Abstract
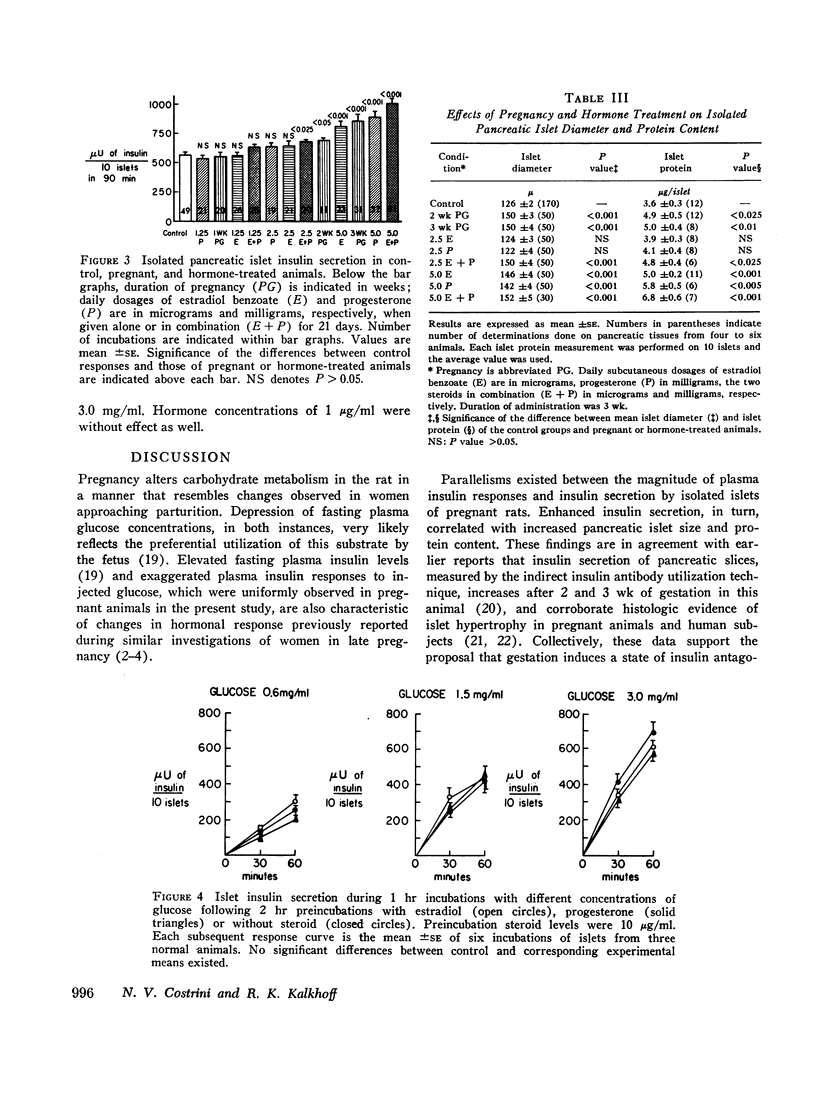
Influences of estrogen and progesterone on the development of hyperinsulinemia and augmented pancreatic islet insulin secretion during pregnancy were assessed in this study. Groups of female rats were injected subcutaneously for 21 days with varying daily dosages of estradiol benzoate or progesterone in oil. On day 21, pancreatic islets were isolated by a collagenase method. Total insulin secretion was measured after 90-min incubations of 10 islets in buffered medium containing glucose. Higher physiologic dosages of estradiol or progesterone, singly or in combination, significantly increased islet secretion above values of untreated control rats and were comparable to augmented islet responses of term, 3-wk pregnant rats. Diameter and protein content of islets obtained from steroid-treated and pregnant rats exceeded control measurements in these instances. However, 2-hr preincubations of control islets with 1 or 10 μg/ml of either steroid did not influence subsequent glucose-stimulated insulin output.
