Abstract
There is ample evidence linking octopamine (OA) and tyramine (TA) to several neurophysiological functions in arthropods. In our laboratory we use the freshwater prawn Macrobrachium rosenbergii to study the neural basis of aggressive behavior. As a first step towards understanding the possible role of these amines and their receptors in the modulation of interactive behaviors, we have cloned a putative octopamine/tyramine receptor. The predicted sequence of the cloned OA/TAMac receptor consists of 1,579 base pairs (bp), with an open reading frame of 1,350 bp that encodes a 450 amino acid protein. This putative protein displays sequence identities of 70% to an Aedes aegypti mosquito TA receptor, followed by 60% to a Stegomyia aegypti mosquito OA receptor, 59% and 58% to the migratory locust TA-1 and -2 receptors respectively, and 57% with the silkworm OA receptor.
We also mapped the OA/TAMac receptor distribution by in-situ hybridization to the receptor’s mRNA, and by immunohistochemistry to its protein. We observed stained cell bodies for the receptor’s mRNA, mainly in the midline region of the thoracic and in the abdominal ganglia, as well as diffuse staining in the brain ganglia. For the receptor’s protein, we observed extensive punctate staining within the neuropil and on the membrane of specific groups of neurons in all ganglia throughout the CNS, including the brain, the midline region and neuropiles of the thoracic ganglia, and ventral part and neuropiles of the abdominal ganglia. The same pattern of stained cells was observed on the thoracic and abdominal ganglia in both in-situ hybridization and immunohistochemistry experiments. Diffuse staining observed with in-situ hybridization also coincides with punctate staining observed in brain, SEG, thoracic, and abdominal ganglia in immunohistochemical preparations. This work provides the first step towards characterizing the neural networks that mediate octopaminergic signaling in prawn.
Keywords: biogenic amines, aggressive behavior, dominance, crustacean
1. Introduction
Crustacean model systems have been used extensively for studying the neural basis of aggressive behavior and the establishment of dominance hierarchies, focusing primarily on the role played by biogenic amines (see reviews by Huber, 2005; Edwards et al., 2003; Kravitz, 2000). However, species such as lobsters and crayfish establish dominance hierarchies based on size and prior experiences, meaning that in their natural habitat status as dominant or subordinate is usually in flux and not set for the long term (Sosa and Baro, 2002). One means to overcome this experimental problem is to use the freshwater prawn Macrobrachium rosenbergii as a model system. This species is similar to other crustaceans in terms of its anatomy, while it establishes fixed dominance hierarchies based primarily on claw type or adult morphotype. Size and prior experience are secondary considerations, becoming primary considerations only among animals of the same morphotype (Barki et al., 1991a,b, 1992). Adult males of M. rosenbergii develop through three different morphotypes, characterized by their claws and behavior. The blue-clawed animals (BC) are dominant, yellow-clawed animals (YC) are subordinate to the BC, and small-clawed animals (SC) are the most submissive of the three morphotypes (Kuris et al., 1987; Ra’anan & Sagi, 1985; Ra’anan & Cohen, 1985).
While it has been shown in the crab that hemolymph concentrations of the biogenic amines octopamine, dopamine and 5-HT are higher following fights (Sneddon et al., 2000; Briffa and Elwood, 2007), the sources and mechanisms of the observed increases are not known. To better understand the actions of these biogenic amines on aggressive behavior, the components of their modulatory systems must be first defined in detail. The 5-HT modulatory system has been fairly well characterized in crustaceans (see reviews by Edwards and Spitzer, 2006; Panksepp et al., 2003; Kravitz, 2000; Beltz, 1999), but much less is known about the octopaminergic system. Norepinephrine has no physiological relevance in protostomes (including insects and crustaceans) and its role is fulfilled by OA, its invertebrate counterpart (Roeder, 2005). In addition to modifying aggressive behavior in crustaceans, it is involved in regulation of the heart beat (Florey and Rathmayer, 1978), setting responsiveness of neurons in the CNS (Glanzman and Krasne, 1983), and regulation of sensory receptor cells (Pasztor and Macmillan, 1990), excitation-contraction coupling (Fischer and Florey, 1987), synaptic transmission at neuromuscular junctions (Dudel, 1965), and escape reflexes (Yeh et al., 1996).
Tyramine (TA) is the synthetic precursor of OA and dopamine (DA), and there is evidence that in invertebrates TA is also involved in a variety of physiological processes independent from OA and DA, including carbohydrate metabolism (Downer, 1979), muscle contraction (Huddart and Oldfield, 1982), locomotion (Sarawasti et al., 2004), excretion (Blumenthal, 2003), reproduction (Sasaki and Nagao, 2002), oviposition (Donini and Lange, 2004), olfaction (Kutsukake et al., 2000), and behavioral sensitization (McClung and Hirsh, 1999). However, the function of TA in crustacean social behavior, if any, is not known, since it has been shown that circulating levels of this amine are not affected during shore crabs’ fights (Sneddon et al., 2000).
The effects of OA are mediated by a conserved family of seven transmembrane G-protein coupled receptors (GPCR; Roeder, 1999). Most members of this family are activated by both OA and TA. There are multiple receptor subtypes that differ in affinity for TA and/or OA, their location and action in the nervous system, and their pharmacology. Several TA and OA receptors from various invertebrate species have been cloned including receptors from the fly (Arakawa et al., 1990; Saudou et al. 1990; Broeck et al., 1995; Reale et al., 1997; Han et al., 1998), roundworm (Rex and Komuniecki, 2002), silkworm (Ohta et al., 2003), tobacco budworm (von Nickisch-Rosenegk et al., 1996), pond snail (Gerhardt et al., 1997a, b), sea hare (Chang et al., 2000), cockroach (Hirashima et al., 2003), and honeybee (Grohmann et al., 2003). However, to date, neither OA nor TA receptors have been identified in crustaceans.
As a first step towards understanding the role of OA signaling mechanisms underlying aggressive behavior and the establishment of dominance hierarchies in the prawn, we cloned and mapped the distribution of a putative OA/TA receptor, referred to as OA/TAMac, from its CNS. To our knowledge, this is the first report of the cloning, sequencing, and mapping of a crustacean OA/TA receptor.
2. Results
Cloning of the OA/TAMac receptor
The full-length sequence of the freshwater prawn OA/TAMac receptor (Accession Number: EU233816) consists of 1,579 base pairs, with an open reading frame of 1,350 base pairs, encoding a 450 amino-acid receptor protein (Fig. 1). The OA/TAMac receptor molecule has seven predicted transmembrane domains.
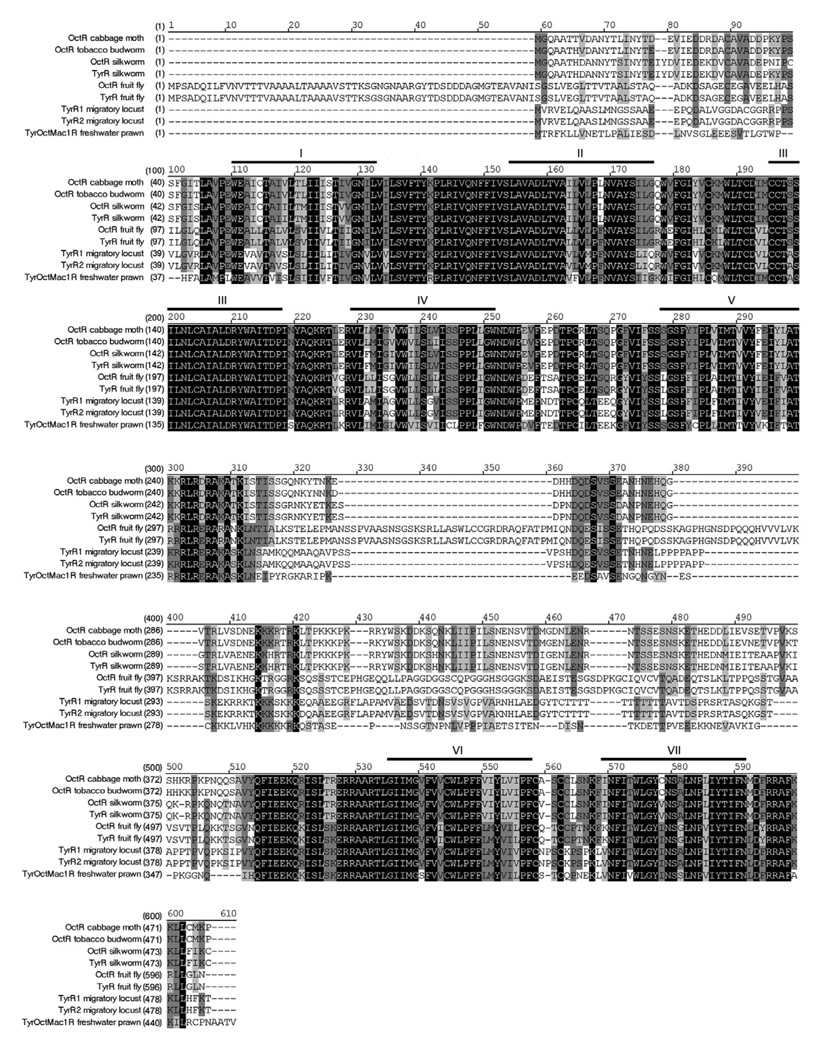
Figure 1. Alignment of OA/TAMac receptor.
