Abstract
Glial cells have several critical roles in the developing and adult olfactory (antennal) lobe of the moth Manduca sexta. Early in development, glial cells occupy discrete regions of the developing olfactory pathway and processes of GABAergic neurons extend into some of these regions. Because GABA is known to have developmental effects in a variety of systems, we explored the possibility that the glial cells express a GABA transporter that could regulate GABA levels to which olfactory neurons and glial cells are exposed. Using an antibody raised against a characterized high-affinity M. sexta GABA transporter with high sequence homology to known mammalian GABA transporters (Mbungu et al., 1995; Umesh and Gill, 2002), we found that the GABA transporter is localized to subsets of centrally derived glial cells during metamorphic adult development. The transporter persists into adulthood in a subset of the neuropil-associated glial cells, but its distribution pattern as determined by light- and electron-microscopic-level immunocytochemistry indicates that it could not serve to regulate GABA concentration in the synaptic cleft. Rather its role is more likely to regulate extracellular GABA levels within the glomerular neuropil. Expression in the sorting zone glial cells disappears after the period of olfactory receptor axon ingrowth, but may be important during ingrowth if GABA regulates axon growth. Glial cells take up GABA, and that uptake can be blocked by DABA. This is the first molecular evidence that the central glial cell population in this pathway is heterogeneous.
Keywords: glia, GAT, invertebrate, insect, antennal
Introduction
Glial cells in vertebrate systems are known to express transporters for various neurotransmitters (Marcaggi and Attwell, 2004). The transporters specific for GABA typically assist in clearing GABA from the synaptic cleft, regulate extracellular levels of GABA, and in some cases, mediate GABA release via transporter reversal (Gadea and Colomé, 2001; Richerson and Wu, 2003). Transporters in invertebrate glial cells are known to take up GABA at peripheral locust (van Marle et al., 1985) and lobster (Orkand and Kravitz, 1971) neuromuscular junctions and at synapses in the lamina of the optic lobe (Campos-Ortega, 1974), suggesting a conserved role in synaptic transmission. Whether they are present during development in invertebrate systems and have similar roles has not been well studied, though a few recent studies suggest transporter involvement in CNS development (e.g., Neckameyer and Cooper, 1998) and more specifically in signaling pathways affecting glial growth (Yager et al., 2001).
In the developing olfactory pathway in the moth Manduca sexta, glial cells are important in axonal pathfinding and in glomerulus construction (Oland et al., 2004; Chotard & Salecker, 2004); the molecular bases for the interactions among glia and neurons are only partially understood. Among the molecules that could be involved is GABA, which appears to have a variety of developmental roles in vertebrate olfactory systems (Priest & Puche, 2004; Gascon et al., 2006; Akiba et al., 2009) and which is present in a population of intrinsic neurons of the olfactory (antennal) lobe of M. sexta from the beginning of the period of ingrowth of olfactory receptor axons (Homberg and Hildebrand, 1994). As part of our study of possible developmental roles for GABA in the antennal lobe, we asked whether glial cells express a GABA transporter. GABA transporters also have been implicated in development of the rat olfactory bulb, with one isoform (GAT3) being expressed in radial glia (Kawamoto et al., 2001).
Mbungu et al. (1995) cloned and expressed a GABA transporter from M. sexta embryo (MasGAT). The MasGAT shows high sequence homology to known mammalian GABA transporters and functional expression in Xenopus oocytes resulted in GABA transport that had the characteristics of a high-affinity, Na+-dependent, saturable process. The transporter is pharmacologically distinct from mammalian transporters. For example, MasGAT is not inhibited by cis-1,3-aminocyclohexame carboxylic acid or β-alanine but is sensitive to L-2,4-diaminobutyric acid (DABA). Drosophila may have several types of Na and Cl-dependent GABA transporters: different patterns of labeling are found with antibodies against rat GAT1, 2, and 3, but the pharmacology of these putative transporters is as yet unknown (Neckameyer and Cooper, 1998). Immuno-localization of the MasGAT transporter in the moth showed stage-specific expression patterns associated with many CNS neuropils at several developmental stages from embryo to adult (Umesh and Gill, 2002).
Here we have studied in detail the expression of the MasGAT in the olfactory pathway of M. sexta during the period of receptor axon ingrowth to the developing antennal lobe. We find that it appears mainly in the cell bodies and processes of a subset of the neuropil glia as well as in a subset of the glia in the axonal sorting zone region of the antennal nerve. This is the first molecular evidence that the central glial cell population in this pathway is heterogeneous.
Some of these results have appeared in abstract form (Mallory et al., 2005; Tolbert et al., 2008).
Methods
Animals
Male and female M. sexta were reared under temperature-, light- and humidity-controlled conditions in a colony housed in the Arizona Research Laboratories Division of Neurobiology. 5th instars were chosen approximately one day prior to wandering. Metamorphosing animals were staged according to features visible through pupal cuticle trans-illuminated with fiber-optic light, and adults were 1-3 days post-eclosion to the moth. Animals were anesthetized either by cold or by exposure to CO2. Adult metamorphic development occurs over 18 stages, each lasting roughly a day (Tolbert et al., 1983).
Deafferentation
After anesthetization by immersion in ice, the antennal anlage on one side of stage-1 animals was removed by methods described previously (Oland and Tolbert, 1987). The contralateral side serves as a control because in this animal the olfactory receptor neurons project only ipsilaterally. Removal of the antennal anlage leaves intact the olfactory input from the labial pit organ receptors, which innervate a single ventrally located glomerulus that lies external to the ring of ordinary glomeruli and does not receive olfactory input from olfactory receptors located on the antenna (Kent et al., 1986).
Immunocytochemistry, laser scanning confocal microscopy and electron microscopy
For light microscopy of anti-MasGAT labeling, brains were dissected from the head and fixed in 4% paraformaldehyde, 4% paraformaldehyde with 0.15% glutaraldehyde, or cold methanol:formalin (9 methanol:1 (37% formalin) solutions. Brains were rinsed in phosphate-buffered saline (PBS, pH 7.4) 2X, vibratome sectioned at 100-μm intervals, incubated in 2% IgG-free BSA in PBS with 0.1% Triton X-100 (PBST) for 1 hr, then incubated 1-3 days at 4°C on a shaker in anti-MasGAT antibody (gift of Dr. Sarjeet Gill) 1:1000 final dilution in PBST. Sections were washed in PBST, then incubated in anti-rabbit secondary for 2 hrs at room temperature (RT), washed in PBST, then in 0.1 M Phosphate buffer, switched to 0.1M Tris buffer (pH 7.4 at RT), incubated in Syto 13 or 59 (1:1000) for 15 min, washed in Tris buffer, cleared in 50:50 then 80:20 glycerol: water, and mounted in 80:20 glycerol:water.
In some cases, the sections also were labeled with anti-HRP antibody after fixation in 4% paraformaldehyde in 0.1M PO4 fixative solution. Because both anti-MasGAT and anti-HRP antibodies were raised in rabbit, the sections were labeled serially, first with anti-MasGAT at 1:500 overnight at 4°C, followed by Cy5-conjugated anti-rabbit Fab’ (Jackson, West Grove, PA) at 1:300 in PBST with 1% Ig-free BSA for 2 hrs. Sections were then washed with PBST 5X5 min, then incubated in rabbit anti-HRP-Rhodamine at 1:50 in PBST overnight at 4°C on a shaker, then washed and processed as above.
Brains used to examine GABA expression were microwave-fixed in 1% glutaraldehyde, 2.5% paraformaldehyde, 1% sodium metabisulfite (SMB), 0.1M sodium cacodylate (Sinakevitch et al., 2001) using a Pelco microwave oven at 24°C at Power 2 for 2 cycles of 2 min on, 2 min off. The brains were placed in fresh fixative solution for an hour at RT, then incubated overnight at 4°C on a shaker. Brains were then washed in Tris buffered saline (TBS), vibratome sectioned at 100- μm intervals, treated for 10-15 min with NaBH4 (0.4 mg/ml in 0.5% SMB, 0.05 M Tris-HCL, pH 7.5), washed in TBS, and blocked in TBSAT (0.1% azide, 0.5% Triton) with 5-10% normal swine serum for 1 hr at RT. Sections were incubated in anti-GABA at 1:1000 in TBSAT on a shaker at RT for 48 hrs, washed 4X15 min in TBSA with 0.1% Triton, and incubated overnight in goat anti-rabbit-Cy3 (Fab’) at 1:200 in TBSA with 0.5% Triton at RT. The sections were washed 3X15 min in TBSA with 0.1%, once in 0.1M Tris buffer, incubated in Syto 13 in Tris buffer a 1:10,000 for 25 min at RT, then washed and mounted as above.
Sections were examined with a Zeiss Meta 510 LSCM. In double-labeled preparations, the instrument’s multi-track line function was used to ensure channel separation. Confocal images were subsequently processed with the Zeiss LSM Image Browser and CorelDraw (for changes in brightness and contrast, for pseudo-coloring, and for panel assembly).
For standard electron microscopy, the brains were placed in fixative solution (4% paraformaldehyde, 0.15% glutaraldehyde, and 0.2% saturated picric acid in 0.1M phosphate buffer, pH 7.4) overnight at 4°C. The tissue was processed subsequently as previously described (Tolbert and Hildebrand, 1981), thin-sectioned and picked up on standard or Formvar-coated slot grids (for stage-7 sections). Sections were examined in a JEOL 1200EX microscope equipped with a Gatan digital camera. Image montages and subsequent tracings of glial processes were made with Corel Draw software and a Wacom Digitizer II digitizing pad. n = one 5th-instar larval brain, n=2 stage-2 brains, n=5 stage-7 brains, and n=3 adult brains.
For immuno-EM with the MasGAT antibody, brains (n=3 stage-6 animals) were fixed as above, vibratome-sectioned at 75-μm intervals, blocked for 1 hr in phosphate-buffered saline with 0.1% Triton X-100 (PBST) with 2% Ig-free BSA (Jackson ImmunoResearch), incubated in anti-MasGAT at 1:750 final dilution in PBST overnight at 4°C on a shaker, then washed 3X10 min in PBST and incubated in goat anti-rabbit IgG Fab’ 1-nm gold (EMS, Hatfield, PA) at 1:50 in PBST with 1% Ig-free BSA for 2 days. The sections then were washed first in PBST 3X10 min, then in distilled water 2X5 min, were silver-enhanced (Ted Pella Silver Enhancing Kit #15718, Redding, CA) for 10 min on ice followed by 5 min at room temperature, then washed in distilled water 2X5 min, post-fixed in 1% OsO4 in 0.1M PO4 buffer, washed in PBS 3X5 min, and dehydrated in graded alcohols followed by propylene oxide washes 2X10 min. The sections were flat-embedded in Epon:Araldite between two sheets of aclar plastic (EMS), cut out and remounted on beem capsules and then thin-sectioned (silver-gold). EM images of the different regions of the antennal nerve and lobe (sorting zone region, nerve layer, glomerular and coarse central neuropil were collected. Antibody penetration in adult tissue was too poor to allow judgment about labeling patterns despite multiple attempts with a variety of protocols.
Antibodies
anti-MasGAT
The polyclonal MasGAT antibody was raised in rabbit against a synthetic peptide corresponding to the C-terminal 14 amino acids plus a cysteine at the N terminus of those 14 residues of the M. sexta GABA transporter (CVRIPEDVPSLRTKM) (Umesh and Gill, 2002). The transporter previously had been cloned from a cDNA library from the M. sexta embryo (Mbungu et al., 1995). The immunizing peptide was conjugated to a maleimide-activated form of KLH. The antibody was affinity-purified from the crude antisera and in Western blot analysis recognized a broad band with mobility between 48 and 52 kDa (Umesh and Gill, 2002). No staining was present in sections of M. sexta CNS when the MasGAT antibody was pre-absorbed with the immunizing peptide (Umesh and Gill, 2002).
