Abstract
Cross talk between the Estrogen Receptor (ER) and ErbB2/HER-2 pathways have long been implicated in breast cancer aetiology and drug response1, yet no direct connection at a transcriptional level has been shown. We now show that estrogen-ER and tamoxifen-ER complexes directly repress ErbB2 transcription via a cis-regulatory element within the ERBB2 gene. We implicate the Paired Box 2 gene product (Pax2), in a novel role, as a crucial mediator of ER repression of ErbB2 by the anti-cancer drug tamoxifen. We show that Pax2 and the ER co-activator AIB-1/SRC-3 compete for binding and regulation of ErbB2 transcription, the outcome of which determines tamoxifen response in breast cancer cells. The repression of ErbB2 by ER-Pax2 links these two important breast cancer subtypes and suggests that aggressive ErbB2 positive tumours can originate from ER positive luminal tumours by circumventing this repressive mechanism. These data provide mechanistic insight into the molecular basis of endocrine resistance in breast cancer.
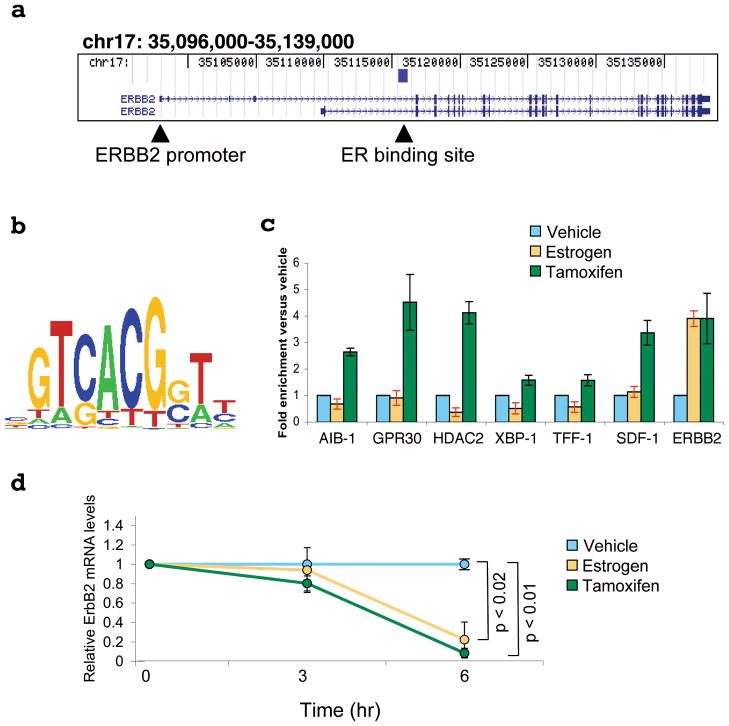
The genomic mapping of Estrogen Receptor binding sites has revealed insight into how ER functions in breast cancer cells, including the finding that ER rarely binds to promoter regions and that ER loading on the chromatin requires the presence of pioneer factors, such as FoxA12–4. We have replicated genome-wide ER Chromatin Immunoprecipitation (ChIP)-on-chip analyses in ER positive MCF-7 cells. Identification of the ER binding sites using a false discovery rate of 5% revealed 8,525 ER sites, with excellent representation (86%) of the published ER binding profile2 (Supplementary data 2). Included within the new, more extensive list, was an ER binding site within the intron of the ERBB2/HER-2 genomic region (Figure 1a). Sequence analysis of all 8,525 ER binding sites revealed a statistical enrichment (p-value < 0.0001) for the Paired Box (Pax) transcription factor motif (GTCANGN(A/G)T) (Figure 1b). Little is known about the role that Pax proteins play in hormone signalling, however, Pax2 was shown to be expressed in a subset of breast cancers and was recently identified as a tamoxifen-regulated effector in endometrial cancer cells5,6.
Figure 1.