The amino acid sequences of OA/TAMac are aligned with homologous invertebrate species. Heavy lines and Roman numbers above the sequence indicate transmembrane regions 1–7. Black boxes with white letters represent areas of identical regions, dark gray boxes with black letters represent conserved regions, and light gray boxes with black letters represent blocks of similar regions. Accession numbers are: cabbage moth Mamestra brasicae, AAK14402; tobacco budworm Heliothis virescens, Q25188; silkworm Bombyx mori, NP_001037504 and BAD11157; fly Drosophila melanogaster, AAA28731 and NP_524419; migratory locust LocusTYR Migratoria, Q25321 and Q25322; freshwater prawn Macrobrachium rosenbergii, EU233816. Abbreviations: octopamine receptor (OctR); tyramine receptor (TyrR); tyramine receptor 1 (TyrR1); tyramine receptor 2 (TyrR2); tyramine/octopamine receptor (Tyr/Oct).
Conservation analysis indicates that the putative OA/TAMac receptor shares 70% identity to an Aedes aegypti mosquito tyramine receptor, followed by 60% identity to a Stegomyia aegypti mosquito octopamine receptor, migratory locust TA 1 and 2 receptors with 59% and 58% of identity respectively, and 57% identity with the silkworm OA receptor. In addition, the OA/TAMac receptor is related to the silkworm and honeybee TA receptors with 55% identity for both, and 54% identity with both the tobacco budworm OA receptor and the yellow swallowtail TA receptor. The lowest identity was shown between the prawn’s OA/TAMac receptor and the fruit fly TA and OA receptors, as well as with the cattle tick OA receptor with 52% identity with all of them.
The putative OA/TAMac receptor has the typical G-protein-coupled receptor (GPCR) transmembrane signature motifs. The transmembrane regions participate in both ligand binding and receptor activation. The most highly conserved regions are seen in the transmembrane domains particularly the third. As expected, the third intracellular loop in the receptor is least conserved, with an amino acid identity as low as 28% between sequences. The carboxy terminus is characteristically short, and the amino terminus of the receptor is also poorly conserved.
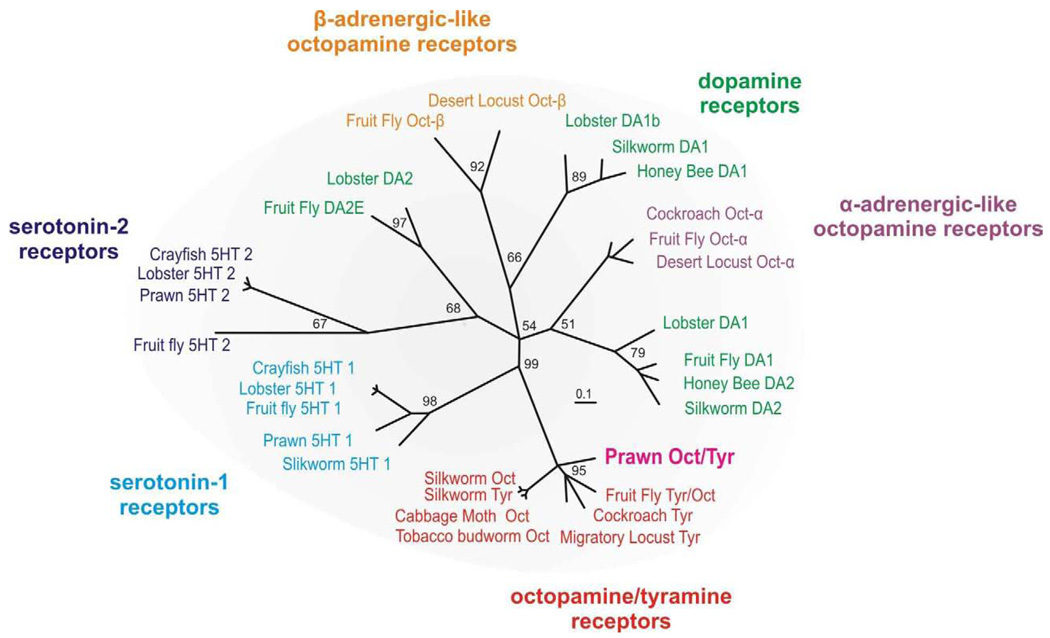
Phylogenetic analysis was used to compare the putative prawn OA/TAMac receptor to biogenic amine receptors from several arthropod species (Fig.2). These receptors include the 5-HT, dopamine, and OA families. It has been proposed that in insects the OA receptor family can be further subdivided into α-adrenergic-like, β-adrenergic-like, and OA/TA type receptors (Evans and Maqueira, 2005). We therefore included representative members from each of these families. Among these closely related receptors, the prawn OA/TAMac sequence clusters with the octopamine/tyramine family, providing strong support for it being an octopamine/tyramine type receptor.
Figure 2. Phylogenetic tree.
The evolutionary relationship of the prawn’s putative OA/TAMac receptor to characterized biogenic amine receptors from other arthropod species indicates it belongs to the octopamine/tyramine family. See Experimental Procedure for the details of tree construction and sequence. Numbers at branches represent support values and the branch-length scale bar represents 0.1 amino acid substitutions per site.
OA/TAMac distribution in the CNS of the prawn
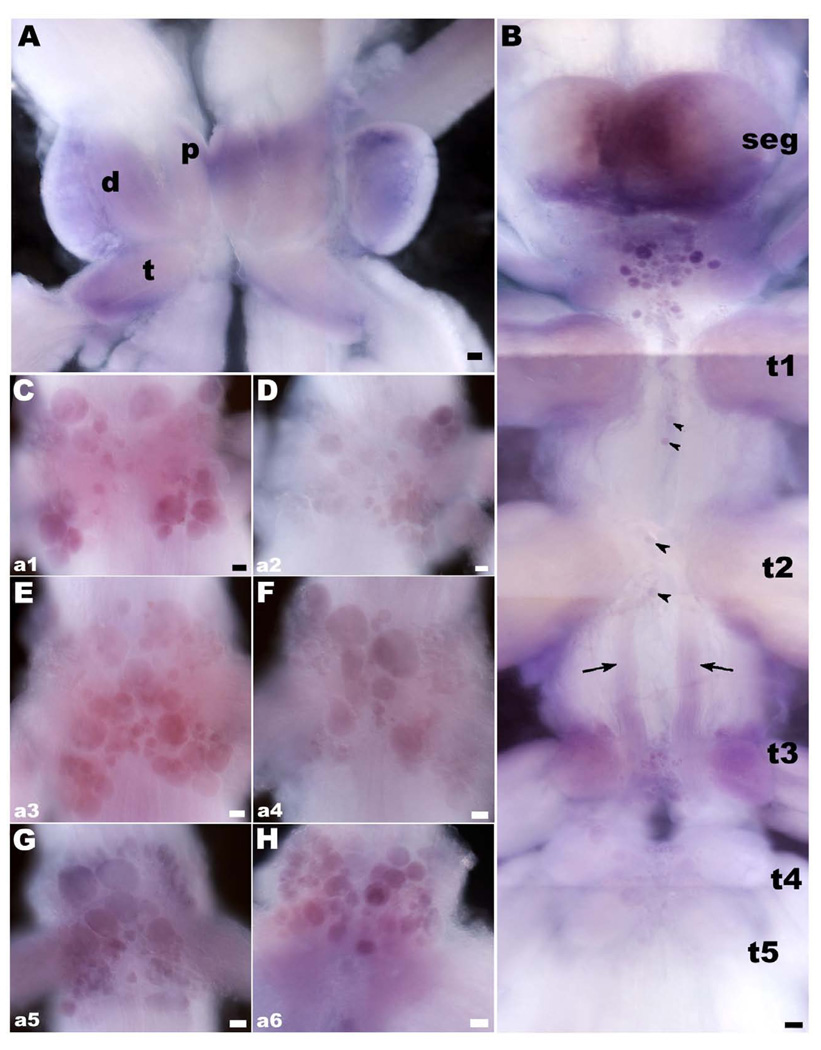
OA/TAMac receptor is widely distributed in the ventral nerve cord. We used in-situ hybridization to localize the receptor’s mRNA in the CNS. It was observed as diffuse staining in the brain and thoracic ganglia, and also as clusters of small- (~10 µm), medium- (~30 µm), and large-sized (~60 µm) cells within the thoracic and abdominal ganglia (n=18). For these experiments, we designed a DIG-labeled probe for the full length of the receptor and found that there are several groups of cell bodies through the CNS that appear to synthesize the receptor in the prawn (Fig. 3). In the brain, OA/TAMac receptor’s mRNA was observed as diffuse staining throughout the neuropil areas of the protocerebrum, deutocerebrum and tritocerebrum (Fig. 3A). Between the subesophageal (SEG) and the first thoracic (T1) ganglia, there is a central cluster of small-, medium-, and large-sized cell bodies that showed different intensities of OA/TAMac staining (Fig. 3B). In the thoracic ganglia, we found from two to four stained cell bodies along the ventral midline, and a group of cells in the ventral midline region of the T3–T5 ganglia (Fig. 3B). Staining was also observed in all the abdominal ganglia (Fig. 3C–H). Cells are arranged in clusters, following a pattern that repeated itself in each of the six ganglia (A1–A6). The pattern consisted of cells located ventrally at the center of each hemiganglion, along with 4 clusters of cells, arranged as the wings of a butterfly, located towards the dorsal lateral-most edges of each ganglion.