In our hands, Western blot analysis of tissue from stage-9 antennal lobes using the Mbungu et al. (1995) protocol revealed a single wide band at ~48 kDA (Fig. 1). For a given lane, four antennal lobes of animals at stage 9 of development were quick-frozen in liquid nitrogen and stored overnight at −80° C. The tissue was homogenized in NuPAGE LDS sample buffer (Invitrogen/Novex, Carlsbad, CA; #NP0007) containing dithiothreitol (Invitrogen, #NP0009) and protease inhibitor cocktail (Sigma, # P2714). Samples were found to be heat labile, so they were incubated at 37 °C for 30 minutes, as in Umesh and Gill (2002), rather than at 70 °C for 10 minutes as recommended by Novex. Proteins were separated using the Novex electrophoresis system with a NuPage 4-12% Bis-Tris polyacrylamide gradient gel and MOPS (3-(N-morpholino) propane sulfonic acid) buffer (Invitrogen, #NP0001), then transferred to PVDF membrane (Immobilon-P, Millipore, Bedford, MA) using NuPAGE Transfer buffer (Invitrogen, #NP0006) and a BioRad Trans-Blot SD semi-dry transfer cell (#170-3940). Blots were incubated for one hr at RT in TBS containing 0.1% Tween 20 and 5% nonfat dry milk (TBSTwM), then incubated overnight in TBSTwM containing the MasGAT antibody at 0.5 μl/ml. Blots were washed 3X 20 min in TBSTwM, then incubated for 3 hr at RT in TBSTw containing 1 μl/5ml HRP-conjugated goat anti-rabbit antibody (Jackson Immunoresearch, # 111-035-144). The blots were washed 2X 10 min, then developed using the Opti-4CN kit (4-chloro-1-naphthol substrate; BioRad, Hercules, CA; #170-8235).
Figure 1.
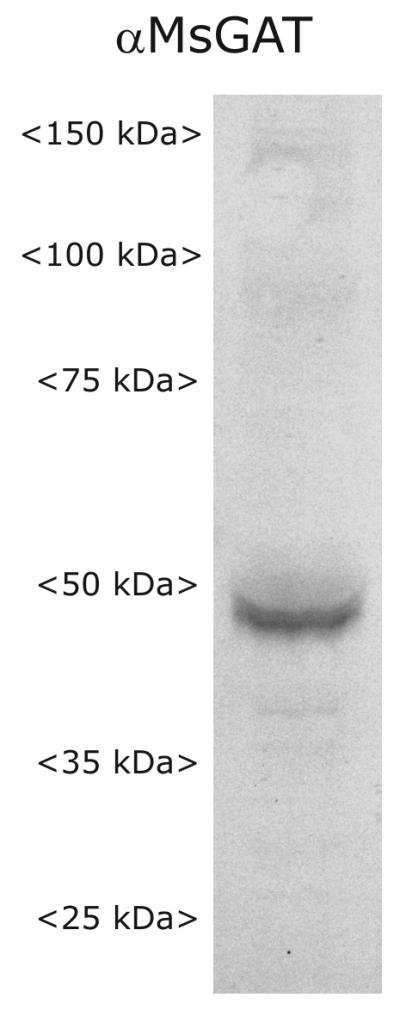
Western blot probed with anti-MasGAT antibody showing single strong band at ~48 kD.
anti-HRP
Anti-HRP antibody has been used for several decades as a neuronal marker in a variety of insects, including Drosophila (Jan and Jan, 1982; Katz et al. 1988, Sun and Salvaterra, 1995), grasshopper (Bentley and Caudy, 1983; Snow et al., 1987), and bee (Kreissl and Bicker, 1992). Immunoprecipitation experiments revealed as many as 17 different membrane glycoproteins from Drosophila embryo and almost as many from grasshopper. Most of these proteins include a neural-specific carbohydrate moiety (Snow et al., 1987; Sun and Salvaterra, 1995) that has since been determined in both Drosophila and C. elegans to be a core α1-3 fucose (Fabini et al. 2001; Paschinger et al., 2004). We were specifically using a rhodamine (TRITC)-conjugated affinity-purified rabbit anti-HRP (Jackson #323-025-621).
anti-GABA
The polyclonal antibody against GABA (Gemacbio, #AP027) was raised in rabbit after immunization with GABA conjugated with glutaraldehyde (G) to BSA. After pre-absorption with the immunizing antigen (Gemacbio, #AG027), labeling was abolished. Antibody specificity was tested by Gemacbio with an ELISA test by competition with β-alanine-G-BSA, glycine-G-BSA, D-aspartate-G-BSA, D-glutamate-G-BSA, yielding, at most, 1/10,000 crossreactivity.
GABA incubation, with and without DABA
The brains of stage-7 animals were removed from the head into insect saline solution (SIS; 150 mM NaCl, 4 mM KCl, 2 mM CaCl2, 10 mM HEPES, 5 mM glucose, pH 7.0, adjusted to 360 mOsm with mannitol), the antennal lobes were desheathed, and the brains incubated for 20 min in control or freshly made GABA-containing solutions that were kept at either RT or 4°C. The 4°C incubation was used to minimize transporter activity. At the end of the incubation period, the brains were washed in cold SIS 4X2 min, and microwave-fixed and processed in accord with the anti-GABA protocol above. Confocal microscope collection parameters were kept constant for all sections imaged.
For in vivo experiments in which diamino-butyric acid (DABA (Sigma)) was used to inhibit the transporter, the brains of stage-7 animals were prepared as above and placed in one of the following conditions: (1) 5-min incubation in 0 DABA at RT or 4°C, 0 GABA, 0 DABA followed by a 20-min incubation in 0 GABA and 0 DABA; (2) 5-min incubation in 0 DABA followed by 20-min incubation in 10 or 50 μm GABA; (3) 5-min incubation in 1 mM DABA followed by 20-min incubation in 0, 10 or 50 μm GABA; and (4) 5-min incubation in 1 mM DABA followed by 20-min incubation in 1 mM DABA with 0, 10 or 50 μm GABA. n=2 for each condition, with the experiment repeated 3X. The 5- and 20-min incubation periods were separated by 2 rinses in cold SIS.
For in vitro inhibition experiments, glial cells were dissociated from the sorting zone and AL neuropil of stage-7 animals and plated as described previously (Tucker et al., 2004), using differential dissection to separate the externally located neuronal cell body clusters from the neuropil where the glial cells are located. The cells were cultured overnight, then exposed for 5 min to 0 or 1 mM DABA made in modified L15 medium (Oland et al., 1996), rinsed 2X in SIS, then incubated in 0 or 1 mM DABA plus 0 or 20 μM GABA in modified L15 medium for 20 min. The experiment was stopped by addition of cold SIS X2 washes, and the cells fixed and processed according to the protocol described above except that the cells were not microwave fixed, fixation time was 30 min, and the anti-GABA antibody incubation time was only 1d.
Results
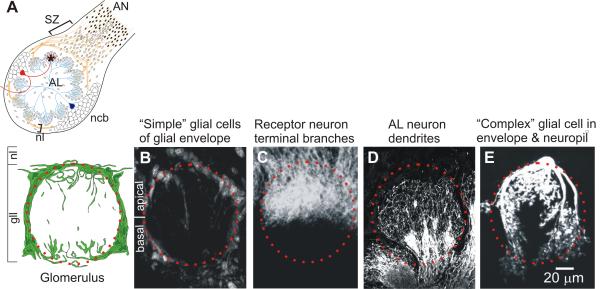
For orientation to this system, Figure 2 illustrates the organization of the primary olfactory pathway in adult M. sexta and shows the spatial relationships among the cellular elements that comprise a typical adult glomerulus. Most of the results in this paper were obtained in animals undergoing adult metamorphic development (18 stages), when the larval nervous system is being reconfigured into the adult nervous system. A few experiments included animals in the final (of 5) larval instars.
Figure 2.
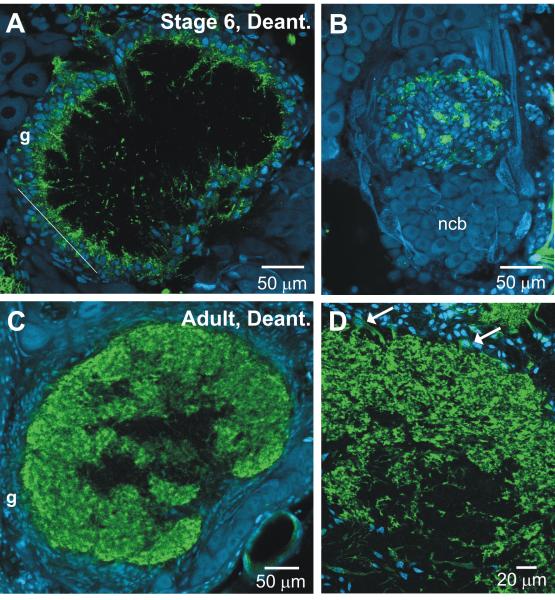
Cellular elements of a glomerulus. A. Schematic diagram of the proximal part of the antennal nerve (AN), which carries receptor axons extending from cell bodies in the sensory epithelium of the antenna, and the antennal lobe (AL). SZ: Sorting Zone; nl: nerve layer; gll: glomerular layer. Neuronal cell bodies (ncb) lie almost entirely in 2 large clusters outside of the neuropil and the nerve layer; a third cluster of ~15 cells (not shown) lies on the anterior surface of the AL. The red neuron is a typical uni-glomerular projection neuron; the blue neuron is a typical multi-glomerular local interneuron. Orange processes are olfactory receptor axons and small ovals are central glial cells. Filled small ovals are peripheral glial cells. Asterisk indicates a glomerulus, a cross-section of which is shown schematically below. Glial envelope and representative glial processes in the neuropil are shown in green. Red dotted circle in diagram and in adjacent images B-E: approximate glomerular boundary. B. Some of the 75-100 simple glial cells that form an almost complete envelope around each glomerulus. C. Terminal branches of some of the sensory neurons (~5000/glomerulus) that enter the apical portion of the glomerulus from the nerve layer. D. Dendrites of some of the AL neurons, which enter from the base of the glomerulus (~ 300/glomerulus). E. One of the complex glial cells (<10/glomerulus). Confocal images were prepared using methods routine in the lab: simple glial cells: propidium iodide with RNAse (Oland et al., 1999); receptor neurons: DiI (Oland et al., 1998); AL neuron dendrites: anti-GABA immunocytochemistry (protocol as in Methods); complex glial cell: Lucifer yellow dye fill (Oland et al., 1999).
The complex glial cell here and in Figure 6L is a slightly different 3-dimensional reconstruction of the cell shown in Oland et al., 1999. Used with permission from Elsevier.
GABA localization during the period of olfactory receptor axon ingrowth (stages 3 to 9 of adult metamorphic development)
At the time that axons of olfactory receptor neurons (ORNs), whose cell bodies are in the antenna, reach the antennal lobe (AL) at stage3/early 4 of adult metamorphic development, the lobe has 3 layers. The core is the neuropil, which comprises the dendritic processes of antennal lobe neurons, mainly local interneurons. The neuropil is surrounded by a several-cell-thick layer of glial cells, and outside this shell of glia are several clusters of neuronal cell bodies. Neuronal cell bodies are never located within the neuropil.
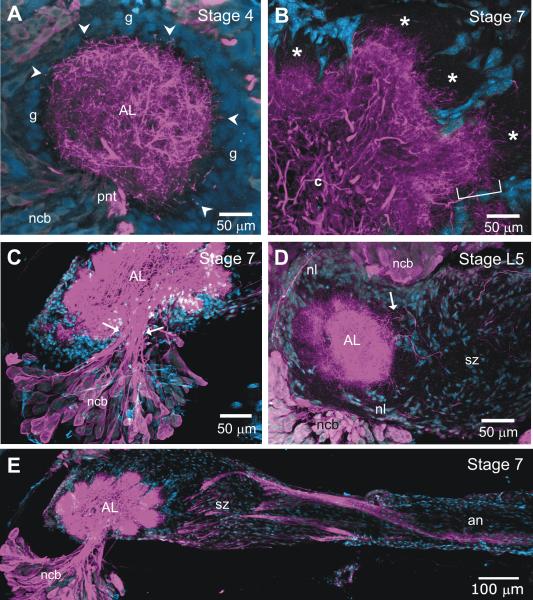
Detailed examination of the localization of GABA in the developing AL (Fig. 3) revealed that at the beginning of the period of axon ingrowth, GABA-positive dendritic processes arborize throughout the neuropil, with some of the distal dendrites extending short distances into the glial shell (Fig. 3A). The terminal branches of the ORN axons grow between the neuronal dendrites and the glial shell (asterisked areas in Fig. 3B) and form protoglomeruli (Oland et al., 1990). The GABAergic dendrites gradually invade these protoglomeruli and eventually arborize throughout the glomerular neuropil. With the exception of a few GABAergic neurons in the tiny anterior cluster, all of the GABAergic neuronal cell bodies are located in the lateral cluster of cell bodies (Fig. 3C). Their primary neurites extend via several sub-fascicles within the primary neurite tract toward the center of the neuropil, then branch to enter most of the glomeruli. The overall dendritic pattern seen in this study is consistent with that originally reported by Homberg and Hildebrand (1994), but they were not able to visualize the finest dendritic branches, and labeling was somewhat less intense, especially at the earlier stages, presumably a function of differences in both antibody used and protocol.
Figure 3.
