Abstract
Objectives
To elucidate the pharmacokinetic/pharmacodynamic (PK/PD) index that predicts colistin efficacy against Acinetobacter baumannii in neutropenic murine thigh and lung infection models, and to determine the extent of the emergence of resistance in vivo to colistin.
Methods
PK/PD of colistin was studied in thigh and lung infection models against A. baumannii ATCC 19606 and two multidrug-resistant clinical isolates (two of the three strains were colistin heteroresistant). Dose fractionation studies were conducted over a daily dose range of 1–160 mg/kg colistin sulphate. Bacterial burden in tissues was measured at 24 h. Non-linear least squares regression analyses were employed to determine the PK/PD index (fAUC/MIC, fCmax/MIC or fT>MIC) best correlating with the efficacy of colistin in each model. Real-time population analysis profiles were conducted for tissue samples to monitor the emergence of resistance.
Results
The fAUC/MIC was the PK/PD index that correlated best with efficacy in both thigh (R2 = 0.90) and lung (R2 = 0.80) infection models. The fAUC/MIC targets required to achieve stasis and 1 log kill against the three strains were 1.89–7.41 and 6.98–13.6 in the thigh infection model, respectively, while the corresponding values were 1.57–6.52 and 8.18–42.1 in the lung infection model. Amplification of colistin-resistant subpopulations was revealed for all strains in both models after 24 h colistin treatment.
Conclusions
This study indicates the importance of achieving adequate time-averaged exposure to colistin and defined target fAUC/MIC values for various magnitudes of kill. Amplification of resistant subpopulations indicates the importance of investigating rational combinations with colistin. The results will facilitate efforts to optimize colistin use in humans.
Keywords: Gram-negative bacteria, emergence of resistance, population analysis profiles, heteroresistance
Introduction
The Infectious Diseases Society of America has listed Acinetobacter baumannii as a difficult-to-treat pathogen and emphasized the need for judicious use of currently available antibiotics.1,2 This microorganism is a nosocomial pathogen and is associated with significant mortality in critically ill patients due to resistance to almost all commercially available antimicrobials, thus limiting therapeutic options.3 In this setting, colistin, a 50-year-old antibiotic, is being used increasingly for the treatment of infections caused by multidrug-resistant (MDR) A. baumannii. Colistin (also known as polymyxin E) is used systemically in the form of its inactive prodrug, colistin methanesulphonate,4 which is converted in vivo to colistin. While resistance to colistin in A. baumannii (MIC > 2 mg/L)5,6 is relatively uncommon, there are disturbing signs that this situation is changing. Colistin heteroresistance (the presence of resistant subpopulations within an isolate that is susceptible based upon its MIC) is a common phenomenon among clinical isolates of A. baumannii7,8 and may be linked to reports of the emergence of colistin resistance.9
Because colistin came into clinical use before the advent of contemporary drug development procedures, there is very little known about its pharmacokinetics (PK) and pharmacodynamics (PD); in particular, the PK/PD index most predictive of antibacterial efficacy against several major Gram-negative pathogens, including A. baumannii, is unknown. Thus, the primary aim of the present study was to elucidate the PK/PD index that predicts colistin efficacy in vivo against A. baumannii. We also determined the extent of the emergence of resistance in vivo to colistin. The studies were conducted in neutropenic murine thigh and lung infection models. To the best of our knowledge, this is the first study specifically designed to elucidate in vivo exposure–response relationships between colistin and A. baumannii.
Materials and methods
Bacterial strains and media
Three strains of A. baumannii were employed in this study: reference strain ATCC 19606 (ATCC, Rockford, MD) and two MDR clinical isolates (248-01-C.248 and N-16870.213). The MICs of colistin (sulphate), as determined by broth microdilution in cation-adjusted Mueller–Hinton broth (CAMHB) according to the Clinical and Laboratory Standards Institute protocol,6 were 1 mg/L for ATCC 19606 and 248-01-C.248, and 0.5 mg/L for N-16870.213; while the MICs for all strains were indicative of susceptibility, the first and third strains were colistin heteroresistant. Colistin heteroresistance was defined as an isolate with colistin MIC ≤ 2 mg/L but with detectable subpopulations able to grow at >2 mg/L colistin.8 All strains were stored in tryptone soy broth with 20% glycerol at −80°C in cryovial storage containers. Prior to each experiment, strains were subcultured onto horse blood agar (Microbiological Media Preparation Unit, University of Melbourne, Victoria, Australia) and incubated at 37°C. A colony was then selected and grown overnight in 10 mL of CAMHB, from which early logarithmic-phase growth was obtained.
