INTRODUCTION
The collection of chapters in this 55th Nebraska Symposium on Motivation Volume clearly highlights that effective strategies for reducing compulsive tobacco use will require a multi-faceted approach in which genetic, neurobiological, individual, and cultural factors are considered. It is difficult, if not impossible, to predict where the next important breakthrough will come from (Bevins & Bardo, 2004; Dethier, 1966; Laidler, 1998). Accordingly, further research that extends and challenges current theory and practice at each of these levels of analysis is needed. The continuing focus of our research program, and the topic of the present chapter, is on the role of Pavlovian conditioning processes involving nicotine. Theoretical and empirical approaches to nicotine dependence that include Pavlovian conditioning processes have lead to important advances in our understanding and treatment of chronic tobacco use (e.g., see Rose, Chapter 8 and Tiffany, Warthen, & Goedecker, Chapter 10 in current Volume). These approaches conceptualize the drug as an unconditioned stimulus (US) or reinforcer. That is, the pharmacological effects of the drug (e.g., reward, analgesia, psychomotor stimulation) enter into an association with stimuli that reliably co-occur with these effects (e.g., paraphernalia, situational cues). Later exposure to these conditioned stimuli (CSs) can evoke conditioned responses (CRs) that increase the chances an individual will seek drug.
More recently, we have suggested that the interoceptive stimulus effects of nicotine might also serve as a CS for other appetitive non-drug outcomes (i.e., USs) and/or a stimulus that occasions whether other CS-US associations will or will not occur (i.e., an occasion setter or facilitator; see Bevins & Palmatier, 2004). We have further suggested that such an associative learning history could impact the tenacity of nicotine addiction—e.g., shorten the time between experimentation and dependence, increase the difficulty of quitting, make sustaining abstinence more difficult, etc. At the current time these suggestions are speculative. With this in mind, the present chapter will review the research in this area, as well as highlight some of its historical precursors and suggest some possible future directions for research. In doing so, hopefully the reader will gain an appreciation for how this approach might lead to further insight into how Pavlovian conditioning processes can alter the motivational function of nicotine in a manner that contributes to chronic tobacco use.
NICOTINE AS A REINFORCER
Most of the research examining the impact of conditioning processes with nicotine has conceptualized nicotine as a reinforcer. For the purposes of the current discussion we mean reinforcer in the sense that both Pavlov (1927) and Skinner (1938) used the term. For Pavlov (1927), reinforcer was used interchangeably with unconditioned stimulus. To quote an example from Pavlov (1927), “Tactile stimulation of the skin is used as a conditioned stimulus for acid. The conditioned stimulus is allowed to act for a period of 3 minutes and is then reinforced, being still continued so as to overlap the action of the acid” [p. 93 (italics added)]. According to this framework, exteroceptive cues that occur in close temporal and spatial relation with tobacco use have the potential to function as conditional stimuli and enter into an association with nicotine (i.e., the reinforcer or US). As a result of this conditioning, a CS acquires the ability to evoke or modify a response. The nature of this CR tends to be more readily predicted from a behavior systems/evolutionary approach to associative learning (cf. Domjan, 2005; Timberlake, 1994). In very general terms, stimuli paired with an appetitive US tend to produce approach and search related CRs along with more US-specific behaviors. In contrast, stimuli paired with an aversive US will come to evoke avoidance and/or anti-predator behaviors. Translated to smoking, stimuli such as throat irritation and smell of cigarette smoke, sight of the cigarette, lighter and ashtray, smoking/work break areas, and/or smoking companions reliably co-occur with the physiological effects of the nicotine US. In smokers, these stimuli come to control changes in reported cravings and urges, as well as a variety of changes in more physiological measures such as heart rate and galvanic skin response (e.g., Geier, Mucha, & Pauli, 2000; Lazev, Herzog, & Brandon, 1999; Pritchard, Robinson, Guy, Davis, & Stiles, 1996; Rose & Levin, 1991; see Tiffany et al., Chapter 10, in this Volume).
To better study the necessary and sufficient conditions for acquisition and expression of nicotine conditioned responding, researchers have developed several preclinical animal models (see Bevins & Palmatier, 2004 for a review). Perhaps the two most widely studied tasks are locomotor conditioning (Bevins, Besheer, & Pickett, 2001; Bevins & Palmatier, 2003; Bevins, Eurek, & Besheer, 2005; Palmatier & Bevins, 2002; Walter & Kuschinsky, 1989) and place conditioning (Grabus, Martin, Brown, & Damaj, 2006; Le Foll & Goldberg, 2005; Shoaib, Stolerman, & Kumar, 1994; see Brunzell & Picciotto, Chapter 3, in this Volume). As an example, in the locomotor conditioning task rats (and less often mice) receive a distinct environment (i.e., a context CS) paired with the psychomotor effect of nicotine. After repeated pairings of the context CS with the nicotine US, the context CS, in the absence of nicotine (i.e., CS-alone test) evokes an increase in activity relative to controls that receive equal exposure to the nicotine and the context in an unpaired fashion. Although a detailed review of this research is tangential to the goal of the present article, we know that acquisition of conditioned hyperactivity is sensitive to nicotine dose (US magnitude), temporal relation between the CS and US (interstimulus interval), and presentation of other excitatory CSs (Bevins et al., 2001; Bevins & Palmatier, 2004; Bevins et al., 2005). Notably, Pavlov (1927) reported that acquisition of conditioned salivation was affected by similar behavioral factors.
For Skinner (1938), “The operation of reinforcement is defined as the presentation of a certain kind of stimulus in a temporal relation with either a stimulus or response. A reinforcing stimulus is defined as such by its power to produce the resulting change” (p. 62). This definition encompasses that of Pavlov’s stimulus-reinforcer relations and extends it to include behavior-reinforcer relations. Current behavioral researchers, albeit not exclusively, tend to use the term reinforcer or reinforcement to refer to the latter relation. As discussed in detail by Caggiula and colleagues in this Volume (Chapter 6) the direct positive reinforcing effects of nicotine, in conjunction with its reinforcer enhancing properties, are important for acquisition and maintenance of tobacco use [see also Chapter 5 by Markou and colleagues that provides a thoughtful discussion of how the removal or avoidance of a withdrawal state (negative reinforcement) also contributes to continued tobacco use].