Figure 3. OA/TAMac receptor’s mRNA in the CNS of the prawn.
A: Brain OA/TAMac receptor mRNA appears as diffuse probe labeling in the neuropil areas of the protocerebrum (p), deutocerebrum (d), and tritocerebrum (t). B: Between the subesophageal ganglion (seg) and the first thoracic ganglia (t1), there is a central cluster of cell bodies of varying sizes that showed OA/TAMac mRNA staining. In the thoracic ganglia, two to four stained cell bodies showed mRNA labeling along the ventral midline (black arrowheads), as did a group of cells in the ventral middle region of the t3–t5 ganglia. There is also diffuse staining in a bilateral bundle of processes extending from t2 to t3 (black arrows). C–H: OA/TAMac mRNA Staining was also observed in all abdominal ganglia. Cells are arranged in clusters, following a pattern that repeated itself in each of the six ganglia (a1–a6). The pattern consisted of cells located ventrally at the center of each hemiganglion, along with 4 clusters of cells, arranged as the wings of a butterfly, located towards the dorsal lateral-most edges of each ganglion. Scale bar = 100 µm.
We used a polyclonal antibody that recognizes a portion of the OA/TAMac receptor to determine the distribution of the protein and compare it with that of its mRNA. We also observed OA/TAMac receptor immunoreactivity (OA/TAMac-ir) widely distributed throughout the prawn’s ventral nerve cord (n= 3 of each morphotype; Fig. 4–5).
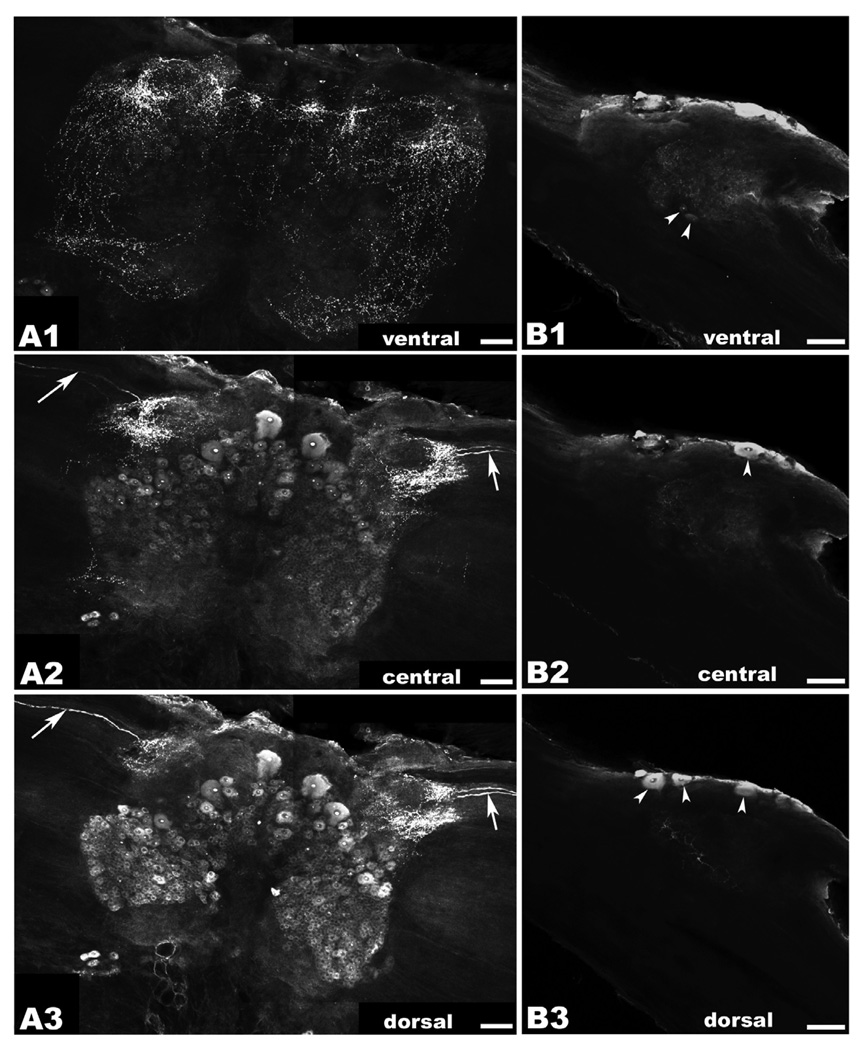
Figure 4. OA/TAMac immunoreactivity (ir) in the brain and circumesophageal (CEG) ganglia.
A1–A3: Brain OA/TAMac-ir was observed as punctate staining in the ventral part of the ganglion (A1), in the protocerebrum, lateralmost aspects of the deutocerebrum, and extending towards the inferior aspects of the tritocerebrum. Moving more deeply towards the center of the ganglion (A2), punctate staining is observed in the lateralmost protocerebrum, while medium- and large-sized cells are found more medially. Highly immunoreactive structures within the nucleus, possibly nucleoli, are observed in all stained cells. Bilateral axons are observed in the optic nerves going towards a region of intense punctate staining in the lateral protocerebrum (white arrows). More dorsally (A3), protocerebrum punctate staining and the bilateral axons (white arrows) are still visible. Smaller-sized cell bodies showing OA/TAMac-ir are also observed in the deutocerebrum. B1–B3: In circumesophageal ganglia, OA/TAMac-ir was observed as punctate staining towards the center of the ventral (B1), middle (B2), and dorsal (B3) aspects of the ganglia. OA/TAMac-ir is also observed in two small cells in the ventral ganglia (white arrowheads; B1), and in one to three medium-sized cells on the outer surface of the ganglia (white arrowheads; B2–B3). Scale bar = 100 µm.
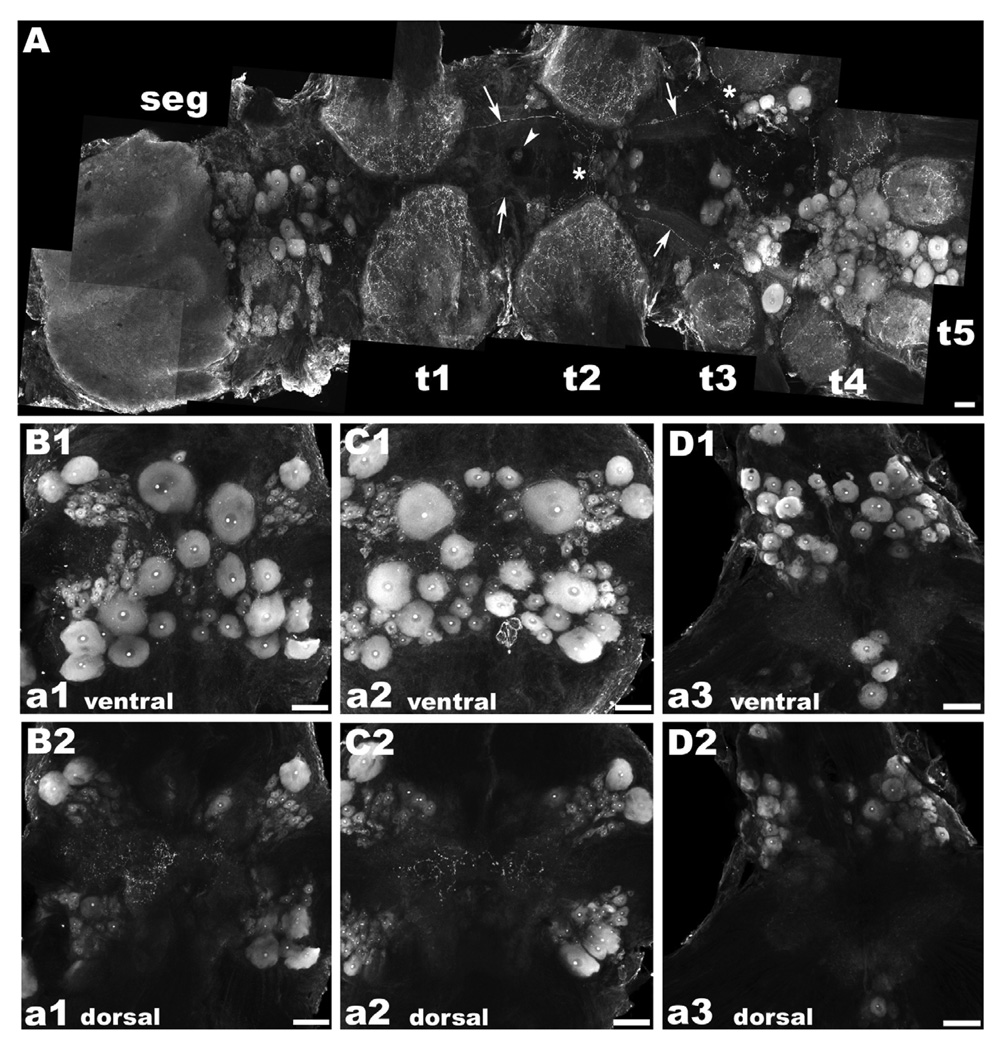
Figure 5. OA/TAMac immunoreactivity (ir) in the subesophageal (seg), thoracic (t), and abdominal (a) ganglia.