Chemicals and reagents
Colistin sulphate (Lot 123K1382; 20 227 U/mg) was purchased from Sigma–Aldrich (St Louis, MO, USA). Prior to each experiment, colistin solutions were freshly prepared in water, sterilized using a 0.2 µm syringe filter and stored at 4°C prior to use. Colistin is stable under these conditions for up to 60 days.10 All other chemicals were from suppliers previously described.11
Neutropenic murine thigh and lung infection models
All animal experimentation was approved by the Monash Institute of Pharmaceutical Sciences animal ethics committee. The neutropenic murine thigh and lung infection models described previously by Dudhani et al.11 were employed. Briefly, 6-week-old, outbred female Swiss albino mice (22–26 g; Monash Animal Services, Clayton, Victoria, Australia) were rendered neutropenic by injecting intraperitoneally cyclophosphamide 4 days (150 mg/kg) and 1 day (100 mg/kg) prior to experimental infection. The animals were provided with food and water ad libitum. Thigh infection was produced by injecting 50 µL of early logarithmic-phase bacterial suspension (∼107 cfu/mL) intramuscularly into each posterior thigh muscle. Lung infection was produced by gradually introducing 50 µL of early logarithmic-phase bacterial suspension (∼108 cfu/mL) into the nares of each anaesthetized mouse. Thereafter, animals were held in a vertical position with their head up for 1 min. In both models, colistin treatment commenced 2 h after inoculation, by which time infection was reproducibly established (see below). The density of the bacterial inoculum was confirmed by quantitative cultures.
PD of colistin in neutropenic mouse thigh and lung infection models
For thigh-infected animals, the colistin (sulphate) regimens involved subcutaneous doses over a range of 1–40 mg/kg and were administered at 6, 8, 12 or 24 h intervals. Because of acute toxicity, the largest dose able to be administered at a given time was 40 mg/kg; the range of daily doses was 1–160 mg/kg/day. Each dosage regimen involved two mice (i.e. four data points). Colistin treatment was initiated 2 h following bacterial inoculation. At 2 or 26 h after bacterial inoculation, untreated mice were humanely killed and thigh homogenates (see below) subjected to quantification of the bacterial load in thigh to define, respectively, the bacterial load at the start time of colistin treatment and overall bacterial growth in the absence of colistin. Colistin-treated mice were humanely killed 24 h after initiation of treatment. Both entire posterior thigh muscles from each mouse were aseptically collected and individually homogenized (Polytron® tissue homogenizer; Kinematica, Switzerland) in 2 mL sterile normal saline in polystyrene round-bottom tubes (Becton Dickinson, NJ, USA). A further 2 mL of sterile saline was added to the homogenate, mixed and filtered through a sterile filter bag (280 µm, Bagpage®; Interscience, France). The filtrate was serially diluted with saline, and 50 µL aliquots were plated (WASP2® spiral plater; Don Whitley Scientific Ltd, England) on nutrient agar plates and incubated at 37°C for 24 h whereupon colonies were counted (Synbiosis protoCOL® colony counter; Don Whitley Scientific Ltd, England). For each thigh, cfu values were expressed as log10 cfu per thigh. The lower limit of quantification was 100 cfu/thigh (equivalent to one colony per plate). For lung-infected mice, the colistin regimens were as described above. At 2 h after inoculation (untreated controls) and 24 h later (untreated controls and colistin-treated mice), animals were sacrificed humanely. Thoracotomy was performed, lungs removed and weighed aseptically, and then homogenized in 2 mL of normal saline as described above. Quantitative cultures (right and left lungs) were conducted as described above. The lower limit of quantification was 220 cfu/lung (equivalent to one colony per plate).
