, could help owners deliver appropriate micronutrient nutrition to their horses. However, this type of monitoring may be too complex for horse owners. Alternatives, such as offering commercially prepared feeds with customized concentrations of Se prescribed by a veterinarian, or offering a Se mineral as a top dress at appropriate amounts to complement the ration, may help improve Se status. Selenium can be offered in an organic form, typically as selenized yeast. Selenium-enriched yeast is more effective in increasing plasma Se concentrations when compared to inorganic Se sources (10). The use of Se-fortified pastures and stored forages offers a method of improving the Se status of livestock which is inexpensive, easy and safe. Application of Se to pastures and crops is widely practiced in countries with Se-deficient soils, such as New Zealand and Finland (37,38).
The daily vitamin E requirement to maintain normal immune function is 1 IU/kg of body weight/day (7). Vitamin E is readily available in fresh pastures such as alfalfa, timothy, meadow fescue and Kentucky bluegrass; however, vitamin E deteriorates over time when these grasses are stored. Thus, in the Atlantic region, one would expect a seasonal effect for the amount of vitamin E intake (4,39). There was, however, no effect of season on vitamin E concentrations in this study; it may be that too few horses were tested in the winter months to detect a seasonal effect, if it exists.
Approximately 87% of the horses that were tested had adequate serum vitamin E concentrations. Only 10 of these horses (1 adult pleasure horse, 1 aged pleasure horse, and 8 racehorses) were supplemented with oral vitamin E. Feeding of fresh pasture, good quality hay, and complete pelleted feeds may explain the relatively high prevalence of adequate vitamin E concentrations (4). The young adult pleasure horses had the highest prevalence of inadequate vitamin E concentration (39%). This may be attributed to feeding and management practices such as feeding minimal and inconsistent amounts of the vitamin E-fortified concentrates.
There was a significant group × Se status interaction for serum vitamin E concentration (Table 2). In racehorses, as Se concentration increased so did the vitamin E concentration. This might be expected since these nutrients are found together in high concentration in the majority of commercial feeds and supplements, and these supplements are likely fed in the greatest quantities to athletic horses.
Thyroid hormones
The racehorses in this study had significantly lower mean serum T4 concentrations compared to the other populations, with 78% of these horses having a serum T4 concentration considered below normal. One reason for this could be the use of exogenous compounds such as phenylbutazone, a frequently used non-steroidal anti-inflammatory (NSAID) that inhibits iodide oxidation and coupling of iodotyrosines resulting in reduced thyroid concentrations for up to 10 d once administration has ceased. Phenylbutazone also competes with T4 for binding sites on plasma proteins, and may decrease production of thyroid stimulating hormone (40,41). Another reason could be the type of diet offered to the racehorses: in one study, high energy diets resulted in lowered serum T4 concentrations with concurrent higher serum T3 concentrations (42).
Fifty-eight percent of the peri-parturient broodmares had low resting T4 concentrations. In late-term pregnancy, mares tend to have normal or decreased serum thyroid hormone concentrations (43,44). The foals had high T3 and T4 concentrations compared to the other groups; foals tend to have much higher concentrations of these hormones at birth, decreasing with age (45). There was a negative correlation between the T3 concentrations of the mares and foals; the reason for this finding is not clear.
In most species, low Se intake is associated with increased serum T4 concentrations (21–23,46). In this study, there was no relationship between Se status and circulating T3; however, there was a positive correlation between Se and T4 concentration, contrary to published data in other livestock species (22). This finding may be related to the iodine content of the mineral supplements and concentrates fed to horses in our study (not recorded) which would tend to increase thyroid hormone production in concert with increasing Se intake. However, it may be that in horses the activity of 3′5′ tri-iodothyronine deiodenase is not as closely related to Se status as in other species, or that the degree of deficiency observed in our population was not sufficiently profound to significantly affect deiodinase activity.
White muscle disease of foals is not an uncommon diagnosis in PEI, this being perhaps the most obvious sequel to poor micronutrient nutrition in broodmares. The clinical significance of the findings of this study, however, is difficult to estimate for several reasons. First, many of the known or proposed effects of Se deficiency in horses are subclinical, and therefore difficult to quantify. The degree of Se deficiency that has been observed in the majority of the horses in this study has been associated with reduced growth rates and milk production in ruminants (47) and reduced immune response to experimental antigen challenge in horses (20); it may be that these covert effects are a greater source of morbidity than the more obvious muscular degenerative disorders. Secondly, the reference ranges used in this report to classify horses as adequate, marginal and deficient for Se status are tentative; unlike the more rigorously developed reference ranges for ruminants (47), reference ranges for horses have been based on limited published literature, primarily related to the occurrence of WMD (30,31). Large-scale production response trials similar to those performed in ruminants would be useful in refining the Se and vitamin E requirements of horses.