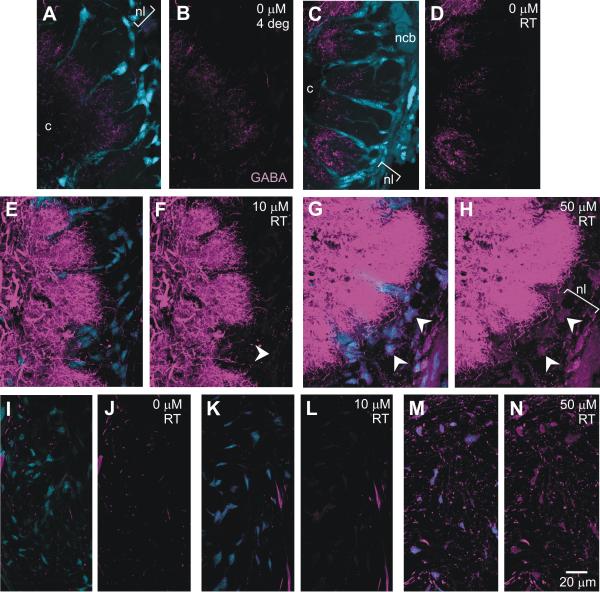
GABA-positive axons and dendrites in the developing antennal nerve and antennal lobe. A. At stage 4, as the first olfactory receptor (ORN) axons enter the nerve layer, GABA-positive dendrites (magenta) fill the antennal lobe (AL) neuropil and a few dendrites (arrowheads) reach into the glial cell layer (blue, g) that surrounds the neuropil. B. By stage 7, ORN axon terminal branches (not labeled in this figure) have filled the outer portion of each glomerulus (*) and GABA-positive dendrites are still mainly in the basal portion (bracket), as well as in the coarse neuropil (c) in the center. C. Arrows indicate borders of the primary neurite tract within which several subtracts carry GABA-positive processes into the neuropil from the lateral cluster of neuronal cell bodies. D-E. As early as late stage 5, the sorting zone (SZ) portion of the antennal nerve and the nerve layer of the AL include a few GABA-positive fibers. GABA-positive fibers in some cases may be dendrites extending from the glomeruli (arrow in D) but also may be axons that by stage 7 appear in bundles in the antennal nerve and sorting zone. an: antennal nerve; ncb: neuronal cell bodies; nl: nerve layer; pnt: primary neurite tract. In this and all subsequent images, the nucleic acid stain Syto 13 were used to label cell nuclei, which appear in blue.
An additional, and unexpected, finding is the presence of GABA-positive processes in the antennal nerve, in the sorting zone region of the nerve, which lies just at the point where ORN axons enter the AL, and in the nerve layer of the AL (Fig. 3D,E). Hoskins et al. (1986) reported a few GABA-positive fibers in the antennal nerve, but these were not identified by source. The images in Figure 3D and E show that some of the GABA-positive processes in the sorting zone and nerve layer appear to be extending from or entering the glomeruli and thus could be either dendrites extending from the AL neurons or axons traveling from the nerve into the glomeruli. By stage 7, however, there are several large bundles of GABA-positive axons that extend the length of the intracranial portion of the antennal nerve (Fig. 3E) A few of these axons travel in the nerve layer of the AL, but most of them turn in the SZ and travel in the antennal mechanosensory tract (not shown). We were not able to follow them in the antenna and cannot say whether the axons extend from mechanosensory neurons in the base of the antenna or from motor neurons in the Antennal Mechanosensory and Motor Center. Their identity is as thus unknown.
Within the AL, the presence of GABAergic dendrites of AL neurons that extend into the glial shell and the looseness of neuropil at early stages as described earlier at the EM level (Oland and Tolbert, 1987) suggested the possibility that GABA could be released from growing dendrites and easily diffuse within the neuropil or glial shell. It could have a direct developmental effect on glial cells, or glial cells under the influence of GABA could in turn affect development of AL neurons. Because glial cells in a variety of species are known to express GABA transporters, we asked whether glial cells in the developing olfactory pathway did so, using an antibody raised against a M. sexta GABA transporter (Umesh and Gill, 2002).
MasGAT localization in larval and pre-innervation adult metamorphic antennal lobes
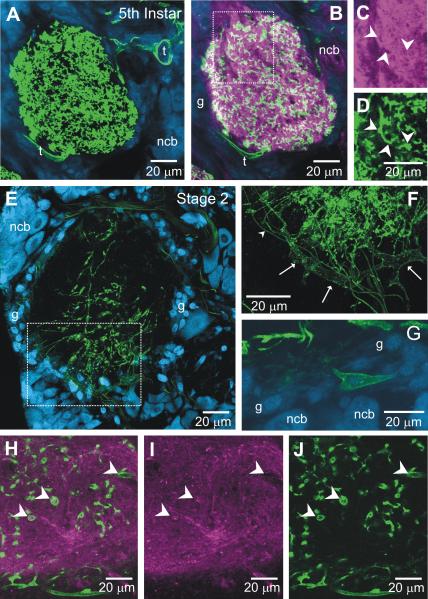
We examined MasGAT expression in 5th instar and stage-2 animals to determine the pattern before axon ingrowth. Consistent with the pattern seen by Umesh and Gill (2002) in larval animals, strong MasGAT labeling was found throughout the neuropil during the 5th instar (Fig. 4A) (n=4), but no labeling was present in any neuronal cell body. Large tracheae near the AL and tracheoles entering the AL neuropil were clearly labeled. Double-labeling with antibodies against MasGAT and HRP (Fig. 4B-D), the latter used as a neuronal marker, showed that some, but not all, MasGAT-positive processes also were HRP-positive while the tracheae were HRP-negative, as expected. Electron microscopy across the entirety of a cross-section of the neuropil of a 5th instar antennal lobe (not shown) revealed only rare processes that had the ultrastructural features of glial cells (Tolbert and Hildebrand, 1981) although such processes were present in other neuropilar regions. Together the data suggest that many of the MasGAT-positive processes in 5th instar AL neuropil are likely to be neuronal.
Figure 4.
MasGAT distribution prior to ORN axon ingrowth to the antennal lobe. A. The 5th-larval instar AL neuropil contains numerous MasGAT-positive processes, some of which are tracheolar (t). B. Double-label with anti-MasGAT (green) and anti-HRP (magenta) antibodies, in a single optical section. C-D. Higher power views of the boxed area in B with the channels separated Right and left arrowheads show possible overlap while lower arrowhead indicates a process in which there is no overlap. E-G. By stage 2 of adult metamorphic development, MasGAT labeling in the neuropil is greatly decreased and mostly within the core of the neuropil. Both thick (arrows in F) and thin (arrowhead in F) tracheolar processes still are MasGAT-positive. G shows a rare MasGAT-positive cell body in the glial layer (g) of the neuropil. H-J. Portion of a stage-2 neuropil double-labeled with anti-HRP and anti-MasGAT antibodies. At this stage, fewer MasGAT-positive processes appear to co-localize with HRP, and some partially ensheathe HRP-positive processes. Arrowheads indicate MasGAT-positive processes in the combined and separate channels.
By stage 2 of metamorphic development (n=4), however, the density of labeled processes in the neuropil had greatly decreased and those still present tended to be located in the core and ventral region of the neuropil (Fig. 4E). The labeled processes were typically long, with a limited amount of branching, and often blebby. A few large tracheae as well as fine tracheoles course within the glial shell and both were MasGAT-positive (Fig. 4F-G). Labeled cell bodies within the glial layer were present but had only a few short processes (Fig. 4E). At this stage, some of the MasGAT-positive processes seen in the neuropil in a single optical section overlapped with HRP-positive neuropil, but others surrounded HRP-positive processes (Fig. 4H-J). Electron microscopy across complete cross-sections of three stage-2 antennal lobes revealed few glial or tracheolar processes in the neuropil (not shown). At this stage, then, the MasGAT-positive processes in the AL neuropil are mainly neuronal processes, but could include a few glial or tracheolar processes, particularly at the neuropil edges.
MasGAT in the developing adult antennal lobe
The distribution pattern for MasGAT reported by Umesh and Gill (2002) in adult animals was described as neuropilar, but showed small cell bodies near the outer rim of each glomerulus. Since these cell bodies lie in the shell of glial cells that surrounds the neuropil and are likely to be glial cells, we tested the hypothesis that glial cells might come to express a GABA transporter as development progressed through the metamorphic period. The timing and distribution of MasGAT were determined during stages 4-9, the period of axon ingrowth, formation of dendritic tufts, and synaptogenesis as well as at stages 12 and 15, a period of dendritic and synaptic refinement that follows glomerulus assembly.
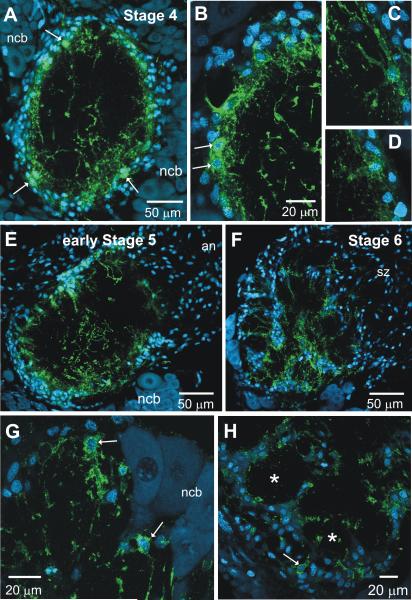
At stage 4 (n=4), glial cells form a layer around the nascent AL neuropil. MasGAT labeling was found on a subset of these glial cells that typically were located within the inner aspect of the glial shell surrounding the neuropil (Fig. 5A-D). Their cell bodies were small compared to the neuronal cell bodies, and the pattern of labeling in the nuclei by the nucleic acid marker Syto 59 was markedly different from that seen in the neurons, and more similar to that seen in the glial cells populating the shell. Their processes extended into the neuropil just below the glial border, forming a short fringe. In addition, a few processes with a very different morphological character that included many blebby regions extended into the core of the neuropil; they could not be traced to specific cell bodies, but resembled those seen at stage 2. Over the next few stages, the blebby processes gradually disappeared from the core neuropil of the lobe (Fig. 5E-F, 6A) raising the possibility that these processes belong to residual larval neurons that die shortly after the transition from larva to pupa (Weeks & Levine, 1995).
Figure 5.
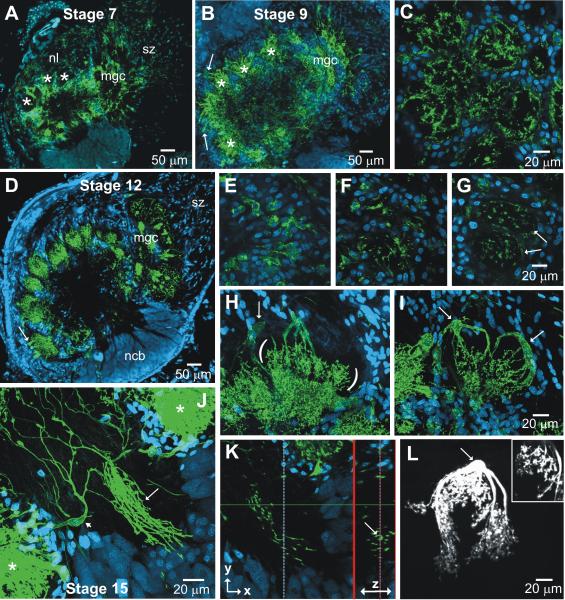
MasGAT distribution during adult metamorphic development of the antennal lobe. A-D. At stage 4, when ORN axon ingrowth has just begun, a few, blebby MasGAT-positive processes are found in the middle of the neuropil. A layer of fine processes extends into the neuropil from a subset of the glial cells (arrows) that forms a shell around the neuropil. C-D shows the morphologies of individual, labeled glial cells at stage 4. During stage 5, MasGAT-positive processes begin to extend into the neuropil (E) and appear in newly developing glomeruli by stage 6 (F) G. Higher power view at stage 6 shows individual MasGAT-positive glial cells (arrows) that are clearly distinct in size and position from the neuronal cell bodies (ncb). Neuronal nuclei usually fail to label with the Syto dyes in M. sexta at these stages, for unknown reasons. H. MasGAT-positive processes begin to line the glial borders forming around each glomeruli. Only a few glial cells are MasGAT-positive.
Figure 6.
