Abstract
Constipation disproportionately affects older adults, with a prevalences of 50% in community-dwelling elderly and 74% in nursing-home residents. Loss of mobility, medications, underlying diseases, impaired anorectal sensation, and ignoring calls to defecate are as important as dyssynergic defecation or irritable bowel syndrome in causing constipation. Detailed medical history on medications and co-morbid problems, and meticulous digital rectal examination may help identify causes of constipation. Likewise, blood tests and colonoscopy may identify organic causes such as colon cancer. Physiological tests such as colonic transit study with radio-opaque markers or wireless motility capsule, anorectal manometry, and balloon expulsion tests can identify disorders of colonic and anorectal function. However, in the elderly, there is usually more than one mechanism, requiring an individualized but multifactorial treatment approach. The management of constipation continues to evolve. Although osmotic laxatives such as polyethylene glycol remain mainstay, several new agents that target different mechanisms appear promising such as chloride-channel activator (lubiprostone), guanylate cyclase agonist (linaclotide), 5HT4 agonist (prucalopride), and peripherally acting μ-opioid receptor antagonists (alvimopan and methylnaltrexone) for opioid-induced constipation. Biofeedback therapy is efficacious for treating dyssynergic defecation and fecal impaction with soiling. However, data on efficacy and safety of drugs in elderly are limited and urgently needed.
Keywords: constipation, elderly, treatment
Introduction
The management of constipation in the elderly is challenging both for patients and healthcare providers. Multiple reasons contribute to this phenomenon, such as the effects of aging on gut physiology, co-morbid illnesses, medications, loss of mobility, inadequate caloric intake, and anorectal sensory changes. Elderly patients, especially those with advanced dementia in nursing homes and those on opioids for palliative care, require an individualized approach for the treatment of constipation.
Definition and epidemiology
Constipation is not a well defined disease entity, but a general term used to describe the difficulties that a subject experiences with moving their bowels.1 Healthcare providers typically define constipation as stool frequency of less than 3 bowel movements per week.2 In contrast, patients define constipation as any form of “difficult defecation”, such as straining, hard stool, feeling of incomplete evacuation, and non-productive urge.3,4 Compared to younger patients, the elderly report more frequent straining, self-digitation, and feelings of anal blockage.4,5 In a study of 531 patients in general practice, 50% gave a different definition of constipation compared to their physicians. 6 Because of these variable definitions of constipation, an international panel of experts proposed the Rome criteria for constipation. The Rome III criteria used a combination of subjective symptoms to define constipation,7 and are currently used widely for performing clinical research in this field.
It is reported that the prevalence of constipation increases with age, especially those over the age of 65 years.8 In elderly patients living in the community, the prevalence of constipation is 50%.4 This number is even higher in nursing home residents, with 74% using daily laxatives.4,9–11 Likewise, elderly women are 2 to 3 times more likely to report constipation than their male counterparts.4 Constipation is also more commonly seen in patients taking multiple medications.12
Health-related quality of life and constipation
Evidence in both disease-specific and generic quality of life (QOL) instruments has shown that constipation is associated with impaired health-related quality of life (HR-QOL). For example, in one study of 126 community-dwelling older adults, respondents with chronic constipation had lower Short-Form 36 (SF-36) scores for physical functioning, mental health, general health perception, and bodily pain when compared to respondents with no constipation.13 Likewise, using the Psychological General Well-Being (PGWB) index, 84 subjects with constipation has lower PGWB total scores and lower domain scores for anxiety, depression, well-being, self-control and general health subscales, indicating worse HR-QOL.14 Furthermore, improvements with HR-QOL were noted with treatment of constipation.15 After laxatives caused significant increases in weekly bowel movements, patients reported fewer urinary symptoms, better sexual function and improved mood and depression.
In addition, constipation is a significant driver of health care costs, as it is ranked among the top 5 most common physician diagnosis for gastrointestinal outpatient visits.4 Using a community survey, the management of constipation is estimated to average $200 per patient within a large HMO.16 Over $821 million dollars (2000 value) was spent on over-the-counter laxatives in the United States alone.8 Other indirect costs of constipation to society include decrease in work related productivity, absences in school, lower quality of life and higher psychological distress.8
Normal continence and defecation
The pelvic floor consists of superficial and deep muscle layers that envelope the rectum, bladder and uterus.17 The superficial muscle layers consist of the internal and external anal sphincters, the perineal body and the transverse perinei muscles. In contrast, the deep pelvic muscles (also known as levator ani) are composed of the pubococcygeus, ileococcygeus and puborectalis muscles.17 These structures are largely innervated by the sacral nerve roots (S2–S4) and the pudendal nerve.
Continence is the ability to retain feces until it is socially conducive to defecate, while defecation is the evacuation of fecal material from the colon. Both functions are regulated by voluntary and involuntary reflex mechanisms, anatomic factors, rectal sensation, and rectal compliance.