Monitoring emergence of colistin resistance
Real-time population analysis profiles (PAPs) were determined for the inoculum and for thigh and lung samples at 24 h in colistin-treated mice, and at the same time in untreated mice. For colistin-treated mice, ‘low’ (1 or 5 mg/kg/day) and ‘high’ (20 or 40 mg/kg/day or 40 mg/kg/6 h) dosage regimens were investigated for each strain in both thigh and lung infection models. The thigh or lung samples and/or their serial saline dilutions were spiral plated on Mueller–Hinton agar plates (Microbiological Media Preparation Unit) without or with various concentrations (0.5, 1, 2, 3, 4, 5, 6, 8 and 10 mg/L) of colistin sulphate. Colonies were counted (as described above) after 24–48 h of incubation at 35°C. The limit of counting for the inoculum was 20 cfu/mL and for tissues as specified above.
Data analysis
The time courses of unbound plasma colistin concentration arising from single subcutaneous doses of 5, 10, 20 or 40 mg/kg in neutropenic infected mice11 were used to generate (via the superposition principle) the unbound concentration profiles for the various dosage regimens administered over the 24 h treatment period in the PD study. For each resulting multiple-dose profile for unbound plasma colistin over the 24 h treatment period, it was possible to determine the PK/PD indices [the percentage of the dosing interval that the unbound drug concentration exceeded the MIC (fT>MIC), the ratio of the area under the unbound concentration–time curve to the MIC (fAUC/MIC) and the ratio of the unbound peak plasma concentration to the MIC (fCmax/MIC)].
The relationship between efficacy and each of the three PK/PD indices was analysed using the inhibitory sigmoid dose–effect model derived from the Hill equation.11 This model is described by the equation:
where E is the measure of effect (i.e. the log10 cfu per thigh or lung at 24 h); E0 is the effect in the absence of drug; Emax is the maximal effect; X is the value of the relevant PK/PD index (fCmax/MIC, fAUC/MIC or fT>MIC); EI50 is the value of the target PK/PD index required to achieve 50% of Emax; and γ is the Hill coefficient of the PK/PD index–effect curve. The relationship between efficacy and each of the three PK/PD indices was determined for each strain in each infection model by non-linear least squares regression (WinNonlin®, Version 5.2.1; Pharsight Corporation, CA, USA). The coefficient of determination (R2) and visual examination of the fit around the experimental data were used to judge the goodness of fit. The magnitude of the most predictive PK/PD index corresponding to various magnitudes of effect [i.e. stasis (suppression of bacterial growth to a level where the number of viable bacterial cells in thigh or lung after 24 h of treatment was equivalent to that at the time of initiation of colistin treatment), and 1 and 2 log10 kill] was estimated from use of the inhibitory effect sigmoid Emax model equation (see above) and the parameters (E0, Emax, EI50 and γ) obtained from the non-linear least squares regression.
From the real-time PAPs for the respective inoculum and the thigh and lung samples from untreated and colistin-treated mice, the percentage of the total population able to grow at each colistin concentration in the PAPs plates (relative to colistin-free plates) was determined.
Results
Relationships between PK/PD indices and antibacterial activity
Thigh infection model
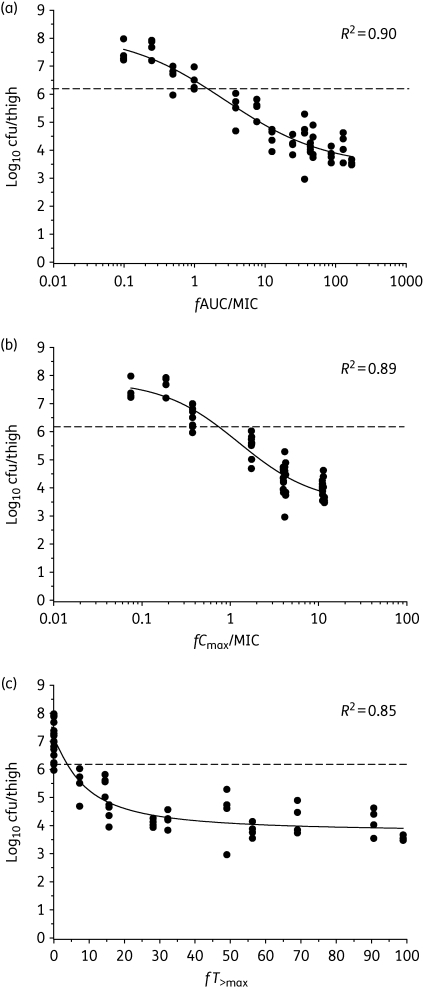
At the start of treatment (2 h after inoculation), the mean ± standard deviation bacterial load in mice was 6.26 ± 0.05, 6.18 ± 0.24 and 6.04 ± 0.16 log10 cfu/thigh for ATCC 19606, 248-01-C.248 and N-16870.213, respectively. Over the next 24 h in untreated control mice, the bacterial numbers increased by 1.12 ± 0.14, 1.03 ± 0.06 and 1.28 ± 0.10 log10 cfu/thigh, respectively. At the upper end of the daily doses of colistin studied, the bacterial burden was decreased, relative to the respective value 2 h after inoculation, by 2.70 ± 0.07, 3.26 ± 0.22 and 2.74 ± 0.10 log10 cfu/thigh for ATCC 19606, 248-01-C.248 and N-16870.213, respectively. Plots of the inhibitory sigmoid Emax model fits of efficacy versus each of the PK/PD indices (fAUC/MIC, fCmax/MIC and fT>MIC) for ATCC 19606 are shown in Figure 1; similar relationships were observed for 248-01-C.248 and N-16870.213 (data not shown). Of the three PK/PD indices, fAUC/MIC was superior to the other two indices for ATCC 19606 (Figure 1) and for the other two strains (data not shown). The PK/PD model parameter estimates for the fAUC/MIC index for each strain in the thigh infection model are shown in Table 1.