A: OA/TAMac-ir was found in a central cluster of large cells in the inferior portion of the seg, superior to t1. Punctate staining was also found in the neuropil of all five thoracic ganglia. Single OA/TAMac-ir large cells were found in the midline of the ventral nerve cord, at the t1 and t2 levels (white arrowhead). The t3–t5 ganglia showed the most prominent staining in large centrally located clusters of multiple-sized cells. A pair of bilateral axons extending from t1 to t3 was observed (white arrows), crossing the ventral midline towards the contralateral side at the t2 level, and other of their ramifications going towards the central, lateral, and inferior parts of the ganglia at the t3 level (white asterisks). B–D: In the abdominal ganglia, staining was observed in cells arranged in clusters, following a “butterfly wing” pattern in the first five ganglia (a1–a5), while a6 also had a pair of clusters of large OA/TAMac-ir cells and four additional cells oriented longitudinally, central and inferior of the neuropil area, extending towards the nerve roots. A representation of the ventral part of a1 (B1), a2 (C1), and a6 (D1) is shown. Punctate OA/TAMac staining was also observed in the neuropil area of all six ganglia. A representation of the dorsal part of a1 (B2), a2 (C2), and a6 (D2) showing the neuropil region is shown. Scale bar = µm.
In the brain, OA/TAMac-ir was observed in the form of punctate staining in the ventral part of the ganglion, in the protocerebrum, lateralmost aspects of the deutocerebrum, and extending towards the inferior aspects of the tritocerebrum (Fig. 4A). Moving more deeply towards the center of the ganglion, punctate staining continues to be observed in the lateralmost protocerebrum, while medium- (~30–50 µm) and large-sized (~60–100 µm) immunoreactive cells are found more medially in this region. More dorsally, punctate staining varies and is observed around the periphery of cells. Highly immunoreactive structures within the nucleus, most likely the nucleoli, are observed in all stained cells. Bilateral immunoreactive axons are observed in the optic nerves going towards a region of intense punctate staining in the lateral protocerebrum (Fig. 4B). In the dorsal part of the ganglion, protocerebrum punctate staining the bilateral axons are still visible. In addition, smaller-sized (~10–20 µm) cell bodies showing OA/TAMac-ir are observed in the deutocerebrum (Fig. 4C).
In the circumesophageal ganglia OA/TAMac-ir was observed in the form of punctate staining towards the center of the ventral (Fig. 4D), middle (Fig. 4E), and dorsal (Fig. 4F) aspects of the ganglia. OA/TAMac-ir is observed in two small-sized cells in the ventral part of the ganglia (Fig. 4D), and in one to three medium-sized cells on the surface of the ganglia (Fig. 4E–F).
In the region between the neuropil of the SEG and T1, OA/TAMac-ir was observed in a central cluster of large-sized cells. We also observed punctuate staining in the neuropil areas of all five thoracic ganglia (Fig. 5A). Single large-sized cells are located in the midline of the ventral nerve cord, at the first and second thoracic levels. The most prominent staining, however, was observed in the T3–T5 ganglia. Each of these ganglia had OA/TAMac staining in large centrally located clusters of multiple-sized cells.
In the abdominal ganglia, staining was observed in the first five ganglia (A1–A5) in cells arranged in clusters, following a the “butterfly wing” pattern mentioned above (Fig. 5B–C), while A6 also had a pair of large clusters of 30–60 µm OA/TAMac stained cells and four immunoreactive cells oriented longitudinally, central and inferior of the neuropil area, extending towards the nerve roots (Fig. 5D). In addition, punctate OA/TAMac staining was observed in the neuropil area of all six ganglia (Fig. 5E–G).
In terms of stained cell bodies, the pattern observed with OA/TAMac-ir is similar to that observed for the receptor’s mRNA. However, as would be expected for a technique that targets sites where transcription and synthesis normally occur, we did not see punctate staining in the neuropil region of the ganglia with in-situ hybridization, nor in processes along the connectives. Instead, we observed a more diffuse staining in the same regions that showed punctate antibody staining, suggesting that there may be mRNA fragments ready to be transcribed as receptors to then be inserted into membranes at synaptic terminals.
3. Discussion
To better understand the role of octopamine and its receptor(s) in establishing dominance hierarchies and modulating aggression in crustaceans, we isolated, cloned, and mapped the distribution of a putative OA/TAMac receptor from the prawn’s CNS.
Cloning of the OA/TAMac receptor
Octopamine is widely considered as the invertebrate counterpart of norepinephrine (NE), and all of its known effects are similar to those observed for activation of adrenergic receptors in vertebrates. One of the most well known functions of adrenergic/octopaminergic signaling is the triggering and modulation of the fight or flight response, which is considered as quick adaptation to energy-demanding situations (Roeder, 2005). Stressful stimuli, such as encounters with predators or rivals, induce this metabolic and behavioral adaptation, leading to enhanced energy supply, increased muscle performance, increased sensory perception, and a corresponding behavioral response (Roeder, 2005).
We cloned a putative OA/TAMac receptor from the CNS of the prawn. Vertebrate and invertebrate biogenic amine receptors have been conserved through evolution in terms of their sequences and signaling pathways (Blenau and Baumann, 2001). We were thus able to take advantage of this sequence conservation to clone this putative receptor from the prawn. Based on its sequence, our cDNA clone encodes a seven transmembrane domain receptor that is most likely a G-protein coupled receptor (GPCR).
Phylogenetic analysis of the OA/TAMac receptor demonstrates a close relationship to other OA/TA-like receptors from arthropods. Specific receptors for OA have been identified through the use of pharmacological and biochemical assays in many invertebrate phyla, including arthropods and mollusks (Axelrod and Saavedra, 1977; Harmer and Horn, 1977; Konishi and Kravitz, 1978; Battelle and Kravitz, 1978; Nathanson, 1979; Battelle et al., 1979; Kravitz et al., 1980; Evans, 1981; Harris-Warrick and Kravitz, 1984). In addition, pharmacological studies in insects have provided evidence for the existence of multiple subtypes of OA receptors (Evans 1980, 1981, 1987; Evans et al, 1988). Even though the number of these identified receptors is growing they are still likely to represent a small fraction of the existing family members. At this time, not enough characterized sequences from across phyla are available to determine a complete evolutionary lineage of the biogenic amine receptors. Recent data suggests that the family is diverse both within and between phyla. Examples of this include the recently identified trace amine-associated receptor family of tyramine-related receptors identified in vertebrates (Hashiguchi and Nishida, 2007) and the appearance of related receptors in sequenced genomes of distant phyla such as Trichoplax adherens (Srivastava et al., 2008). Based on the close relationship between our protein sequence with that of characterized insect’s OA and TA receptors, we can preliminarily identify it as an OA/TA type, the first known in a crustacean species.
OA/TAMac distribution in the CNS of the prawn
To better understand the role played by crustacean OA receptors in aggressive behavior and the establishment of dominance hierarchies, we designed a DIG-labeled probe and raised a polyclonal antibody that recognize this receptor’s mRNA and protein, respectively, and used both to determine its CNS distribution in the prawn.
In terms of specific regions within the prawn’s CNS that showed mRNA expression of the OA/TAMac receptor, both diffuse staining and stained cell bodies were observed in the brain, the lower portion of the SEG ganglion, between it and the first thoracic ganglia (T1), in the midline section and roots of the five thoracic ganglia, in bilateral processes extending from T1 to T3 ganglia, and in the six abdominal ganglia. Likewise, OA/TAMac receptor immunoreactivity (OA/TAMac–ir) is observed as extensive punctuate staining within the neuropil, and on the membrane of groups of neurons in all ganglia, and a bilateral process between T2–T3 ganglia.
Diffuse staining observed with the mRNA probe matches with punctate staining observed when using an anti-OA/TAMac receptor antibody. The diffuse staining pattern observed with in-situ hybridization is indicative of the receptor’s mRNA ready to synthesize the receptor at synaptic sites along neural processes or synaptic terminals. Similarly, punctate antibody staining around the periphery of somata and/or along processes may represent functional aggregations of the receptor in regions where it will perform its signaling role. Localization to the cell body may be indicative that functional receptor here may be responsible for mediating signals that result in rapid changes in the transcriptional activity of the cell. Although we observed punctuate OA/TAMac–ir located on the membrane of groups of neurons, we also observed cytoplasmic staining through all ganglia. This may indicate newly synthesized receptor ready for shipping to the cell membrane or vesicles containing recycled receptor. Similar punctuate localization of serotonin and other GPCRs in somata has been previously reported for the nervous systems of other species, such as rat, fruit fly and spider (Gérard et al., 1994; Ramaekers et al., 2001; Panek et al., 2003).
On the basis of previous reports observed in two different lobster species (Schneider et al., 1993; Antonsen and Paul, 2001), and observations from our own laboratory (Reyes-Colón et al., submitted), there is a relative small number of octopaminergic neurons in crustaceans. Of these octopaminergic cells, only a single pair is located in both A4 and A5 in the American lobster (Schneider et al., 1993), and no cells are found in the abdominal ganglia of neither the squat lobster (Antonsen and Paul, 2001), nor the prawn (Reyes-Colón et al., submitted). Similarly, only a small number of neurons contain OA in the entire nervous system of different insects (Roeder, 2005). However, they are enough to supply most neuropils of the brain and most parts of the thoraco-abdominal nervous system with fine meshworks of OA-containing dendritic structures (Roeder, 2005).