Defecation starts when the cerebral cortex receives an awareness and perception of critical level of filling in the rectum. When the individual adopts a sitting or squatting position, the anal sphincters and the puborectalis relax, straightening the anorectal angle. Simultaneously, the voluntary efforts of bearing down increases the intra-abdominal pressure, facilitating the development of a stripping wave, resulting in stool evacuation.
Common causes of constipation in the elderly
In the elderly, constipation most likely has a multifactorial etiology, with more than one mechanism present in a single patient, such as co-morbid illnesses or medication side effects (Table 1). In the elderly, living in hospice with advanced cancer and pain, opioid-induced constipation is common.
Table 1.
Common causes of constipation in the elderly
Medications
|
Endocrine and metabolic diseases
|
Neurologic disorders
|
Myopathic disorders
|
Others
|
Furthermore, there are psychosocial and behavioral factors that may predispose the elderly to develop constipation, such as decreased mobility, inadequate caloric intake, and anorectal sensation changes. Ignoring calls to defecate, can lead to fecal retention in the elderly.4 Suppression of rectal sensation follows chronic fecal retention. As a result, only large stools will be perceived, leading to difficulty with defecation.4
In the elderly, chronic constipation can lead to fecal impaction and fecal incontinence. Fecal impaction is the accumulation of hardened feces in the colon or rectum.18 Liquid stools from the proximal colon can bypass the impacted stool, causing overflow incontinence, often mistaken for diarrhea. Fecal impaction has been identified in 40% of hospitalized older patients in the UK.18 It has been linked to acute states of confusion in this population. In severe cases, fecal impaction can cause stercoral ulcerations, intestinal obstruction or bowel perforation.18 If left untreated, these complications can be life threatening.
Disorders of colonic and anorectal function causing constipation in the elderly
In the absence of alarm symptoms, such as weight loss, bleeding, change in bowel habit, the two most commonly seen subtypes of primary constipation in the elderly are slow transit constipation (STC) and dyssynergic defecation (DD), with a less common subtype being irritable bowel syndrome with constipation (IBS-C).
Slow transit constipation
STC is defined as the delay of stool transit through the colon, due to a myopathy, neuropathy or secondary to an evacuation disorder such as DD.8
In the elderly, age related neurodegenerative changes in the enteric nervous system have been previously noted. There was a 37% loss of enteric neurons in older people (more than 65 years old) when compared with younger people (20–35 years old).4 This was associated with an increase in the elastic and collagen fibers in the myenteric ganglia of older subjects.4
Similarly, a recent study showed the selective age related loss of neurons expressing choline acetyltransferase with sparing of neuronal nitric oxide in human colon.20 These findings suggest an increase in inhibitory neurons in the aging colon, affecting gut motility. However, the significance of these studies is unclear since these findings could suggest either a primary entity or secondary to chronic use of laxatives and/or behavioral changes of constipated patients through the years.
In fact, gut transit time and colonic motility are similar between healthy older and younger participants.1 In contrast, elderly people with chronic illness reporting constipation have a prolonged total gut transit time of 4 to 9 days (normal is less than 3 days).1 In the least mobile of nursing home residents, transit times are prolonged up to 3 weeks.1 It appears that factors related to aging, such as chronic medical conditions and immobility, impact gut motility, rather than aging itself.
Dyssynergic defecation
DD is characterized by difficulty of expelling stool from the anorectum.8
DD is believed to be caused by failure of recto-anal coordination, either by impaired rectal contraction, paradoxical anal contraction, or inadequate anal relaxation.17 Anorectal physiologic changes, such as reductions in internal anal sphincter pressure, pelvic muscle strength, and changes in rectal sensitivity have been reported in the elderly.4
Women, especially those who had sustained injuries during vaginal deliveries, have larger decrease in anorectal squeeze pressures.4 Taken together, these may predispose the elderly to develop DD.
Irritable bowel syndrome with constipation
IBS-C is largely defined by chronic or recurrent abdominal pain or discomfort associated with altered bowel habits, with ≥25% of stools being hard or lumpy.19 These patients may or may not have STC or DD. Although rare, some elderly subjects have IBS-C.
Diagnosis of constipation in the elderly
Medical history and physical examination
Constipated patients present with several symptoms. As a healthcare provider, it is important to ascertain the patient’s complaint regarding what they mean by constipation. A careful medical history, noting medical conditions and medications that affect colonic transit should be conducted (Table 1).
The history should include an assessment of stool frequency, stool consistency, stool size, degree of straining during defecation, and a history of ignoring a call to defecate. A dietary history should assess the amount of fiber and water intake, and the number of meals and when they are consumed. The history should also include the number, type and frequency of laxatives used. In the elderly, fecal seepage and incontinence may be presenting symptoms of fecal impaction.