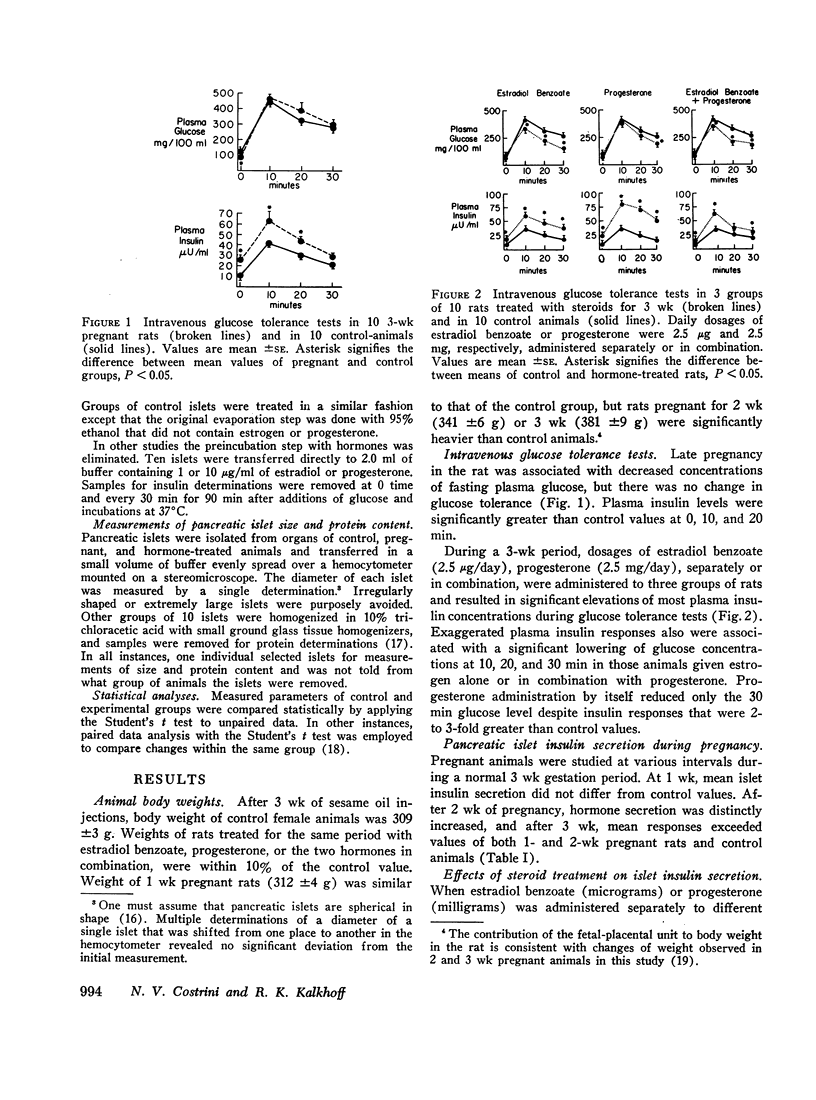
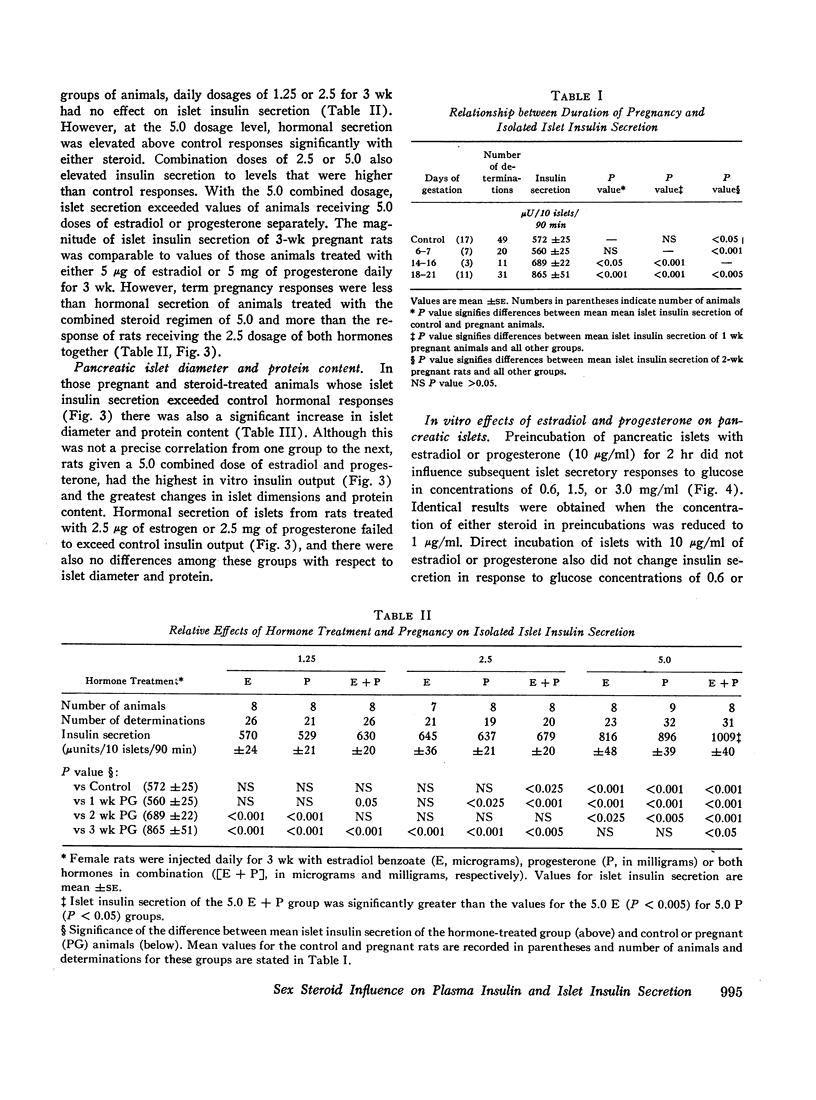
In related studies, plasma insulin responses during 30 min intravenous glucose tolerance tests were significantly above control responses in term-pregnant rats and animals receiving comparable dosages of steroids for 3 wk. Unlike pregnancy or progesterone treatment, estradiol administration alone or with progesterone significantly lowered postchallenge plasma glucose concentrations.