MasGAT distribution during adult metamporphic development of the antennal lobe (continued). A,B, D. During stages 7, 9, and 12, MasGAT is most strongly associated with the bases of the glomeruli and with the glial borders around each glomerulus (*). Cell bodies of individual glial cells (arrows) are most often associated with the apical (outer) edge of the glomeruli. mgc: male-specific macroglomerular complex. C. Stage 9, Tangential section through the glomerular layer at approximately the midpoint (apex to base) of the glomeruli shows MasGAT-positive processes forming an incomplete lining of the glial border around each glomerulus as well as a small number of processes in the glomerular cores. E-G. Three individual sections from a series taken from the nerve layer into the centers of 2 adjacent glomeruli (arrows). H-I. Large MasGAT-positive cells show large cell bodies (arrows) in the glial border, stout arms and fine branches in the bottom portion of the glomeruli. Parentheses in H flank the highly branched processes in the basal part of the glomerulus. Glial cell in L was filled with Lucifer Yellow during a whole cell recording and belongs to the “complex” glial cell class. Inset shows details of fine branches. J. MasGAT-positive processes in the primary neurite tract of the lateral neuronal cell body cluster (arrow). Small arrow in J indicates a large MasGAT-positive glial cell contributing to the processes in the tract. *, Glomeruli adjacent to the tract. Were the primary neurites of the neurons extending in the track visible (see Fig. 7E), they would be seen traveling in parallel with the MasGAT-labeled processes. K. A smaller stack of 20 optical sections (z=0.4) taken from the stack in J (38 sections). The view to the right of the bold vertical line was generated in the z axis of the optical stack at the position of the thin dotted vertical line on the left of the panel. It shows an image of the tract perpendicular to its long axis (arrow) and shows that the MasGAT-positive processes run in parallel with the (unlabeled) primary neurites, but do not ensheathe them; only one labeled processes has a ‘c’ shape, and none has the circular sheath that would be expected if the process enwrapped an axon(s)
During stage 5 (n=4), a large number of receptor axons grow into the lobe and, on reaching their target area, form axonal protoglomeruli. These protoglomeruli become surrounded by the processes of neuropil-associated glial cells. MasGAT was expressed by a subset of cells in the glial border and labeled processes extended toward the central core of the neuropil (Fig. 5E). By stage 6 (n=10), when developing glomeruli are well surrounded by glial processes, MasGAT-positive cell bodies and processes were found within the glial envelope and also within the developing glomerular neuropil (Fig.5 F-H).
All of the cellular elements of mature glomeruli – receptor axon terminals, projection and local interneuron dendrites, and a glial envelope - are present in each developing glomerulus by stage 7. The glial envelope is robust, particularly between glomeruli. Between stage 7 and 9, when synaptogenesis proceeds and dendritic arbors become more highly branched and tufted (Oland et al., 1990), the density of MasGAT-positive processes in the glomeruli also increased (Fig. 6A-C) (stage 7, n=6; stage 9, n=2). MasGAT-positive cell bodies, still a small subset of the cells in the glial envelopes (<10 of the 75-100 glia that form each envelope), were located mostly at the apex of each glomerulus (just inside the nerve layer). A cross-section through the middle of the glomerular layer (Fig. 6C) shows that MasGAT-positive processes line the inner wall of the glial envelopes and some processes penetrate the core of the glomerular neuropil.
This pattern was especially clear at stage 12 (Fig. 6D) (n=7) and stage 15 (n=2) when tracking MasGAT-positive processes through a glomerulus, beginning at the apical glial border (Fig.6E-G). Figure 6H-I shows the most common morphology for these cells - several large unbranched processes extending from the cell body, with sprays of short branches in the basal portion of the glomerulus. By their morphology, these cells are likely to belong to the class of neuropil-associated glial cells as identified by Strausfeld (1976), and in particular to the subclass of these cells in the AL that we have called “complex” glial cells (Oland et al., 1999). The image shown in Figure 6L is an example of a complex glial cell from the previous study in which neuropilar glial cells were filled with Lucifer Yellow dye during whole cell recordings (Oland et al., 1998). In that study, we estimated that each glomerulus included between 1 and 5 of these cells, a number roughly consistent with the number of MasGAT-positive cells/ glomerulus estimated in the current study.
One additional consistent feature in older (≥ stage 7) ALs was the presence of MasGAT-positive processes interleaved with the primary neurites of neurons with cell bodies in the lateral cluster (Fig. 6J-K, 7E). They traveled in parallel with the primary neurites and usually did not ensheathe them. The processes were found from the base of the neuronal cluster to the coarse neuropil at the center of the lobe and could never be traced to neuronal cell bodies. In the entire set of preparations of developing antennal lobes, fewer than 10 neuronal cell bodies in the AL neuronal cell body clusters had any detectable MasGAT labeling, and none showed any labeling in their primary neurites when it was possible to follow them from the cell body to the primary neurite tract. MasGAT-positive processes sometimes were found in more than one sub-tract of the primary neurite tract at stage-7, but by stage 12, the labeled processes usually were confined to a single sub-tract. We do not know the identity of the neurons whose primary neurites extend in this tract; the lateral cluster contains both local and projection neurons and the cell bodies of neurons with particular transmitter phenotypes typically are scattered within the cluster (Kent et al., 1987; Homberg et al., 1990; Homberg and Hildebrand, 1994). Also, as shown in Figure 3C, GABAergic primary neurites travel in several, if not all, of the sub-tracts, so there is no necessary spatial relationship between GABAergic processes and MasGAT-positive processes.
Figure 7.
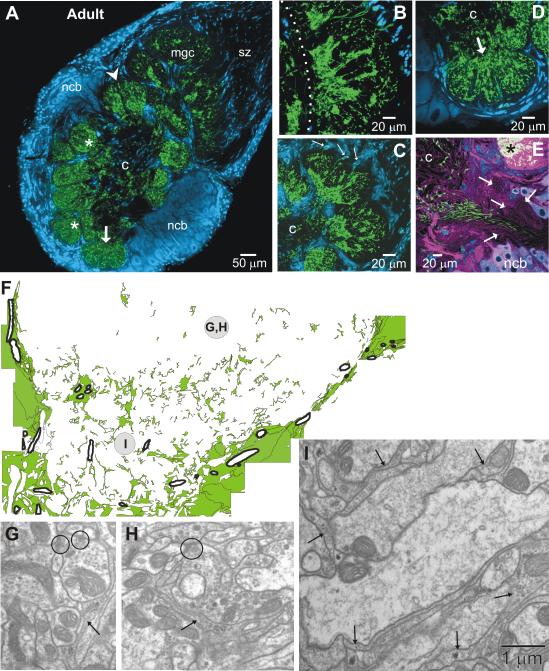
MasGAT in the adult antennal lobe. A. MasGAT persists in the adult, typically most densely expressed in the basal region of each glomerulus (*), though the distribution within glomeruli varies somewhat. Labeled processes also appear in the coarse neuropil (c) in the center of the lobe. No glial cells in the SZ express MasGAT in the adult. Expression is strong in the basal regions of each compartment of the male-specific macroglomerular complex (mgc), shown at higher power in (B) and in the pair of large female glomeruli (C). The cumulus portion of the mgc is to the right of the dotted line. D. Expression in the labial pit organ glomerulus (arrow in A and D) is more diffuse. Cell body labeling is less intense, but when present can be traced to labeled processes in the neuropil (arrows in C). E. Primary neurite tract of the lateral cluster of neuronal cell bodies, double-labeled with anti-MasGAT (green) and anti-HRP (magenta), shows MasGAT-positive processes in a limited region of the tract. Several sub-tracts (arrows) show no MasGAT-positive processes. F. Glial processes (green) traced from an EM montage of a complete cross-section of an ordinary glomerulus with a glial process distribution like that in the glomerulus in A indicated by the arrowhead. The basal half of the glomerulus is shown. Tracheolar processes are outlined with thick black line. The glial envelope that surrounds the glomerulus consists of multiple fine glial processes that were not individually traced, but rather were combined and represented as large regions. The upper region of the tracing (near the center of the glomerulus) has few glial processes. Synapses (circles) in this region (G-H) or in the basal region (I) have no necessary relationship to glial processes (arrows in G-I). In the basal region (I), large dendritic processes are typically partially or fully ensheathed by glial processes.
The distribution of MasGAT in the developing and adult brain was not limited to the ALs, but included strong labeling in the neuropil of the optic lobes, the protocerebrum, and the sub-esophageal ganglion, and had the same glial pattern as in the AL (not shown). The cell bodies of a few very large neurons appeared in the sub-esophageal ganglion.
MasGAT in the adult antennal lobe
The adult olfactory neuropil in the moth appears in frontal sections as an array of roughly spheroidal glomeruli that form a ring around a core of coarse neuropil. Each glomerulus is almost completely surrounded by glial cells and some glial cells are located within the coarse neuropil. The cell bodies of the AL neurons now lie in distinct clusters entirely outside of the neuropil.
In the adult (n=4), MasGAT-positive processes were found in both the glomerular and the coarse central neuropil (Fig. 7A). All glomeruli, including the male- (Fig. 7B) and female-specific (Fig. 7C) glomeruli and the labial pit organ glomerulus (Fig. 7D), contained labeled processes, but the density and pattern of labeling varied across the population of glomeruli (Fig. 7A). Sampling across the full glomerular array showed that the greatest density of MasGAT labeling was usually in the basal part of a glomerulus, the portion that lies closest to the central coarse neuropil. In the male- and female-specific glomeruli in particular, the basal regions were very densely labeled. In contrast, the MasGAT-positive processes in the labial pit organ glomerulus, which receives its sensory input from the CO2-sensitive pit organ that sits at the tip of the labial palp, were distributed rather homogeneously within the lobular sub-regions of the glomerulus. Within the adult AL, the only cell bodies labeled, and then only faintly, were cell bodies distributed very sparsely within the glial envelopes around the glomeruli.
Processes were not always smoothly labeled but instead showed regions or patches that labeled more brightly. At higher power (not shown), the MasGAT-positive processes followed the pattern described near the end of the period of axon ingrowth (as in Fig. 6) – robust branches that bifurcated extensively and often had very thin flattened regions, consistent with the thin profiles seen at the electron-microscopic level (Fig. 7F-I).
Using a montage of electron-microscopic images acquired across the entire lower half of an adult glomerulus, we traced all the processes that could be identified as glial by the presence of ribosomes, rough ER and glycogen particles (Tolbert and Hildebrand, 1981), to see if it matched the labeling pattern seen with the antibody against MasGAT. The resulting map of the distribution of glial processes (Fig. 7F) shows a distribution that closely matches the pattern of dense basal MasGAT labeling seen at the light-microscopic level. In the middle part of the glomerulus, which has a high density of synapses, there are few glial processes (this study and Oland et al., 1999). In the basal part, where large dendritic branches of AL neurons enter the glomerulus, glial processes fully or partially surrounded almost all of the dendrites. Although many synapses are present in this region (Sun et al., 1997), neither here nor in the middle region were glial processes associated with the synapses (Fig. 7F-I).
Identity of MasGAT-positive processes
Figures 5-7 provide strong evidence that MasGAT-positive cells in stage-4 and older antennal lobes are glial cells: (1) the cell bodies lie in the glial layer around the neuropil or, later, around the glomerular compartments of the neuropil; (2) the labeled cell bodies are much smaller than neuronal cell bodies, which in the insect CNS lie in clusters separate from the neuropil, and the size and appearance of their nuclei match those of the other cells in the glial layer; (3) the morphology of mature individual MasGAT-positive cells, as well as their number in each glomerulus is consistent with the morphology and number of complex glial cells described previously (Oland et al., 1999), and (4) the distribution matched the distribution of glial processes determined by electron microscopy.
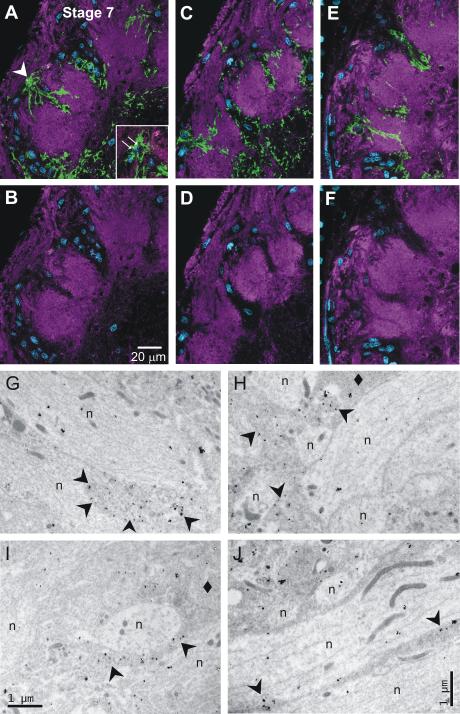
We also double labeled sections from developing tissue with anti-MasGAT and anti-HRP (n=13; 5th instar, stage 2, stage 7 and stage 18). Figure 8 A-F, from a stage-7 AL, provides a comparison of single optical sections that revealed almost complete spatial separation between MasGAT-positive processes and HRP-positive neuropil. Thick MasGAT-positive processes showed no overlap with HRP-positive processes. Some of the finest processes do appear to overlap, but this is not a clear case of co-localization because most distal MasGAT-positive processes are very thin, as are the fine dendritic branches of neurons arborizing in the glomeruli. Thus both HRP-positive and MasGAT-positive processes can appear within the same volume in a single optical section. In addition, biochemical studies have shown that neuroglian, which in the M. sexta olfactory pathway is expressed in the plasma membranes of both receptor axons and central glial cells (Gibson and Tolbert, 2006), also carries the anti-HRP epitope (Snow et al. 1987; Desai et al. 1994) raising a small possibility that some glial processes could be HRP- positive.