Estrogen Receptor ChIP-on-chip reveals a binding site within the ERBB2 gene region that is bound by Pax2. a. Schematic representation of the ERBB2 gene locus and the intronic ER binding site, as defined by ER ChIP-on-chip experiments. b. Pax motif enriched within ER binding sites. c. Pax2 ChIP after vehicle, estrogen or tamoxifen treatment. d. Changes in ErbB2 mRNA by real time RT-PCR after vehicle, estrogen or tamoxifen treatment. All experiments are the average of three independent replicates ± Std Dev.
Tamoxifen is one of the most successful and effective therapies in the treatment of breast cancer, but tamoxifen resistance is inevitable7. Tamoxifen resistant breast tumours are characterised by elevated ErbB2 levels8 and ER positive cell line models over expressing ErbB2 acquire resistance to tamoxifen9. We assessed Pax2 binding to a select number of ER binding sites adjacent to important estrogen regulated genes, including the newly identified binding site within the ERBB2 gene. Pax2 was generally recruited only after tamoxifen treatment, with the exception of the ER binding site within ERBB2 (Figure 1c), where Pax2 was recruited to the ER binding site after both estrogen and tamoxifen treatment. Given previous evidence that ErbB2 could be repressed by both estrogen10 and tamoxifen11, we hypothesised that Pax2 may be functioning as a general ER-associated transcriptional repressor and that the ER binding site within ERBB2 may be a cis-regulatory element for active repression by ER. Indeed, analysis confirmed that ErbB2 mRNA levels are decreased by estrogen and tamoxifen in our MCF-7 cells (Figure 1d). Co-IP experiments showed that ER and Pax2 form a complex after tamoxifen treatment (Supplementary data 3) and Re-ChIP experiments confirmed that ER and Pax2 co-occupy the ER binding site within the ERBB2 gene simultaneously, after treatment with tamoxifen (Supplementary data 3). Furthermore, we experimentally verified this ER binding site as the cis-regulatory element for the ERBB2 gene (Supplementary data 4). This cis-regulatory region is independent of a previously identified regulatory region10, although this previously characterised region may also play an indirect role in the regulation of ErbB2 transcription.
ER positive luminal tumours with the poorest prognosis tend to have elevated ErbB2 levels12 and up to half of ErbB2 positive tumours are also positive for ER13. As such, we hypothesised that the anti-proliferative effects of tamoxifen treatment require repression of ERBB2, and that breast cancers can potentially acquire tamoxifen resistance by amplifying the ERBB2 locus, or by deregulating the control mechanisms that normally repress ErbB2 transcription. Unlike tamoxifen, repression of ERBB2 by estrogen may not be a critical event, since cell proliferation by estrogen likely results from the estrogen-mediated up-regulation of numerous oncogenes.
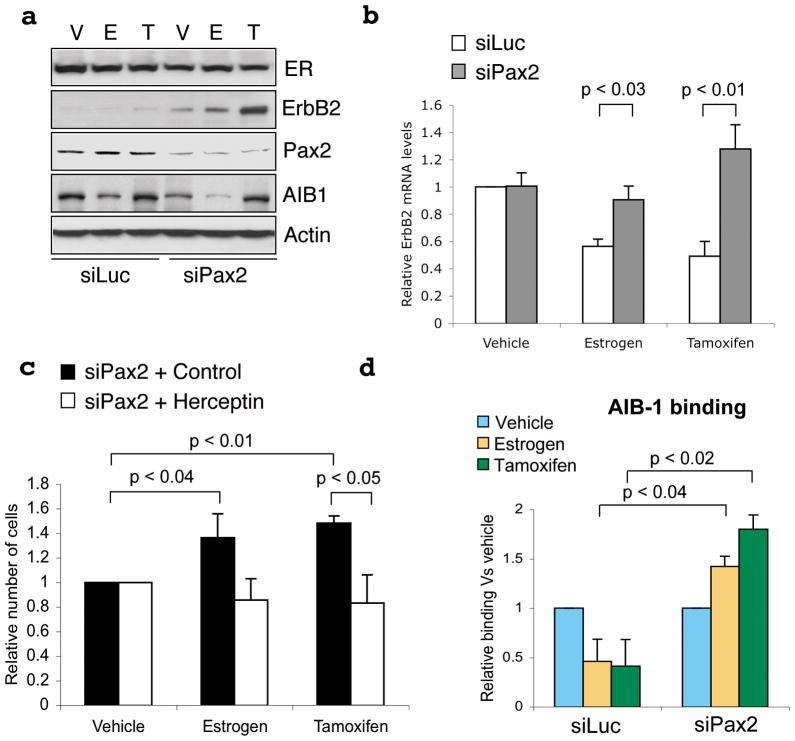