An instrumental response (e.g., lever press) followed by intravenous (IV) nicotine can maintain and/or increase the frequency of that response (Corrigall & Coen, 1989; Donny, Caggiula, Mielke, Jacobs, Rose, & Sved, 1998). This preclinical self-administration model is one of the most widely used to study the reinforcing effects of abused drugs, including nicotine. In our laboratory, we have recently established nicotine self-administration in rats. Briefly, rats were surgically prepared with an IV catheter following a lever press autoshaping protocol with sucrose designed to engender a high operant level on both levers before starting the self-administration phase. The initiation of daily 1 h self-administration session was signaled by onset of the houselights and insertion of both levers. If the rat pressed the active lever, the levers were immediately withdrawn and nicotine was infused across 1 sec; illumination of the cue lights above each lever signaled the infusion. After a 60-sec timeout, the levers were reinserted. Notably, the house light remained on during the timeout. Inactive lever presses were recorded, but did not have any programmed consequence. Rats were started on 0.06 mg base/kg/infusion of nicotine and then switched to 0.03 mg base/kg/infusion. Figure 7.1 shows the active and inactive responses for each rat across the acquisition phase. All rats pressed more on the active than the inactive lever by the end of training with the 0.06 mg/kg dose of nicotine. This difference was enhanced when the dose was dropped to 0.03 mg/kg nicotine suggesting that rats were sensitive to the dose of nicotine in this protocol (Figure 7.1). This point was further supported by each rat’s behavior during a subsequent extinction phase where saline replaced nicotine as the infused solution; all remaining procedural details remained the same. That is, all rats increased presses on the inactive lever on the first day of extinction (3 to 23, 0 to 10, and 0 to 3 for rats 4897, 4990, and 4991, respectively; data not shown). Additionally, active lever responding on average decreased across repeated extinction sessions.
Figure 7.1.
Each panel shows data for a rat in a nicotine self-administration experiment conducted by Jennifer Murray in my laboratory. The main narrative includes a description of the procedures. All rats readily self-administered nicotine as indicated by more responding on the active (nicotine) than inactive lever.
NICOTINE AS A DISCRIMINATIVE STIMULUS
As described in the previous section, nicotine is clearly able to function as reinforcer. This conceptualization and its theoretical extensions have lead to many important advances in our understanding of the addictive qualities of nicotine involved in tobacco addiction. Also contributing to our understanding of tobacco use and addiction is the research on the discriminative stimulus (SD) effect of nicotine. That is, the pharmacological action of nicotine on the nervous system has perceptible interoceptive effects that can gain stimulus control over instrumental responding. Studying nicotine as a SD has provided important insight into behavioral and neuropharmacological processes underlying the subjective effects of nicotine (e.g., Damaj, Creasy, Grove, Rosecrans, & Martin, 1994; Damaj, Creasy, Welch, Rosecrans, Aceto, & Martin, 1995; Perkins, DiMarco, Grobe, Scierka, & Stiller, 1994; Stolerman, 1989; see Perkins, Chapter 9, in this Volume).
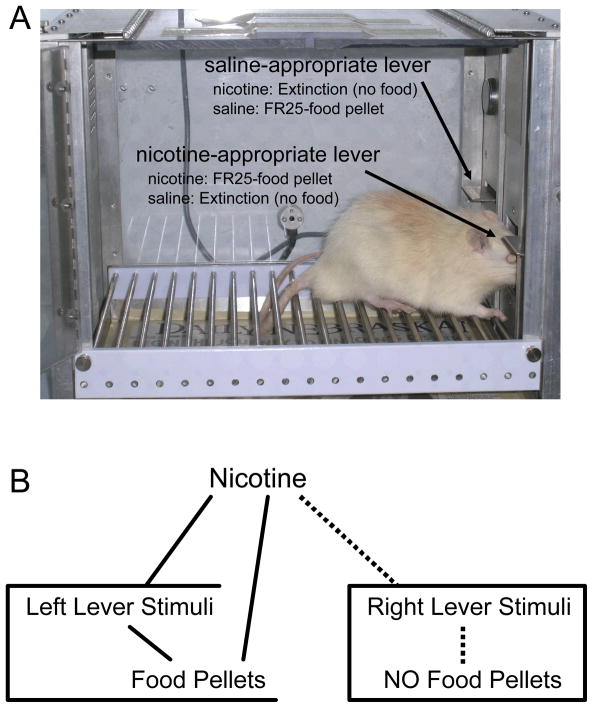
Of interest for the present discussion is the two-lever operant drug discrimination task widely used by behavioral pharmacologists to study the SD effects of nicotine in rodents (see Figure 7.2A). In this example, on sessions (days) when nicotine is administered presses on the right lever will be reinforced with a food pellet after a fixed ratio (FR) 25 schedule is completed. At the same time, nicotine occasions non-reinforcement (i.e., extinction) of left lever presses. On saline sessions, the schedules are reversed. Left lever presses are reinforced on an FR25 and right lever presses are now under extinction. With sufficient training, nicotine functions as a SD/SΔ as evidenced by better than 80% responding on the drug-appropriate lever before any reinforcer is delivered—right lever on nicotine sessions and left lever for saline sessions.
Figure 7.2.
Panel A shows a typical conditioning chamber set up to conduct two-lever operant drug discrimination. The text associated with each arrow describes the response contingency in force under a prototypical drug discrimination experiment using nicotine and saline as the injected solutions to be discriminated. Panel B shows hypothetical stimulus associations that are imbedded within the response contingencies of an operant drug discrimination study. Although only a nicotine session is shown for simplicity sake, it is clear that there are many direct and higher-order associations possible (see narrative for more detail).
In contrast to neuropharmacological processes, potential behavioral (conditioning) processes involved in this discrimination have not been as well studied. As described in the previous paragraph, the interoceptive effects of nicotine simultaneously function to occasion responding (SD) as well as inhibit responding (SΔ). Figure 7.2B diagrams some of the additional associative structures that could be of empirical and theoretical interest. For simplicity sake this diagram just shows a nicotine session and does not include the instrumental response (i.e., only stimuli are diagramed). Notably, the SD and SΔ function of nicotine are associated with different stimuli such as spatial location of the right versus left lever. Thus, on nicotine sessions exteroceptive and proprioceptive stimuli affiliated with the right lever are paired with food; stimuli associated with the left lever are not. Further, the interoceptive stimulus effects of nicotine are paired with intermittent access to food. From a broadly defined conditioning perspective, a glance at these potential associative structures described in Figure 7.2B prompts several important questions. For example, does nicotine function as a contextual stimulus and acquire conditioned reinforcing value by being paired with food pellets? If so, does this contribute to discrimination performance? Alternatively, perhaps nicotine functions as a negative and/or positive facilitator (occasion setter) that disambiguates the stimulus relation between the lever stimuli and availability of food. On this latter point, observations of a well-trained rat will reveal that it engages in many food-related behaviors such as gnawing, licking, and/or nosing the lever while performing the instrumentally-trained response (Bevins, 2001; Peterson, Ackil, Frommer, & Hearst, 1972; see also Kintsch & Witte, 1962). They also display goal-tracking behavior such as orienting and moving to the food trough or dipper (Bevins, 2001; Farwell & Ayres, 1979). Such behaviors indicate acquisition of a lever CS–food US association and suggest that the pharmacological effects of nicotine are likely occasioning that the lever stimuli will be paired with food.