Octopaminergic cells of the thoraco-abdominal nervous system of insects supply peripheral effectors organs with this transmitter (Roeder, 2005). Likewise, OA-neurons are found within the connective tissue sheath of the second roots of lobster thoracic ganglia (Kravitz et al., 1976). These cells can release OA into the hemolymph at two points, and they are likely to serve a neurosecretory function. One site of release is near the point where the hemolymph passes from the ventral sinus to the base of the gills. The second site is close to the lateral ends of the second roots, where they enlarge to form pericardial organs. The pericardial organs lie within the pericardial sinus and are believed to contain distal endings for at least some OA cells (Evans et al., 1976). Freshly oxygenated hemolymph courses by this second site immediately before it enters the heart to then be distributed to all tissues.
Peripheral targets of amines have been shown to have no serotonergic innervation, and are supplied exclusively via the circulation by means of serotonin-containing neurosecretory neurons (Kravitz, 2000). Thus the fact that we found expression of the OA/TAMac receptor in regions where there is no apparent OA innervation lead us to believe that this is most likely also true for OA in the prawn. Immunohistochemical studies in the prawn conducted in our laboratory showed a group of octopaminergic neurons between thoracic ganglia three and four in an area surrounding a small artery connecting two of the main arteries of the prawn (Reyes-Colón et al., submitted). This group of cells may have a similar role in supplying the hemolymph with circulating OA and, subsequently, all peripheral organs.
In summary, by using diverse techniques, we obtained the full sequence of an octopamine/tyramine receptor (OA/TAMac) in the freshwater prawn, and mapped its mRNA and protein expression in the prawn’s CNS. This study constitutes the first report of the cloning, sequencing and mapping of a crustacean OA/TA receptor, and is also the first description of the receptor’s distribution in the crustacean CNS. Phylogenetic analysis indicates that this receptor is most closely related to those of insects. Through in-situ hybridization and immunohistochemistry, we observed the OA/TAMac receptor around and inside somata of specific subsets of neurons, and as diffuse or punctate staining in the neuropil of the brain and all thoracic and abdominal ganglia.
In vertebrates, trace amines are a family of endogenous compounds with structural similarity to classical monoamine neurotransmitters found at low concentrations in the mammalian brain (Berry, 2007; Sotnikova et al., 2009a,b). These monoamines include tyramine, tryptamine, synephrine, octopamine, and β-phenylethylamine. Although their functional role in mammals remains largely unknown, it has been noted that trace amine levels can be altered in various human disorders (Sotnikova et al., 2009a,b). The discovery of a specific family of GPCRs known as trace amines-associated receptors, which are capable of being activated by these trace amines, promotes the idea to use them as targets to correct monoaminergic processes that could be dysfunctional in various disorders of brain and peripheral nervous system (Sotnikova et al., 2009a,b). To do this, it is necessary to further characterize their functional roles and biochemical properties, and identify their endogeous and exogenous ligands (Sotnikova et al., 2009a,b).
These experiments represent important steps to better understand the octopaminergic system in the CNS of crustaceans. These findings will lay down the basis for elucidating the mechanisms linking biogenic amine synaptic functions with interactive behaviors. Further experiments will examine if this receptor is differentially expressed in specific areas of the CNS in a manner that can be correlated with the characteristic levels of aggression or the dominance status of the prawn’s three male morphotypes.
4. Experimental Procedure
Experimental animals
Male prawns (Macrobrachium rosenbergii) measuring 8–15 cm in length, from eyestalk to telson, were obtained from the Experimental Aquaculture Station of the University of Puerto Rico and/or from private suppliers in the North coast of Puerto Rico. They were maintained in 30-gallon tanks with continuously filtered and aerated water under a 12h light/12h dark cycle. Water temperature was maintained at 26–28°C and the pH adjusted to 7.7 (safe range= 6.9–8.5). Animals were fed a high protein (>40%) pelleted purina chow once every other day.
Dissection
Prawns in the inter-molt (C) stage of the molting cycle (Peebles, 1977) were used, with no missing or recently regenerated limbs and no signs of parasitism. They were anesthetized and euthanized by cooling on ice from 10–15 minutes, until they became fully unresponsive (i.e. movement of swimmerets/walking legs and heartbeat stopped). This method is considered acceptable for crustaceans (Coyle et al., 2004), being faster and producing less distress than exposure to chemical anesthetics, which usually require higher concentrations than those used in fish and other vertebrates. It has also been deemed less disruptive of central nervous system function and structure than exposure to hypertonic saline solutions (Battison et al., 2000). After being weighed and measured, the prawns were transected between the thorax and abdomen. Their claws, walking legs, and carapaces were removed and one segment of the animal was placed in cold (4°C) prawn saline solution (PSS), while the other segment was dissected. The PSS had the following composition in (mM): NaCl 220; KCl 5.5, CaCl2 13.5; MgCl2 2.5; Tris 5; pH=7.4 (Miller et al., 1985). The dissection was carried out on ice. The thoracic and abdominal ventral nerve cord and the brain were isolated quickly with forceps by removing all surrounding organs and muscles and cutting all nerves.
For in-situ hybridization experiments, all tissues were immediately treated in Protease (1 mg/mL PSS) at 34° C for 5–10 minutes, rinsed four times in PSS, and fixed by immersion in freshly prepared 4% paraformaldehyde in 0.1 M phosphate buffer saline (PBS) for three hours. Preparations were washed in 0.1M PBS three times for 15 minutes each, and fully desheathed with fine forceps and scissors.
All procedures involving the use of animals were approved by the University of Puerto Rico Medical Sciences Campus Institutional Animal Care and Use Committee prior to the start of the experiments.
Cloning of OA/TAMac receptor
Total RNA was extracted from the central nervous system of the freshwater prawn according to prodedures previously described by Chomczynski and Sacchi (1987). Briefly, a magnasphere kit (Promega, Madison, WI, USA) was used to isolate mRNA from total RNA. First-strand cDNA was produced in a standard 20-µl reaction using Superscript II (Life Technologies, Gaithersburg, MD, USA) according to the directions provided by the manufacturer: 1 × first-strand buffer, 1–5 µg total RNA, 250 ng random hexamer, 500 µM dNTP, 10 Nm DTT. Amino acid sequences from OA/TA receptors cloned previously in other species were aligned and conserved regions were chosen as templates for the synthesis of degenerate primers. A standard PCR was performed using 5 µl of first strand cDNA and the following degenerate primers:
Forward: 5’-CCCAATCTGGTTCCTCCTCCCATAGCCG-3’
Reverse: 5’-CCCATTCTGTCCATTCTCACTGACGGC-3’
This reaction produced a 759-base pair PCR product that was purified, subcloned using a TOPO cloning kit (Invitrogen, La Jolla, CA, USA), and sequenced (Cornell BioResource Center, Ithaca, NY, USA).
RACE to obtain the OA/TAMac receptor termini
We performed 5’ and 3’ rapid amplification of cDNA ends (RACE) using the following RACE primers:
5’ RACE: 5’-AATGGCTGTGCTATAATGATCC-3’
3’ RACE: 5’-AGGTCGATACACTCATCCACCG-3’
The prawn’s mRNA was purified from nervous system total RNA with Oligotex (Qiagen, Chatsworth, CA, USA). 5’ and 3’ RACE reactions were performed with the SMART RACE amplification kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. RACE products were cloned with a TA cloning kit (Invitrogen, La Jolla, CA, USA) and sequenced (Cornell BioResource Center, Ithaca, NY, USA).
BLAST analysis followed by paired alignments with invertebrate TYR- and OA-like receptors was conducted to confirm the identity of the prawn’s OA/TAMac receptor. The prawn’s OA/TAMac predicted amino acid sequence was then aligned with TYR-, and OA-like receptors from other species using ClustalX (Jeanmougin et al., 1998; Thompson et al., 1997) with default parameters. Accession numbers for sequences used in this alignment were: Macrobrachium rosenbergii, MrTyrOctMacR, EU233816; Bombyx mori, BmTyrR, BAD11157; BmOctR, NP_001037504; Heliothis virescens, HvOctR, Q25188; Mamestra brassicae, MbOctR, AAK14402; LocusTYR migratoria, LmTyrR2, Q25322; LmTyrR1, Q25321; Drosophila melanogaster, DmTyrR NP_524419; DmOctR AAA28731.
Phylogenetic analysis
The phylogenetic tree was generated using the maximum likelihood algorithm implemented in the program TREE-PUZZLE (Schmidt et al., 2002) (http://www.tree-puzzle.de). The JTT (Jones et al, 1992) model of amino acid substitution, exact parameter estimates, and 10,000 puzzling steps were used in the calculation. The initial multiple alignment was done using ClustalX (Jeanmougin et al., 1998; Thompson et al., 1997) with default parameters. Gaps in the alignment were removed manually in GeneDoc (Nicholas et al., 1997) prior to tree construction. The tree image was first generated using Treeview (Page, 1996) and the final graphic was produced with Corel Draw.