Finally, a social history with emphasis on the patient’s current living situation, such as living with family or alone; nursing home; or in hospice are important. Furthermore, information about a patient’s activities of daily living, such as dressing and eating, and instrumental activities of daily living, such as grocery shopping and housework, can provide clues on the patient’s functional capacity and level of cognition. Taking note of the patients’ psychiatric co-morbidities and psychosocial stressors are especially important in dealing with IBS patients.
A thorough anorectal and digital rectal exam is essential. It should go beyond looking at skin erosions, skin tags, anal fissures, or hemorrhoids. Using a cotton bud or a blunt needle, gently stroke the four quadrants of the perineal skin. Neuropathy is suspected if this maneuver failed to invoke a reflex contraction of the external anal sphincter. Finally, patients should be asked to bear down as if to defecate. It is important for the examiner to perceive relaxation of the external anal sphincter together with perineal descent. If these features are absent, one should suspect DD.
Metabolic and structural evaluation
Since constipation may be caused by an underlying metabolic and pathologic disorder, routine blood tests, such as a complete blood count, biochemical profile, calcium levels and thyroid functions are usually performed. Structural tests including a flexible sigmoidoscopy or a colonoscopy can provide evidence for chronic laxative use, such as melanosis coli, or mucosal lesions such as solitary rectal ulcer, inflammatory bowel disease, or malignancy. In the absence of a clear explanation, a functional disorder should be considered.
Physiological tests
In order to diagnose STC and DD, several additional Physiological tests are usually employed.
Colonic transit study
The colonic transit study provides a physician with a better understanding of the rate of stool movement through the colon. The test involves the ingestion of a single Stizmarks® capsule (Konsyl Pharmaceuticals, Fort Worth, Texas) containing 24 radio-opaque markers on day 1 and by Obtaining a plain radiograph on day 6 (after 120 hours).
Normal transit is when there are less than 5 markers remaining in the colon.21 STC is diagnosed when 6 or more markers are scattered throughout the colon. Recently, a wireless motility capsule has been tested and found to be useful and safe in the elderly. This not only provides colonic and whole gut transit time but also provides regional transit time such as gastric emptying using a standardized protocol and is free of radiation.22
Anorectal manometry
The anorectal manometry (ARM) provides pressure readings in the rectum and anal sphincters, as well as data on rectal sensation, rectoanal reflexes, and rectal compliance. 8 In normal defecation, the rectal pressure rises with a synchronized fall in anal sphincter pressures. The inability to coordinate these anorectal processes underlies the main pathophysiological abnormality in patients with DD.23 These patients are thought to have impaired rectal contraction, paradoxical anal contraction, impaired relaxation, or a combination of these mechanisms.23,24 Finally, the ARM provides information on anorectal sensory dysfunction, as exemplified by higher thresholds for first sensation and thresholds for desire to defecate.23
Balloon expulsion test
This test is performed by inserting a silicon filled stool-like device called the fecom or a 4 cm long balloon filled with 50 mL of warm water inside the patient’s rectum. Most normal subjects can expel the stool-like device within 1 minute. Inability to expel the device within one minute is highly suggestive of DD.23
Prevention and management of constipation in the elderly
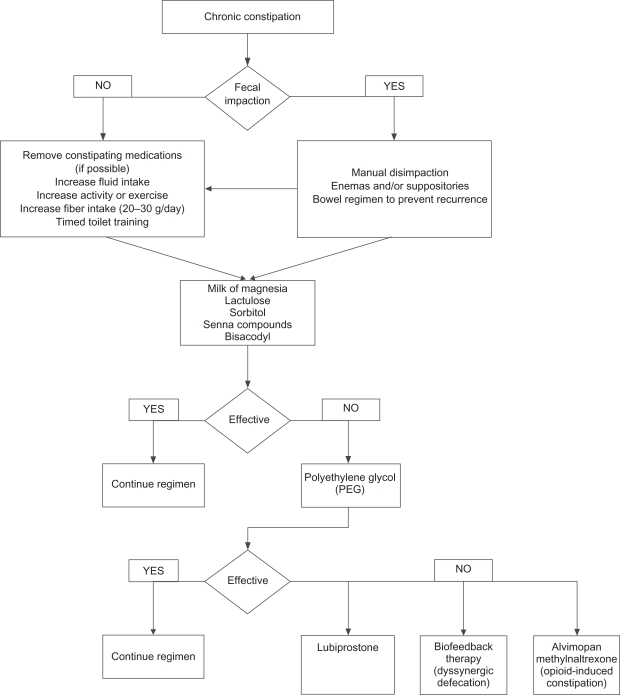
Figure 1 shows a convenient treatment algorithm to assist the practitioner in devising a suitable treatment modality for a given patient. Specific options and treatments are discussed below.
