Abstract
Objectives
To assess long-term virological efficacy and the emergence of drug resistance in children who receive antiretroviral treatment (ART) in rural Tanzania.
Patients and methods
Haydom Lutheran Hospital has provided ART to HIV-infected individuals since 2003. From February through May 2009, a cross-sectional virological efficacy survey was conducted among children (<15 years) who had completed ≥6 months of first-line non-nucleoside reverse transcriptase inhibitor (NNRTI)-based ART. Genotypic resistance was determined in those with a viral load of >200 copies/mL.
Results
Virological response was measured in 19 of 23 eligible children; 8 of 19 were girls and median age at ART initiation was 5 years (range 2–14 years). Median duration of ART at the time of the survey was 40 months (range 11–61 months). Only 8 children were virologically suppressed (≤40 copies/mL), whereas 11 children had clinically relevant resistance mutations in the reverse transcriptase gene. The most frequent mutations were M184V (n = 11), conferring resistance to lamivudine and emtricitabine, and Y181C (n = 4), G190A/S (n = 4) and K103N (n = 4), conferring resistance to NNRTIs. Of concern, three children had thymidine analogue mutations, associated with cross-resistance to all nucleoside reverse transcriptase inhibitors. Despite widespread resistance, however, only one child experienced a new WHO stage 4 event and none had a CD4 cell count of <200 cells/mm3.
Conclusions
Among children on long-term ART in rural Tanzania, >50% harboured drug resistance. Results for children were markedly poorer than for adults attending the same programme, underscoring the need for improved treatment strategies for children in resource-limited settings.
Keywords: HIV infections, antiretroviral therapy, sub-Saharan Africa, child
Introduction
Currently, 2.1 million children are living with HIV/AIDS, of whom ∼90% reside in sub-Saharan Africa. Access to antiretroviral treatment (ART) has increased dramatically over the past years, and by the end of 2008, 275 700 children were receiving ART in low- and middle-income countries.1
There are numerous obstacles to paediatric ART in resource-limited settings, including: lack of reliable HIV tests for infants; limited laboratory capacity to monitor treatment efficacy; variable pharmacokinetics in children; and lack of paediatric antiretroviral formulations. Most studies evaluating paediatric ART in resource-limited settings have been limited by short follow-up time and few have provided genotypic resistance results.2–6 Furthermore, there is a paucity of research from rural settings, which often face shortages of health workers, transport difficulties and other logistical constraints. Therefore, we aimed to assess long-term virological efficacy and the emergence of drug resistance among children who received first-line ART at a rural Tanzanian hospital.
Patients and methods
Haydom Lutheran Hospital is a 400 bed hospital located in northern Tanzania. Since 2003, free ART has been offered to HIV-infected children in accordance with guidelines from the WHO.7 ART eligibility was based solely on clinical criteria (WHO paediatric stage 3 or 4) until 2006, when automated CD4 cell counts became available (FACSCount flow cytometer, Becton Dickinson, San Jose, CA, USA). First-line treatment comprised stavudine or zidovudine, combined with lamivudine, and either nevirapine or efavirenz. Paediatric second-line treatment was not available at the time of the survey. After ART initiation, patients were seen by a clinical officer every 3 months and CD4 cell counts were performed every 3–6 months.
From February through May 2009, a cross-sectional survey was carried out among children who had completed ≥6 months of first-line ART. Children aged <15 years who presented at the clinic during the survey period were considered eligible. Ethical approval was granted by the National Institute for Medical Research (Tanzania) and Regional Committee for Medical Research Ethics (Norway), and patients or their carers gave written informed consent to participate in the study.
Plasma specimens for HIV viral load were analysed at Muhimbili National Hospital, Dar es Salaam, Tanzania, using the COBAS AmpliPrep/COBAS TaqMan 48 Analyzer (Roche Diagnostics, Branchburg, NJ, USA) with a lower detection limit of 40 copies/mL. Specimens with a viral load of >200 copies/mL were sent to Oslo University Hospital, Ulleval, Oslo, Norway, for subtyping and genotypic resistance testing using the ViroSeq HIV-1 Genotyping System (Abbott Molecular, De Plains, IL, USA). Drug resistance mutations listed in the December 2009 update from the International AIDS Society were considered in this study.8 Subtyping and resistance profiles to antiretroviral drugs were interpreted according to the Stanford University HIV Drug Resistance Database (HIVdb Program, http://hivdb.stanford.edu).