The scientific name, common name, abbreviation, and accession number for all species and sequences used in the phylogenetic analysis are listed as follows: Apis mellifera, Honey bee (DA1, NP_001011595.1; DA2, NP_001011567.1); Bombyx mori, Silkworm (DA1, NP_001108459.1; DA2, NP_001108338.1; Tyr, BAD11157; Oct, NP_001037504; 5-HT1, NP_001037502.1); Drosophila melanogaster, Fruit fly (DA1, AAC47161.1; DA2E, NP_001027080.1; Oct-α, AAF55798.2; Oct-β, ABC66171.2; Tyr/Oct, P22270.2; 5-HT1, NP_476802; 5-HT2, NP_649806; DA1, AAC47161.1; DA2E, NP_001027080.1); Heliothis virescens, Tobacco budworm (Oct, Q25188); Locust migratoria, Migratory Locust (TA, Q25321) ; Machrobrachium rosenbergii, Prawn (5-HT1, EU363466; 5-HT2, EF033662; OA/TAmac, EU233816); Mamestra brassicae, Cabbage Moth (Oct, AAK14402); Panularis interruptus, Lobster (DA1, ABB87182.1; DA1b, ABB87183.1; DA2, ABI64137.1; 5-HT1, AAS18607.1; 5-HT2, AAS57919); Periplaneta americana, Cockroach (Oct-α, AAP93817.1; TA, CAQ48240.1); Procambarus clarkii, Crayfish (5-HT1, ABX10973.1; 5-HT2, ABX10972.1); Schistocerca gregaria, Desert Locust ( Oct-α, ADD91574.1; Oct-β, ADD91575.1).
In-situ hybridization of the OA/TAMac receptor
The in-situ hybridization protocol was modified from those previously published by Bogdanov et al., 1996 and Ono et al., 2000. After all ganglia were fully desheathed with fine forceps and scissors, we transferred them to 24-well plates, and proceeded with washes in 10× PBS/Tween-20 (PTW) and Triton X-100 for ten minutes each, PTW for five minutes and treatment in Proteinase K at room temperature for one hour. Tissue was post-fixed in 4% paraformaldehyde in 0.1 M PBS, washed in Glycine/PTW two times, PTW three times, and Triethanolamine hydrochloride acid (TEA-HCl) pH 8 two times. Acetic anhydride was added (2.5 ul) to the TEA-HCl followed by shaking for five minutes two times, and then the tissue was washed in PTW four times. Preparations were placed in Hybridization buffer (50% formamide, 5mM EDTA, 5× SSC, 1× Denhardt’s solution (0.02% ficoll, 0.02% polyvynilpirrolidon, 0.02% bovine serum albumin (BSA)), 0.1% Tween 20, 0.5 mg/ml yeast tRNA) for 6–8 hrs at 50°C. The probe for OA/TAMac receptor was added to same well (1ul of probe/mL of Hybridization buffer) and incubated overnight at 50°C. Tissue was washed in 50% formamide/5× saline sodium citrate (SSC)/1% sodium dodecyl sulfate (SDS) for 30 min at 60°C, then in 50% formamide/2× SSC/1% SDS for 30 min at 60°C, and then in 0.2× SSC for 30 min at 55°C two times. Tissue was washed in 1× PBS, 0.1% Triton X-100, 2 mg/ml BSA (PBT) four times, and blocked with 10% normal goat serum in PBT for 90–120 min at 4°C, and incubated with alkaline phosphatase-conjugated Digoxigenin(DIG)-antibody (1:1000) in 1% normal goat serum in PBT for 12–14 hrs at 4°C. Preparations were washed in PBT for 30 minutes three times, and in detection buffer (100 mM NaCl, 50 mM MgCl2, 0.1% Tween-20, 1 mM levamisol, 100 mM TrisHCl, pH=9.5) twice for five minutes. In the same well, we added 20 ul/ml of nitro-blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) solution to the detection buffer, incubated in the dark at 4°C, post-fixed the samples with 4% Paraformaldehyde/MeOH for 30–60 minutes at 4°C, and washed twice in PBT. The tissue was exposed to a Ethanol/0.1M PBS series (100%, 70%, 50%, 30%; 5 minutes each), followed by a 0.1M PBS/Glycerol series (100%, 70%, 50%, 30%; 5 minutes each). We then placed the tissue in Glycerol Buffer and mounted it in a slide with a cover slip to take pictures with a Zeiss Axioscop microscope. Controls included use of the sense probe instead of the anti-sense, and omission of the probe.
Generation of affinity-purified anti-OA/TAMac antibodies
The amino acids sequences of OA/TA receptors from the cabbage moth (Accession Number: AAK14402), tobacco budworm (Accession Number: Q25188), silkworm (Accession Numbers: NP_001037504, BAD11157), fly (Accession Numbers: AAA28731, NP_524419), migratory locust (Accession Numbers: Q25321, Q25322), and freshwater prawn (Accession Number: EU233816) were aligned (Figure 1) and two conserved, highly charged, non-transmembrane regions were identified:
Peptide A: CQFIEEKQKISLSKERRAART
Peptide B: DWPDVFTEDTPCILTEEKGF
To determine if any known proteins other than OA/TA receptors contain a similar peptide sequence, each peptide was used in a protein-protein blast (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi), and no such instances were found. Peptides representing these two regions were synthesized and each was used as an antigen in the production of affinity-purified sheep polyclonal antibodies. For peptide A, a cysteine was included at the beginning of the sequence in order to conjugate it to the carrier, KLH. Peptide synthesis, conjugation to KLH, antibody production, and affinity purification were performed by Bethyl Laboratories (Montgomery, TX, USA).
OA/TAMac immunoreactivity
Ventral nerve cords were removed in PSS as described above. They were immediately fixed by immersion in freshly prepared 4% paraformaldehyde in 0.1M PBS at room temperature for three hours with constant shaking, and rinsed overnight in 0.1M PBS containing 0.2% Triton X-100 (PBST). Preparations were incubated in normal donkey serum (1:20) in 0.1M PBST at 4°C for 3–5 hours, followed by the primary policlonal sheep anti-OA/TAMac-B antibody (Bethyl Laboratories, TX, USA), at a concentration of 5 µg/ml in PBST for 2–3 days. After washing 12 times with 0.1M PBST, 30 minutes each, tissue was incubated in the secondary antibody donkey anti-sheep Alexa 488 (Molecular Probes, Carlsbad, CA, USA) at a dilution of 1:200 in PBST at 4°C overnight. Preparations were washed in 0.1M PBST 6 times, 1 hour each, then in 0.1M PBS 5 times, 15 minutes each, and left overnight in 90% glycerol/PBS buffer. The next day they were mounted with Polyaquamount, coverslipped, and viewed with a Zeiss Axioskop using epifluorescent excitation and/or a Zeiss LSM Pascal confocal microscope. The brightness and contrast of the digital images obtained by the Zeiss LSM Pascal software were adjusted so that they would be uniform in the figures presented here. Confocal stacks were reconstructed and analyzed in Adobe Photoshop 7.0 on a Dell PC computer. Controls included preadsorption of the primary antibody with the peptide used to generate the antibody for 3–4 hrs at room temperature before incubation with the preparation (peptide to antibody: 1:20, w/w).
Acknowledgements
We would like to thanks Ms. Yelena Bobkova from the Whitney Laboratory for Marine Bioscience for technical support.
This work was supported by NIH/MBRS-SCORE S06GM008224 (Sosa), NIMH/MRISP MH48190 (Sosa), NIH/RCMI G12RR03051 (Institute of Neurobiology), NSF DBI 0115825 (Institute of Neurobiology), NIH RO1 NS39103 (Moroz), NIH/MBRS-RISE R25-GM061838 (School of Medicine).