Figure 1.
Treatment algorithm for the management of chronic constipation in the elderly.
Fluid intake and exercise, caloric intake and timed toilet training
Although useful, there is little evidence to support maintenance of adequate hydration and regular nonstrenuous exercise in the management of constipation. In a study involving 6 test and 9 control subjects, consumption of extra fluid did not show significant differences in stool output.25 Although epidemiologic studies show sedentary people are 3 times more likely to report constipation, studies on the effect of exercise and gut transit time are inconsistent. 8 In elderly patients, fluid intake should be monitored closely especially in those with cardiac and renal disease. In contrast, evidence suggests that elderly patients consuming fewer meals and caloric intake are more prone to constipation.26
Patients who have a normal bowel pattern usually move their bowels at the same time every day, suggesting that defecation is partly a conditioned reflex.8 Likewise, colonic motor activity increases after waking and after a meal (gastrocolonic reflex). These suggest that constipated patients may establish a regular pattern of defecation by ritualizing a bowel habit that takes advantage of this normal physiologic stimulus.8 Using the same principle, timed toiled training consists of educating patients to attempt a bowel movement at least twice a day, usually 30 minutes after meals, and to strain no more than 5 minutes.
Diet and fiber
Previous studies have shown that a high fiber diet increases stool weight and decreases colon transit time, while low fiber diet leads to constipation.27,28 However, patients with either STC or DD do not respond well with a dietary fiber of ≥30 g/day.29 In contrast, constipated patients without an underlying motility disorder have improved or became symptom free with this amount of supplemental fiber.29 A systematic review showed that bulk laxatives or fibers showed an average weighted increase of 1.4 (95% CI, 0.6–2.2) bowel movements per week.30 A fiber intake of 20–30 g of fiber a day is generally recommended. A recent randomized controlled trial (RCT) showed that dried plums were more effective than psyllium in the management of mild to moderate constipation.31
Laxatives
Several recent reviews have discussed common classification of laxatives, their mode of action, the recommended dosage, and potential side effects. In the elderly, use of laxatives must be individualized with special attention to patient’s medical history (cardiac and renal co-morbid conditions), drug interactions, costs, and side effects.32 Laxatives most commonly used in clinical practice include milk of magnesia, lactulose, senna compounds, bisacodyl and polyethylene glycol (PEG) preparations.8 In a 4-week study involving constipated elderly patients, 70% sorbitol was as efficacious as lactulose, but was cheaper and better tolerated.33
Similarly, a senna fiber combination (Agiolax®) in elderly nursing home residents improved stool consistency, frequency and ease of passage, when compared with lactulose. 34 The senna fiber was also 40% cheaper. In a long term randomized, multi-center study of polyethylene glycol (PEG), 17 grams once a day was better at achieving treatment success at 6 months, when compared with placebo (PEG 52% vs 11% placebo; P < 0.001).35 Treatment success was defined as relief of modified Rome criteria for constipation for 50% or more of their treatment weeks. Furthermore, similar efficacy was seen in the study’s subgroup analysis involving 75 elderly subjects. Lastly, in a short term study of 100 patients with medication induced constipation, PEG at 17 g daily for 28 days was more effective than placebo in achieving treatment success (PEG 78.3% vs placebo 39.1%; P < 0.001).36 Similar results were also observed in the subgroup of 28 elderly patients.
Despite efforts in including the elderly in RCTs, most studies on the use of laxatives in the elderly are limited because of small sample size and problems with methodology. Side effects of laxatives such as abdominal discomfort, electrolyte imbalances, allergic reactions and hepatotoxicity have been previously reported.4
Stool softeners, suppositories and enemas
Although widely practiced, stool softeners have limited clinical efficacy.4,37 Suppositories may be used in institutionalized patients with obstructed defecation to help with rectal evacuation.4
Similarly, enemas are used in this population group to prevent fecal impaction. Side effects such as electrolyte imbalances have been noted with phosphate enemas and rectal mucosal damage with soapsuds enema. When necessary, tap water enema is the safest one to use.
Newer and upcoming treatment options
Lubiprostone
Lubiprostone is an oral bicyclic fatty acid that activates type 2 chloride channels on the intestinal epithelial cells, secreting chloride and water in the gut lumen.38 In several multi-center RCTs, lubiprostone, when compared to placebo, has consistently shown to increase complete spontaneous bowel movements per week, as well as improved stool consistency, straining, constipation severity and patient-reported treatment effectiveness.39–41 In one of the study, 10% of the studies participants were elderly.40
Prucalopride
Prucalopride, a dihydrobenzofurancarboxamide derivative, is a selective high-affinity 5HT4 receptor agonist.42 Unlike other drugs in its class, such as tegaserod, mosapride and renzapride, prucalopride has a lower affinity for the human Ether-a-go-go Related Gene protein (hERG).42 It is believed that the effects on the hERG channel may have led to the unfavorable cardiovascular profile seen with tegaserod. Recently, in a double-blind RCT with 84 elderly nursing home residents with chronic constipation, 2 mg prucalopride once daily for 4 weeks was safe and well tolerated.43 Currently, prucalopride has been released in Europe, but not in the USA.