The main outcomes of interest were on-treatment viral suppression and clinically significant genotypic resistance. Weight-for-age Z-scores were calculated using software from WHO (WHO AntroPlus for personal computers, http://www.who.int/growthref/tools/en/). Adherence was classified as good (>95%), variable (80%–95%) or poor (<80%), based on clinician estimates. Student t-tests were used to compare mean CD4 cell counts between those with and without resistance. Logistic regression was used to study associations between baseline characteristics and the emergence of drug resistance. Variables with P < 0.1 in univariable analyses were selected for multivariable regression analysis. Data were analysed with SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL, USA), except for 95% confidence intervals (CIs) for proportions, which were calculated with NCSS version 2007 (NCSS, Kaysville, UT, USA). All tests were two-sided and the level of significance was set at P < 0.05.
Results
Of 48 children who enrolled in the HIV programme and started ART before 1 August 2008, 11 (23%) died, 3 (6%) were lost to follow-up, 9 (19%) were transferred to another health facility and 2 (4%) discontinued treatment. Of the remaining 23 children who were eligible for virological testing, 19 attended the clinic during the survey period and were included in this study (Table 1).
Table 1.
Genotypic resistance results for 19 HIV-infected children on long-term ART, Haydom Lutheran Hospital, Tanzania
| ID no. | Sex | Age at ART start (years) | ART regimen: initial (currenta) | Duration of ART (months) | Most recent CD4 cell count (cells/mm3)b | Viral load (copies/mL) | NRTI mutations | NNRTI mutations | Resistance to antiretroviral drugs |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 6 | ZDV/3TC/EFV (d4T/3TC/NVP) | 40 | 595 | 640 000 | M184V | V108I, Y181CV | 3TC, FTC, all NNRTIs |
| 2 | M | 7 | d4T/3TC/NVP | 11 | 667 | 300 000 | M184V | G190S | 3TC, FTC, all NNRTIs |
| 3 | M | 5 | ZDV/3TC/NVP (d4T/3TC/NVP) | 18 | 650 | 220 000 | D67N, K70R, M184V, T215Y, K219EQ | K101E, Y181C, G190A | all NRTIs and NNRTIs |
| 4 | M | 11 | d4T/3TC/NVP | 58 | 550 | 28 000 | V75I, M184V, T215Y | K103N, V108I | all NRTIs, NVP, EFV |
| 5 | M | 3 | d4T/3TC/NVP | 59 | 575 | 23 000 | M184V | K101E, G190A | 3TC, FTC, all NNRTIs |
| 6 | M | 5 | ZDV/3TC/EFV (d4T/3TC/NVP) | 25 | 899 | 6400 | M184V | Y181C | 3TC, FTC, all NNRTIs |
| 7 | F | 2 | ZDV/3TC/NVP | 35 | 748 | 5000 | M184V | Y181C | 3TC, FTC, all NNRTIs |
| 8 | F | 4 | d4T/3TC/NVP (ZDV/3TC/EFV) | 56 | 264 | 1200 | D67N, K70R, M184V, L210W, T215F, K219Q | V179T, G190A | all NRTIs and NNRTIs |
| 9 | M | 14 | d4T/3TC/EFV (d4T/3TC/NVP) | 37 | 398 | 980 | M184V | K103N, V108I | 3TC, FTC, NVP, EFV |
| 10 | M | 8 | d4T/3TC/NVP | 54 | 713 | 870 | M184V | K103N | 3TC, FTC, NVP, EFV |
| 11 | F | 2 | ZDV/3TC/NVP (d4T/3TC/NVP) | 36 | 496 | 350 | A64V, M184V | V90I, K103N | 3TC, FTC, NVP, EFV |
| 12 | F | 2 | ZDV/3TC/NVP (ZDV/3TC/EFV) | 27 | >2000 | NDc | none | none | none |
| 13 | M | 5 | ZDV/3TC/NVP (d4T/3TC/NVP) | 51 | 820 | 40 | — | — | — |
| 14 | F | 9 | d4T/3TC/NVP | 61 | 712 | <40 | — | — | — |
| 15 | M | 10 | ZDV/3TC/NVP (d4T/3TC/NVP) | 53 | 800 | <40 | — | — | — |
| 16 | F | 5 | ZDV/3TC/EFV (d4T/3TC/NVP) | 43 | 1261 | <40 | — | — | — |
| 17 | F | 5 | ZDV/3TC/EFV (d4T/3TC/NVP) | 40 | 1050 | <40 | — | — | — |
| 18 | F | 3 | ZDV/3TC/NVP (d4T/3TC/EFV) | 23 | 1599 | <40 | — | — | — |
| 19 | M | 5 | d4T/3TC/NVP | 11 | 672 | <40 | — | — | — |
d4T, stavudine; ZDV, zidovudine; 3TC, lamivudine; FTC, emtricitabine; NVP, nevirapine; EFV, efavirenz.