These results indicate that estradiol and progesterone contribute to enhanced islet insulin secretion and plasma insulin responses to glucose administration during pregnancy. This change is not acutely produced but can be related to hypertrophy of islets following chronic hormonal administration. Although the data do not distinguish between direct and indirect beta-cytotrophic effects of these sex steroids, metabolic actions of estradiol and progesterone may differ, since estrogen treatment lowers plasma glucose curves following the induction of hyperinsulinemia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLEICHER S. J., O'SULLIVAN J. B., FREINKEL N. CARBOHYDRATE METABOLISM IN PREGNANCY. V. THE INTERRELATIONS OF GLUCOSE, INSULIN AND FREE FATTY ACIDS IN LATE PREGNANCY AND POST PARTUM. N Engl J Med. 1964 Oct 22;271:866–872. doi: 10.1056/NEJM196410222711702. [DOI] [PubMed] [Google Scholar]
- BROLIN S., HELLMAN B. New methods for determination of the pancreatic islet volume. Diabetes. 1963 Jan-Feb;12:62–65. doi: 10.2337/diab.12.1.62. [DOI] [PubMed] [Google Scholar]
- Beck P., Daughaday W. H. Human placental lactogen: studies of its acute metabolic effects and disposition in normal man. J Clin Invest. 1967 Jan;46(1):103–110. doi: 10.1172/JCI105503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck P., Parker M. L., Daughaday W. H. Radioimmunologic measurement of human placental lactogen in plasma by a double antibody method during normal and diabetic pregnancies. J Clin Endocrinol Metab. 1965 Nov;25(11):1457–1462. doi: 10.1210/jcem-25-11-1457. [DOI] [PubMed] [Google Scholar]
- Beck P. Progestin enhancement of the plasma insulin response to glucose in Rhesus monkeys. Diabetes. 1969 Mar;18(3):146–152. doi: 10.2337/diab.18.3.146. [DOI] [PubMed] [Google Scholar]
- Bullock D. W., Cook B. Concentration of progesterone in the blood. J Endocrinol. 1967 Apr;37(4):382–384. [PubMed] [Google Scholar]
- Csapo A. I., Wiest W. G. An examination of the quantitative relationship between progesterone and the maintenance of pregnancy. Endocrinology. 1969 Oct;85(4):735–746. doi: 10.1210/endo-85-4-735. [DOI] [PubMed] [Google Scholar]
- Forbes T. R. Synergisms and antagonisms of estrogens and progesterone in a mouse uterine bio-assay. Endocrinology. 1966 Aug;79(2):420–423. doi: 10.1210/endo-79-2-420. [DOI] [PubMed] [Google Scholar]
- GALLETTI F., KLOPPER A. THE EFFECT OF PROGESTERONE ON THE QUANTITY AND DISTRIBUTION OF BODY FAT IN THE FEMALE RAT. Acta Endocrinol (Copenh) 1964 Jul;46:379–386. doi: 10.1530/acta.0.0460379. [DOI] [PubMed] [Google Scholar]
- GROSVENOR C. E., TURNER C. W. Effect of estrogen upon thyroxine secretion rate in intact female rats. Proc Soc Exp Biol Med. 1959 May;101(1):194–196. doi: 10.3181/00379727-101-24880. [DOI] [PubMed] [Google Scholar]
- Gusdon J. P., Jr, Leake N. H., Atkins W., Van Dyke A. H. Immunochemical comparison of human placental lactogen and placental proteins from other species. Am J Obstet Gynecol. 1970 Jun 1;107(3):441–444. doi: 10.1016/0002-9378(70)90573-9. [DOI] [PubMed] [Google Scholar]
- HELLMAN B. The islets of Langerhans in the rat during pregnancy and lactation, with special reference to the changes in the B/A cell ratio. Acta Obstet Gynecol Scand. 1960;39:331–342. doi: 10.3109/00016346009159930. [DOI] [PubMed] [Google Scholar]