In order to implicate a role for Pax2 in the estrogen and tamoxifen-mediated repression of ErbB2, we specifically silenced Pax2. Immunoblotting revealed efficient knockdown of Pax2 protein levels, but no significant effect on ER levels (Figure 2a). In control transfected cells, estrogen and tamoxifen both rapidly repressed ErbB2 mRNA (Figure 2b), but siPax2 abrogated this inhibition and consequently ErbB2 transcription and protein levels were elevated in the presence of both estrogen and tamoxifen treatment (Figures 2a and 2b). This coincided with an accumulation of phospho-RNA PolII at the promoter (the longer isoform) of ERBB2 following treatment with both estrogen and tamoxifen in the presence of siPax2 (Supplementary data 5). Relative to control, tamoxifen treatment of siPax2 transfected cells resulted in an increase in cell number (Figure 2c), thereby reversing the growth arrest observed after tamoxifen treatment. These experiments were reproduced using an additional Pax2 siRNA (Supplementary data 6). Importantly, pre-treatment of cells with an anti-ErbB2 antibody (Herceptin) blocked the siPax2 mediated cell growth, confirming that the increased cell growth following Pax2 silencing was primarily due to the increase in ErbB2 levels (Figure 2c).
Figure 2.
Specific silencing of Pax2 reverses the tamoxifen-mediated repression of ErbB2 and growth arrest. a. siRNA to Pax2 was transfected into hormone-depleted MCF-7 cells and stimulated with vehicle (V), estrogen (E) or tamoxifen (T) and total protein was immunoblotted b. Control siRNA (siLuc) (white bars) or siPax2 (gray bars) was transfected and ErbB2 mRNA was assessed. c. siPax2 was transfected into cells in the presence of control or Herceptin (ErbB2 antibody) after which cells were collected and total viable cell number was determined. The control transfection (siLuc) data is in Supplementary figure 7. d. Control siRNA (siLuc) or siPax2 was performed as described and cells were treated with vehicle, estrogen or tamoxifen. AIB-1 binding to the ERBB2 enhancer was determined by ChIP. All experiments are the average of three independent replicates ± Std Dev.
We recapitulated these findings in another ER positive breast cancer cell line (ZR75-1 cells). Tamoxifen repressed ErbB2 mRNA and protein levels in ZR75-1 cells, and this repression was inhibited by transfection of siPax2. Similar to MCF-7 cells, siPax2 reversed the growth inhibitory effects of tamoxifen, such that ZR75-1 cells acquired tamoxifen resistance in the absence of Pax2 (Supplementary data 8).
In combination with elevated ErbB2 levels, tamoxifen resistant breast cancers are also characterised by increased levels of the ER co-activator Amplified in Breast Cancer-1 (AIB-1/SRC-3)8. AIB-1 promotes tumourigenesis14,15 and is essential for ErbB2 driven oncogenesis in mice16. We assessed whether AIB-1 could compete with Pax2 for binding to the ErbB2 cis-element; an event that may contribute to the elevated ErbB2 levels associated with tamoxifen resistant tumours8. ChIP showed decreased AIB-1 binding at the ErbB2 cis-element following estrogen and tamoxifen treatment (Figure 2d), likely due to displacement by Pax2. This was proven by inhibiting Pax2 by siRNA which consequently allowed for estrogen and tamoxifen-mediated recruitment of AIB-1 to the ERBB2 cis-regulatory element (Figure 2d).