aOnly specified if different from initial regimen.
bWithin ±6 months of viral load/resistance testing.
cND, not done; venipuncture failed and dried blood spots were used for resistance testing.
Eight of the 19 children were girls and the median age at ART initiation was 5 years (range 2–14 years). Only eight children were taken care of by their parents. Most patients had signs and symptoms of severe immunodeficiency at baseline: 10 children were classified as WHO stage 4; 8 as WHO stage 3; and one as WHO stage 2. Median weight-for-age Z-score was −2.67 (range −5.66 to −0.19) and 9 of 18 had a haemoglobin level of <10 g/dL. Only five patients had a pre-ART CD4 cell count; the median absolute CD4 cell count was 311 cells/mm3 (range 245–426 cells/mm3) and the median CD4% (CD4 cell count divided by total lymphocyte count) was 11.5% (range 6.1%–21.3%). There were no significant differences between children who remained in care and those lost to the programme with regard to gender, age, WHO stage, weight-for-age Z-scores, haemoglobin or CD4 cell count.
Median duration of ART at the time of the survey was 40 months (range 11–61 months). The initial ART regimen was zidovudine/lamivudine/nevirapine in seven patients, stavudine/lamivudine/nevirapine in seven patients, zidovudine/lamivudine/efavirenz in four patients and stavudine/lamivudine/efavirenz in one patient. All but one received syrup formulations as part of their initial regimen. Only one child (Patient 18) had previously been exposed to antiretroviral drugs through prevention of mother-to-child transmission interventions.
A plasma viral load result was available in 18 children, of whom 7 had ≤40 copies/mL (Table 1). In one patient venipuncture failed, but genotyping from dried blood spots succeeded. Assuming viral suppression in this patient, who had a CD4 cell count of >2000 cells/mm3 and no drug resistance, still only 8 of 19 patients (42%; 95% CI: 20%–67%) were classified as virologically suppressed.
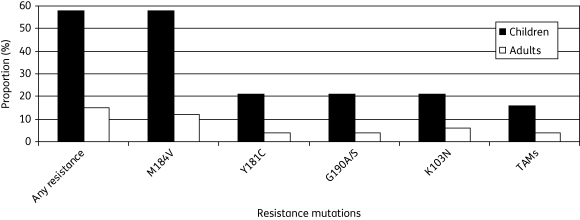
Eleven plasma samples with a viral load of >200 copies/mL were genotyped, in addition to the dried blood spot specimen. HIV-1 subtypes were: A (n = 5); C (n = 3); D (n = 2); CRF01_AE (n = 1); and inconclusive (n = 1). Eleven of 19 patients (58%; 95% CI: 34%–80%) had clinically relevant resistance mutations in the reverse transcriptase (RT) gene (Table 1), all of whom had resistance to both nucleoside reverse transcriptase inhibitors (NRTIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs). None had clinically relevant resistance mutations in the protease gene. The most frequent RT mutations were M184V (n = 11), conferring resistance to lamivudine and emtricitabine, and Y181C (n = 4), G190A/S (n = 4) and K103N (n = 4), conferring resistance to NNRTIs. Of concern, three children (16%; 95% CI: 3%–40%) had thymidine analogue mutations, associated with cross-resistance to all NRTIs. Figure 1 gives the prevalence of drug resistance RT mutations in children, compared with adults from the same programme with ≥30 months (median 36 months, range 30–53 months) follow-up time on first-line ART.9
Figure 1.