Figure 8.
Identity of MasGAT-positive processes. A-B, C-D, E-F. Glial nuclei in blue. Upper panels (A,C,E) show a portion of stage-7 ALs double-labeled single optical sections with anti-MasGAT (green) and anti-HRP (magenta); lower panels (B,D,F) show only anti-HRP labeling. Comparison of upper and lower panels reveals no overlap between MasGAT-positive and HRP-positive processes, except possibly at the distal tips of the MasGAT-positive processes. These processes are extremely thin so that glial and neuronal processes can occupy the same Z volume. Inset: The labeled processes in A were traced to the cell body that appeared in the position in the glial border indicated by the arrowhead but that was obscured by overlying labeled processes in the full 3-D reconstruction. G-J. Electron micrographs taken in glomerular neuropil with MasGAT indicated by the presence of silver-enhanced gold particles. Some (arrowheads), but not all (diamonds), glial processes contain gold particles. Neuronal processes (n) have no labeling above background.
To resolve definitively the question of whether MasGAT-positive processes in the developing (post stage 5) AL neuropil and SZ are glial and/or neuronal, we used immuno-EM (Fig. 8G-J). While some processes identifiable ultrastructurally as glial were unlabeled, as expected from the labeling pattern in the confocal microscope, the highest density of label was found in clearly glial processes (~5 particles/cm2). Neuronal processes bore only background levels of immunogold (~0.4 particles/cm2). We conclude that the cells labeled by the MasGAT antibody are glial cells by the criteria of morphology, size, location, and features characteristic of glial cells at the EM level.
MasGAT in the antennal nerve, sorting zone, and nerve layer of the antennal lobe
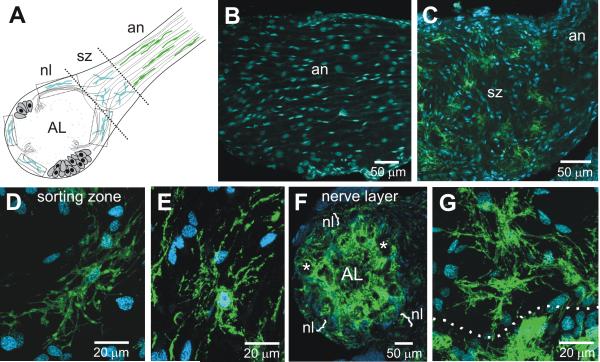
Figure 9A shows schematically the distribution of the several types of glial cells in the nerve and nerve layer portions of the olfactory pathway. The olfactory (antennal) nerve is populated by both peripherally and centrally derived glial cells. The former migrate from the antennal sensory epithelium, following the receptor axons (Rössler et al., 1999). The latter arise from a group of glial cells located at the site where the receptor axons enter the lobe; they migrate into the most proximal part of the nerve to form the axon sorting zone (SZ) region (Rössler et al., 1999). All cell bodies in the SZ are glial except for a very small population of tracheolar cells. Shortly after receptor axons enter the SZ, they change their axonal associations and their trajectories, and they leave the SZ fasciculated in bundles that target particular regions of the neuropil, or sometimes particular glomeruli (Oland et al., 1998; Rössler et al., 1999).
Figure 9.
MasGAT in the developing nerve and nerve layer of the antennal lobe from a stage-7 animal. A. Schematic diagram of the M. sexta nerve and antennal lobe. Peripheral glial cells populate most of the length of the antennal nerve (an), migrating from the antennal sensory epithelium. Centrally derived glial cells populate the sorting zone region (sz), the nerve layer (nl), and form the glomerular borders. B. No MasGAT-positive glial cells were found among the peripheral glial cells. A subset of glial cells in the SZ (C-E) and in the nerve layer (F-G) were MasGAT-positive during the period of ORN axon ingrowth. D-E and G show higher power views of the morphology of individual MasGAT-positive glial cells. Dotted line in G indicates the border between the nerve layer, above, and the apical edge of a glomerulus, below. *, glomerulus.
No MasGAT labeling was found at any stage in any portion of the antennal nerve distal to the SZ (Fig. 9B). Within the SZ, however, a subset of the glial cells expressed MasGAT beginning after the SZ glial cells begin to populate the region (stage 5) and continuing until at least stage 9 (Fig. 9C). Thereafter, MasGAT expression among the SZ glia was detected infrequently and was completely absent at the end of metamorphic development and in the adult. MasGAT-positive SZ glia typically were distributed rather evenly within the SZ, their multipolar processes spreading widely (Fig. 9D-E).
MasGAT-positive glia also could be found in the nerve layer of the AL, again labeling a subset of glial cells (Fig. 9F-G). Their processes were branched, extending generally in parallel with the axons traveling around the outside of the neuropil, but also extending toward the glomerular layer, presumably accompanying fascicles of axons that have turned from the nerve layer toward the apical edge of a glomerulus.
MasGAT labeling in deafferented antennal lobes
The presence of MasGAT was not dependent on the presence of olfactory receptor axons. When the antennal anlage is removed prior to axon outgrowth, the neuropil of the ipsilateral AL fails to become glomerular (Hildebrand et al., 1979; Oland and Tolbert, 1987). Glial cells divide, but remain in their immature position, forming a thick shell around the neuropil (Oland and Tolbert, 1987). Nevertheless, a subset of the glial cells forming the shell (Fig. 10A-B) (n=4 stage-7 animals) was strongly labeled. The cell bodies of most of the MasGAT-positive glial cells were found on the inner edge of the shell. Their morphology resembled that in normal lobes of roughly the same age, with branches extending into the neuropil. A few processes with a blebby morphology appeared in the coarse central neuropil.
Figure 10.
MasGAT in antennal lobes that developed in the chronic absence of ORN input. A. Glial cells have failed to migrate into the neuropil, but MasGAT-positive glial processes do extend into the neuropil from a subset of the glial cells in the glial border (g). MasGAT-positive process outgrowth is similar to that in a normal lobe at about stage 5-6 of development. B. A tangential section through the glial border of another preparation in roughly the position indicated by the line in A. As in normally developing ALs, only a subset of the glial cells are MasGAT-positive. C, D. Adult. Although lacking glomerular structure, the distribution and amount of MasGAT labeling in the fine-textured neuropil that forms the outer layer of the deafferented AL is about the same as in a normally developing AL. Arrows in D show MasGAT-positive glial cell bodies. ncb: neuronal cell bodies.
In the adult, however, there is extensive MasGAT labeling in the antennal lobe neuropil (Fig. 10C-D). As in the normal adult antennal lobe, only a few glial cell bodies were labeled; among the population of the glial cells with labeled cell bodies, several had processes that could be traced into the neuropil. These branched into fine processes that often were clustered and had vellate expansions. Except that these cells had no glial envelope within which to arborize, the overall amount and type of branching was similar to that in the normal adult lobe.
GABA uptake into glial cells
If the MasGAT-positive glial cells have functional GABA transporters, they would be expected to take up GABA. Immunocytochemical labeling for GABA in stage-7 ALs after standard fixation, using collection parameters that optimized images of the dendrites of GABAergic neurons, revealed no evidence of GABA in glial cells, even if the time of incubation in anti-GABA antibody was increased to 3 days. It was possible to see faint labeling in glial cells if the gain was increased significantly (not shown). To test whether these glial cells would take up GABA, we exposed freshly dissected desheathed antennal lobes to 0, 10 or 50 μM GABA (n=62), for 20 minutes at either room temperature, or 4°C to minimize transporter function. At 0 μM, using the same collection parameters, no GABA labeling could be detected in the glial cells (Fig. 11A-D); neuronal labeling was slightly greater in preparations incubated at RT than at 4°C. After incubation in 10 μM GABA, faint labeling could be detected in some glial cells and the intensity of labeling in the neuronal processes increased markedly (Fig. 11E-F). At 50 μM, however, all glial cells showed GABA labeling and the labeling of neuronal processes became dramatically more intense (Fig. 11G-H), especially after room-temperature exposure. A similar pattern was seen among the SZ glial cells (Fig. 11I-N).
Figure 11.
Incubation in GABA. First panel of each pair shows GABA (magenta) and glial (blue) labeling; second panel shows only GABA labeling. A-D Glial cells in the nerve layer (nl) and in the glomerular borders showed no GABA labeling under resting conditions (0 uM at 4°C or at RT). E-F. After 20-min incubation in 10 μM GABA, a few glial cells in the AL show faint labeling (arrowhead) and neuronal labeling intensity is increased. G-H. In 50 μM GABA, most glial cells show GABA uptake (arrowheads) and neuronal labeling intensity was greatly increased. The same pattern was found in the SZ region of the nerve (I-N). M-N shows GABA-positive axons, mostly in cross-section, in the SZ. Image collection parameters and 3-D stack sizes were kept constant.
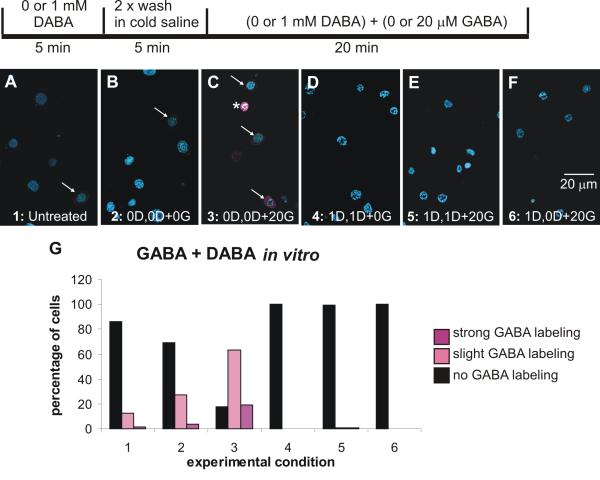
Because the in vivo environment complicates understanding of direct and indirect effects of GABA exposure, we exposed cultured SZ and neuropil glial cells to GABA (Sigma) and then to GABA in the presence of diamino-butyric acid (DABA (Sigma)), which had been shown by Mbungu et al. (1995) to effectively (~85%) block the M. sexta transporter expressed in Xenopus oocytes. Figure 12 shows that a few glial cells are GABA-positive even under untreated (no solution changes) or control conditions, no 5-min pre-exposure to DABA and incubation for 20 min in medium containing neither DABA nor GABA. These results suggest that the glial cells may be taking up GABA from the medium, which contains fetal bovine serum. When incubated in GABA (Condition 3), many of the glial cells became lightly or strongly labeled with GABA, uptake that was essentially completely blocked by DABA whether the DABA was present for both the 5-min pre-exposure and the 20-min incubation periods (Condition 5), or only for the 5- min pre-exposure period (Condition 6).
Figure 12.
GABA uptake and blockade of uptake by DABA in cultured sorting zone and neuropil glial cells. A-F. Confocal images of cultured glial cells after exposure to GABA and DABA in accord with the experimental protocol shown schematically above. G. Histogram showing that a small percentage of glial cells were GABA-positive (magenta) under control conditions (Conditions 1 and 2). In the presence of 20 mM GABA without DABA (Condition 3), many glial cells became GABA-positive. When DABA was present throughout the experiment, GABA labeling was eliminated, whether or not cells were exposed to 20 μM GABA (Conditions 4 and 5). If DABA was given only for 5-min before GABA treatment (Condition 6), GABA labeling again was eliminated. 982 cells divided among the conditions were examined. D: DABA; G: GABA. Glial cell nuclei labeled with Syto 13 (blue). Lightly GABA-positive cells indicated by arrows; a strongly positive cell by an asterisk.
Discussion
We have shown a GABA transporter localizes to a subset of glial cells in the developing olfactory pathway of the moth and that glial cells in the SZ and associated with the AL neuropil are capable of taking up GABA.