Based on the results of this study, we suggest that owners be well-informed about likely trace element deficiencies that occur in their region, and be encouraged to read feed and supplement labels, familiarize themselves with micronutrient requirements, and frequently review their supplementation practices. Education for horse owners and trainers on correct micronutrient nutrition of horses should be readily available.
In conclusion, it appears that Se deficiency is widespread in PEI horse populations, especially in pleasure horses. Vitamin E deficiency is also relatively common in pleasure horses, especially younger pleasure horses. Micronutrient supplementation practices employed by PEI horse owners appear inadequate to ensure sufficiency for vitamin E and Se, putting horses at risk for a variety of Se- and vitamin E-responsive conditions. The high prevalence of Se deficiency in broodmares is of particular concern because it places their foals at higher risk of muscular degeneration and impaired response to pathogens. Correct micronutrient nutrition of horses should receive more attention from horse owners and their nutritional advisors. CVJ
Footnotes
This study was funded by the Sir James Dunn Animal Welfare Center, Charlottetown, Prince Edward Island.
Reprints will not be available from the authors.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Underwood EL, Suttle NF. The Mineral Nutrition of Livestock. 3rd ed. New York: BABI Publ; 2001. Selenium; pp. 421–475. [Google Scholar]
- 2.Winter K, Gupta U, Nass H, Kunelius H. Selenium content of feedstuffs produced in Prince Edward Island. Can J Anim Sci. 1973;53:113–124. [Google Scholar]
- 3.Winter K, Gupta U. Selenium content of forages grown in Nova Scotia, New Brunswick, and Newfoundland. Can J Anim Sci. 1979;59:107–111. [Google Scholar]
- 4.Lewis LD. Feeding and Care of the Horse. 2nd ed. New York: Lippincott Williams and Wilkins; 1996. [Google Scholar]
- 5.Lofstedt J. White muscle disease of foals. Vet Clin North Am Equine Pract. 1997;13:169–185. doi: 10.1016/s0749-0739(17)30262-6. [DOI] [PubMed] [Google Scholar]
- 6.Gupta U, Winter K. Selenium content of soils and crops and the effects of lime and sulphur on plant selenium. Can J Soil Sci. 1975;55:161–166. [Google Scholar]
- 7.National Research Council. Nutrient Requirements of Horses. 6th ed. Washington: The National Academies Press; 2007. [Google Scholar]
- 8.Stowe HD, Pagan JD, editors. Advances in Equine Nutrition. UK: Nottingham Univ Pr; 1998. Selenium supplementation for horse feed; pp. 97–103. [Google Scholar]
- 9.Pagan JD. Micromineral requirements in horses. Advances in Equine Nutrition; Proc for the Equine Nutrition Conf for Feed Manufacturers; Lexington, Kentucky. 2000. p. 107. [Google Scholar]
- 10.Richardson SM, Siciliano PD, Engle TE, Larson CK, Ward TL. Effect of selenium supplementation and source on the selenium status of horses. J Anim Sci. 2006;84:1742–1748. doi: 10.2527/jas.2005-413. [DOI] [PubMed] [Google Scholar]
- 11.Van Vleet JF. Retention of selenium in tissues of calves, lambs, and pigs after parenteral injection of a selenium-vitamin E preparation. Am J Vet Res. 1975;36:1335–1340. [PubMed] [Google Scholar]
- 12.Wichtel JJ, Grace ND, Firth EC. The effect of injectable barium selenate on the selenium status of horses on pasture. N Z Vet J. 1998;46:186–190. doi: 10.1080/00480169.1998.36087. [DOI] [PubMed] [Google Scholar]
- 13.Wichtel JJ, Keefe GP, Van Leeuwen JA, Spangler E, McNiven MA, Ogilvie TH. The selenium status of dairy herds in Prince Edward Island. Can Vet J. 2004;45:124–132. [PMC free article] [PubMed] [Google Scholar]