Linaclotide
Linaclotide is a guanylate cyclase C receptor agonist that stimulates intestinal fluid secretion and transit; it also has been shown to reduce pain in animal models.44 In a multicenter RCT, 310 patients with chronic constipation were randomly assigned to receive 75, 150, 300, or 600 μg oral linaclotide or placebo daily for 4 weeks.44 Compared with placebo, there was a significant dose related increase in weekly rate of spontaneous bowel movements (SBMs) in the linaclotide groups.
Linaclotide also proved effective in improving secondary endpoints, such as stool consistency, straining, abdominal discomfort, bloating, global assessments and quality of life. Diarrhea was the most common adverse event.
Colchicine
Colchicine, an alkaloid substance usually used to treat gout, is an anti-inflammatory agent that inhibits microtubule assembly in white blood cells. However, it is known to induce diarrhea when taken in higher doses. The mechanism of inducing diarrhea by colchicine is unknown. It has been reported that colchicine increases prostaglandin synthesis, intestinal secretion and gastrointestrial motility.45 It also reduces water and electrolyte absorption in the intestine and increases secretion through a cyclic AMP mediated activity.
In a double-blind, placebo-controlled study of patients with STC (n = 60), colchicine was shown to be effective in lowering Knowles-Eccersly-Scot symptoms (KESS) scores.45 KESS is a valid technique in diagnosing and evaluating symptoms of constipation. The mean KESS scores at 2 months were 11.67 and 18.66 for colchicine and placebo groups, respectively (P = 0.0001). The authors concluded that low-dose colchicine (1 mg daily) is effective in the treatment of STC.45
Alvimopan and methylnaltrexone
Recently, alvimopan46–48 and methylnaltrexone49 have been introduced for the treatment of opioid-induced constipation. Both agents are peripherally acting μ-opioid receptor antagonists that do not cross the blood–brain barrier. As a result, these agents have the advantage of not inhibiting the analgesic effects of opioids.
In a 21-day randomized trial involving 168 patients, alvimopan, in a dose response manner, significantly produced at least 1 bowel movement in 8 hours.48 Furthermore, in a randomized, parallel-group, repeated dose trial involving methylnaltrexone, 5 mg methylnaltrexone produced a 50% laxation response within 4 hours of administration.49 Furthermore, this class of agents has potential uses for other narcotic induced side effects, such as opioid-related nausea and vomiting, urinary retention, pruritus or post-operative ileus.
Dyssynergic defecation and fecal impaction with soiling
The treatment of DD consists of fiber rich diet, laxatives, timed toilet training and biofeedback therapy. The purpose of biofeedback is to restore the normal pattern of defecation by using an instrument based learning process. In biofeedback therapy, patients are taught diaphragmatic breathing techniques to improve their abdominal push efforts and to synchronize this with anal relaxation. A manometric probe is inserted into the patient’s rectum, capturing anal and rectal pressure readings on a monitor. Auditory and visual feedback is provided to the patients as they attempt defecation. The patient’s posture and breathing techniques are also corrected. For sensory rectal training, a balloon in the rectum is distended with 60 mL of air to provide the patient a sensation of rectal fullness or a desire to defecate.
Four RCTs that evaluated the efficacy of biofeedback therapy in the treatment of DD concluded that biofeedback is consistently superior to laxatives, standard therapy, sham therapy, placebo and diazepam.50–53 A preliminary study also showed that home biofeedback is a cost effective alternative when compared to office biofeedback.54
However, the efficacy of biofeedback in the elderly remains unclear. Since biofeedback is based on operant learning conditioning techniques, an evaluation of the patient’s physical and mental capabilities is important in assessing its usefulness in the elderly with significant co-morbidities and advanced dementia.
Surgery
In patients with constipation that is refractory to medical therapy, surgery can be an option. Subtotal colectomy with ileorectal anastomosis is the treatment of choice in patients with refractory slow transit constipation, provided that DD has been excluded.55,56 Results with using segmental colonic resection in constipation are always disappointing.4,57
It is also important to emphasize that in patients with DD, surgery does not improve symptoms unless the dyssynergia has been corrected with biofeedback.8
Reported side effects of surgery include diarrhea, incontinence and bowel obstruction.4
Furthermore, the elderly might be unfit for surgery due to advanced age and significant co-morbidities.
Summary
Constipation is a common polysymptomatic disorder affecting up to 74% of elderly nursing-home residents. It leads to considerable economic burden, loss of work-related productivity, as well as decreased HR-QOL.