Characteristics of GABA transporters
The MasGABA transporter (Mbungu et al, 1995) belongs to the Na+/Cl−-dependent transmitter transporter superfamily (Gadea and Colomé, 2001). In rat and human CNS, molecular cloning studies have revealed three high-affinity types of GABA transporters - GAT-1, −2 and −3 (Borden et al., 1992, 1995; Guastell et al., 1990) - as well as a low-affinity sub-type called BGT-1, each with a distinct pharmacology. Each has a distinct expression pattern, with GAT-1 appearing in neurons and astrocytes, GAT-3 mainly in glial cells, and GAT-2 in epithelial, neuronal and glial cells. GAT-2 is present throughout the astrocytes while GAT-1 in astrocytes mainly appears in the distal branches.
Among the insects, Drosophila’s genome includes 21 genes that are putative members of the SLC6 transporter family (Thimgan et al., 2006). Of these, only DmCG1732 belongs to the GABA transporter subfamily, which also includes MasGAT, TnGAT, AgCP8499, and CeSNF11. inebriated (ine), originally proposed to be a GABA transporter (Stern and Ganetzky, 1992; Burg et al., 1996; Soehnge et al., 1996), does not fall within this subfamily and its substrate has not yet been identified (Huang et al., 2002) although recent data suggest that it may transport carcinine (Gavin et al., 2007). Drosophila synaptic plasma membrane vesicles isolated from adult flies are capable of transporting [3H]-GABA in a time- and Na+ and CL−-dependent manner. Their function was blocked by the GABA-transporter inhibitor DL-2,4-diaminobutyric acid (DABA), and at somewhat lower levels by nipecotic acid (Leal and Neckameyer, 2002). Interestingly, the riboprobe for DmCG1732 labeled only a subset of the neuropil-associated glial cells and not neuronal cell bodies in the CNS (Thimgan et al., 2006) and [3H] GABA is taken up into glial cells of the lamina of both housefly and Drosophila, but not into neurons (Campos-Ortega, 1974). The possibility that multiple isoforms will be recognized in Drosophila is suggested by the finding that antibodies specific for the mammalian GABA transporters revealed distinct temporal and spatial patterns of expression in the Drosophila CNS (Neckameyer and Cooper, 1998).
The moth, Trichoplusia ni, also expresses a high-affinity, Na+ and Cl−-dependent GABA transporter in the brain. Its deduced amino acid sequence shows 95% identity with the M. sexta GAT, and its pharmacology also resembles that of the M. sexta GAT (Gao et al., 1999). The MasGAT transporter most closely resembles the mouse GAT-1, with 58.5% sequence identity, but shows a different pharmacology from the vertebrate GAT-1 when tested in Xenopus oocytes (Mbungu et al., 1995). Nipecotic acid reduced transport by ~50% and DABA reduced it by more than 80%, while β-alanine, guvacine, ACHC, BABA, and hemicholinium had no effect. The MasGAT was shown to be highly selective for GABA with a possible very low level of choline transport. In other insects for which sequence data are available, one GAT has been identified to date for the beetle Tribolium and 2 isoforms have been found in the bee Apis mellifera (Entrez Nucleotide Database, www.ncbi.nlm.nih.gov). It should be noted that while characterization of the M. sexta GABA transporter revealed it to be a high-affinity saturable transporter (Mbungu et al., 1995), the reported affinity of MasGAT for GABA, like that of anther moth, T. ni, falls at the low end of the high-affinity range, similar to lower affinity component reported for GABA transport in locust neurons (Breer and Heilgenberg, 1985).
Na+-dependent GABA uptake mechanisms also have been identified in locust (Shepherd and Tyrer, 1985; Breer and Heilgenberg, 1985), cockroach (Bermudez et al., 1988), and lobster (Iverson and Kravitz, 1968), but they have not been characterized at the molecular level.
Developmental labeling pattern and role of MasGAT
MasGAT was expressed in the olfactory pathway of M. sexta during metamorphic adult development and in the adult in centrally derived glial cells, but importantly, only in a subset. In the neuropil-associated glia, we see two morphological types, the complex glial cells whose branches extend within the neuropil and the simple glial cells that contribute to the glial envelopes that surround glomeruli in the ALs (Oland et al., 1998). The results here suggest that it is the complex glial cells that express the MasGABA transporter. In the sorting zone region of the nerve, we have not previously identified morphological subtypes, but again, only a subset expresses the transporter. This is the first molecular marker in this system that distinguishes differentiated glial cell sub-types and is the first strong indication of functional diversity among them. Similarly, an excitatory amino acid transporter found in embryonic CNS and a vesicular monoamine transporter in the visual system are some of the few markers of subsets among mature Drosophila glial cells (Soustelle et al., 2002; Romero-Calderón et al., 2008).
The first glial cells expressing the MasGABA transporter in the metamorphosing M. sexta antennal lobe appear along the innermost edge of the shell of glial cells surrounding the neuropil, in a position that places them directly adjacent to the dendrites of GABAergic AL neurons. While we do not yet know if the expression of the transporter is regulated by these GABAergic neurons, we do know that it is not regulated by the sensory afferent axons as MasGAT-positive glial cells appear in lobes developing in the chronic absence of afferent axons.
While the glial cells expressing the transporter become spatially separated from the GABAergic dendrites as the ingrowing receptor axons interpose themselves between the glia and the dendrites during the early stages of glomerulus formation, they eventually extend into and around a neuropil rich in GABAergic dendrites, branching heavily in the more basal region of the glomeruli. The transporter appears to be distributed throughout the glial cells, from cell body to distalmost processes
Transporters have long been described in vertebrates as having a role in transmitter recovery at synapses and in regulation of extracellular levels of transmitter (Sakatani et al., 1992; Isaacson et al, 1993; Minelli et al., 1995; Otis et al., 1996; Marcaggi and Attwell, 2004; DiGregorio et al., 2002; Marcaggi et al., 2003; Overstreet and Westborook, 2003; Takayasu et al., 2006), and more recently studies have shown non-vesicular release of GABA through reversal of the GABA transporter GAT-1 (Richerson and Wu, 2003; Wu et al., 2007). Their distribution is consistent with these roles, with GABA transporters prominently located on pre-synaptic neuronal membranes as well as on peri-synaptic glial membranes (Radian et al., 1990; Ribak et al., 1996). But, since we found no specific association of glial processes with synapses in the glomerular neuropil of the M. sexta AL, the transmitter recovery role classically associated with transporters in perisynaptic glia is not a role carried out by the MasGAT-positive glia.
Some of the MasGAT-positive glial cells in the AL also form a thin layer of processes just inside the glial envelope that almost surrounds each glomerulus. The location of these processes, as well as those along the processes that enwrap large basal dendrites, as well as the demonstrated ability of the glial cells to take up GABA, suggests that these transporters are likely to be important in limiting extra-synaptic spread of GABA within the neuropil of an individual glomerulus. At this point, we do not yet have behavioral data in adult moths to indicate the effect of blocking the transporter on olfactory function. In experiments with Drosophila larvae, however, blocking the GABA transporter(s) with nipecotic acid elicited changes in odor preference and in frequency of mouth hook contractions (Neckameyer and Cooper, 1998), while in adult females DABA and nipecotic acid induced deficits in locomotion, in the righting reflex, and in geotactic behavior, but not in feeding behavior or sexual receptivity (Leal and Neckameyer, 2002).
MasGAT also appears in the SZ, a glia-rich region of the M. sexta antennal nerve where receptor axons dramatically change their growth behavior - in trajectory, in axonal partners, and in growth cone size and complexity (Oland et al., 1998; Rössler et al., 1999; Tucker and Tolbert, 2003). With the findings of the current study, we have added a potential GABA-MasGAT-mediated influence to the array of molecular interactions between axons and glial cells already under study, including nitric oxide (Gibson et al., 2001), the adhesion molecules neuroglian and fasciclin (Gibson and Tolbert, 2006; Higgins et al., 2002), and the RTKs Eph/Ephrin (Kaneko and Nighorn, 2003), and the FGFR and EGFR (Gibson and Tolbert, 2006; Oland et al., 2008).
MasGAT labels a subset of SZ glia, but only transiently, from shortly after glial cells begin to migrate out into the nerve to approximately the end of the period of axon ingrowth. The transient presence of the transporter in this group of glial cells implies a developmental role for the transporter in glial regulation of GABA levels in this region, which in turn suggests a role for GABA itself in regulating the growth of olfactory receptor axons. GABA now has been well documented to affect neuronal process outgrowth during development (e.g., Barker et al., 1998; Spoerri 1988; Barbin et al., 1993; Ben-Ari et al., 1994; Owens & Kriegstein, 2002). Ongoing experiments in M. sexta to test the role of GABA on axon outgrowth and the effect of the GABA transporter DABA will reveal whether regulation of GABA in the developing olfactory pathway, especially in the sorting zone might facilitate the process of locating and fasciculating with like axons.
SZ glia can take up GABA as shown by the incubation experiments, but unlike the neuropilar glial cells in both the developing and adult AL that are close to or within a neuropil heavily invested with GABAergic processes, the MasGAT-positive glial cells in the SZ do not have an obvious major source of GABA. To date, no synapses have been identified in this region, even transiently, again ruling out a synaptic function. The antennal nerve does contain a small population of GABA-positive axons, though we do not yet know where their cell bodies reside. Although most of the GABA-positive axons eventually travel within the mechanosensory tract, part of their trajectory is through the SZ where they could release GABA from their growth cones. GABA has been shown to be released from isolated growth cones in a Ca++- and vesicleindependent manner that can be blocked by incubation in a GAT inhibitor (Taylor et al., 1990; Taylor and Gordon-Weeks, 1991), and GABA also can be released spontaneously by a vesicular mechanism from the growth cones of developing axons prior to synapse formation (Gao and van den Pol, 2000). GABA labeling during the early period of glomerulus development (Fig. 3) suggests the possibility that the dendrites of GABAergic AL neurons can extend transiently beyond the glomerular borders and into the nerve layer. If so, especially in the case of lateral glomeruli, these dendrites need extend only a short distance to reach into the SZ where they could serve as an additional source of GABA to the SZ glia residing there.
That most of the glial cells in the antennal lobe and in the SZ took up GABA when incubated in it was unexpected because the MasGAT labeling was so clearly limited to a rather small subset of the glial cells in either location. Under normal resting conditions, little or no GABA was detectable in any glial cells We cannot rule out the presence of an efficient GABA breakdown pathway within these glial cells that limits our ability to detect GABA in these cells except under the test conditions. It also is possible that GABA can be moved across the glial network as both SZ and neuropilar glial cells are extensively dye-coupled during roughly the first half of metamorphic adult development (Oland et al., 1999). This would limit the concentration detectable in any one cell when the extra-cellular level of GABA is low, and show widespread GABA labeling when a high extra-cellular concentration drives the transporter in one cell to take up GABA. The rather high percentage of glial cells that took up GABA in the cell culture experiments suggests however, that this can be only a partial explanation; although these glial cells do become coupled in vitro (Oland, unpublished), at the density at which they were plated, processes would not typically come in contact, reducing the likelihood of coupling. Some of the labeling may also have been non-specific despite the results of the GABA control condition.
The incubation experiment also revealed an increase in the intensity of GABA labeling among the AL neurons. Taking together this finding and the finding that the MasGAT antibody labeled at least some neurons during 5th-instar larval development and during the early metamorphic stages, it is possible that the neuronal uptake is simply the result of residual transporter expression. It also is reasonable to suspect that another GABA transporter, or alternatively spliced isoforms differentially distributed among neurons and glial cells, is yet to be found in M. sexta.
Finally, when we extended our developmental series back into the last larval instar to examine the idea that the blebby MasGAT-positive processes in the central neuropil of the AL at early stages of metamorphic development were the remnants of a larval population of MasGAT-positive neurons, the data were consistent with the hypothesis. The GABA transporter recognized by the MasGAT antibody was distributed mainly in AL neurons during the 5th instar and mainly in a subset of glial cells during metamorphic development and in the adult. We did not pursue the study to earlier larval times nor address the role of the transporter in the larval neurons as the current study was focused on understanding the pattern of labeling in the glial cells of the metamorphosing AL, but developmental roles for neuronal GATs could reflect the need for developing systems to carefully regulate extracellular GABA because of GABA’s effects on many developmental processes, including morphogensis, regulation of cell proliferation, axonal growth and synapse formation (e.g., Ben-Ari, 2002; Owens and Kriegstein, 2002; Takayama and Inoue, 2005; Akerman and Cline, 2007; Huang, 2009). We do not know why the localization of the GABA transporter recognized by the MasGAT antibody in M. sexta changes from mainly neuronal to mainly glial during the period around the onset of metamorphosis. Nevertheless, the GABA uptake experiment suggests that the AL neurons continue to use a GABA transporter during metamorphosis to regulate GABA levels in the developing glomerular neuropil, and that this may be an important function especially in regions of the glomerular neuropil that are nearly devoid of GAT-bearing glial processes.