Proportion (%) of children on ART who harboured drug resistance mutations in the RT gene. For comparison, adults from the same programme with ≥30 months (median 36 months, range 30–53 months) follow-up time on first-line ART are shown.9 TAMs, thymidine analogue mutations.
In spite of widespread resistance in >50% of the patients, only one child experienced a new WHO stage 4 event. CD4 cell counts at the time of resistance testing (±6 months) were available for all 19 patients and none had a CD4 cell count of <200 cells/mm3. However, mean CD4 cell count was significantly lower in patients with resistance compared with those without resistance (596 versus 1114 cells/mm3; P = 0.018).
Only adherence was significantly associated with emergence of drug resistance (poor/variable versus good; odds ratio 12.0; 95% CI: 1.3–111.3; P = 0.029). No significant associations were found for sex, age, WHO stage, weight-for-age Z-score, haemoglobin level, total lymphocyte count, carer (parents versus others) or duration of ART. Since all patients with viral non-suppression also had resistance, and vice versa, the same analysis also applies to predictors of viral non-suppression.
Discussion
Only 42% of the children in this study had a suppressed HIV viral load and >50% harboured drug resistance after a median of 40 months on first-line ART. These results were markedly poorer than for adults attending the same clinic, of whom 15% had drug resistance after 3–4 years on first-line ART.9
Only a few previous studies have reported long-term virological results of paediatric ART in Africa. Rouet et al.10 found that only 46.5% of children had a suppressed viraemia (<300 copies/mL) after 42 months on mainly protease inhibitor-based ART in Abidjan, Côte d'Ivoire. In contrast, Davies et al.11 recently reported excellent treatment outcomes in a large paediatric multicentre cohort in South Africa, with viral suppression (<400 copies/mL) in 82.4% of children after 3 years on ART. Our study, however, is the first to describe long-term resistance results from a rural African paediatric ART programme. The resistance pattern observed in our cohort, with high-level resistance to lamivudine and NNRTIs in patients with viral non-suppression, is similar to recent data from paediatric cohorts in Thailand and China,5,6,12 and might provide a useful forecast of drug resistance and demand for second-line paediatric antiretroviral drugs in resource-limited settings in the coming years.
In the present study, the smaller children were prescribed syrups and/or tablets, whereas those >15 kg received an adult fixed-dose combination tablet divided into halves and quarters. Both these approaches have serious weaknesses. The syrup formulations may be difficult to administer correctly, foul tasting, require refrigeration and have short shelf-lives once opened.7 With regard to the use of adult fixed-dose combination tablets, accurate cutting can be difficult to achieve. Moreover, the ratio between the three drugs is not suitable for paediatric use, since children have a faster nevirapine metabolism than adults.13 Given the low genetic barrier of the NNRTIs, selection of resistance can occur rapidly in patients with suboptimal adherence or reduced serum concentrations. Poor adherence was a strong and significant predictor of resistance in our study, and, hence, in order to promote adherence, it is imperative to increase access to fixed-dose combination tablets specifically designed for children. This should include a variety of drug combinations, including regimens based on ritonavir-boosted protease inhibitors, for which the genetic barrier to resistance is higher.
There were certain limitations to our study. First, the study was small and the estimates of drug resistance have wide CIs. Second, the lack of objective measures of adherence was a weakness of our study design. Third, there was a selection bias of older children, who represent long-time non-progressors with a better prognosis and whose adherence might differ from younger children. On the other hand, since ART initiation was based mostly on clinical criteria, there was a selection bias towards more advanced immunodeficiency at baseline, which increases the risk of drug resistance. However, these biases reflect the realities in many African ART programmes.2–4,10,11 Finally, this was a cross-sectional survey of patients alive and in care, leaving out a high number of patients who died, were lost to follow-up or transferred out; hence, our data cannot be extrapolated to all patients starting ART. Nevertheless, we managed to include 19 of 23 eligible children and, thus, we believe our results are representative of children who receive long-term ART in this setting.
In conclusion, we found an alarmingly high prevalence of drug resistance among children who received long-term ART in rural Tanzania. Results for children were markedly poorer than for adults attending the same clinic. Improved treatment strategies for children, such as access to a variety of fixed-dose combination tablets for all age groups, should be prioritized in the global efforts to scale-up ART in resource-limited settings.