We subsequently showed that expression of AIB-1 competes with Pax2 for binding to the ErbB2 cis-element and that this results in an increase in ErbB2 transcription and an increase in cell proliferation in the presence of tamoxifen (Supplementary data 9). Elevated AIB-1 levels block Pax2 binding and ERBB2 gene repression, thereby reversing the antiproliferative effects of tamoxifen. This suggests that a stoichiometric balance between the co-activator AIB-1 and the putative repressor Pax2, impinge on the binding and regulation of ErbB2, providing mechanistic insight into the important role that AIB-1 plays in the tamoxifen response17. MCF-7 cells already have elevated AIB-1 levels due to a genomic amplification of the AIB-1 locus14, but they also have increased Pax2 protein levels (data not shown), potentially explaining why they retain sensitivity to tamoxifen treatment. However, we could also show that AIB-1 expression could reverse the anti-proliferative effects of tamoxifen in T47D cells, a cell line that does not already have elevated AIB-1 levels14 (Supplementary data 9). These data confirm a general role for AIB-1 in reversing tamoxifen responsiveness in ER positive breast cancer cell lines.
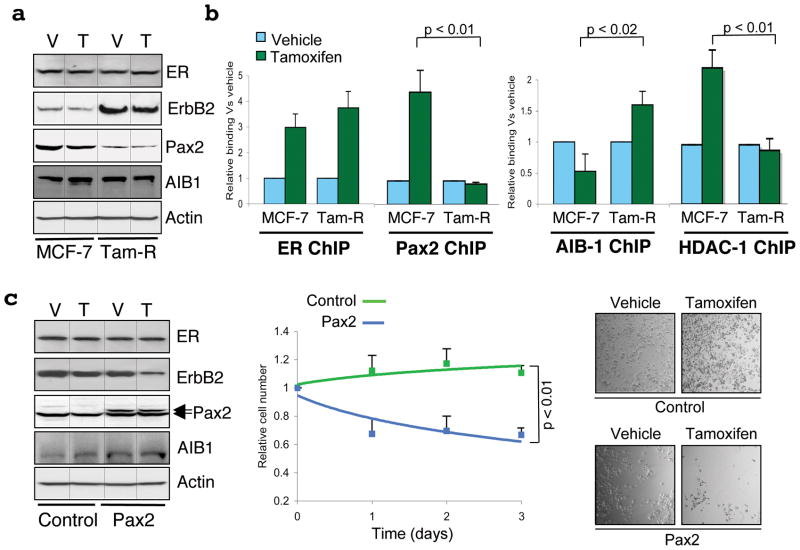
The role of ErbB2 in tamoxifen resistance is demonstrated by data showing that tamoxifen resistant breast cancer cell lines can be inhibited by treatment with anti-ErbB2 antibodies (Herceptin)18. We investigated the hypothesis that Pax2 is required for repression of ErbB2 and that tamoxifen resistance may be due to alterations in this pathway. We utilised an MCF-7 cell line model that had been grown in the presence of tamoxifen and had acquired resistance18. These tamoxifen-resistant cells (Tam-R) have elevated ErbB2 levels but do not have amplification of the ERBB2 locus18. In wild type MCF-7 cells, tamoxifen repressed ErbB2 mRNA levels (Figure 2b) and protein levels (by 40%) (Figure 3a) as expected, but ErbB2 levels were elevated in Tam-R cells and did not decrease in response to tamoxifen treatment (Figure 3a). Western blot analysis comparing wild type and Tam-R MCF-7 cells revealed no changes in ER protein levels, supporting clinical studies showing that changes in ER levels are not a general mechanism for tamoxifen resistant breast cancers19,20. AIB-1 protein levels were also unaltered, but interestingly Pax2 protein levels were lower in Tam-R cells (Figure 3a), providing a potential explanation for the elevated ErbB2 levels in these tamoxifen-resistant cells.
Figure 3.