Abbreviations
- 5-HT
serotonin
- OA
octopamine
- BC
blue-clawed male
- YC
yellow-clawed male
- SC
small-clawed male
- CNS
central nervous system
- TA
tyramine
- DA
dopamine
- OA/TAMac
Macrobrachium rosenbergii putative octopamine/tyramine receptor
- GPCR
G-protein coupled receptor
- Pro
protocerebrum
- Tri
tritocerebrum
- CEG
circumesophageal ganglia
- SEG
subesophageal ganglion
- T1–T5
first to fifth thoracic ganglia
- A1–A6
first to sixth abdominal ganlia
- RACE
rapid amplification of cDNA ends
- PSS
prawn saline solution
- PBS
phosphate buffer solution
- PTW
PBS with Tween-20
- BSA
bovine serum albumin
- TEA-HCl
triethanolamine hydrochloride acid
- SSC
saline sodium citrate
- SDS
sodium dodecyl sulfate
- PBT
PBS with Triton X-100 and BSA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Antonsen BL, Paul DH. Serotonergic and octopaminergic systems in the squat lobster Munida quadrispina (Anomura, Glatheidae) J Comp Neurol. 2001;439:450–468. doi: 10.1002/cne.1362. [DOI] [PubMed] [Google Scholar]
- Arakawa S, Gocayne JD, McCombie WR, Urquhart DA, Hall LM, Fraser CM, Vanter JC. Cloning, localization, and permanent expression of a Drosophila octopamine receptor. Neuron. 1990;4:343–354. doi: 10.1016/0896-6273(90)90047-j. [DOI] [PubMed] [Google Scholar]
- Axelrod J, Saavedra JM. Octopamine. Nature. 1977;265:501–504. doi: 10.1038/265501a0. [DOI] [PubMed] [Google Scholar]
- Barki A, Karplus I, Goren M. The agonistic behavior of the three male morphotypes of the freshwater prawn Macrobrachium rosenbergii (Crustacea, Palaemonidae) Behavior. 1991a;116:252–277. [Google Scholar]
- Barki A, Karplus I, Goren M. Morphotype related dominance hierarchies in males of Macrobrachium rosenbergii (Crustacea, Palaemoidae) Behavior. 1991b;117:145–160. [Google Scholar]
- Barki A, Karplus I, Goren M. Effects of size and morphotype on domiance hierarachies and resource competition in the freshwater prawn Macrobrachium rosenbergii. Animal Behavior. 1992;44:547–555. [Google Scholar]
- Battelle BA, Kravitz EA, Sitieve H. Neurotransmitter synthesis in Limulus ventral nerve photoreceptors. Experientia. 1979;35:778. doi: 10.1007/BF01968242. [DOI] [PubMed] [Google Scholar]
- Battelle BA, Kravitz EA. Targets of octopamine action in the lobster: Cyclic nucleotide changes and physiological effects in hemolymph, heart and exoskeletal muscle. J Pharmacol. Exp. Ther. 1978;205:438–448. [PubMed] [Google Scholar]
- Battison A, MacMillan R, Mackenzie A, Rose P, Cawthorn R, Horney B. Use of injectable potassium chloride for euthanasia of American lobsters (Homarus americanus) Comp. Med. 2000;50:545–550. [PubMed] [Google Scholar]
- Beltz BS. Distribution and functional anatomy of amine-containing neurons in decapod crustaceans. Microsc. Res. Tech. 1999;44:105–120. doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<105::AID-JEMT5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Berry MD. The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev Recent Clin Trials. 2007;2:3–19. doi: 10.2174/157488707779318107. [DOI] [PubMed] [Google Scholar]
- Blenau W, Baumann A. Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera. Arch. Insect Biochem. Physiol. 2001;48:13–38. doi: 10.1002/arch.1055. [DOI] [PubMed] [Google Scholar]
- Blumenthal EM. Regultion of chloride permeability by endogenously produced tyramine in the Drosophila Malpighian tubule. Am. J. Physiol. Cell. Physiol. 2003;284:C718–C726. doi: 10.1152/ajpcell.00359.2002. [DOI] [PubMed] [Google Scholar]
- Bogdanov YD, Balaban PM, Zakharov IS, Poteryaev DA, Belyavsky AV. Identification of two novel genes specifically expressed in the D-group neurons of the terrestrial snail CNS. Invertebr. Neurosci. 1996;2:61–69. doi: 10.1007/BF02336661. [DOI] [PubMed] [Google Scholar]
- Briffa M, Elwood RW. Monoamines and decision making during contests in the hermit crab Pagurus bernhardus. Animal Behaviour. 2007;73:605–612. [Google Scholar]
- Broeck JV, Vulsteke V, Huybrechts R, De Loof A. Characterization of a cloned locust tyramine receptor cDNA by functional expression in permanently transformed Drosophila S2 cells. Journal of Neurochemistry. 1995;64:2387–2395. doi: 10.1046/j.1471-4159.1995.64062387.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidium thiocycanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coyle SD, Durborow RM, Tidwell JH. Anesthesia and aquaculture. Vol. 3900. Southern Regional Aquaculture Center (SRAC) Publication; 2004. pp. 1–6. [Google Scholar]
- Donini A, Lange AB. Evidence for a possible neurotransmitter/neuromodulator role of tyramine on the locust oviducts. J. Insect Physiol. 2004;50:351–361. doi: 10.1016/j.jinsphys.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Downer RGH. Trehalose production in isolated fat body of the American cockroach, PeriplaneTYR americana. Comp. Biochem. Physiol. 1979;62C:31–34. doi: 10.1016/0306-4492(79)90096-0. [DOI] [PubMed] [Google Scholar]
- Dudel J. Facilitatory effects of 5-hydroxy-tryptamine on the crayfish neuromuscular junction. Nauyn Schmiedeberg’s Arch Pharmacol. 1965;249:515–528. doi: 10.1007/BF00246558. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Issa FA, Herberholz J. The neural basis of dominance hierarchy formation in crayfish. Microsc Res. Tech. 2003;60:369–376. doi: 10.1002/jemt.10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DH, Spitzer N. Social dominance and serotonin receptor genes in crayfish. Curr. Top. Dev. Biol. 2006;74:177–199. doi: 10.1016/S0070-2153(06)74006-6. 6. [DOI] [PubMed] [Google Scholar]
- Evans PD, Maqueira D. Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert. Neurosci. 2005;5:111–118. doi: 10.1007/s10158-005-0001-z. [DOI] [PubMed] [Google Scholar]
- Evans PD, Thonoor M, Midgeley JM. Activities of octopamine and synephrine stereoisomers on octopaminergic receptor subtypes in locust skeletal muscle. J. Pharm. Pharmacol. 1988;40:855–861. doi: 10.1111/j.2042-7158.1988.tb06288.x. [DOI] [PubMed] [Google Scholar]
- Evans PD, Kravitz EA, Talamo BR. Octopamine release at two points along lobster nerve trunks. J Physiol. 1976;262:71–89. doi: 10.1113/jphysiol.1976.sp011586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PD. Biogenic amines in the insect nervous system. Adv. Insect Physiol. 1980;15:317–473. [Google Scholar]
- Evans PD. Multiple receptor types for octopamine in the locust. J Physiol. (Lond) 1981;318:99–122. doi: 10.1113/jphysiol.1981.sp013853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PD. Phenyliminoimidalolidine derivatives activate both octopamine1 and octopamine2 receptor subtypes in locust skeletal muscle. J Exp. Biol. 1987;129:239–250. [Google Scholar]
- Fischer L, Florey E. Octopamine action on the contractile system of crustacean skeletal muscle. Comp Biochem Physiol. 1987;88C:335–342. doi: 10.1016/0742-8413(87)90131-9. [DOI] [PubMed] [Google Scholar]
- Florey E, Rathmayer M. The effects of octopamine and other amines on the heart and on neuromuscular transmission in decapod crustaceans: further evidence for a role as a neurohormone. Comp Biochem Physiol. 1978;61C:229–237. doi: 10.1016/0306-4492(78)90136-3. [DOI] [PubMed] [Google Scholar]
- Gérard C, Langlois X, Gingrich J, Doucet E, Vergé D, Kia HK, Raisman R, Gozlan H, el Mestikawy S, Hamon M. Production and characterization of polyclonal antibodies recognizing the intracytoplasmic third loop of the 5-hydroxytryptamine1A receptor. Neuroscience. 1994;62(3):721–739. doi: 10.1016/0306-4522(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Gerhardt CC, Barker RA, Piedk GJ, Planta RJ, Vreugdenhil E, Leysen JE, van Heerlkhuizen H. Molecular cloning and pharmacological characterization of a molluscan octopamine receptor. Mol. Pharmacol. 1997a;51:293–300. doi: 10.1124/mol.51.2.293. [DOI] [PubMed] [Google Scholar]
- Gerhardt CC, Lodder HC, Vincent M, Bakker RA, Planta RJ, Vreugdenhil E, Kits KS, van Heerlkhuizen H. Cloning and expression of a complementary DNA encoding a molluscan octopamine receptor that couples to chloride channels in HEK293 cells. J. Biol. Chem. 1997b;272:6201–6207. doi: 10.1074/jbc.272.10.6201. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Krasne FB. Serotonin and octopamine have opposite modulatory effects on the crayfish’s lateral giant escape reaction. J. Neurosci. 1983;3:2263–2269. doi: 10.1523/JNEUROSCI.03-11-02263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann L, Blenau W, Erber J, Erbert PR, Strunker T, Baumann A. Molecular and functional characterization of an octopamine receptor form honeybee (Apis mellifera) brain. J. Neurochem. 2003;86:725–735. doi: 10.1046/j.1471-4159.2003.01876.x. [DOI] [PubMed] [Google Scholar]
- Han KA, Millar NS, Davis RL. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J. Neurosci. 1998;18:3650–3658. doi: 10.1523/JNEUROSCI.18-10-03650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer AJ, Horn AS. Octopamine-sensitive adenylate cyclase in cockroach brain: effects of agonist, antagonists and guanylyl nucleotides. Mol. Pharmacol. 1977;13:512–520. [PubMed] [Google Scholar]