Multiple conditions and causes predispose the elderly to constipation and many factors are usually present in one single individual.
The past decade has given us significant mechanistic insights in the pathophysiology of constipation, providing us with newer therapeutic agents and modalities such as lubiprostone, prucalopride, linaclotide, methylnaltrexone and biofeedback therapy. However, data on their efficacy, safety and real-life applicability in the elderly are still limited.
More active recruitment of the elderly in clinical trials is needed to provide better evidence-based management of constipation in this population.
Footnotes
Disclosures
Dr Rao has served as an Advisory Board member, and has received research support from, SmartPill Corporation, Ironwood Pharmaceuticals, and Takeda Pharmaceuticals.
Dr Satish Rao is supported by NIH grant RO1 DK 57100-05.
References
- 1.McCrea GL, Miaskowski C, Stotts NA, et al. Pathophysiology of constipation in the older adult. World J Gastroenterol. 2008;14(17):2631–2638. doi: 10.3748/wjg.14.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drossman DA, Sandler RS, McKee DC, et al. Bowel patterns among subjects not seeking health care. Use of a questionnaire to identify a population with bowel dysfunction. Gastroenterology. 1982;83(3):529–534. [PubMed] [Google Scholar]
- 3.Pare P, Ferrazzi S, Thompson WG, et al. An epidemiological survey of constipation in Canada: Definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol. 2001;96(11):3130–3137. doi: 10.1111/j.1572-0241.2001.05259.x. [DOI] [PubMed] [Google Scholar]
- 4.Bouras EP, Tangalos EG. Chronic constipation in the elderly. Gastroenterol Clin North Am. 2009;38(3):463–480. doi: 10.1016/j.gtc.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Talley NJ, Fleming KC, Evans JM, et al. Constipation in an elderly community: A study of prevalence and potential risk factors. Am J Gastroenterol. 1996;91(1):19–25. [PubMed] [Google Scholar]
- 6.Herz MJ, Kahan E, Zalevski S, et al. Constipation: A different entity for patients and doctors. Fam Pract. 1996;13(2):156–159. doi: 10.1093/fampra/13.2.156. [DOI] [PubMed] [Google Scholar]
- 7.Longstreth GF. Functional bowel disorders: Functional constipation. In: Drossman DA, editor. The functional gastrointestinal disorders. 3rd ed. Lawrence (KS): Allen Press Inc.; 2006. p. 515. [Google Scholar]
- 8.Rao SS. Constipation: Evaluation and treatment of colonic and anorectal motility disorders. Gastroenterol Clin North Am. 2007;36(3):687, 711, x. doi: 10.1016/j.gtc.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Harari D, Gurwitz JH, Avorn J, et al. Constipation: Assessment and management in an institutionalized elderly population. J Am Geriatr Soc. 1994;42(9):947–952. doi: 10.1111/j.1532-5415.1994.tb06585.x. [DOI] [PubMed] [Google Scholar]
- 10.Talley NJ. Def initions, epidemiology, and impact of chronic constipation. Rev Gastroenterol Disord. 2004;4(Suppl 2):S3–S10. [PubMed] [Google Scholar]
- 11.Primrose WR, Capewell AE, Simpson GK, et al. Prescribing patterns observed in registered nursing homes and long-stay geriatric wards. Age Ageing. 1987;16(1):25–28. doi: 10.1093/ageing/16.1.25. [DOI] [PubMed] [Google Scholar]
- 12.Whitehead WE, Drinkwater D, Cheskin LJ, et al. Constipation in the elderly living at home. definition, prevalence, and relationship to lifestyle and health status. J Am Geriatr Soc. 1989;37(5):423–429. doi: 10.1111/j.1532-5415.1989.tb02638.x. [DOI] [PubMed] [Google Scholar]
- 13.O’Keefe EA, Talley NJ, Tangalos EG, et al. A bowel symptom questionnaire for the elderly. J Gerontol. 1992;47(4):M116–M121. doi: 10.1093/geronj/47.4.m116. [DOI] [PubMed] [Google Scholar]
- 14.Glia A, Lindberg G. Quality of life in patients with different types of functional constipation. Scand J Gastroenterol. 1997;32(11):1083–1089. doi: 10.3109/00365529709002985. [DOI] [PubMed] [Google Scholar]
- 15.Charach G, Greenstein A, Rabinovich P, et al. Alleviating constipation in the elderly improves lower urinary tract symptoms Gerontology200;47272–76. [DOI] [PubMed] [Google Scholar]