Figure 1.
Relationships for A. baumannii ATCC 19606 between the log10 cfu/thigh at 24 h and the PK/PD indices (a) fAUC/MIC, (b) fCmax/MIC and (c) fT>MIC. Each symbol represents the mean datum per mouse from two thighs. R2 is the coefficient of determination. The dotted line represents the mean bacterial burden in thighs at the start of treatment.
Table 1.
The PK/PD model parameter estimates for the fAUC/MIC index of colistin against all three strains of A. baumannii in thigh and lung infection models
| Strain | Emax (log10 cfu/organ) | E0 (log10 cfu/organ) | EI50 | γ |
|---|---|---|---|---|
| Thigh infection model | ||||
| ATCC 19606 | 3.95 (10.1%)a | 7.59 (2.8%) | 3.78 (27.9%) | 0.79 (21.0%) |
| 248-01-C.248b | 4.13 (6.8%) | 6.97 (2.1%) | 16.0 (13.5%) | 1.68 (24.5%) |
| N-16870.213b | 4.53 (6.3%) | 7.47 (2.1%) | 2.72 (18.4%) | 0.60 (12.3%) |
| Lung infection model | ||||
| ATCC 19606 | 3.55 (13.9%) | 7.36 (3.8%) | 4.05 (36.2%) | 0.71 (24.4%) |
| 248-01-C.248b | 4.33 (8.8%) | 6.93 (2.8%) | 17.6 (17.9%) | 1.70 (28.8%) |
| N-16870.213b | 2.67 (16.4%) | 7.76 (3.1%) | 8.12 (38.5%) | 0.93 (36.7%) |
aData in parentheses are the percentage relative standard error.
bMultidrug-resistant clinical strain.
Lung infection model
Two hours after inoculation (i.e. the time of commencement of colistin treatment), bacterial burden was 6.15 ± 0.12, 6.32 ± 0.06 and 6.56 ± 0.10 log10 cfu/lung for ATCC 19606, 248-01-C.248 and N-16870.213, respectively. Over the next 24 h in untreated animals, bacterial numbers increased by 0.97 ± 0.30, 1.15 ± 0.18 and 1.17 ± 0.14 log10 cfu/lung, respectively. The most effective colistin dosage regimens resulted in a reduction, relative to bacterial numbers at the start of colistin treatment (i.e. 2 h after inoculation), of 2.67 ± 0.21, 3.39 ± 0.13 and 1.23 ± 0.18 log10 cfu/lung, respectively. For each of the three strains, the strongest relationship between the antibacterial effect and each of the PK/PD indices occurred for fAUC/MIC (data not shown). The PK/PD model parameter estimates for the fAUC/MIC index for each strain in the lung infection model are in Table 1.
Magnitude of fAUC/MIC associated with various magnitudes of antibacterial effect
Table 2 shows the values of fAUC/MIC required for bacteriostasis and for a 1 and 2 log10 reduction of the bacterial burden. The fAUC/MIC values associated with various magnitudes of effect were generally similar for a given strain between the two infection models and also across the three strains within a given model.