Pax2 restores tamoxifen sensitivity in tamoxifen resistant breast cancer cells. a. Total protein from wild type MCF-7 or tamoxifen resistant MCF-7 cells (Tam-R) was immunoblotted. b. ChIP of ER, Pax2, AIB-1 and HDAC-1 at the ER binding site in the ERBB2 gene in wild type and Tam-R cells after vehicle or tamoxifen treatment. c. Control or Pax2 expressing plasmids were transfected into Tam-R cells, followed by vehicle (V) or tamoxifen (T) treatment. Total protein was immunoblotted. Following Pax2 expression in Tam-R cells, total viable cell numbers were determined after vehicle or tamoxifen treatment. The immunoblots were cropped and the original figures are in Supplementary data 11. All experiments are the average of three independent replicates ± Std Dev.
Tamoxifen-mediated ER recruitment to the ERBB2 cis-regulatory element was assessed in the Tam-R cells and was shown to be similar to wild type MCF-7 cells (Figure 3b). However, as suspected from the lower Pax2 levels in Tam-R cells, Pax2 binding was significantly reduced in the Tam-R cells. Similarly, Histone Deacetylase 1 (HDAC-1) binding was shown to occur only in the wild type cells and not in the Tam-R cells, confirming that active repression occurs in wild type cells at the ERBB2 cis-regulatory element but not in the Tam-R cells. In contrast, tamoxifen-mediated AIB-1 recruitment was elevated in Tam-R cells (Figure 3b), despite unaltered AIB-1 levels. In order to test the hypothesis that the decreased Pax2 levels contribute to the increased expression of ErbB2 and the altered response to tamoxifen in the Tam-R cells, we re-introduced Pax2 into these cells (Figure 3c). After over-expression of Pax2, tamoxifen was now able to repress ErbB2 mRNA (Supplementary data 12) and protein levels (Figure 3c) in Tam-R cells. The over expression of Pax2 resulted in decreased RNA PolII binding to the ErbB2 promoter and decreased AIB-1 binding to the ER binding site (Supplementary data 12), strengthening the hypothesis that AIB-1 and Pax2 compete for binding and regulation of the ERBB2 gene. Active tamoxifen gene repression was restored by Pax2 expression as indicated by the recruitment of HDAC-1 (Supplementary data 12). Pax2 was subsequently shown to be a critical regulator of cellular proliferation, since expression of Pax2 restored the ability of tamoxifen to inhibit cell growth in these previously resistant cells (Figure 3c).
We recapitulated these findings in BT-474 breast cancer cells, which are ER positive, but resistant to tamoxifen21, likely due to a genomic amplification of the ERBB2 locus22. Therefore, these cells represent another possible mechanism of acquired tamoxifen resistance, whereby amplification of the ErbB2 locus can overcome the growth inhibitory effects imposed by tamoxifen in ER positive breast cancers23,24. Expression of Pax2 in BT-474 cells resulted in tamoxifen mediated repression of ErbB2 mRNA and protein levels (Supplementary data 13) and resulted in tamoxifen dependent inhibition of cell growth (Supplementary data 13), such that growth inhibitory effects of tamoxifen were restored, even in the presence of the amplified ERBB2 locus.
Our findings suggest that Pax2 is a key deterministic component in the tamoxifen response. To confirm these findings in primary breast cancer, we performed Pax2 immunohistochemistry on 109 ER positive breast cancer cases25, all of which had been treated with tamoxifen. Of these 109 tumours, 68 were Pax2 positive and 41 Pax2 negative. Tumours with positive Pax2 staining corresponded to a significantly improved recurrence free survival in patients, relative to Pax2 negative tumours (p-value < 0.0001) (Supplementary data 14). Furthermore, within the Pax2 positive tumours only, those that were also positive for AIB-1 had a worse clinical outcome than the tumours that were AIB-1 negative (Figure 4). The tumours that were Pax2 positive and AIB-1 negative, had the best prognosis of all, with a recurrence rate of only 5.8% (Figure 4). Cox regression analysis confirmed an inverse dependent relationship between Pax2 and AIB-1 levels in determining relapse (p-value < 0.03). Interestingly, the Pax2 positive, AIB-1 negative tumours also had the lowest percentage of ErbB2 positive staining (Figure 4), supporting our hypothesis that a balance between Pax2 and AIB-1 ultimately dictates ErbB2 expression and determines tamoxifen efficacy.