Summary
During development of the primary olfactory pathway in M. sexta, MasGAT is present in a subset of glial cells in the sorting zone region of the antennal nerve, in the nerve layer of the antennal lobe, and in the glial envelopes that surround the olfactory glomeruli. Labeling in the sorting zone disappears roughly coincident with the completion of receptor axon ingrowth while that in the nerve layer and in the glial envelopes persists into adulthood. Immuno-EM showed that MasGAT-positive glial processes in the neuropil of the antennal lobe had no specific spatial relationship to synapses. Incubation in GABA showed that both neurons and glial cells were capable of taking up GABA. Our data suggest that MasGAT in the sorting zone and in certain neuropil glial cells may have a role in regulating extracellular levels of GABA and that glia could interact with neurons via GABA to exert developmental effects on neurons.
Acknowledgments
The authors are grateful to: Patricia Jansma, for her advice on the immuno-EM and her expert management of the ARLDN Imaging Facility; Suzanne Mackzum and Margaret Marez for maintaining a robust M. sexta colony; Dr. Sarjeet Gill, UC-Riverside, for his generous gift of the MasGAT antibody; and to the members of our laboratory for many critical discussions of this work.
Footnotes
Funded by NIH R01DC008597.
Literature Cited
- Akerman CJ, Cline HT. Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci. 2007;30:382–389. doi: 10.1016/j.tins.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Sasaki H, Huerta PT, Estevez AG, Baker H, Cave JW. gamma-Aminobutyric acid-mediated regulation of the activity-dependent olfactory bulb dopaminergic phenotype. J Neurosci Res. 2009;87:2211–2221. doi: 10.1002/jnr.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbin G, Pollard H, Gaïarsa JL, Ben-Ari Y. Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons. Neurosci Lett. 1993;152:150–154. doi: 10.1016/0304-3940(93)90505-f. [DOI] [PubMed] [Google Scholar]
- Barker JL, Behar T, Li YX, Liu QY, Ma W, Maric D, Maric I, Schaffner AE, Serafini R, Smith SV, Somogyi R, Vautrin JY, Wen XL, Xian H. GABAergic cells and signals in CNS development. Perspect Dev Neurobiol. 1998;5:305–322. [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory acitons of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Tseeb V, Raggozzino D, Khazipov R, Gaiarsa JL. gamma-Aminobutyric acid (GABA): a fast excitatory transmitter which may regulate the development of hippocampal neurones in early postnatal life. Prog Brain Res. 1994;102:261–273. doi: 10.1016/S0079-6123(08)60545-2. [DOI] [PubMed] [Google Scholar]
- Bentley D, Caudy M. Pioneer axons lose directed growth after selective killing of guidepost cells. Nature. 1983;304:62–65. doi: 10.1038/304062a0. [DOI] [PubMed] [Google Scholar]
- Bermudez I, Bothan RP, Beadle DJ. High- and low-affinity uptake of amino acid transmitters in cultured neurons and muscle cells of the cockroach, Periplaneta Americana. Insect Biochem. 1988;18:242–262. [Google Scholar]
- Borden LA, Smith KE, Gustafson EL, Branchek TA, Weinshank RL. Cloning and expression of a betaine/GABA transporter from human brain. J Neurochem. 1995;64:977–984. doi: 10.1046/j.1471-4159.1995.64030977.x. [DOI] [PubMed] [Google Scholar]
- Borden LA, Smith KE, Hartig PR, Branchek TA, Weinshank RL. Molecular heterogeneity of the gamma-aminobutyric acid (GABA) transport system. Cloning of two novel high affinity GABA transporters from rat brain. J Biol Chem. 1992;267:21098–21104. [PubMed] [Google Scholar]
- Breer H, Heilgenberg H. Neurochemistry of GABAergic activities in the central nervous system of Locusta migratoria. J Comp Physiol A. 1985;157:343–354. [Google Scholar]
- Brookes N, Kettenmann H, Ransom BR. Neuroglia. 2nd edition Oxford University Press; NY: 2005. Mechanisms of solute transport in glia; pp. 163–176. [Google Scholar]
- Burg MG, Geng C, Guan Y, Koliantz G, Pak WL. Drosophila rosA gene, which when mutant causes aberrant photoreceptor oscillation, encodes a novel neurotransmitter transporter homologue. J Neurogenet. 1996;11:59–79. doi: 10.3109/01677069609107063. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA. Autoradiographic localization of 3H-gamma-aminobutyric acid uptake in the lamina ganglionaris of Musca and Drosophila. Z Zellforsch Mikrosk Anat. 1974;147:415–431. doi: 10.1007/BF00307474. [DOI] [PubMed] [Google Scholar]
- Chotard C, Salecker I. Neurons and glia: team players in axon guidance. Trends Neurosci. 2004;27:655–661. doi: 10.1016/j.tins.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Desai CJ, Popova E, Zinn K. A Drosophila receptor tyrosine phosphatase expressed in the embryonic CNS and larval optic lobes is a member of the set of proteins bearing the “HRP” carbohydrate epitope. J Neurosci. 1994;14:7272–7283. doi: 10.1523/JNEUROSCI.14-12-07272.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGregorio DA, Nusser Z, Silver RA. Spillover of glutamate onto synaptic AMPA receptors enhances fast transmission at a cerebellar synapse. Neuron. 2002;35:521–533. doi: 10.1016/s0896-6273(02)00787-0. [DOI] [PubMed] [Google Scholar]; Neuron. 2002 Sep 26;36:187. Erratum in: [Google Scholar]
- Fabini G, Freilinger A, Altmann F, Wilson IBH. Identification of core alpha 1,3-fucosylated glycans and cloning of the requisite fucosyltransferase cDNA from Drosophila melanogaster. Potential basis of the neural anti-horseadish peroxidase epitope. J Biol Chem. 2001;276:28058–28067. doi: 10.1074/jbc.M100573200. [DOI] [PubMed] [Google Scholar]
- Gadea A, López-Colomé AM. Glial transporters for glutamate, glycine, and GABA: II. GABA transporters. J Neuroscience Research. 2001;63:461–468. doi: 10.1002/jnr.1040. [DOI] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. GABA release from mouse axonal growth cones. J Physiol. 2000;523:629–637. doi: 10.1111/j.1469-7793.2000.t01-1-00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, McLean H, Caveney S, Donly C. Molecular cloning and functional characterization of a GABA transporter from the CNS of the cabage looper, Trichoplusia ni. Insect Biochem Mol Biol. 1999;29:609–623. doi: 10.1016/s0965-1748(99)00039-9. [DOI] [PubMed] [Google Scholar]
- Gascon E, Dayer AG, Sauvain M-O, Potter G, Jennoy B, DeRoo M, Zgraggen E, Demaurex N, Muller D, Kiss JZ. GABA regulates dendritic growth by stabilizing lamellipodia in newly generated interneurons of the olfactory bulb. J Neurosci. 2006;26:12956–12966. doi: 10.1523/JNEUROSCI.4508-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin BA, Arruda SE, Dolph PJ. The role of carcinine in signaling at the Drosophila photoreceptor synapse. PLoS Genet. 2007;3:e206. doi: 10.1371/journal.pgen.0030206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson NJ, Rössler W, Nighorn AJ, Oland LA, Hildebrand JG, Tolbert LP. Neuron-glia communication via nitric oxide is essential in establishing antennal-lobe structure in Manduca sexta. Dev Biol. 2001;240:326–339. doi: 10.1006/dbio.2001.0463. [DOI] [PubMed] [Google Scholar]
- Gibson NJ, Tolbert LP. Activation of epidermal growth factor receptor mediates receptor axon sorting and extension in the developing olfactory system of the moth Manduca sexta. J Comp Neurol. 2006;495:554–572. doi: 10.1002/cne.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella J, Nelson N, Nelson H, Czyzyk L, Keynan S, Miedel MC, Davidson N, Lester HA, Kanner BI. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Higgins MR, Gibson NJ, Eckholdt PA, Nighorn A, Copenhaver PF, Nardi J, Tolbert LP. Different isoforms of fasciclin II are expressed by a subset of developing olfactory receptor neurons and by olfactory-nerve glial cells during formation of glomeruli in the moth Manduca sexta. Dev Biol. 2002;244:134–154. doi: 10.1006/dbio.2002.0583. [DOI] [PubMed] [Google Scholar]
- Hildebrand JG, Hall LM, Osmond BC. Distribution of binding sites for 125I-labeled alpha-bungarotoxin in normal and deafferented antennal lobes of Manduca sexta. Proc Natl Acad Sci U S A. 1979;76:499–503. doi: 10.1073/pnas.76.1.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg U, Hildebrand JG. Postembryonic development of gamma-aminobutyric acid-like immunoreactivity in the brain of the sphinx moth Manduca sexta. J Comp Neurol. 1994;339:132–149. doi: 10.1002/cne.903390112. [DOI] [PubMed] [Google Scholar]
- Homberg U, Kingan TG, Hildebrand JG. Distribution of FMRFamide-like immunoreactivity in the brain and suboesophageal ganglion of the sphinx moth Manduca sexta and colocalization with SCPB-, BPP-, and GABA-like immunoreactivity. Cell Tissue Res. 1990;259:401–419. doi: 10.1007/BF01740767. [DOI] [PubMed] [Google Scholar]
- Hoskins SG, Homberg U, Kingan TG, Christensen TA, Hildebrand JG. Immunocytochemistry of GABA in the antennal lobes of the sphinx moth Manduca sexta. Cell Tiss Res. 1986;244:243–252. doi: 10.1007/BF00219199. [DOI] [PubMed] [Google Scholar]
- Huang ZJ. Activity-dependent development of inhibitory synapses and innervation pattern: role of GABA signaling and beyond. J Physiol. 2009;587:1881–1888. doi: 10.1113/jphysiol.2008.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Huang Y, Chinnappan R, Bocchini C, Gustin MC, Stern M. The Drosophila inebriated-encoded neurotransmitter/osmolyte transporter: dual roles in the control of neuronal excitability and the osmotic stress response. Genetics. 2002;160:561–569. doi: 10.1093/genetics/160.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Solís JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Iversen LL, Kravitz EA. The metabolism of gamma-aminobutyric acid (GABA) in the lobster nervous system--uptake of GABA in the nerve-muscle preparations. J Neurochem. 1968;15:609–620. doi: 10.1111/j.1471-4159.1968.tb08960.x. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. PNAS (USA) 1982;79:2700–2704. doi: 10.1073/pnas.79.8.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Nighorn A. Interaxonal Eph-ephrin signaling may mediate sorting of olfactory sensory axons in Manduca sexta. J Neurosci. 2003;23:11523–11538. doi: 10.1523/JNEUROSCI.23-37-11523.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz F, Moats W, Jan YN. A carbohydrate epitope expressed uniquely on the cell surface of Drosophila neurons is altered in the mutant nac (neurally altered carbohydrate) EMBO J. 1988;7:3471–3477. doi: 10.1002/j.1460-2075.1988.tb03222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto M, Ohno K, Kuriyama K, Kubo T, Sato K. Developmental changes in GABA transporter (GAT1 and GAT3) mRNA expressions in the rat olfactory bulb. Devel Br Res. 2001;126:137–145. doi: 10.1016/s0165-3806(00)00137-1. [DOI] [PubMed] [Google Scholar]
- Kent KS, Harrow ID, Quartararo P, Hildebrand JG. An accessory olfactory pathway in Lepidoptera: the labial pit organ and its central projections in Manduca sexta and certain other sphinx and silk moths. Cell Tissue Res. 1986;245:237–245. doi: 10.1007/BF00213927. [DOI] [PubMed] [Google Scholar]
- Kent KS, Hoskins SG, Hildebrand JG. A novel serotonin-immunoreactive neuron in the antennal lobe of the sphinx moth Manduca sexta persists throughout postembryonic life. J Neurobiol. 1987;18:451–465. doi: 10.1002/neu.480180506. [DOI] [PubMed] [Google Scholar]
- Kreissl S, Bicker G. Dissociated neurons of the pupal honeybee brain in cell culture. J Neurocytol. 1992;21:545–556. doi: 10.1007/BF01187116. [DOI] [PubMed] [Google Scholar]
- Leal SM, Neckameyer WS. Pharmacological evidence for GABAergic regulation of specific behaviors in Drosophila melanogaster. J Neurobiol. 2002;50:245–261. doi: 10.1002/neu.10030. [DOI] [PubMed] [Google Scholar]
- Mallory HS, Nighorn AJ, Hayshi JH, Oland LA. Neuropil glia and interneurons in the olfactory (antennal) lobe of the moth Manduca sexta respond to GABA during early glomerulus development. Soc NSci Abstr. 2005 [Google Scholar]