Table 2.
Target values of colistin fAUC/MIC for stasis, and for 1 and 2 log10 kill against all three A. baumannii strains in thigh and lung infection models
| Kill effect |
fAUC/MIC |
||
|---|---|---|---|
| ATCC 19606 | 248-01-C.248a | N-16870.213a | |
| Thigh infection model | |||
| Static effect | 1.89 | 6.75 | 7.41 |
| 1 log10 kill | 6.98 | 13.6 | 11.9 |
| 2 log10 kill | 43.0 | 24.7 | 17.5 |
| Lung infection model | |||
| Static effect | 1.57 | 6.08 | 6.52 |
| 1 log10 kill | 8.18 | 12.9 | 42.1 |
| 2 log10 kill | 95.0 | 22.5 | b |
aMultidrug-resistant clinical strain.
b2 log10 kill was not achieved for this strain in the lung infection model.
Emergence of colistin resistance
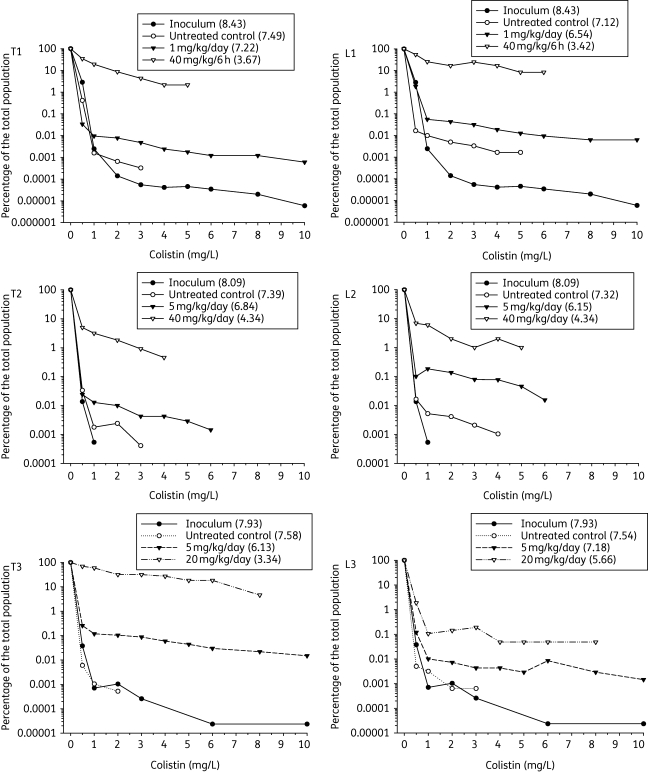
Real-time PAPs demonstrated that 24 h of colistin treatment was associated with the amplification of resistant subpopulations for all strains in both models. Figure 2 shows that while there was a dose-dependent reduction in bacterial burden in thigh or lungs at 24 h relative to the respective untreated control animals, the percentage of the total bacterial population able to grow at various colistin concentrations was increased as a result of colistin treatment.
Figure 2.
Percentage of the total bacterial population able to grow at each colistin concentration (0.5, 1, 2, 3, 4, 5, 6, 8 and 10 mg/L) on the PAP plates (relative to colistin-free plates) determined from real-time PAPs of thigh (T) and lung (L) samples of (1) ATCC 19606, (2) 248-01-C.248 and (3) N-16870.213 after exposure to selected dosage regimens of colistin for 24 h or no colistin treatment in the PD study. Corresponding data for the respective inocula are also shown. The numbers in parentheses in the symbol keys are the log10 cfu per thigh or lung, or log10 cfu/mL for the inocula. Data missing for certain colistin concentrations correspond to values below the limit of bacterial counting.
Discussion
In the current study, two murine infection models were used to identify the PK/PD index most predictive of the antibacterial activity of colistin against A. baumannii and the magnitude of the predictive index required for various magnitudes of effect. Colistin (sulphate) was used in the present study, since it is the active antibacterial agent formed in vivo after administration of its inactive prodrug, colistin methanesulphonate.12–14 We identified that fAUC/MIC was the PK/PD index most predictive of the antibacterial effect of colistin against A. baumannii. In both the thigh and lung infection models, the fAUC/MIC ratio appeared to be slightly more predictive of in vivo bacterial killing than fCmax/MIC and fT>MIC, based upon R2 values and visual examination of the fits. The fAUC/MIC ratio has also been shown to be the PK/PD index most predictive of antibacterial effect against P. aeruginosa in the same murine thigh and lung infection models.11 The fact that antibacterial activity of colistin against A. baumannii in vivo was well correlated with the fAUC/MIC ratio in the present study is consistent with the potent concentration-dependent killing observed in vitro against this bacterial species.15,16 The in vivo relationship between bacterial killing and the fAUC/MIC ratio suggests that it will be important to achieve adequate time-averaged exposure to colistin across the day.