Figure 4.
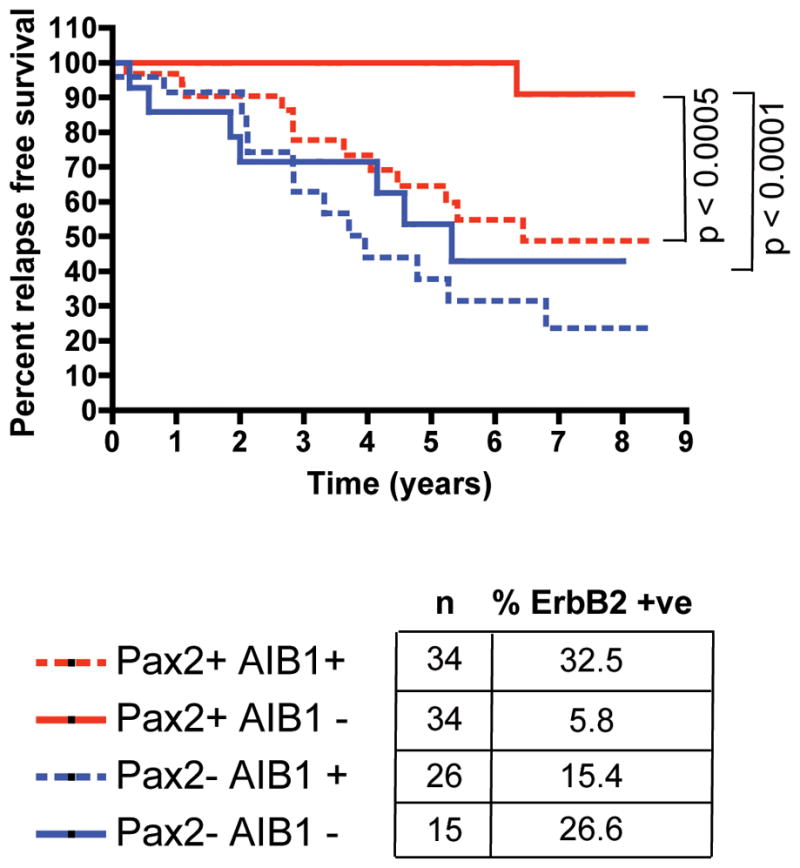
Pax2 predicts clinical outcome in breast cancer patients. Kaplan-Meier curve representing percent relapse free survival in tumours based on Pax2 and AIB-1 staining (n = 109). The percentage of ErbB2 over-expressing tumours within each category is provided.
Endocrine resistance is a significant problem in breast cancer treatment. One of the few validated clinical features of tamoxifen-resistant breast cancer is the combined elevation of AIB-1 and ErbB2 pathways8. We now provide evidence that Pax2 is a critical tamoxifen-recruited transcriptional repressor of the ERBB2 gene and that increased AIB-1 expression can out compete Pax2 binding, directly resulting in increased ErbB2 expression. Alterations in AIB-1-Pax2 stoichiometry dictate the efficacy of tamoxifen in breast cancers (a schematic model of these events is shown in Supplementary data 1). These new data suggest an intrinsic transcriptional link between tumours driven by ER and those driven by ErbB2, which together account for a significant majority of all breast cancers. The role of Pax2 as a repressor is unexpected since Pax2 is generally a transcriptional activator and was shown to be a tamoxifen regulated gene that can induce endometrial cancer6. Given the fact that tamoxifen has antiproliferative effects in the breast but possesses agonist properties in the endometrium26, it is possible that Pax2 may have tissue specific effects and may be one of the primary determinants for SERM action in female reproductive tissue.