- 16.Singh G, Lingala V, Wang H, et al. Use of health care resources and cost of care for adults with constipation. Clin Gastroenterol Hepatol. 2007;5(9):1053–1058. doi: 10.1016/j.cgh.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Rao SS, Go JT. Treating pelvic floor disorders of defecation: Management or cure? Curr Gastroenterol Rep. 2009;11(4):278–287. doi: 10.1007/s11894-009-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallagher P, O’Mahony D. Constipation in old age. Best Pract Res Clin Gastroenterol. 2009;23(6):875–887. doi: 10.1016/j.bpg.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Videlock EJ, Chang L. Irritable bowel syndrome: Current approach to symptoms, evaluation, and treatment. Gastroenterol Clin North Am. 2007;36(3):665–685. doi: 10.1016/j.gtc.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, et al. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil. 2009;21(7):746–E46. doi: 10.1111/j.1365-2982.2008.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bharucha AE, Phillips SF. Slow transit constipation. Gastroenterol Clin North Am. 2001;30(1):77–95. doi: 10.1016/s0889-8553(05)70168-0. [DOI] [PubMed] [Google Scholar]
- 22.Rao SS, Paulson J, Saad R, et al. Assessment of colonic, whole gut and regional transit in elderly constipated and healthy subjects with novel wireless pH/pressure capsule (SmartPill®). Gastroenterology. 2009;136:A950. [Google Scholar]
- 23.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: A failure of rectoanal coordination. Am J Gastroenterol. 1998;93(7):1042–1050. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 24.Rao SS, Hatfield R, Soffer E, et al. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol. 1999;94(3):773–783. doi: 10.1111/j.1572-0241.1999.00950.x. [DOI] [PubMed] [Google Scholar]
- 25.Chung BD, Parekh U, Sellin JH. Effect of increased fluid intake on stool output in normal healthy volunteers. J Clin Gastroenterol. 1999;28(1):29–32. doi: 10.1097/00004836-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Towers AL, Burgio KL, Locher JL, et al. Constipation in the elderly: Influence of dietary, psychological, and physiological factors. J Am Geriatr Soc. 1994;42(7):701–706. doi: 10.1111/j.1532-5415.1994.tb06527.x. [DOI] [PubMed] [Google Scholar]
- 27.Tucker DM, Sandstead HH, Logan GM, Jr, et al. Dietary fiber and personality factors as determinants of stool output. Gastroenterology. 1981;81(5):879–883. [PubMed] [Google Scholar]
- 28.Burkitt DP, Walker AR, Painter NS. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet. 1972;2(7792):1408–1412. doi: 10.1016/s0140-6736(72)92974-1. [DOI] [PubMed] [Google Scholar]
- 29.Voderholzer WA, Schatke W, Muhldorfer BE, et al. Clinical response to dietary fiber treatment of chronic constipation. Am J Gastroenterol. 1997;92(1):95–98. [PubMed] [Google Scholar]
- 30.Tramonte SM, Brand MB, Mulrow CD, et al. The treatment of chronic constipation in adults. A systematic review. J Gen Intern Med. 1997;12(1):15–24. doi: 10.1046/j.1525-1497.1997.12103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao SSC, Paulson J, Donahoe R, et al. Investigation of dried plums in constipation – a randomized controlled trial. AM J Gastroenterol. 2009;104:S496. [Google Scholar]
- 32.Locke GR, 3rd, Pemberton JH, Phillips SF. American gastroenterological association medical position statement: Guidelines on constipation. Gastroenterology. 2000;119(6):1761–1766. doi: 10.1053/gast.2000.20390. [DOI] [PubMed] [Google Scholar]
- 33.Lederle FA, Busch DL, Mattox KM, et al. Cost-effective treatment of constipation in the elderly: A randomized double-blind comparison of sorbitol and lactulose. Am J Med. 1990;89(5):597–601. doi: 10.1016/0002-9343(90)90177-f. [DOI] [PubMed] [Google Scholar]
- 34.Passmore AP, Davies KW, Flanagan PG, et al. A comparison of agiolax and lactulose in elderly patients with chronic constipation. Pharmacology. 1993;47(Suppl 1):249–252. doi: 10.1159/000139865. [DOI] [PubMed] [Google Scholar]
- 35.Dipalma JA, Cleveland MV, McGowan J, et al. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol. 2007;102(7):1436–1441. doi: 10.1111/j.1572-0241.2007.01199.x. [DOI] [PubMed] [Google Scholar]
- 36.DiPalma JA, Cleveland MB, McGowan J, et al. A comparison of polyethylene glycol laxative and placebo for relief of constipation from constipating medications. South Med J. 2007;100(11):1085–1090. doi: 10.1097/SMJ.0b013e318157ec8f. [DOI] [PubMed] [Google Scholar]
- 37.Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: Systematic review. Am J Gastroenterol. 2005;100(4):936–971. doi: 10.1111/j.1572-0241.2005.40925.x. [DOI] [PubMed] [Google Scholar]