The data in Table 2 for all three strains (two of which were MDR clinical strains) in a given infection model reveal a relatively low degree of interstrain variability (<4–5-fold) in the fAUC/MIC values associated with bacterial stasis, and 1 and 2 log10 kill. The fAUC/MIC ratios were of the same order as those observed previously for P. aeruginosa in the same models.11 For example, the fAUC/MIC associated with 1 log10 kill of three strains of P. aeruginosa in the thigh model ranged from 15.6 to 22.8, while the corresponding range for the lung infection model was 12.2–16.7.11 In the present study, similar Hill coefficients (Table 1) and similar fAUC/MIC ratios associated with various magnitudes of effect (Table 2) were observed for each A. baumannii strain in the thigh and lung infection models. This contrasts with previous findings for P. aeruginosa in the same models where the Hill coefficient for a given strain was substantially lower in lung infection.11 This may possibly be due to differences in the interplay between the relative proportions of colistin-susceptible and -resistant subpopulations, and differences in the growth dynamics of the respective subpopulations of A. baumannii and P. aeruginosa between the two infection sites. It is likely that the relative access of colistin into the respective infection sites (thigh versus lung) would be similar for infections caused by the two bacterial species, although there is no direct evidence to prove that this is the case.
It is not possible to compare the fAUC/MIC values required for various magnitudes of antibacterial effect against A. baumannii observed in the murine models in this study with the fAUC/MIC values for colistin achieved with currently used colistin methanesulphonate dosage regimens in patients. While there is increasing information on the total plasma colistin concentrations occurring in colistin methanesulphonate-treated patients,13,14,17–19 there is no information on the unbound plasma concentrations of colistin. The plasma binding of colistin in mice has been shown recently20 to be very complex, being influenced by its own concentration and those of its binding proteins, albumin and α-1-acid glycoprotein (AAG). The binding of colistin in plasma of infected patients is very likely to be similarly complex, especially since the concentration of AAG in plasma fluctuates in response to various pathophysiological stresses, including infections.21,22
In dosage regimen design, consideration must be given to the potential for the emergence of resistance. It has been shown in an in vitro PK/PD model that simulated human dosing regimens of colistin against two colistin-heteroresistant strains of A. baumannii resulted in extensive initial killing followed by regrowth as early as 6 h after initiation of the regimens.16 In that study, real-time PAPs conducted 24, 48 and 72 h after the start of colistin treatment revealed extensive emergence of resistant subpopulations with all of the colistin regimens. The present study is the first to examine the emergence of resistance to colistin in animal infection models. While the MICs for all three A. baumannii strains were indicative of susceptibility, the first and third strains at baseline were overtly colistin heteroresistant, as determined by PAPs of the initial inoculum. The concentrations of colistin (sulphate) used in the PAPs studies (0.5–10 mg/L) were chosen based upon the relative magnitude of the MICs and the plasma concentrations of colistin typically achieved (0.5–3.5 mg/L) after intravenous administration of colistin methanesulphonate in patients13,14,17–19 together with the breakpoints for colistin against A. baumannii.6,23 The real-time PAPs revealed the emergence of resistance after 24 h of colistin treatment in both murine infection models for all three strains, including that which was not colistin heteroresistant at baseline (Figure 2). While the absolute number of bacteria able to grow at ≥4 mg/L colistin in PAPs was lower after the high colistin dose regimen as compared with the low colistin dose regimen, there was a higher proportion of bacterial cells able to grow at these colistin concentrations after the high dose regimen, because of killing of a larger proportion of the colistin-susceptible subpopulation. The results from the present study are consistent with recent clinical reports.9,24 Hawley et al.9 used PAPs to identify resistant Acinetobacter subpopulations from colistin-susceptible clinical isolates; the proportion of cells exhibiting resistance was significantly higher among isolates recovered from patients treated with colistin methanesulphonate. David and Gill24 suggested that suboptimal colistin methanesulphonate