Methods summary
MCF-7, T47D, BT-474 and ZR75-1 cells were grown as previous described27. Tam-R cells were derived by long term exposure to tamoxifen18 and grown in the same conditions as wild type MCF-7. Genome-wide Estrogen Receptor ChIP-on-chip experiments were performed in MCF-7 cells in duplicate, as previously described2. ER binding sites analysis were determined using MAT28, with a False Discovery Rate (FDR) of 5%. The analysis of motif enrichments was performed using the CEAS program (http://ceas.cbi.pku.edu.cn/). For mRNA experiments, cells were deprived of hormones as previously described29. Total RNA was collected and RT-PCR was performed as previously described2. ChIP experiments were performed in hormone depleted media as previously described3. Proliferations assays were performed in full media. siRNA experiments were performed as previously described2 in hormone-depleted media. For Immunohistochemistry, 109 ER positive breast cancer sections were collected and processed as previously described25. Immunohistochemistry for Pax2 was performed on an automated BondMax Immunostainer (Leica, Germany) using Pax2 antibody (Abcam: ab38738) and AIB-1 antibody (611105; Transduction laboratories, UK). Immunohistochemistry for ErbB2 was previously described25. Statistical analysis was performed using two tailed paired T-tests and p-values lower than 0.05 were considered statistical. In all figures, the data are the average of a minimum of three independent replicates ± Std Dev. Kaplan-Meier curve statistics were determined using log-rank test. Pax2 and AIB-1 relationships were established using Cox regression analysis.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Danielle Carroll, Suet-Feung Chin, Ian Mills, Charlie Massie and Paul Edwards for discussions. The authors would also like to thank Joanne Mitchell for histology work and Dr Matthew Eldridge and Sarah Vowler, CRUK and Dr Boris Adryan, MRC Laboratory of Molecular Biology, Cambridge, for bioinformatic help. The authors would like to thank Dr. Stefano Buttiglieri, Torino, Italy, for the p-TARGET-Pax2 construct and Dr. Jerome Eeckhoute, University of Rennes, CNRS, France, for the pcDNA-AIB-1 construct. Herceptin was a kind gift from Dr. Mahesh Iddawela, Cancer Research UK, Cambridge. This work was supported by grants from the NIDDK (R01DK074967 to M.B.) and the NCI (P01CA8011105 and the DF/HCC Breast Cancer SPORE Grant to M.B.). We would like to acknowledge the support of The University of Cambridge, Cancer Research UK and Hutchison Whampoa Limited.
References
- 1.Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nature reviews. 2002;2(2):101. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 2.Carroll JS, Meyer CA, Song J, et al. Genome-wide analysis of estrogen receptor binding sites. Nature genetics. 2006;38(11):1289. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 3.Carroll JS, Liu XS, Brodsky AS, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122(1):33. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Lupien M, Eeckhoute J, Meyer CA, et al. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132(6):958. doi: 10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muratovska A, Zhou C, He S, et al. Paired-Box genes are frequently expressed in cancer and often required for cancer cell survival. Oncogene. 2003;22(39):7989. doi: 10.1038/sj.onc.1206766. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Chen Y, Liang J, et al. Hypomethylation-linked activation of PAX2 mediates tamoxifen-stimulated endometrial carcinogenesis. Nature. 2005;438(7070):981. doi: 10.1038/nature04225. [DOI] [PubMed] [Google Scholar]
- 7.Clarke R, Leonessa F, Welch JN, et al. Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol Rev. 2001;53(1):25. [PubMed] [Google Scholar]
- 8.Osborne CK, Bardou V, Hopp TA, et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. Journal of the National Cancer Institute. 2003;95(5):353. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 9.Benz CC, Scott GK, Sarup JC, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24(2):85. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 10.Bates NP, Hurst HC. An intron 1 enhancer element mediates oestrogen-induced suppression of ERBB2 expression. Oncogene. 1997;15(4):473. doi: 10.1038/sj.onc.1201368. [DOI] [PubMed] [Google Scholar]