The discussion in the previous paragraph is not meant to imply that the response-reinforcer relation is not an important variable in operant drug discrimination with nicotine or any other drug. Indeed, the schedule of reinforcement has been shown to alter acquisition and generalization in a two-lever drug discrimination task with nicotine (e.g., Stolerman, 1989). Rather, this discussion is meant to highlight that there are many relatively complex stimulus-stimulus and stimulus-reinforcer (i.e., Pavlovian) relations embedded in the task that could also affect the functioning of nicotine as an interoceptive stimulus. In fact, Pavlovian relations covary with the response-reinforcer relations and might as readily account for changes in the discriminative qualities of nicotine with changes in the reinforcement schedule. Given the importance of Pavlovian conditioning processes in nicotine addiction prescribed by theorists and researchers (e.g., Bevins & Palmatier, 2004; Conklin & Tiffany, 2002; Geier et al., 2000; Henningfield, Schuh, & Jarvik, 1995; Lazev et al., 1999; Rose & Levin, 1991; see Tiffany et al., Chapter 10, in this Volume), there is surprisingly little research investigating the interoceptive stimulus effects of nicotine from this theoretical perspective.
INTEROCEPTIVE PAVLOVIAN CONDITIONING: A HISTORICAL FRAMEWORK
There has been a long history in the Pavlovian conditioning field of studying interoceptive stimuli as CSs. The early research was interested in stimulation of the viscera (stomach, intestine) or brain as the CS (Bykov, 1957; Doty, 1961; Loucks, 1938). For example, Bykov prepared a dog surgically so that water flowed in and then out of the stomach (i.e., the interoceptive CS). This irrigation of the stomach, which produced very little salivation alone, was then paired with access to meat powder and bread US. As described by Bykov (1957), “After several such combinations we found that if water was allowed to flow into the stomach 20 seconds in advance of the reinforcement, the irrigation alone caused the dog to start licking its lips and turning its head to the food box while there was a copious salivary secretion” (p. 249). This example is especially notable given our interest in Pavlovian appetitive conditioning using interoceptive stimuli produced by drug states (see later). That is, Bykov’s dog displayed food-related CRs to the interoceptive CS that included licking lips and salivation (see also Pavlov, 1927), as well as sign/goal tracking (i.e., turning toward food box).
The study of interoceptive stimuli was later extended to the peripheral administration of ligands (e.g., Cook, Davidson, Davis, & Kelleher, 1960). Of particular relevance to the present discussion is the extension of this research to the pharmacological effects of abused drugs. This type of research can be categorized as either drug–drug conditioning or drug–non-drug US conditioning. A recent example of drug–drug conditioning comes from Shepard Siegel’s laboratory (e.g., Kim, Siegel, & Patenall, 1999; Sokolowska, Siegel, & Kim, 2002) investigating the ability of the early pharmacological effects of morphine (early onset cues) to serve as a CS for its later, more profound, analgesic effects in rats (for similar research with ethanol see Greeley, Lê, Poulos, & Cappell, 1984). Other drug–drug conditioning research has used one drug as the CS for the later delivery of a different drug (e.g., Revusky, Davey, & Zagorski, 1989). In drug–non-drug US conditioning, the drug state serves as the CS for delivery of a non-pharmacological US. A well-controlled example of this under studied area was conducted by Bormann and Overton (1993). In that conditioned suppression experiment rats had an IP injection of morphine repeatedly paired with a foot-shock US. Relative to controls, the morphine CS came to evoke a conditioned fear response as measured by drink suppression. Turner and Altshuler (1976) reported a similar result in rats using amphetamine as the CS and a decrease in lever pressing as the measure of conditioned fear.
NICOTINE AS AN INTEROCEPTIVE CS
Until recent research from our laboratory (see also Troisi, 2006), there has been very little research directly assessing the role of nicotine as a CS. A notable exception to this statement is a study in humans by Clements, Glautier, Stolerman, White, and Taylor (1996). Clements and colleagues, inspired by some of the early drug–drug conditioning research with rats, sought to test whether nicotine could function as a CS for an ethanol US. In that study, one set of smokers received 8 conditioning sessions. On half the sessions, a subcutaneous injection of nicotine (0.6 mg) into the upper arm was followed by a drink containing 9.4% alcohol. For the remaining sessions, a saline injection was followed by a placebo drink that used the same base as in the nicotine sessions (i.e., red angostura). Measures of conditioning included skin conductance, inter-beat interval of the heart, as well as mood/urge ratings. In summarizing their results Clements et al. (1996) concluded that “the study provided inconclusive evidence for the ability of one drug to act as a CS for the presentation of another in human subjects” (p. 94).
In retrospect, the lack of evidence for conditioning to the nicotine CS was not surprising for several reasons. For example, Clements and colleagues acknowledged the route and dose of nicotine may not have been sufficiently salient, or the proper temporal dynamics, to function as a CS. This point is especially notable given that the participants were smokers. That is, from a Pavlovian conditioning perspective, the CS effects of nicotine likely already have a rich conditioning history that might make it difficult to see any effect of a few conditioning trials in the laboratory. As an example, the individuals in this study smoked an average of 15.3 cigarettes per day. Although the duration of smoking is not reported, it is probably an underestimate to say that the participants with a mean age of 27 (range= 21 to 44) years old were smoking at this rate for at least 9 years (i.e., since they were 18 years old). If so, the average number of cigarettes consumed by an individual is estimated at just over 50,000. Thus, in this example there were at least 50,000 potential conditioning trials in which the interoceptive stimulus effects of nicotine could have been paired with other appetitive stimuli (e.g., alcohol, food, socialization, work break, peer acceptance, etc.). The four conditioning trials used by Clements et al. (1996) seem few in comparison to an individual’s experience before entering the experiment.
We do not mean to imply that the CS effects of a drug cannot be studied in the laboratory situation with human participants. Rather, experiments will simply need to take such history into account. Indeed, in a more recent and cleverly designed study Alessi, Roll, Reilly, and Johanson (2002) clearly demonstrated the feasibility of studying a drug state as CS capable of entering into an association with a reward. Briefly, human participants had a non-preferred drug (typically diazepam) paired with increased pay during a subsequent computer task. The monetary outcome (US) induced a preference for the interoceptive effects of diazepam (CS). Or, in the word of the authors “drug (diazepam) may have acquired the properties of a conditioned reinforcer as a result of its association with money” (p. 81).