- Harris-Warrick RM, Kravitz EA. Cellular mechanisms for modulation of posture by octopamine and serotonin in the lobster. J. Neurosci. 1984;4:1976–1993. doi: 10.1523/JNEUROSCI.04-08-01976.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi Y, Nishida M. Evolution of trace amine-associated receptor (TAAR) gene family in vertebrates: lineage-specific expansions and degradations of a second class of vertebrate chemosensory receptors expressed in the olfactory epithelium. Mol. Biol. Evol. 2007;24(9):2099–2107. doi: 10.1093/molbev/msm140. [DOI] [PubMed] [Google Scholar]
- Hirashima A, Morimoto M, Kuwano E, Eto M. Octopaminergic agonists for the cockroach neuronal octopamine receptor. Journal of Insect Science. 2003;3:1–9. doi: 10.1093/jis/3.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R. Amines and motivated behaviors: A simpler systems approach to complex behavioral phenomena. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2005;191:231–239. doi: 10.1007/s00359-004-0585-5. [DOI] [PubMed] [Google Scholar]
- Huddart H, Oldfield AC. Spontaneous activity of foregut and hindgut: visceral muscle of the locust, LocusTYR migratoria-II. The effect of biogenic amines. Comp. Biochem. Physiol. 1982;73C:303–311. [Google Scholar]
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 1998;23:403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- Konishi S, Kravitz EA. The physiological properties of amine-containing neurons in the lobster nervous system. J. Physiol. 1978;279:215–229. doi: 10.1113/jphysiol.1978.sp012341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz EA, Battelle BA, Evans PD, Talamo BR, Wallace BG. Octopamine neurons in lobsters. In: Ferrendelli JA, McEwen BS, Snyder SH, editors. Society for Neuroscience Symposia. Vol. 1. 1976. pp. 67–81. [Google Scholar]
- Kravitz EA, Glusman S, Harris-Warrick RM, Livingstone MS, Schwarz T, Goy MF. Amines and a peptide as neurohormones in lobsters: actions on neuromuscular preparations and preliminary behavioral studies. J. Exp. Biol. 1980;89:159–175. doi: 10.1242/jeb.89.1.159. [DOI] [PubMed] [Google Scholar]
- Kravitz EA. Serotonin and aggression: insights gained from a lobster model system and speculations on the role of amine neurons in a complex behavior. J Comp Physiol A. 2000;186:221–238. doi: 10.1007/s003590050423. [DOI] [PubMed] [Google Scholar]
- Kuris AM, Ra’anan Z, Sagi A, Cohen D. Morphotypic differentiation of male Malaysian giant prawns Macrobrachium rosenbergii. J. Crust. Biol. 1987;7:219–237. 1. [Google Scholar]
- Kutsukake M, Komatsu A, Yamamoto D, Ishiwa-Chigusa S. A tyramine receptor gene mutation causes a defective olfactory behavior in Drosophila melanogaster. Gene. 2000;245:31–42. doi: 10.1016/s0378-1119(99)00569-7. [DOI] [PubMed] [Google Scholar]
- McClung C, Hirsh J. The trace amine tyramine is essential for sensitization to cocaine in Drosophila. Curr. Biol. 1999;9:853–860. doi: 10.1016/s0960-9822(99)80389-3. [DOI] [PubMed] [Google Scholar]
- Miller MW, Parnas H, Parnas I. Dopaminergic modulation of neuromuscular transmission in the prawn. J Physiol. 1985;363:363–375. doi: 10.1113/jphysiol.1985.sp015716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson JA. Octopamine receptors, adenosine 3',5'-monophosphate, and neural control of firefly flashing. Science. 1979;203(4375):65–68. doi: 10.1126/science.214856. [DOI] [PubMed] [Google Scholar]
- Nicholas KB, Nicholas HB, Jr, Deerfield DW., II GeneDoc: analysis and visualization of genetic variation. Embnew News. 1997;4:14. [Google Scholar]
- Ohta H, Utsumi T, Ozoe Y. B96Bom encodes a Bombyx mori tyramine receptor negatively coupled to adenylate cyclase. Insect Mol. Biol. 2003;12:217–223. doi: 10.1046/j.1365-2583.2003.00404.x. [DOI] [PubMed] [Google Scholar]
- Page RD. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Panek I, Meisner S, Torkkeli PH. Distribution and function of GABAB receptors in spider peripheral mechanosensilla. J Neurophysiol. 2003;90(4):2571–2580. doi: 10.1152/jn.00321.2003. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Yue Z, Drerup C, Huber R. Amine neurochemistry and aggression in crayfish. Micros. Res. Tech. 2003;60:360–368. doi: 10.1002/jemt.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasztor VM, Macnillan DL. The actions of proctolin, octopamine and serotonin on crustacean proprioceptors show species and neurone specificity. J Exp Biol. 1990;152:485–504. [Google Scholar]
- Peebles JB. A rapid technique for molt staging in live Macrobrachium rosenbergii. Aquaculture. 1977;12:173–180. [Google Scholar]
- Ra’anan Z, Sagi A. Alternative mating strategies in male morphotypes of the freshwater prawn Macrobrachium rosenbergii (de Man) Biol. Bull. 1985;169:592–601. [Google Scholar]
- Ra’anan Z, Cohen D. The ontogeny of social structure and population dynamics in the freshwater prawn Macrobrachium rosenbergii (de Man) In: Schram FM, Wenner A, editors. Crustacean Issues II. Crustacean Growth. 1985. pp. 277–311. 1. [Google Scholar]
- Ramaekers A, Parmentier ML, Lasnier C, Bockaert J, Grau Y. Distribution of metabotropic glutamate receptor DmGlu-A in Drosophila melanogaster central nervous system. J Comp Neurol. 2001;438(2):213–225. doi: 10.1002/cne.1310. [DOI] [PubMed] [Google Scholar]
- Reale V, Hannan F, Midgley JM. The expression of a cloned Drosophila octopamine/tyramine receptor in Xenopus oocytes. Brain Res. 1997;769:309–320. doi: 10.1016/s0006-8993(97)00723-3. [DOI] [PubMed] [Google Scholar]
- Rex E, Komuniecki RW. Characterization of a tyramine receptor from Caenorhabditis elegans. Journal of Neurochemistry. 2002;82:1352–1359. doi: 10.1046/j.1471-4159.2002.01065.x. [DOI] [PubMed] [Google Scholar]
- Roeder T. Octopamine in invertebrates. Progress in Neurobiology. 1999;59:533–561. doi: 10.1016/s0301-0082(99)00016-7. [DOI] [PubMed] [Google Scholar]
- Roeder T. Tyramine and octopamine: Ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- Sarawasti S, Fox LE, Soil DR, Wu CF. Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. J. Neurobiol. 2004;58:425–441. doi: 10.1002/neu.10298. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Nagao T. Brain tyramine and reproductive states of workers in honeybees. J Insect Physiol. 2002;48:1075–1085. doi: 10.1016/s0022-1910(02)00200-7. [DOI] [PubMed] [Google Scholar]
- Saudou F, Amlaiky N, Plassat JL, Borelli E, Hen R. Cloning and characterization of a Drosophila tyramine receptor. EMBO J. 1990;9:3611–3617. doi: 10.1002/j.1460-2075.1990.tb07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HA, Strimmer K, Vingron M, von Haeseler A. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics. 2002;18:502–504. doi: 10.1093/bioinformatics/18.3.502. [DOI] [PubMed] [Google Scholar]
- Schneider H, Trimmer BA, Rapus J, Eckert M, Valentine DE, Kravitz EA. Mapping of octopamine-immunoreactive neurons in the central nervous system of the lobster. J. Comp. Neurol. 1993;329:129–142. doi: 10.1002/cne.903290109. [DOI] [PubMed] [Google Scholar]
- Sneddon LU, Taylor AC, Huntingford FA, Watson DG. Agonistic behaviour and biogenic amines in shore crabs Carcinus maenas. J. Exp. Biol. 2000;203:537–545. doi: 10.1242/jeb.203.3.537. [DOI] [PubMed] [Google Scholar]
- Sosa MA, Baro DJ. Amine effects on aggression in the giant tropical freshwater prawn Macrobrachium rosenbergii. In: Wiese K, editor. The Crustacean Nervous System. Springer-Verlag Berlin-Heidelberg; 2002. pp. 143–155. [Google Scholar]
- Sotnikova LS, Zhdanov VV, Udut VV, Dygai AM. Influence of poetam preparation on the state of autonomic nervous system in patients with severe anemia caused by dysfunctional uterine bleedings. Bull Exp Biol Med. 2009a;148:508–510. doi: 10.1007/s10517-010-0749-y. [DOI] [PubMed] [Google Scholar]
- Sotnikova LS, Shevtsova NM, Sherstoboev EY, Zhdanov VV, Dygai AM. Complex application of preparation containing ultralow doses of antibodies for the treatment of anemia caused by pubertal uterine bleedings. Bull Exp Biol Med. 2009b;148:505–507. doi: 10.1007/s10517-010-0748-z. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Begovic E, Chapman J, Putnam NH, Hellsten U, Kawashima T, Kuo A, Mitros T, Salamov A, Carpenter ML, Signorovitch AY, Moreno MA, Kamm K, Grimwood J, Schmutz J, Shapiro H, Grigoriev IV, Buss LW, Schierwater B, Dellaporta SL, Rokhsar DS. The Trichoplax genome and the nature of placozoans. Nature. 2008;454(7207):955–960. doi: 10.1038/nature07191. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The Clustal_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Nickisch-Rosenegk E, Krieger J, Kubick S, Laage R, Strobel J, Strotmann J, Breer H. Cloning of biogenic amine receptors from moths (Bombyx mori and Heliothis virescens) Insect. Biochem. Mol. Biol. 1996;26:817–827. doi: 10.1016/s0965-1748(96)00031-8. [DOI] [PubMed] [Google Scholar]
- Yeh SR, Fricke RA, Edwards DH. The effects of social experiences on serotonergic modulation of the escape circuit of crayfish. Science. 1996;271:366–369. doi: 10.1126/science.271.5247.366. [DOI] [PubMed] [Google Scholar]