- 11.Frasor J, Stossi F, Danes JM, et al. Selective estrogen receptor modulators: discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer research. 2004;64(4):1522. doi: 10.1158/0008-5472.can-03-3326. [DOI] [PubMed] [Google Scholar]
- 12.Kun Y, How LC, Hoon TP, et al. Classifying the estrogen receptor status of breast cancers by expression profiles reveals a poor prognosis subpopulation exhibiting high expression of the ERBB2 receptor. Human molecular genetics. 2003;12(24):3245. doi: 10.1093/hmg/ddg347. [DOI] [PubMed] [Google Scholar]
- 13.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. The New England journal of medicine. 2005;353(16):1659. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 14.Anzick SL, Kononen J, Walker RL, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science (New York, NY) 1997;277(5328):965. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 15.Torres-Arzayus MI, De Mora JF, Yuan J, et al. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer cell. 2004;6(3):263. doi: 10.1016/j.ccr.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 16.Fereshteh MP, Tilli MT, Kim SE, et al. The nuclear receptor coactivator amplified in breast cancer-1 is required for Neu (ErbB2/HER2) activation, signaling, and mammary tumorigenesis in mice. Cancer research. 2008;68(10):3697. doi: 10.1158/0008-5472.CAN-07-6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louie MC, Zou JX, Rabinovich A, et al. ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Molecular and cellular biology. 2004;24(12):5157. doi: 10.1128/MCB.24.12.5157-5171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knowlden JM, Hutcheson IR, Jones HE, et al. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144(3):1032. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 19.Johnston SR, Saccani-Jotti G, Smith IE, et al. Changes in estrogen receptor, progesterone receptor, and pS2 expression in tamoxifen-resistant human breast cancer. Cancer research. 1995;55(15):3331. [PubMed] [Google Scholar]
- 20.Green KA, Carroll JS. Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nature reviews. 2007;7(9):713. doi: 10.1038/nrc2211. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Eppenberger-Castori S, Eppenberger U, et al. The NFkappaB pathway and endocrine-resistant breast cancer. Endocrine-related cancer. 2005;12(Suppl 1):S37. doi: 10.1677/erc.1.00977. [DOI] [PubMed] [Google Scholar]
- 22.Clark J, Edwards S, John M, et al. Identification of amplified and expressed genes in breast cancer by comparative hybridization onto microarrays of randomly selected cDNA clones. Genes, chromosomes & cancer. 2002;34(1):104. doi: 10.1002/gcc.10039. [DOI] [PubMed] [Google Scholar]
- 23.Dowsett M. Overexpression of HER-2 as a resistance mechanism to hormonal therapy for breast cancer. Endocrine-related cancer. 2001;8(3):191. doi: 10.1677/erc.0.0080191. [DOI] [PubMed] [Google Scholar]
- 24.Dowsett M, Harper-Wynne C, Boeddinghaus I, et al. HER-2 amplification impedes the antiproliferative effects of hormone therapy in estrogen receptor-positive primary breast cancer. Cancer research. 2001;61(23):8452. [PubMed] [Google Scholar]
- 25.Jiang J, Sarwar N, Peston D, et al. Phosphorylation of estrogen receptor-alpha at Ser167 is indicative of longer disease-free and overall survival in breast cancer patients. Clin Cancer Res. 2007;13(19):5769. doi: 10.1158/1078-0432.CCR-07-0822. [DOI] [PubMed] [Google Scholar]
- 26.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. Journal of the National Cancer Institute. 2005;97(22):1652. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 27.Neve RM, Chin K, Fridlyand J, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer cell. 2006;10(6):515. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson WE, Li W, Meyer CA, et al. Model-based analysis of tiling-arrays for ChIP-chip. Proc Natl Acad Sci U S A. 2006;103(33):12457. doi: 10.1073/pnas.0601180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carroll JS, Prall OW, Musgrove EA, et al. A pure estrogen antagonist inhibits cyclin E-Cdk2 activity in MCF-7 breast cancer cells and induces accumulation of p130-E2F4 complexes characteristic of quiescence. The Journal of biological chemistry. 2000;275(49):38221. doi: 10.1074/jbc.M004424200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.