- 38.Cuppoletti J, Malinowska DH, Tewari KP, et al. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol. 2004;287(5):C1173–C1183. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- 39.Johanson JF, Morton D, Geenen J, et al. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am J Gastroenterol. 2008;103(1):170–177. doi: 10.1111/j.1572-0241.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 40.Johanson JF, Ueno R. Lubiprostone, a locally acting chloride channel activator, in adult patients with chronic constipation: A double-blind, placebo-controlled, dose-ranging study to evaluate efficacy and safety. Aliment Pharmacol Ther. 2007;25(11):1351–1361. doi: 10.1111/j.1365-2036.2007.03320.x. [DOI] [PubMed] [Google Scholar]
- 41.Johanson JF, Drossman DA, Panas R, et al. Clinical trial: Phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2008;27(8):685–696. doi: 10.1111/j.1365-2036.2008.03629.x. [DOI] [PubMed] [Google Scholar]
- 42.Camilleri M, Kerstens R, Rykx A, et al. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358(22):2344–2354. doi: 10.1056/NEJMoa0800670. [DOI] [PubMed] [Google Scholar]
- 43.Camilleri M, Beyens G, Kerstens R, et al. Safety assessment of prucalopride in elderly patients with constipation: A doubleblind, placebo-controlled study. Neurogastroenterol Motil. 2009;21(12):1256–1263. doi: 10.1111/j.1365-2982.2009.01398.x. [DOI] [PubMed] [Google Scholar]
- 44.Lembo AJ, Kurtz CB, Macdougall JE, et al. Linaclotide is effective for patients with chronic constipation. Gastroenterology. 2010;138:886–895. doi: 10.1053/j.gastro.2009.12.050. [DOI] [PubMed] [Google Scholar]
- 45.Taghavi SA, Shabani S, Mehramiri A, et al. Colchicine is effective for short-term treatment of slow transit constipation: A double-blind placebo-controlled clinical trial. Int J Colorectal Dis. 2010;25(3):389–394. doi: 10.1007/s00384-009-0794-z. [DOI] [PubMed] [Google Scholar]
- 46.Gonenne J, Camilleri M, Ferber I, et al. Effect of alvimopan and codeine on gastrointestinal transit: A randomized controlled study. Clin Gastroenterol Hepatol. 2005;3(8):784–791. doi: 10.1016/s1542-3565(05)00434-9. [DOI] [PubMed] [Google Scholar]
- 47.Camilleri M. Alvimopan, a selective peripherally acting mu-opioid antagonist. Neurogastroenterol Motil. 2005 Apr;17(2):157–165. doi: 10.1111/j.1365-2982.2005.00640.x. [DOI] [PubMed] [Google Scholar]
- 48.Paulson DM, Kennedy DT, Donovick RA, et al. Alvimopan: An oral, peripherally acting, mu-opioid receptor antagonist for the treatment of opioid-induced bowel dysfunction – a 21-day treatment-randomized clinical trial. J Pain. 2005;6(3):184–192. doi: 10.1016/j.jpain.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Portenoy RK, Thomas J, Moehl Boatwright ML, et al. Subcutaneous methylnaltrexone for the treatment of opioid-induced constipation in patients with advanced illness: A double-blind, randomized, parallel group, dose-ranging study. J Pain Symptom Manage. 2008;35(5):458–468. doi: 10.1016/j.jpainsymman.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007;5(3):331–338. doi: 10.1016/j.cgh.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 51.Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;130(3):657–664. doi: 10.1053/j.gastro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005;129(1):86–97. doi: 10.1053/j.gastro.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 53.Heymen S, Scarlett Y, Jones K, et al. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Dis Colon Rectum. 2007 Apr;50(4):428–441. doi: 10.1007/s10350-006-0814-9. [DOI] [PubMed] [Google Scholar]
- 54.Go JT, Brown K, Schneider J, et al. Cost-effectiveness analysis of office vs home biofeedback in the treatment of dyssynergic defecation. AM J Gastroenterol. 2009;104:S488. [Google Scholar]
- 55.Nyam DC, Pemberton JH, Ilstrup DM, et al. Long-term results of surgery for chronic constipation. Dis Colon Rectum. 1997;40(3):273–279. doi: 10.1007/BF02050415. [DOI] [PubMed] [Google Scholar]
- 56.Hassan I, Pemberton JH, Young-Fadok TM, et al. Ileorectal anastomosis for slow transit constipation: Long-term functional and quality of life results J Gastrointest Surg 2006101013306; discussion 1336–1337 [DOI] [PubMed] [Google Scholar]
- 57.Rotholtz NA, Wexner SD. Surgical treatment of constipation and fecal incontinence. Gastroenterol Clin North Am. 2001;30(1):131–166. doi: 10.1016/s0889-8553(05)70171-0. [DOI] [PubMed] [Google Scholar]