- Herrera E., Knopp R. H., Freinkel N. Carbohydrate metabolism in pregnancy. VI. Plasma fuels, insulin, liver composition, gluconeogenesis, and nitrogen metabolism during late gestation in the fed and fasted rat. J Clin Invest. 1969 Dec;48(12):2260–2272. doi: 10.1172/JCI106192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALKHOFF R., SCHALCH D. S., WALKER J. L., BECK P., KIPNIS D. M., DAUGHADAY W. H. DIABETOGENIC FACTORS ASSOCIATED WITH PREGNANCY. Trans Assoc Am Physicians. 1964;77:270–280. [PubMed] [Google Scholar]
- KREBS H. A. Body size and tissue respiration. Biochim Biophys Acta. 1950 Jan;4(1-3):249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- Kalkhoff R. K., Jacobson M., Lemper D. Progesterone, pregnancy and the augmented plasma insulin response. J Clin Endocrinol Metab. 1970 Jul;31(1):24–28. doi: 10.1210/jcem-31-1-24. [DOI] [PubMed] [Google Scholar]
- Kalkhoff R. K., Richardson B. L., Beck P. Relative effects of pregnancy, human placental lactogen and prednisolone on carbohydrate tolerance in normal and subclinical diabetic subjects. Diabetes. 1969 Mar;18(3):153–163. doi: 10.2337/diab.18.3.153. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Landau R. L., Lugibihl K. The effect of progesterone on the concentration of plasma amino acids in man. Metabolism. 1967 Dec;16(12):1114–1122. doi: 10.1016/0026-0495(67)90057-1. [DOI] [PubMed] [Google Scholar]
- Lyngset O., Velle W. In vitro metabolism of oestrogens in liver tissue from domestic animals. Acta Endocrinol (Copenh) 1968 Dec;59(4):682–688. doi: 10.1530/acta.0.0590682. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Picard C., Flament-Durand J. Effects of pregnancy and chorionic growth hormone upon insulin secretion. Endocrinology. 1969 Jan;84(1):41–44. doi: 10.1210/endo-84-1-41. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr, Steiner D. F. The effect of estrogen on sugar transport in the rat uterus. Biochim Biophys Acta. 1967 Sep 9;135(4):717–726. doi: 10.1016/0005-2736(67)90102-2. [DOI] [PubMed] [Google Scholar]
- SPELLACY W. N., GOETZ F. C. Plasma insulin in normal late pregnancy. N Engl J Med. 1963 May 2;268:988–991. doi: 10.1056/NEJM196305022681805. [DOI] [PubMed] [Google Scholar]
- Samaan N., Yen S. C., Gonzalez D., Pearson O. H. Metabolic effects of placental lactogen (HPL) in man. J Clin Endocrinol Metab. 1968 Apr;28(4):485–491. doi: 10.1210/jcem-28-4-485. [DOI] [PubMed] [Google Scholar]
- Song C. S., Kappas A. The influence estrogens, progestins, and pregnancy on the liver. Vitam Horm. 1968;26:147–195. doi: 10.1016/s0083-6729(08)60754-2. [DOI] [PubMed] [Google Scholar]
- Spellacy W. N. A review of carbohydrate metabolism and the oral contraceptives. Am J Obstet Gynecol. 1969 Jun 1;104(3):448–460. doi: 10.1016/s0002-9378(16)34204-1. [DOI] [PubMed] [Google Scholar]
- TALAAT M., HABIB Y. A., ABDELNABY S., HAMDI H., MALEK A. Y., IBRAHIM Z. A., SAAD A. F. EFFECT OF OESTRADIOL DIPROPIONATE ON THE GLUCOSE TOLERANCE AND INSULIN SENSITIVITY CURVES IN NORMAL AND OVARECTOMIZED FEMALE RABBITS. Arch Int Pharmacodyn Ther. 1965 Feb;153:290–299. [PubMed] [Google Scholar]
- Talaat M., Habib Y. A., Higazy A. M., Abdel Naby S., Malek A. Y., Ibrahim Z. A. Effect of sex hormones on the carbohydrate metabolism in normal and diabetic women. Arch Int Pharmacodyn Ther. 1965 Apr;154(2):402–411. [PubMed] [Google Scholar]
- Yoshinaga K., Hawkins R. A., Stocker J. F. Estrogen secretion by the rat ovary in vivo during the estrous cycle and pregnancy. Endocrinology. 1969 Jul;85(1):103–112. doi: 10.1210/endo-85-1-103. [DOI] [PubMed] [Google Scholar]
- Zinneman H. H., Seal U. S., Doe R. P. Urinary amino acids in pregnancy, following progesterone, and estrogen-progesterone. J Clin Endocrinol Metab. 1967 Mar;27(3):397–405. doi: 10.1210/jcem-27-3-397. [DOI] [PubMed] [Google Scholar]