- Marcaggi P, Attwell D. Role of glial amino acid transporters in synaptic transmission and brain energetics. Glia. 2004;47:217–225. doi: 10.1002/glia.20027. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Billups D, Attwell D. The role of glial glutamate transporters in maintaining the independent operation of juvenile mouse cerebellar parallel fibre synapses. J Physiol. 2003;552(Pt 1):89–107. doi: 10.1113/jphysiol.2003.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle J, Piek T, Lammertse T, Lind A, Van Weeren-Kramer J. Selectivity of the uptake of glutamate and GABA in two morphologically distinct insect neuromuscular synapses. Brain Res. 1985;348:107–111. doi: 10.1016/0006-8993(85)90365-8. [DOI] [PubMed] [Google Scholar]
- Mbungu D, Ross LS, Gill SS. Cloning, functional expression, and pharmacology of a GABA transporter from Manduca sexta. Arch Biochem Biophys. 1995;318:489–497. doi: 10.1006/abbi.1995.1258. [DOI] [PubMed] [Google Scholar]
- Minelli A, Brecha NC, Karschin C, DeBiasi S, Conti F. GAT-1, a high-affinity GABA plasma membrane transporter, is localized to neurons and astroglia in the cerebral cortex. J Neurosci. 1995;15:7734–7746. doi: 10.1523/JNEUROSCI.15-11-07734.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer WS, Cooper RL. GABA transporters in Drosophila melanogaster: molecular cloning, behavior, and physiology. Invert Neurosci. 1998;3:279–294. doi: 10.1007/BF02577688. [DOI] [PubMed] [Google Scholar]
- Oland LA, Gibson NJ, Pearson JT, Tolbert LP. Neuroglian and FGFR interactions in development of the glia-rich axon sorting zone in the moth olfactory pathway. International Symposium Olfact Taste. 2008 Abstr#97. [Google Scholar]
- Oland LA, Marrero HG, Burger I. Glial cells in the developing and adult olfactory lobe of the moth Manduca sexta. Cell Tissue Res. 1999;297:527–545. doi: 10.1007/s004410051379. [DOI] [PubMed] [Google Scholar]
- Oland LA, Müller T, Kettenmann H, Hayashi J. Preparation of primary cultures and acute slices of the nervous system of the moth Manduca sexta. J NSci Methods. 1996;69:103–112. doi: 10.1016/S0165-0270(96)00025-8. [DOI] [PubMed] [Google Scholar]
- Oland LA, Orr G, Tolbert LP. Construction of a protoglomerular template by olfactory axons initiates the formation of olfactory glomeruli in the insect brain. J Neurosci. 1990;10:2096–2112. doi: 10.1523/JNEUROSCI.10-07-02096.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oland LA, Pott WM, Higgins MR, Tolbert LP. Targeted ingrowth and glial relationships of olfactory receptor axons in the primary olfactory pathway of an insect. J Comp Neurol. 1998;398:119–138. [PubMed] [Google Scholar]
- Oland LA, Tolbert LP. Glial patterns during early development of antennal lobes of Manduca sexta: a comparison between normal lobes and lobes deprived of antennal axons. J Comp Neurol. 1987;255:196–207. doi: 10.1002/cne.902550204. [DOI] [PubMed] [Google Scholar]
- Orkand PM, Kravitz EA. Localization of the sites of gamma-aminobutyric acid (GABA) uptake in lobster nerve-muscle preparations. J Cell Biol. 1971;49:75–89. doi: 10.1083/jcb.49.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Wu YC, Trussell LO. Delayed clearance of transmitter and the role of glutamate transporters at synapses with multiple release sites. J Neurosci. 1996;16:1634–1644. doi: 10.1523/JNEUROSCI.16-05-01634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet LS, Westbrook GL. Synapse density regulates independence at unitary inhibitory synapses. J Neurosci. 2003;23:2618–2626. doi: 10.1523/JNEUROSCI.23-07-02618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Paschinger K, Rendic D, Lochnit G, Jantsch V, Wilson IB. Molecular basis of anti-horseradish peroxidase staining in Caenorhabditis elegans. J Biol Chem. 2004;279:49588–49598. doi: 10.1074/jbc.M408978200. [DOI] [PubMed] [Google Scholar]
- Priest CA, Puche AC. GABAB receptor expression and function in olfactory receptor neuron axon growth. J Neurobio. 2004;60:154–165. doi: 10.1002/neu.20011. [DOI] [PubMed] [Google Scholar]
- Radian R, Ottersen OP, Storm-Mathisen J, Castel M, Kanner BI. Immunocytochemical localization of the GABA transporter in rat brain. J Neurosci. 1990;10:1319–1330. doi: 10.1523/JNEUROSCI.10-04-01319.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Tong WM, Brecha NC. GABA plasma membrane transporters, GAT-1 and GAT-3, display different distributions in the rat hippocampus. J Comp Neurol. 1996;367:595–606. doi: 10.1002/(SICI)1096-9861(19960415)367:4<595::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wu Y. The dynamic equilibrium of neurotransmitter transporters: Not just for reuptake anymore. J Neurophysiol. 2003;90:1363–1374. doi: 10.1152/jn.00317.2003. [DOI] [PubMed] [Google Scholar]
- Romero-Calderón R, Uhlenbrock G, Borycz J, Simon AF, Grygoruk A, Yee SK, Shyer A, Ackerson LC, Maidment NT, Meinertzhagen IA, Hovemann BT, Krantz DE. A glial variant of the vesicular monoamine transporter is required to store histamine in the Drosophila visual system. PLoS Genet. 2008;4:e1000245. doi: 10.1371/journal.pgen.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössler W, Oland LA, Higgins MR, Hildebrand JG, Tolbert LP. Development of a glia-rich axon-sorting zone in the olfactory pathway of the moth Manduca sexta. J Neurosci. 1999;19:9865–9877. doi: 10.1523/JNEUROSCI.19-22-09865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakatani K, Black JA, Kocsis JD. Transient presence and functional interaction of endogenous GABA and GABAA receptors in developing rat optic nerve. Proc Biol Sci. 1992;247:155–161. doi: 10.1098/rspb.1992.0022. [DOI] [PubMed] [Google Scholar]
- Shepherd D, Tyrer NM. Inhibition of GABA uptake potentiates the effects of exogenous GABA on locust skeletal muscle. Comp Biochem Physiol C. 1985;82:315–321. doi: 10.1016/0742-8413(85)90169-0. [DOI] [PubMed] [Google Scholar]
- Sinakevitch I, Farris SM, Strausfeld NJ. Taurine-, aspartate- and glutamate-like immunoreactivity identifies chemically distinct subdivisions of Kenyon cells in the cockroach mushroom body. J Comp Neurol. 2001;439:352–367. doi: 10.1002/cne.1355. [DOI] [PubMed] [Google Scholar]
- Snow PM, Patel NH, Harrelson AL, Goodman CS. Neural-specific carbohydrate moiety shared by many surface glycoproteins in Drosophila and grasshopper embryos. J Neurosci. 1987;7:4137–4144. doi: 10.1523/JNEUROSCI.07-12-04137.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehnge H, Huang X, Becker M, Whitley P, Conover D, Stern M. A neurotransmitter transporter encoded by the Drosophila inebriated gene. Proc Natl Acad Sci U S A. 1996;93:13262–13267. doi: 10.1073/pnas.93.23.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soustelle L, Besson MT, Rival T, Birman S. Terminal glial differentiation involves regulated expression of the excitatory amino acid transporters in the Drosophila embryonic CNS. Dev Biol. 2002;248:294–306. doi: 10.1006/dbio.2002.0742. [DOI] [PubMed] [Google Scholar]
- Spoerri PE. Neurotrophic effects of GABA in cultures of embryonic chick brain and retina. Synapse. 1988;2:11–22. doi: 10.1002/syn.890020104. [DOI] [PubMed] [Google Scholar]
- Stern M, Ganetzky B. Identification and characterization of inebriated, a gene affecting neuronal excitability in Drosophila. J Neurogenet. 1992;8:157–172. doi: 10.3109/01677069209083445. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ. Atlas of an insect brain. Springer; New York: 1976. [Google Scholar]
- Sun B, Salvaterra PM. Characterization of Nervana, a Drosophila melanogaster neuron-specific glycoprotein antigen recognized by anti-horseradish peroxidase antibodies. J Neurochem. 1995;65:434–443. doi: 10.1046/j.1471-4159.1995.65010434.x. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Tolbert LP, Hildebrand JG. Synaptic organization of the uniglomerular projection neurons of the antennal lobe of the moth Manduca sexta: a laser scanning confocal and electron microscopic study. J Comp Neurol. 1997;379:2–20. doi: 10.1002/(sici)1096-9861(19970303)379:1<2::aid-cne2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Takayama C, Inoue Y. Developmental expression of GABA transporter-1 and 3 during formation of the GABAergic synapses in the mouse cerebellar cortex. Dev Br Res. 2005;158:41–49. doi: 10.1016/j.devbrainres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Takayasu Y, Iino M, Shimamoto K, Tanaka K, Ozawa S. Glial glutamate transporters maintain one-to-one relationship at the climing fiver-Purkinje cell synapse by preventing glutamate spillover. J Neuroscience. 2006;26:6563–6572. doi: 10.1523/JNEUROSCI.5342-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Docherty M, Gordon-Weeks PR. GABAergic growth cones: release of endogenous gamma-aminobutyric acid precedes the expression of synaptic vesicle antigens. J Neurochem. 1990;54:1689–1699. doi: 10.1111/j.1471-4159.1990.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Taylor J, Gordon-Weeks PR. Calcium-independent gamma-aminobutyric acid release from growth cones: role of gamma-aminobutyric acid transport. J Neurochem. 1991;56:273–280. doi: 10.1111/j.1471-4159.1991.tb02592.x. [DOI] [PubMed] [Google Scholar]
- Thimgan MS, Berg JS, Stuart AE. Comparative sequence analysis and tissue localization of members of the SLC6 family of transporters in adult Drosophila melanogaster. J Experimental Biol. 2006;209:3383–3404. doi: 10.1242/jeb.02328. [DOI] [PubMed] [Google Scholar]
- Tolbert LP, Gibson NJ, Oland LA. A GABA transporter is expressed by subsets of glial cells in the developing and adult olfactory lobe of Manduca sexta. Soc NSci. 2008 Abstr #625.17. [Google Scholar]
- Tolbert LP, Hildebrand JG. Organization and synaptic ultrastructure of glomeruli in the antennal lobes of the moth Manduca sexta: A Study using thin sections and freeze-fracture. Proc Roy Soc Lond [Biol] 1981;213:279–301. [Google Scholar]
- Tolbert LP, Matsumoto SG, Hildebrand JG. Development of synapses in the antennal lobes of the moth Manduca sexta during metamorphosis. J Neurosci. 1983;3:1158–1175. doi: 10.1523/JNEUROSCI.03-06-01158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert LP, Oland LA, Tucker ES, Gibson NJ, Higgins MR, Lipscomb BW. Bidirectional influences between neurons and glial cells in the developing olfactory system. Prog Neurobiol. 2004;73:73–105. doi: 10.1016/j.pneurobio.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Tucker ES, Oland LA, Tolbert LP. in vitro analyses of interactions between olfactory receptor growth cones and glial cells that mediate axon sorting and glomerulus formation. J Neurobio. 2004;56:24–40. doi: 10.1002/cne.20058. [DOI] [PubMed] [Google Scholar]
- Tucker ES, Tolbert LP. Reciprocal interactions between olfactory receptor axons and olfactory nerve glia cultured from the developing moth Manduca sexta. Dev Biol. 2003;260:9–30. doi: 10.1016/s0012-1606(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Umesh A, Gill SS. Immunocytochemical localization of a Manduca sexta gamma-aminobutyric acid transporter. J Comp Neurol. 2002;448:388–398. doi: 10.1002/cne.10271. [DOI] [PubMed] [Google Scholar]
- Weeks JC, Levine RB. Steroid hormone effects on neurons subserving behavior. Curr Opin Neurobiol. 1995;5:809–815. doi: 10.1016/0959-4388(95)80110-3. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang W, Díez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron. 2007;56:851–865. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager J, Richards S, Hekmat-Scafe DS, Hurd DD, Sundaresan V, Caprette DR, Saxton WM, Carlson JR, Stern M. Control of Drosophila perineurial glial growth by interacting neurotransmitter-mediated signaling pathways. Proc Natl Acad Sci U S A. 2001;98:10445–10450. doi: 10.1073/pnas.191107698. [DOI] [PMC free article] [PubMed] [Google Scholar]