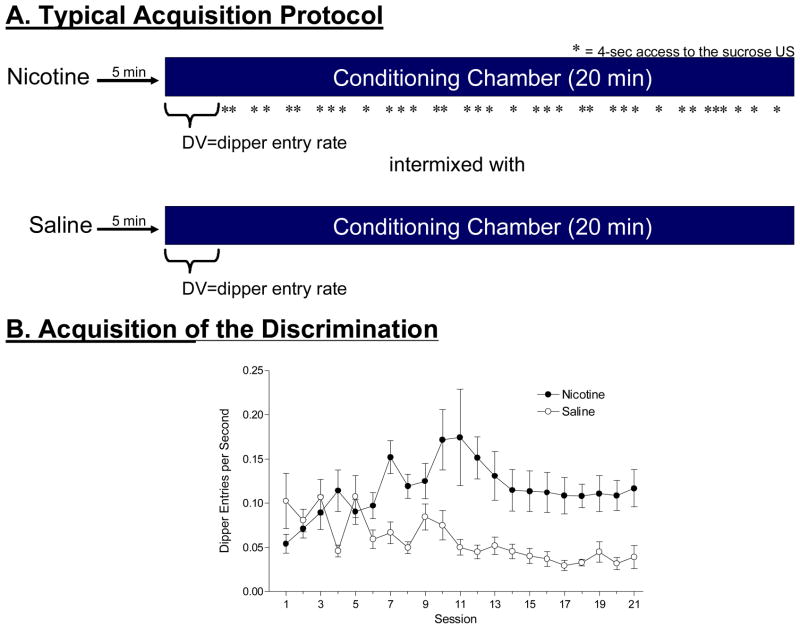
More recently, we have developed a preclinical animal model to study the ability of the pharmacological effects of nicotine to serve as an interoceptive contextual CS for a non-drug appetitive US (i.e., sucrose) in rats (Besheer, Palmatier, Metschke, & Bevins, 2004; Bevins and Palmatier, 2004; Bevins, Penrod, & Reichel, 2007; Murray & Bevins, 2007; Reichel, Linkugel, & Bevins, in press; Wilkinson, Murray, Li, Wiltgen, Penrod, Berg, & Bevins, 2006). In this Pavlovian appetitive conditioning task, rats received a subcutaneous (SC) injection of nicotine (i.e., the CS) paired with intermittent access to liquid sucrose (i.e., the US) across a 20-min session. Intermixed with these nicotine sessions were saline sessions in which rats were injected with saline, placed into the same conditioning chambers, but sucrose was withheld (see Figure 7.3A for procedural schematic). Relative to saline (no drug), nicotine evokes differential approach and head entry into the dipper receptacle (see Figure 7.3B). This increase in behaviors directed at the location where the reinforcer has occurred in the past has been referred to as ‘goal tracking’ (Boakes, 1977; Farwell & Ayres, 1979) and is a widely used measure of Pavlovian conditioning (e.g., Bouton & Sunsay, 2003; Delamater, 1995; Rescorla, 2006).
Figure 7.3.
Panel A shows a schematic of a typical protocol used to train the interoceptive stimulus effects of nicotine as an excitatory CS. In brief, nicotine sessions are intermixed with saline session. On nicotine sessions, rats receive intermittent access to sucrose in a dipper receptacle; sucrose is withheld on saline sessions. Panel B shows acquisition of conditioned responding (i.e., dipper entries before first sucrose delivery or equivalent time in saline sessions) to the nicotine CS. In this study conducted by Jill Rosno in my laboratory, the nicotine CS dose was (0.4 mg base/kg, SC) and the US was 26% sucrose. In a given nicotine session, There were 36 separate 4-sec deliveries of the sucrose US.
Ongoing research in the laboratory has focused on neuropharmacological and behavioral processes underlying nicotine’s ability to function as an interoceptive context CS in this appetitive drug discrimination procedure. For instance, Wilkinson et al. (2006) found that the magnitude of the goal-tracking CR increased with the number of nicotine CS–sucrose US pairings and that this more robust CR was more resistant to extinction (i.e., more nicotine CS presentations without sucrose to decrease the CR toward control). CR magnitude also increased with higher concentrations of sucrose (unpublished data). A nicotine dose as low as 0.1 mg/kg can serve as a CS using a fading-dose procedure (Bevins & Palmatier, 2004) or as the dose used from the initiation of training (Murray & Bevins, 2007). Although acquisition rate is similar with lower (0.1 and 0.2 mg/kg) and higher (0.4 mg/kg) doses of nicotine, resistance to extinction increased with nicotine CS dose (Murray & Bevins, in press). Importantly, nicotine’s ability to evoke this appetitive CR does not reflect state-dependent learning (Bevins et al., 2007).
Besheer et al. (2004) established that the CS effects of nicotine were blocked by pretreatment with the central and peripheral nicotinic acetylcholine receptor (nAChR) antagonist mecamylamine, but not the mostly peripheral nAChR antagonist hexamethonium, suggesting a role of central nervous system (CNS) receptors. Additional neuropharmacological research published or in progress in our laboratory has implicated the α4β2* nAChR, the dopamine and norepinephrine transporter, the glutamatergic NMDA receptor, and the cannabinoid CB1 receptor in the CS effects of nicotine. Dopamine D1, D2, and D3 receptors, as well as the metabotropic glutamate receptor subtype 5 receptor and the α7* nAChR appear to have minimal role in nicotine’s ability to function as a CS [Murray & Bevins, 2007; unpublished data from experiments in progress; see Chapters 2, 3, and 4 of present Volume (Placzek & Dani; Brunzell & Picciotto; Dwoskin, Pivavarchyk, Joyce, Neugebauer, Zheng, Zhang, Bardo, & Crooks, respectively) for a discussion of nAChRs]. In sum, the specificity exemplified by the agonist and antagonist research just described, along with the consistency of the behavioral manipulations with past learning research highlights the utility of this Pavlovian drug discrimination task for studying the underlying behavioral and neural processes of the interoceptive conditional stimulus effects of nicotine.
In more “standard” Pavlovian discrimination tasks, auditory and visual stimuli are often used as CSs. These type of discrete stimuli—versus situational or static apparatus cues—can be readily turned on and off during a conditioning session. Further, several presentations can be programmed in each session allowing one to track acquisition of conditioned responding trial-by-trial. To date, all of our published research on the CS effects of nicotine has used SC injections of nicotine. As such, our empirical efforts have employed manipulations comparable to those used with exteroceptive contextual or static apparatus cues. Recent advances in our laboratory, however, have extended this conceptualization of the CS effects of nicotine to include more discrete stimulus properties. Those advances are based on pairing a low dose of nicotine infused IV with brief access to sucrose in long daily sessions. More specifically, food restricted male Sprague Dawley rats were dipper trained for 3 days and then surgically prepared with IV catheters. Acquisition training followed the surgical recovery period. For acquisition, rats received ten IV infusions of nicotine (0.01 mg base/kg) in a 2-h session. Each 1-sec nicotine infusion (i.e., the CS) was followed 30 sec later by 4-sec access to 26% (w/v) sucrose (i.e., the US); nicotine infusions were separated by an average of 11 min. This protocol was repeated daily for 12 days. The last day of acquisition was followed 24 h later by the first of seven extinction sessions in which the nicotine CS was still infused, but the sucrose US was withheld.