- 14.Wichtel JJ, Grace ND, Firth EC. Selenium in the horse: New developments. Newsletter of New Zealand Veterinary Association. 1997 [Google Scholar]
- 15.Kirschvink N, de Moffarts B, Lekeux P. The oxidant/antioxidant equilibrium in horses. Vet J. 2008;177:178–191. doi: 10.1016/j.tvjl.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Portier K, de MB, Fellman N, et al. The effects of dietary N-3 and antioxidant supplementation on erythrocyte membrane fatty acid composition and fluidity in exercising horses. Equine Vet J Suppl. 2006;36:279–284. doi: 10.1111/j.2042-3306.2006.tb05553.x. [DOI] [PubMed] [Google Scholar]
- 17.Wilson TM, Morrison HA, Palmer NC, Finley GG, van Dreumel AA. Myodegeneration and suspected selenium/vitamin E deficiency in horses. J Am Vet Med Assoc. 1976;169:213–217. [PubMed] [Google Scholar]
- 18.Higuchi T, Ichijo S, Osame S, Ohishi H. Studies on serum selenium and tocopherol in white muscle disease of foal. Nippon Juigaku Zasshi. 1989;51:52–59. doi: 10.1292/jvms1939.51.52. [DOI] [PubMed] [Google Scholar]
- 19.Arthur JR, McKenzie RC, Beckett GJ. Selenium in the immune system. J Nutr. 2003;133(5 Suppl 1):1457S–1459S. doi: 10.1093/jn/133.5.1457S. [DOI] [PubMed] [Google Scholar]
- 20.Knight DA, Tyznik WJ. The effect of dietary selenium on humoral immunocompetence of ponies. J Anim Sci. 1990;68:1311–1317. doi: 10.2527/1990.6851311x. [DOI] [PubMed] [Google Scholar]
- 21.Awadeh FT, Kincaid RL, Johnson KA. Effect of level and source of dietary selenium on concentrations of thyroid hormones and immunoglobulins in beef cows and calves. J Anim Sci. 1998;76:1204–1215. doi: 10.2527/1998.7641204x. [DOI] [PubMed] [Google Scholar]
- 22.Wichtel JJ, Craigie AL, Freeman DA, Varela-Alvarez H, Williamson NB. Effect of selenium and iodine supplementation on growth rate and on thyroid and somatotropic function in dairy calves at pasture. J Dairy Sci. 1996;79:1865–1872. doi: 10.3168/jds.s0022-0302(96)76554-2. [DOI] [PubMed] [Google Scholar]
- 23.Donald GE, Langlands JP, Bowles JE, Smith AJ. Subclinical selenium insufficiency. 4. Effects of selenium, iodine, and thiocyanate supplementation of grazing ewes on their selenium and iodine status, and on the status and growth of their lambs. Aus J Exp Agric. 1993;33:411–416. [Google Scholar]
- 24.Muirhead TL, McClure JT, Wichtel JJ, et al. The effect of age on serum antibody titers after rabies and influenza vaccination in healthy horses. J Vet Intern Med. 2008;22:654–661. doi: 10.1111/j.1939-1676.2008.0091.x. [DOI] [PubMed] [Google Scholar]
- 25.McClure JT, DeLuca JL, Lunn DP, Miller J. Evaluation of IgG concentration and IgG subisotypes in foals with complete or partial failure of passive transfer after administration of intravenous serum or plasma. Equine Vet J. 2001;33:681–686. doi: 10.2746/042516401776249273. [DOI] [PubMed] [Google Scholar]
- 26.Feurstein M, Schlemmer G. Determination of Selenium in Human Serum by GFAAS with Transverse Heated Graphite Atomizer and Longitudinal Zeeman Effect Background Correction. 20 ed. Bodenseewerk, Uberlingen, Germany: Perkin Elmer Bodenseewerk; 1999. [Google Scholar]
- 27.Stahr HM. Analytical Methods in Toxicology. New York: Jon Wiley and Sons; 1991. pp. 239–245. [Google Scholar]
- 28.Eitenmiller R, Landen WO. Vitamin Analysis for the Health and Food Sciences. Boca Raton, Florida: CRC press; 1999. pp. 109–148. [Google Scholar]
- 29.Bourgeious C. Determination of Vitamin E: Tocopherols and Tocotrienols. New York: Elsevier Applied Science; 1992. pp. 14–65. [Google Scholar]
- 30.Puls R. Mineral Levels in Animal Health: Diagnostic Data. 2nd ed. Clearbrook, British Columbia: Sherpa International; 1994. [Google Scholar]