Funding
This work was supported by a grant from the Regional Health Authority of South-Eastern Norway (Helse Sor-Ost RHF).
Transparency declarations
None to declare.
Acknowledgements
We are indebted to the patients who participated in the study. We acknowledge the staff at Haydom HIV Care and Treatment Centre, the hospital management, the Ministry of Health and the National AIDS Control Program for collaboration and support.
References
- 1.WHO. Towards Universal Access: Scaling Up Priority HIV/AIDS Interventions in the Health Sector. Progress Report 2009. Geneva: WHO; 2009. [Google Scholar]
- 2.Chaix ML, Rouet F, Kouakoussui KA, et al. Genotypic human immunodeficiency virus type 1 drug resistance in highly active antiretroviral therapy-treated children in Abidjan, Cote d'Ivoire. Pediatr Infect Dis J. 2005;24:1072–6. doi: 10.1097/01.inf.0000190413.88671.92. [DOI] [PubMed] [Google Scholar]
- 3.Song R, Jelagat J, Dzombo D, et al. Efficacy of highly active antiretroviral therapy in HIV-1 infected children in Kenya. Pediatrics. 2007;120:e856–61. doi: 10.1542/peds.2006-1122. doi:10.1542/peds.2006-1122. [DOI] [PubMed] [Google Scholar]
- 4.Germanaud D, Derache A, Traore M, et al. Level of viral load and antiretroviral resistance after 6 months of non-nucleoside reverse transcriptase inhibitor first-line treatment in HIV-1-infected children in Mali. J Antimicrob Chemother. 2010;65:118–24. doi: 10.1093/jac/dkp412. doi:10.1093/jac/dkp412. [DOI] [PubMed] [Google Scholar]
- 5.Jittamala P, Puthanakit T, Chaiinseeard S, et al. Predictors of virologic failure and genotypic resistance mutation patterns in Thai children receiving non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Pediatr Infect Dis J. 2009;28:826–30. doi: 10.1097/INF.0b013e3181a458f9. [DOI] [PubMed] [Google Scholar]
- 6.Zhang F, Haberer J, Wei H, et al. Drug resistance in the Chinese National Pediatric Highly Active Antiretroviral Therapy Cohort: implications for paediatric treatment in the developing world. Int J STD AIDS. 2009;20:406–9. doi: 10.1258/ijsa.2008.008357. doi:10.1258/ijsa.2008.008357. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Antiretroviral Therapy of HIV Infection in Infants and Children in Resource-limited Settings: Towards Universal Access. Recommendations for a Public Health Approach. Geneva: WHO; 2006. [PubMed] [Google Scholar]
- 8.Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: December 2009. Top HIV Med. 2009;17:138–45. [PubMed] [Google Scholar]
- 9.Johannessen A, Naman E, Kivuyo SL, et al. Virological efficacy and emergence of drug resistance in adults on antiretroviral treatment in rural Tanzania. BMC Infect Dis. 2009;9:108. doi: 10.1186/1471-2334-9-108. doi:10.1186/1471-2334-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouet F, Fassinou P, Inwoley A, et al. Long-term survival and immuno-virological response of African HIV-1-infected children to highly active antiretroviral therapy regimens. AIDS. 2006;20:2315–9. doi: 10.1097/QAD.0b013e328010943b. doi:10.1097/QAD.0b013e328010943b. [DOI] [PubMed] [Google Scholar]
- 11.Davies MA, Keiser O, Technau K, et al. Outcomes of the South African National Antiretroviral Treatment Programme for children: the IeDEA Southern Africa collaboration. S Afr Med J. 2009;99:730–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Puthanakit T, Jourdain G, Hongsiriwon S, et al. HIV-1 drug resistance mutations in children after failure of first-line nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy. HIV Med. 2010 doi: 10.1111/j.1468-1293.2010.00828.x. in press. doi:10.1111/j.1468-1293.2010.00828.x. [DOI] [PubMed] [Google Scholar]
- 13.Ellis JC, L'homme RF, Ewings FM, et al. Nevirapine concentrations in HIV-infected children treated with divided fixed-dose combination antiretroviral tablets in Malawi and Zambia. Antivir Ther. 2007;12:253–60. [PubMed] [Google Scholar]