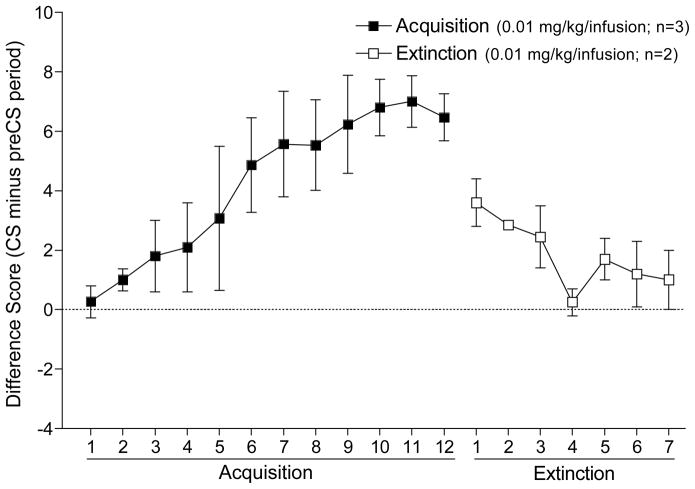
Figure 7.4 shows the results from this study examining the ability of IV nicotine to function as a CS. The main dependent measure is number of dipper entries in the 30 sec following the nicotine infusion (CS period) minus number of entries in the 30 sec immediately before the infusion (pre-CS period). A positive value indicates an increase in dipper entries; 0 indicates no change. Nicotine readily acquired control of conditioned responding (i.e., goal tracking). Further, this conditioned responding decreased systematically across sessions when sucrose was withheld (i.e., extinction). These findings are notable for several reasons. First, they demonstrate that a dose of nicotine on the lower end of the self-administration dose-effect curve has sufficient stimulus properties to function as a CS. Second, by the end of acquisition training, dipper entries increased after each nicotine infusion (trial-by-trial data not shown) suggesting that IV nicotine can be used in a manner more similar to a discrete cue. Finally, in the extinction phase nicotine infusions continued, but there was a progressive decrease in dipper entries across sessions. Because nicotine was infused to the same extent in acquisition and extinction, a psychomotor stimulant account of the increased dipper entries in the acquisition phase is untenable. That is, a psychomotor account predicts that the pattern of responding should not change in the extinction phase; this obviously did not occur. Accordingly, the increase in dipper entries in acquisition reflects a conditioned association between nicotine and sucrose. Indeed, we recently conducted an unpaired control group in which nicotine and sucrose occurred in the same session, but their presentations were separated by at least 4 min. This unpaired control did not display an increase in dipper entries immediately following nicotine infusion (data not shown). This result indicates that temporal contiguity between the nicotine and sucrose is required; a conclusion consistent with the extinction results and the implication of conditioning processes (Pavlov, 1927; Wasserman & Miller, 1997).
Figure 7.4.
This figure shows results of an experiment conducted by Jennifer Murray in my laboratory using a 1-sec intravenous administration of 0.01 mg base/kg nicotine as the CS; 4 sec access to the sucrose US followed 30 sec later. Intravenous nicotine acquired control over conditioned responding and this conditioning was susceptible to extinction.
NICOTINE AS AN INTEROCEPTIVE OCCASION SETTER
The research examining the ability of nicotine to function as a CS assumes that the interoceptive effects of nicotine enter into a direct association with the sucrose US. Differential control of a goal-tracking CR by nicotine provides evidence for this conditioned association (see later discussion). A natural extension of this associative analysis is that the nicotine drug state should also be able to serve as a positive or negative occasion setter (i.e., also termed ‘facilitator’ or ‘modulator’ in the Pavlovian conditioning literature). A positive occasion setter is a stimulus that sets the occasion upon which each presentation of a CS will be paired with the US; a negative occasion setter indicates that presentations of the CS will not be reinforced [see Schmajuk & Holland (1998) and Swartzentruber (1995) for reviews]. Research from our laboratory has shown that nicotine can function in both capacities (Bevins, Wilkinson, Palmatier, Siebert, & Wiltgen, 2006; Palmatier, Peterson, Wilkinson, & Bevins, 2004; Palmatier, Wilkinson, & Bevins, 2005; Palmatier & Bevins, 2007). For example, as a positive drug feature (i.e., occasion setter) nicotine disambiguated the relation between a brief light cue and sucrose delivery. That is, the discrete light CS was paired with the sucrose US when nicotine was administered before the start of the session. In contrast, on saline sessions the same light CS was present, but access to sucrose was withheld. As a negative feature, the interoceptive effects of nicotine indicate that the light CS will not be followed by the sucrose US. Rather, the light CS will be paired with sucrose on saline (no drug) sessions.
Although we have not conducted nearly the amount of neuropharmacological research within this nicotine occasion setting task as with the CS task, the Pavlovian discrimination is quickly acquired, mediated by central nicotinic acetylcholine receptors, and is pharmacologically specific (Bevins et al., 2006; Palmatier et al., 2004; Palmatier et al., 2005). Rather, our empirical efforts in this area have focused more on the underlying behavioral processes mediating nicotine’s ability to modulate responding. For the positive occasion setting research, this has entailed asking whether nicotine (or other drug states such as chlordiazepoxide) facilitate conditioned responding to a CS through simple excitatory processes such as a direct association with the US. Or, is it necessary to infer a non-associative or higher-order associative process to account for its modulatory control over conditioned responding. One account of occasion setting suggests that the discrete CS (e.g., light in our situation) acquires weak excitatory strength from being paired with the sucrose US on half the sessions. Although this excitation is not sufficient to evoke conditioned responding alone, when the CS is combined with the ‘occasion setter’ that has also been paired with the US on half the sessions, excitation passes some threshold and conditioned responding is observed (cf. Rescorla, 1986). Note that this explanation assumes that the CS and the occasion setter enter into separate excitatory associations with the US that will ‘summate’ when the two are presented together. This summation account predicts that nicotine will lose its ability to modulate (facilitate) responding to the CS after extensive presentation of the nicotine occasion setter without the sucrose US—i.e., procedural extinction.
We recently tested this account using nicotine as the occasion setter (see Experiment 1 in Palmatier & Bevins, 2007). Briefly, nicotine was trained as a positive occasion setter as described earlier. In a subsequent phase, nicotine was presented repeatedly without the discrete CS or sucrose US. This phase was meant to decrease excitation controlled directly by nicotine. Then, the light was re-introduced. Even though there were as many nicotine extinction sessions as there were original nicotine training sessions, conditioned responding to the discrete CS was still facilitated by the nicotine. A similar pattern was observed when amphetamine or chlordiazepoxide functioned as the occasion setter (Palmatier & Bevins, 2007). Combined, this research strains any summation type account. Further, it suggests that the nicotine drug state is modulating responding to the CS via a higher order associative or non-associative process. Currently unpublished research from our laboratory has confirmed this assumption. That is, nicotine trained as an occasion setter for one discrete CS (e.g., light) was able to transfer its modulatory control to a completely separate and distinct CS (e.g., white noise) that has been separately trained with chlordiazepoxide as the occasion setter. Notably, the pharmacological effects of chlordiazepoxide do not substitute for a nicotine occasion setter in the absence of this associative training. Additionally, a novel drug state (amphetamine) did not prompt conditioned responding to either the discrete CS indicating that training two Pavlovian occasion setting discriminations within subject does not merely result in a drug versus no drug discrimination where the default is to respond when in a drug state. Thus, we are left to conclude that transfer of modulatory control of conditioned responding between nicotine and chlordiazepoxide reflects a common underlying higher-order associative or non-associative processes that allows for generalization. That is, related conditioning histories allows for functional substitution (versus pharmacological substitution) by drug states (Palmatier, 2004; see Bonardi & Hall, 1994 for comparable results with exteroceptive stimuli).