- 31.Puls R. Vitamin levels in Animal Health: Diagnostic Data and Bibliograhics. Clearbrook, British Columbia: Sherpa International; 1994. [Google Scholar]
- 32.Messer NT, Riddle WT, Traub-Darbatz DA, Rafsal KJ, Thompson DL. Thyroid hormone levels in thoroughbred mares and their foals at parturition. Proc Ann Conv AAEP. 1998:248. [Google Scholar]
- 33.Mahan DC, Parrett NA. Evaluating the efficacy of selenium-enriched yeast and sodium selenite on tissue selenium retention and serum glutathione peroxidase activity in grower and finisher swine. J Anim Sci. 1996;74:2967–2974. doi: 10.2527/1996.74122967x. [DOI] [PubMed] [Google Scholar]
- 34.Maas J, Peauroi JR, Tonjes T, Karlonas J, Galey FD, Han B. Intramuscular selenium administration in selenium-deficient cattle. J Vet Intern Med. 1993;7:342–348. doi: 10.1111/j.1939-1676.1993.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 35.Stowe HD, Herdt TH. Clinical assessment of selenium status of livestock. J Anim Sci. 1992;70:3928–3933. doi: 10.2527/1992.70123928x. [DOI] [PubMed] [Google Scholar]
- 36.Roneus B. Glutathione peroxidase and selenium in the blood of healthy horses and foals affected by muscular dystrophy. Nord Vet Med. 1982;34:350–353. [PubMed] [Google Scholar]
- 37.Whelan BR, Peter DW, Barrow NJ. Selenium fertilizers for pasture grazed by sheep. 1. Selenium concentration in whole blood and plasma. Aus J Agric Res. 1994;45:863–875. [Google Scholar]
- 38.Varo P, Alfthan G, Ekholm P, Aro A, Koivistoinen P. Selenium intake and serum selenium in Finland: Effects of soil fertilization with selenium. Am J Clin Nutr. 1988;48:324–329. doi: 10.1093/ajcn/48.2.324. [DOI] [PubMed] [Google Scholar]
- 39.Ullrey DE. Vitamin E for swine. J Anim Sci. 1981;53:1039–1056. doi: 10.2527/jas1981.5341039x. [DOI] [PubMed] [Google Scholar]
- 40.Ramirez S, Wolfsheimer KJ, Moore RM, Mora F, Bueno AC, Mirza T. Duration of effects of phenylbutazone on serum total thyroxine and free thyroxine concentrations in horses. J Vet Intern Med. 1997;11:371–374. doi: 10.1111/j.1939-1676.1997.tb00483.x. [DOI] [PubMed] [Google Scholar]
- 41.Morris DD, Garcia M. Thyroid-stimulating hormone: Response test in healthy horses, and effect of phenylbutazone on equine thyroid hormones. Am J Vet Res. 1983;44:503–507. [PubMed] [Google Scholar]
- 42.Glade MJ, Luba NK. Serum triiodothyronine and thyroxine concentrations in weanling horses fed carbohydrate by direct gastric infusion. Am J Vet Res. 1987;48:578–582. [PubMed] [Google Scholar]
- 43.Katovich M, Evans JW, Sanchez O. Effects of season, pregnancy and lactation on thyroxine turnover in the mare. J Anim Sci. 1974;38:811–818. doi: 10.2527/jas1974.384811x. [DOI] [PubMed] [Google Scholar]
- 44.Flisinska-Bojanowska A, Komosa M, Gill J. Influence of pregnancy on diurnal and seasonal changes in cortisol, T3 and T4 levels in the mare blood serum. Comp Biochem Physiol A. 1991;98:23–30. doi: 10.1016/0300-9629(91)90571-s. [DOI] [PubMed] [Google Scholar]
- 45.Chen CL, Riley AM. Serum thyroxine and triiodothyronine concentrations in neonatal foals and mature horses. Am J Vet Res. 1981;42:1415–1417. [PubMed] [Google Scholar]
- 46.Arthur JR, Nicol F, Hutchinson AR, Beckett GJ. The effects of selenium depletion and repletion on the metabolism of thyroid hormones in the rat. J Inorg Biochem. 1990;39:101–108. doi: 10.1016/0162-0134(90)80018-s. [DOI] [PubMed] [Google Scholar]
- 47.Wichtel JJ. A review of selenium deficiency in grazing ruminants. Part 2: Towards a more rational approach to diagnosis and prevention. N Z Vet J. 1998;46:54–58. doi: 10.1080/00480169.1998.36056. [DOI] [PubMed] [Google Scholar]