therapy in a burns patient may have resulted in amplification of a colistin-resistant subpopulation leading to elevation of the colistin MIC from ≤0.5 to 4 mg/L. The difficulty in eradicating colistin-resistant subpopulations as observed in an in vitro PK/PD model,16 in patients treated with colistin9,24 and also in the current murine infection models suggests the need for care with the use of colistin monotherapy for serious A. baumannii infections, especially in immunocompromised patients. Recently, it has been demonstrated that the colistin-resistant subpopulations in A. baumannii clinical isolates have substantially increased susceptibility to antibiotics that are inactive (e.g. those normally considered active only against Gram-positive bacteria) or have borderline activity against the parent colistin-heteroresistant clinical isolates,25 a finding that has been confirmed by others.26 That unexpected finding, together with the demonstration in this study of the amplification of colistin-resistant subpopulations upon treatment with colistin, raises the possibility of rationally selecting combinations to increase colistin efficacy against A. baumannii while minimizing the potential for the emergence of resistance. Such an approach would be different from conventional microbiological synergy concepts (i.e. against a homogeneous bacterial population); rather, it may rely on colistin targeting its susceptible subpopulation while the second antibiotic targets the colistin-resistant subpopulation.
In conclusion, we have shown in two mouse infection models that fAUC/MIC is the PK/PD index that is most predictive of antibacterial effect against A. baumannii. This indicates the importance of achieving adequate time-averaged exposure to colistin across the day. The study has also defined fAUC/MIC targets for achieving various magnitudes of bacterial kill. These targets will facilitate the design of dosage regimens for patients, as more information becomes available on the unbound plasma colistin concentrations in patients. Colistin-resistant subpopulations were amplified during colistin treatment, highlighting the importance of research to systematically investigate rational combination therapy to target both the colistin-susceptible and -resistant subpopulations.
Funding
This work was supported by the Australian National Health and Medical Research Council (NHMRC), and the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (Award Number R01AI079330 and R01AI070896). JL is an Australian NHMRC R. Douglas Wright Research Fellow.
Transparency declarations
None to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Acknowledgements
This study was presented in part at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 12–15 September 2009.
The assistance of Mr Chun-Hong Tan of the Facility for Anti-infective Drug Development and Innovation, Monash University, Melbourne, is gratefully acknowledged.
References
- 1.Talbot GH, Bradley J, Edwards JE, Jr, et al. Bad bugs need drugs: an update on the development pipeline from the antimicrobial availability task force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42:657–68. doi: 10.1086/499819. doi:10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. doi:10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 3.Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med. 2008;358:1271–81. doi: 10.1056/NEJMra070741. doi:10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 4.Bergen PJ, Li J, Rayner CR, et al. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50:1953–8. doi: 10.1128/AAC.00035-06. doi:10.1128/AAC.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The European Committee on Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) 2010. Clinical Breakpoint Tables for Interpretation of MICs and Zone Diameters (Version 1.1 April 2010). http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/EUCAST_breakpoints_v1.1.pdf. (07 May 2010, date last accessed) [DOI] [PubMed]
- 6.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 17th Informational Supplement M100-S17. Wayne, PA, USA: CLSI; 2007. [Google Scholar]