IMPLICATIONS FOR THE MOTIVATIONAL FUNCTION OF NICOTINE
We suggest that a more complete analysis of nicotine dependence will also include nicotine in the role of a CS. An explicit assumption in our research is that nicotine’s ability to control a CR reflects an acquired excitatory association with the sucrose US (Bevins & Palmatier, 2004). Although the inferred nature of the association may vary with one’s theoretical preference, this assumption of acquired excitation is held in some form by most Pavlovian conditioning theorists (Bouton 2002; Domjan, 2005; Konorski, 1948; Miller & Escobar, 2002; Pavlov, 1927; Pearce, 1987; Rescorla 1988; Timberlake, 1994; Wagner & Brandon, 2001) and is supported by the research described in this chapter. If nicotine acquires additional appetitive properties by virtue of its conditioning history, then not only are the stimulus properties of the drug changed as evidenced by its control of a CR, but its ability to function in other capacities (e.g., reward, reinforcer, US, etc.) might also be changed. Such changes in the motivational function of nicotine for the smoker could affect the trajectory of nicotine dependence and suggest modifications to current intervention strategies. In less technical and more speculative terms, if the “meaning” of nicotine is altered by an individual’s experiences while using nicotine, these associated experiences could alter the progression to dependence, affect the tenacity of the addiction, change the difficulty of quitting, alter the likelihood of relapse, and/or change the magnitude and duration of the relapse. Although in our current research and in the present proposal we focus on positive or appetitive experiences which would change these addiction outcomes for the worse, there is a clear prediction that negative or aversive experiences could also change the trajectory for dependence (e.g., prevent further experimentation and hence development of dependence, decrease likelihood of relapse, etc.).
The research reviewed in this chapter clearly establishes that interoceptive effects of nicotine function as a CS that comes to evoke an appetitive CR. However, the possibility that the motivational impact of nicotine would change as a function of conditioning history has not been directly assessed. Widely studied phenomena such as second-order conditioning (Bevins, Delzer, & Bardo, 1996; Holland & Rescorla, 1975; Pavlov, 1927), counterconditioning (Brooks, Hale, Nelson, & Bouton, 1995; Lovibond & Dickinson, 1982; Pearce & Dickinson, 1975), and revaluation (Holland & Straub, 1979; Molina, Bannoura, Chotro, McKinzie, Arnold, & Spear, 1996; Yin & Knowlton, 2002) support the idea that a cue paired with a biologically relevant outcome will acquire additional appetitive or aversive properties depending on the nature of the US. Additionally, there are a few scattered but important published reports more directly related to this suggestion. Perhaps the most directly relevant is a very clever experiment by Molina et al. (1996). In that study, they reported that a tactile aversion conditioned by an ethanol US was reversed if ethanol was later paired with sucrose. That is, 10-day-old rat pups had a distinct tactile CS paired with intragastrically administered ethanol (2 g/kg, 16.8% v/v). Relative to Unpaired controls, this conditioning history produced a clear aversion for the tactile CS. If rat pups had this same dose of ethanol subsequently paired with 10 min of a sucrose solution (15.3% w/v) infused through an intra-oral cannula then they did not display this tactile aversion. Merely exposing the pups to unpaired ethanol and sucrose or providing an alternative learning history was not sufficient to alter the previously acquired tactile aversion. In the authors’ words, “after pups in the present experiments acquired an aversion to the texture as a consequence of its pairing with alcohol US properties, the pup’s representation of these properties was changed (devalued) during Phase 2 by pairing the state of alcohol intoxication with an appetitive sucrose infusion” (p. 130). Notably, ongoing research in our laboratory indicates that an appetitive conditioning history with nicotine as a CS appears to enhance its rewarding US effects as measured in a place conditioning task.
We also suggest that a more complete analysis of nicotine dependence will include nicotine in the role of an occasion setter. As such, nicotine disambiguates when other stimuli will be paired with a US. Although functioning as an occasion setter does not preclude also serving as a CS, it will be of interest to determine if some conditions are more likely to encourage higher-order associations rather than direct associations with nicotine. The motivational impact of Pavlovian conditioning history where nicotine serves as an occasion setter was highlighted by the functional substitution research described earlier (Palmatier, 2004). In that research, a drug pharmacologically distinct from nicotine (i.e., chlordiazepoxide), facilitated conditioned responding to the CS (e.g., light) that was paired with sucrose only in the nicotine state. This substitution only occurs when chlordiazepoxide is trained as an occasion setter for a different CS (e.g., white noise). That is, transfer of motivational function was based on learning histories and not on an overlap in the pharmacological effects of the drugs. This functional substitution could have important implications for smoking relapse. Seemingly unrelated stimuli could prompt craving, urges, and/or drug seeking because they share a common conditioning history with nicotine.
In closing, better intervention and prevention programs for nicotine dependence will require a multi-faceted and translational approach in which genetic, neurobiological, individual, and cultural factors are considered. In the present chapter we have focused on interoceptive Pavlovian conditioning processes in which nicotine’s motivational function could be altered by conditioning history. Such conditioning history could importantly affect nicotine addiction. Albeit speculative, alterations in nicotine’s effects resulting from Pavlovian conditioning could speed the transition between experimentation and dependence, make quitting more difficult, and/or contribute to the high relapse rate. Clearly, more research is required to test these possibilities, as well as to better understand interoceptive Pavlovian conditioning processes with nicotine. This understanding will no doubt enhance the effectiveness of intervention and prevention programs for tobacco use.
Acknowledgments
I want to thank all the individuals that have worked so hard over the years on the research discussed in this Chapter. That research and the uncountable discussions prompted by this work have shaped my thinking in important ways. The research and the author while writing this Chapter were partially supported by DA018114.
References
- Alessi SM, Roll JM, Reilly MP, Johanson CE. Establishment of a diazepam preference in human volunteers following differential-conditioning history of placebo versus diazepam choice. Experimental and Clinical Psychopharmacology. 2002;10:77–83. [PubMed] [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: A Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology. 2004;172:108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Delzer TA, Bardo MT. Second-order conditioning detects unexpressed morphine-induced salt aversion. Animal Learning & Behavior. 1996;24:221–229. [Google Scholar]
- Bevins RA. Should we essentially ignore the role of stimuli in a general account of operant selection? Behavioral & Brain Sciences. 2001;24:528–529. [Google Scholar]
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: Dopaminergic and GABAergic influences on conditioned expression. Pharmacology, Biochemistry and Behavior. 2001;68:135–145. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Bardo MT. Introduction: Motivation, drug abuse, and 50 years of theoretical and empirical inquiry. In: Bevins RA, Bardo MT, editors. Motivational Factors in the Etiology of Drug Abuse, Volume 50 of the Nebraska Symposium on Motivation. Lincoln NE: University of Nebraska Press; 2004. pp. ix–xv. [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Nicotine-conditioned locomotor sensitization in rats: Assessment of the US-preexposure effect. Behavioural Brain Research. 2003;143:65–74. doi: 10.1016/s0166-4328(03)00009-3. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behavioral and Cognitive Neuroscience Reviews. 2004;3:143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Eurek S, Besheer J. Timing of conditioned response in a nicotine locomotor conditioning preparation: Manipulations of the temporal arrangement between context cues and drug administration. Behavioural Brain Research. 2005;159:135–143. doi: 10.1016/j.bbr.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Wilkinson JL, Palmatier MI, Siebert HL, Wiltgen SM. Characterization of nicotine’s ability to serve as a negative feature in a Pavlovian appetitive conditioning task in rats. Psychopharmacology. 2006;184:470–481. doi: 10.1007/s00213-005-0079-3. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Penrod RD, Reichel CM. Nicotine does not produce state-dependent effects on learning in a Pavlovian appetitive goal-tracking task with rats. Behavioural Brain Research. 2007;177:134–141. doi: 10.1016/j.bbr.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz HMB, editors. Operant-Pavlovian interactions. Hillsdale NJ: Erlbaum; 1977. pp. 67–97. [Google Scholar]
- Bonardi C, Hall G. Occasion-setting training renders stimuli more similar: Acquired equivalence between the targets of feature-positive discriminations. Quarterly Journal of Experimental Psychology. 1994;47B:63–81. [PubMed] [Google Scholar]
- Bormann NM, Overton DA. Morphine as a conditioned stimulus in a conditioned emotional response paradigm. Psychopharmacology. 1993;112:277–284. doi: 10.1007/BF02244922. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Sunsay C. Importance of trial versus accumulating time across trials in partially reinforced appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:62–77. [PubMed] [Google Scholar]
- Brooks DC, Hale B, Nelson JB, Bouton ME. Reinstatement after counterconditioning. Animal Learning & Behavior. 1995;23:383–390. [Google Scholar]
- Bykov KM. The cerebral cortex and the internal organs. New York: Chemical Publishing Company; 1957. [Google Scholar]
- Clements K, Glautier S, Stolerman IP, White JAW, Taylor C. Classical conditioning in humans: Nicotine as CS and alcohol as US. Human Psychopharmacology. 1996;11:85–95. [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Cook L, Davidson A, Davis DJ, Kelleher RT. Epinephrine, norepinephrine, and acetylcholine as conditioned stimuli for avoidance behavior. Science. 1960;131:990–991. doi: 10.1126/science.131.3405.990. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Dethier VG. Insects and the concept of motivation. In: Levine D, editor. Nebraska Symposium on Motivation, 1966. Lincoln NE: University of Nebraska Press; 1966. pp. 105–136. [Google Scholar]
- Damaj MI, Creasy KR, Grove AD, Rosecrans JA, Martin BR. Pharmacological effects of epibatidine optical enantiomers. Brain Research. 1994;664:34–40. doi: 10.1016/0006-8993(94)91950-x. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Creasy KR, Welch SP, Rosecrans JA, Aceto MD, Martin BR. Comparative pharmacology of nicotine and ABT-418, a new nicotinic agonist. Psychopharmacology. 1995;120:483–490. doi: 10.1007/BF02245822. [DOI] [PubMed] [Google Scholar]
- Delamater AR. Outcome-selective effects of intertrial reinforcement in a Pavlovian appetitive conditioning paradigm with rats. Animal Learning & Behavior. 1995;23:31–39. [Google Scholar]
- Domjan M. Pavlovian conditioning: A functional perspective. Annual Review of Psychology. 2005;56:179–206. doi: 10.1146/annurev.psych.55.090902.141409. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology. 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Doty RW. Conditioned reflexes formed and evoked by brain stimulation. In: Sheer DE, editor. Electrical stimulation of the brain: An interdisciplinary survey of neurobehavioral integrative systems. Austin TX: University of Texas Press; 1961. pp. 397–412. [Google Scholar]
- Farwell BJ, Ayres JJB. Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking (“goal tracking”) in rats. Learning and Motivation. 1979;10:295–312. [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology. 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology. 2006;184:456–463. doi: 10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- Greeley J, Lê DA, Poulos CX, Cappell H. Alcohol is an effective cue in the conditioned control of tolerance to alcohol. Psychopharmacology. 1984;83:159–162. doi: 10.1007/BF00429726. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Schuh LM, Jarvik ME. Pathophysiology of tobacco dependence. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The fourth generation of progress. New York: Raven Press; 1995. pp. 1715–1729. [Google Scholar]
- Holland PC, Rescorla RA. Second-order conditioning with food unconditioned stimulus. Journal of Comparative and Physiological Psychology. 1975;88:459–467. doi: 10.1037/h0076219. [DOI] [PubMed] [Google Scholar]
- Holland PC, Straub JJ. Differential effects of two ways of devaluing the unconditioned stimulus after Pavlovian appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5:65–78. doi: 10.1037//0097-7403.5.1.65. [DOI] [PubMed] [Google Scholar]
- Kim JA, Siegel S, Patenall VRA. Drug-onset cues as signals: Intraadministration associations and tolerance. Journal of Experimental Psychology: Animal Behavior Processes. 1999;25:491–504. [PubMed] [Google Scholar]
- Kintsch W, Witte RS. Concurrent conditioning of bar press and salivation response. Journal of Comparative and Physiological Psychology. 1962;55:963–968. doi: 10.1037/h0046131. [DOI] [PubMed] [Google Scholar]
- Konorski J. Conditioned reflexes and neuron organization. Cambridge: Cambridge University Press; 1948. [Google Scholar]
- Laidler KJ. To light such a candle: Chapters in the history of science and technology. Oxford: Oxford University Press; 1998. [Google Scholar]
- Lazev AB, Herzog TA, Brandon TH. Classical conditioning of environmental cues to cigarette smoking. Experimental and Clinical Psychopharmacology. 1999;7:56–63. doi: 10.1037//1064-1297.7.1.56. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology. 2005;178:481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Loucks RB. Studies of neural structures essential for learning. II: The conditioning of salivary and striped muscle responses to faradization of cortical sensory elements, and the action of sleep upon such mechanisms. Journal of Comparative Psychology. 1938;25:315–332. [Google Scholar]