- 7.Li J, Rayner CR, Nation RL, et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2006;50:2946–50. doi: 10.1128/AAC.00103-06. doi:10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yau W, Owen RJ, Poudyal A, et al. Colistin hetero-resistance in multidrug-resistant Acinetobacter baumannii clinical isolates from the Western Pacific region in the SENTRY antimicrobial surveillance programme. J Infection. 2009;58:138–44. doi: 10.1016/j.jinf.2008.11.002. doi:10.1016/j.jinf.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Hawley JS, Murray CK, Jorgensen JH. Colistin heteroresistance in Acinetobacter and its association with previous colistin therapy. Antimicrob Agents Chemother. 2008;52:351–2. doi: 10.1128/AAC.00766-07. doi:10.1128/AAC.00766-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Milne RW, Nation RL, et al. Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob Agents Chemother. 2003;47:1364–70. doi: 10.1128/AAC.47.4.1364-1370.2003. doi:10.1128/AAC.47.4.1364-1370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudhani RV, Turnidge JD, Coulthard K, et al. Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob Agents Chemother. 2010;54:1117–24. doi: 10.1128/AAC.01114-09. doi:10.1128/AAC.01114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Milne RW, Nation RL, et al. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother. 2004;53:837–40. doi: 10.1093/jac/dkh167. doi:10.1093/jac/dkh167. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Coulthard K, Milne R, et al. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J Antimicrob Chemother. 2003;52:987–92. doi: 10.1093/jac/dkg468. doi:10.1093/jac/dkg468. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Rayner CR, Nation RL, et al. Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother. 2005;49:4814–5. doi: 10.1128/AAC.49.11.4814-4815.2005. doi:10.1128/AAC.49.11.4814-4815.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owen RJ, Li J, Nation RL, et al. In vitro pharmacodynamics of colistin against Acinetobacter baumannii clinical isolates. J Antimicrob Chemother. 2007;59:473–7. doi: 10.1093/jac/dkl512. doi:10.1093/jac/dkl512. [DOI] [PubMed] [Google Scholar]
- 16.Tan C-H, Li J, Nation RL. Activity of colistin against heteroresistant Acinetobacter baumannii and emergence of resistance in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2007;51:3413–5. doi: 10.1128/AAC.01571-06. doi:10.1128/AAC.01571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plachouras D, Karvanen M, Friberg LE, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by Gram-negative bacteria. Antimicrob Agents Chemother. 2009;53:3430–6. doi: 10.1128/AAC.01361-08. doi:10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markou N, Markantonis SL, Dimitrakis E, et al. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, Gram-negative bacilli infections: a prospective, open-label, uncontrolled study. Clin Ther. 2008;30:143–51. doi: 10.1016/j.clinthera.2008.01.015. doi:10.1016/j.clinthera.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Reed MD, Stern RC, O'Riordan MA, et al. The pharmacokinetics of colistin in patients with cystic fibrosis. J Clin Pharmacol. 2001;41:645–54. doi: 10.1177/00912700122010537. [DOI] [PubMed] [Google Scholar]
- 20.Dudhani RV, Li J, Nation RL. Plasma binding of colistin involves multiple proteins and is concentration dependent: potential clinical implications. Abstracts of the Forty-ninth Interscience Conference on Antimicrobial Agents and Chemotherapy; 2009; San Francisco, CA. Washington, DC, USA: American Society for Microbiology; Abstract A1-576, p. 41. [Google Scholar]
- 21.Morita K, Yamaji A. Changes in the serum protein binding of vancomycin in patients with methicillin-resistant Staphylococcus aureus infection: the role of serum α 1-acid glycoprotein levels. Ther Drug Monit. 1995;17:107–12. doi: 10.1097/00007691-199504000-00001. doi:10.1097/00007691-199504000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Voulgari F, Cummins P, Gardecki TI, et al. Serum levels of acute phase and cardiac proteins after myocardial infarction, surgery, and infection. Br Heart J. 1982;48:352–6. doi: 10.1136/hrt.48.4.352. doi:10.1136/hrt.48.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.British Society for Antimicrobial Chemotherapy. BSAC Methods for Antimicrobial Susceptibility Testing, Version 8 [online]. http://www.bsac.org.uk/_db/_documents/Version_8_-_January_2009.pdf. (07 May 2010, date last accessed)
- 24.David MD, Gill MJ. Potential for underdosing and emergence of resistance in Acinetobacter baumannii during treatment with colistin. J Antimicrob Chemother. 2008;61:962–4. doi: 10.1093/jac/dkn009. doi:10.1093/jac/dkn009. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Nation RL, Owen RJ, et al. Antibiograms of multidrug-resistant clinical Acinetobacter baumannii: promising therapeutic options for treatment of infection with colistin-resistant strains. Clin Infect Dis. 2007;45:594–8. doi: 10.1086/520658. doi:10.1086/520658. [DOI] [PubMed] [Google Scholar]
- 26.Mendes RE, Fritsche TR, Sader HS, et al. Increased antimicrobial susceptibility profiles among polymyxin resistant Acinetobacter baumannii clinical isolates. Clin Infect Dis. 2008;46:1324–6. doi: 10.1086/533476. doi:10.1086/533476. [DOI] [PubMed] [Google Scholar]