- Lovibond PF, Dickinson A. Counterconditioning of appetitive and defensive CRs in rabbits. The Quarterly Journal of Experimental Psychology B: Comparative and Physiological Psychology. 1982;34B:115–126. doi: 10.1080/14640748208400880. [DOI] [PubMed] [Google Scholar]
- Miller R, Escobar M. Learning: Laws and models of basic conditioning. In: Pashler H, Gallistel R, editors. Steven's handbook of experimental psychology (3rd ed.), Vol. 3: Learning, motivation, and emotion. Hoboken NJ: John Wiley & Sons, Inc; 2002. pp. 47–102. [Google Scholar]
- Molina JC, Bannoura MD, Chotro MG, McKinzie DL, Arnold HM, Spear NE. Alcohol-mediated tactile conditioned aversions in infant rats: Devaluation of conditioning through alcohol-sucrose associations. Neurobiology of Learning and Memory. 1996;66:121–132. doi: 10.1006/nlme.1996.0053. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of a nicotine conditioned stimulus. European Journal of Pharmacology. 2007;561:91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. The conditional stimulus effects of nicotine vary as a function of training dose. Behavioural Pharmacology. doi: 10.1097/FBP.0b013e3282f14ec6. in press. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA. Examination of GABAergic and dopaminergic compounds in the acquisition of nicotine-conditioned hyperactivity in rats. Neuropsychobiology. 2002;45:87–94. doi: 10.1159/000048682. [DOI] [PubMed] [Google Scholar]
- Palmatier MI. Unpublished Dissertation. Department of Psychology, University of Nebraska-Lincoln; 2004. Drug modulators in appetitive Pavlovian conditioning. [Google Scholar]
- Palmatier MI, Peterson JL, Wilkinson JL, Bevins RA. Nicotine serves as a feature-positive modulator of Pavlovian appetitive conditioning in rats. Behavioural Pharmacology. 2004;15:183–194. [PubMed] [Google Scholar]
- Palmatier MI, Wilkinson JL, Bevins RA. Stimulus properties of nicotine, amphetamine, and chlordiazepoxide as positive features in a Pavlovian appetitive discrimination task in rats. Neuropsychopharmacology. 2005;30:731–741. doi: 10.1038/sj.npp.1300629. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA. Facilitation by drug states does not depend on acquired excitatory strength. Behavioural Brain Research. 2007;176:292–301. doi: 10.1016/j.bbr.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Pearce JM, Dickinson A. Pavlovian counterconditioning: Changing the suppressive properties of shock by association with food. Journal of Experimental Psychology: Animal Behavior Processes. 1975;2:170–177. doi: 10.1037//0097-7403.1.2.170. [DOI] [PubMed] [Google Scholar]
- Pearce JM. A model of stimulus generalisation for Pavlovian conditioning. Psychological Review. 1987;84:61–73. [PubMed] [Google Scholar]
- Perkins KA, DiMarco A, Grobe JE, Scierka A, Stiller RL. Nicotine discrimination in male and female smokers. Psychopharmacology. 1994;116:407–413. doi: 10.1007/BF02247470. [DOI] [PubMed] [Google Scholar]
- Peterson GB, Ackil JE, Frommer GP, Hearst ES. Conditioned approach and contact behavior toward signals for food or brain-stimulation reinforcement. Science. 1972;177:1009–1011. doi: 10.1126/science.177.4053.1009. [DOI] [PubMed] [Google Scholar]
- Pritchard WS, Robinson JH, Guy TD, Davis RA, Stiles MF. Assessing the sensory role of nicotine in cigarette smoking. Psychopharmacology. 1996;127:55–62. doi: 10.1007/BF02805975. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Nicotine as a conditioned stimulus: Impact of ADHD medications. Experimental and Clinical Psychopharmacology. doi: 10.1037/1064-1297.15.5.501. in press. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Extinction of facilitation. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:16–24. [Google Scholar]
- Rescorla RA. Behavioral studies of Pavlovian conditioning. Annual Review of Neuroscience. 1988;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Deepened extinction from compound stimuli presentation. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:135–144. doi: 10.1037/0097-7403.32.2.135. [DOI] [PubMed] [Google Scholar]
- Revusky S, Davey V, Zagorski M. Heart rate conditioning with pentobarbital as a conditioned stimulus and amphetamine as an unconditioned stimulus. Behavioral Neuroscience. 1989;103:296–307. doi: 10.1037//0735-7044.103.2.296. [DOI] [PubMed] [Google Scholar]
- Rose JE, Levin ED. Inter-relationships between conditioned and primary reinforcement in the maintenance of cigarette smoking. British Journal of Addiction. 1991;86:605–609. doi: 10.1111/j.1360-0443.1991.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Schmajuk NA, Holland PC. Occasion setting: Associative learning and cognition in animals. Washington DC: American Psychological Association; 1998. [Google Scholar]
- Shoaib M, Stolerman IP, Kumar RC. Nicotine-induced place preferences following prior nicotine exposure in rats. Psychopharmacology. 1994;113:445–452. doi: 10.1007/BF02245221. [DOI] [PubMed] [Google Scholar]
- Skinner BF. The behavior of organisms. New York: Appleton Century Crofts; 1938. [Google Scholar]
- Sokolowska M, Siegel S, Kim JA. Intraadministration associations: Conditional hyperalgesia elicited by morphine onset cues. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:309–320. [PubMed] [Google Scholar]
- Stolerman IP. Discriminative stimulus effects of nicotine in rats trained under different schedules of reinforcement. Psychopharmacology. 1989;97:131–138. doi: 10.1007/BF00443427. [DOI] [PubMed] [Google Scholar]
- Swartzentruber DE. Modulatory mechanisms in Pavlovian conditioning. Animal Learning & Behavior. 1995;23:123–143. [Google Scholar]
- Timberlake W. Behavior systems, associationism, and Pavlovian conditioning. Psychonomic Bulletin & Review. 1994;1:405–420. doi: 10.3758/BF03210945. [DOI] [PubMed] [Google Scholar]
- Troisi JR., II Pavlovian-instrumental transfer of the discriminative stimulus effects of nicotine and ethanol in rats. The Psychological Record. 2006;56:499–512. [Google Scholar]
- Turner EG, Altshuler HL. Conditioned suppression of an operant response using d-amphetamine as the conditioned stimulus. Psychopharmacology. 1976;50:139–143. doi: 10.1007/BF00430482. [DOI] [PubMed] [Google Scholar]
- Wagner AR, Brandon SE. A componential model of Pavlovian conditioning. In: Mower RR, Klein SB, editors. Handbook of contemporary learning theories. Mahwah, NJ: LEA; 2001. pp. 23–64. [Google Scholar]
- Walter S, Kuschinsky K. Conditioning of nicotine effects on motility and behaviour in rats. Naunyn-Schmiedeberg's Archives of Pharmacology. 1989;339:208–213. doi: 10.1007/BF00165145. [DOI] [PubMed] [Google Scholar]
- Wasserman EA, Miller RR. What’s elementary about associative learning? Annual Review of Psychology. 1997;48:573–607. doi: 10.1146/annurev.psych.48.1.573. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Murray JE, Li C, Wiltgen SM, Penrod RD, Berg SA, Bevins RA. Interoceptive Pavlovian conditioning with nicotine as the conditional stimulus varies as a function of number of conditioning trials and unpaired sucrose deliveries. Behavioural Pharmacology. 2006;17:161–172. doi: 10.1097/01.fbp.0000197456.63150.cd. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. Reinforcer devaluation abolishes conditioned cue preference: Evidence for stimulus-stimulus associations. Behavioral Neuroscience. 2002;116:174–177. doi: 10.1037//0735-7044.116.1.174. [DOI] [PubMed] [Google Scholar]