Abstract
In order to determine whether an adrenergic mechanism is involved in the secretion of growth hormone and insulin, the effect of adrenergic-blocking or -stimulating agents on plasma human growth hormone (HGH), immunoreactive insulin, blood free fatty acids (FFA), and glucose levels was studied in normal human subjects.
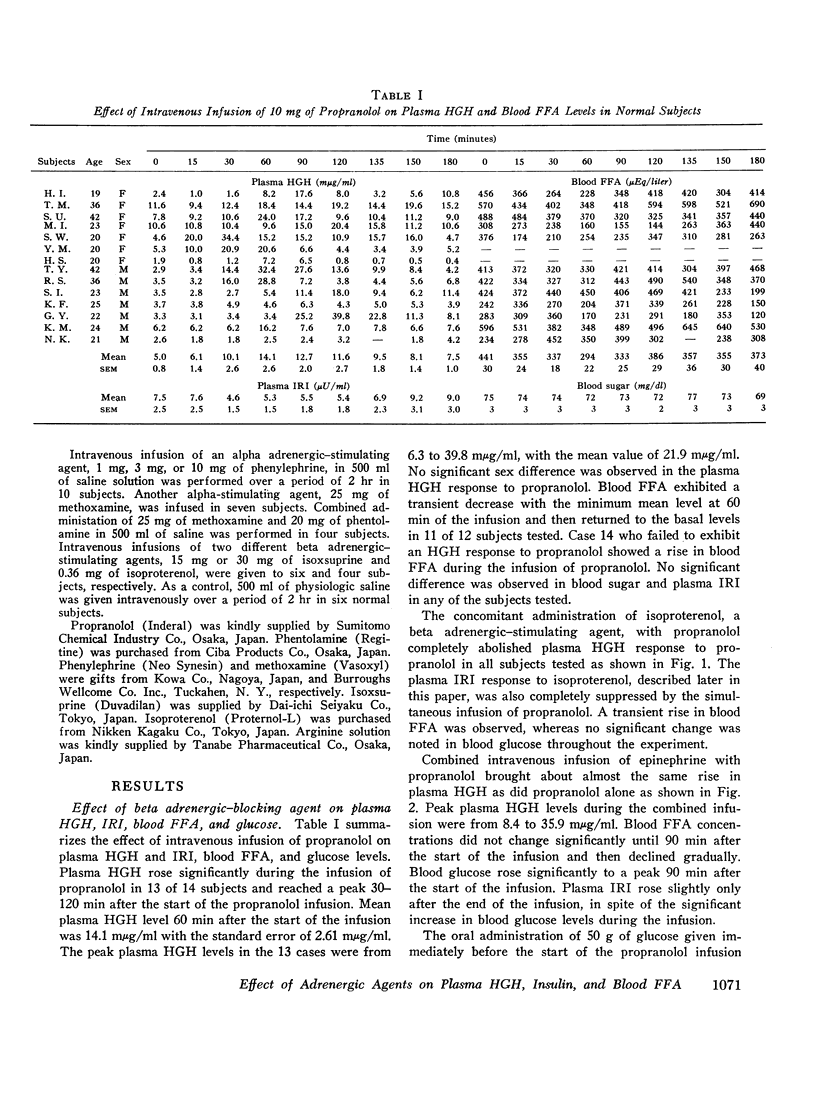
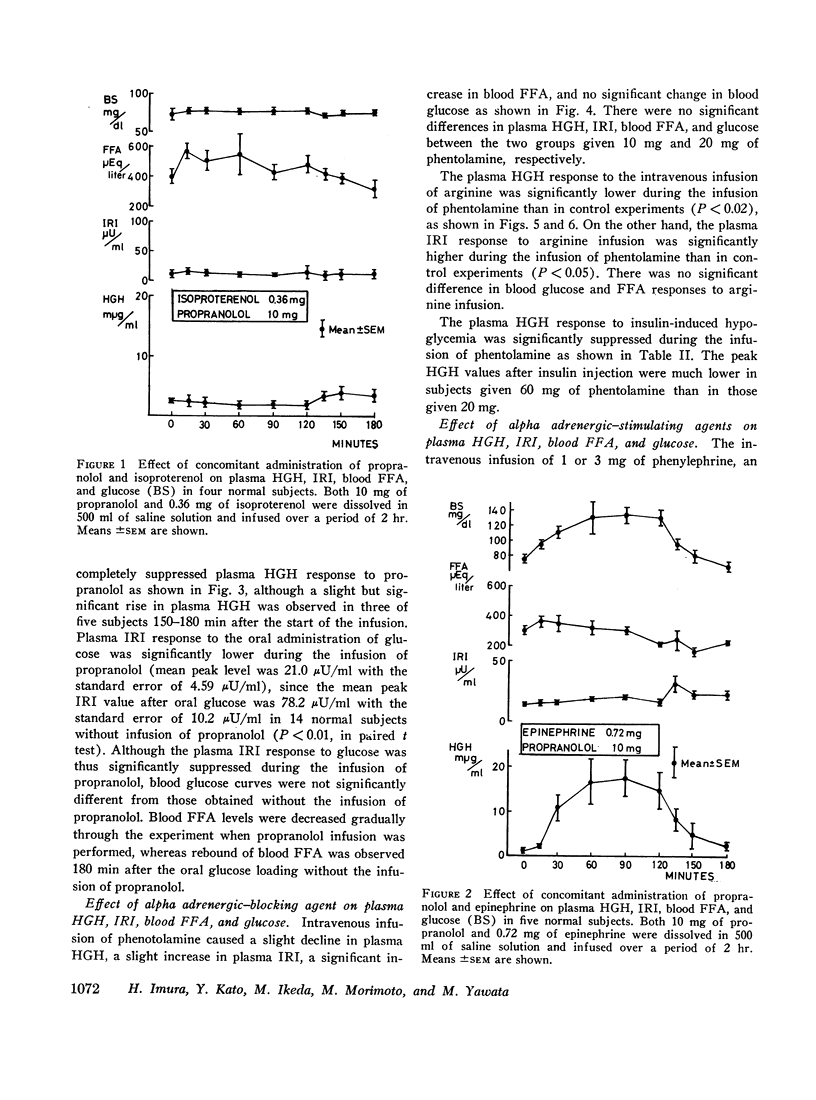
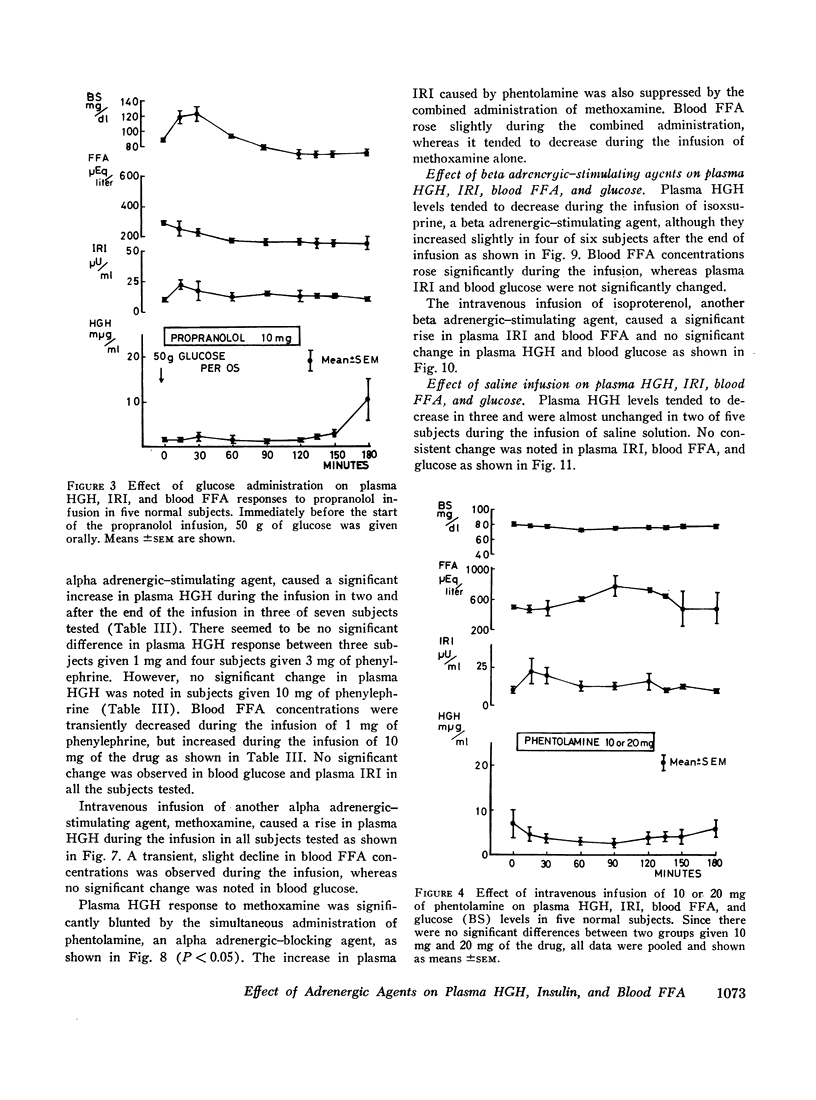
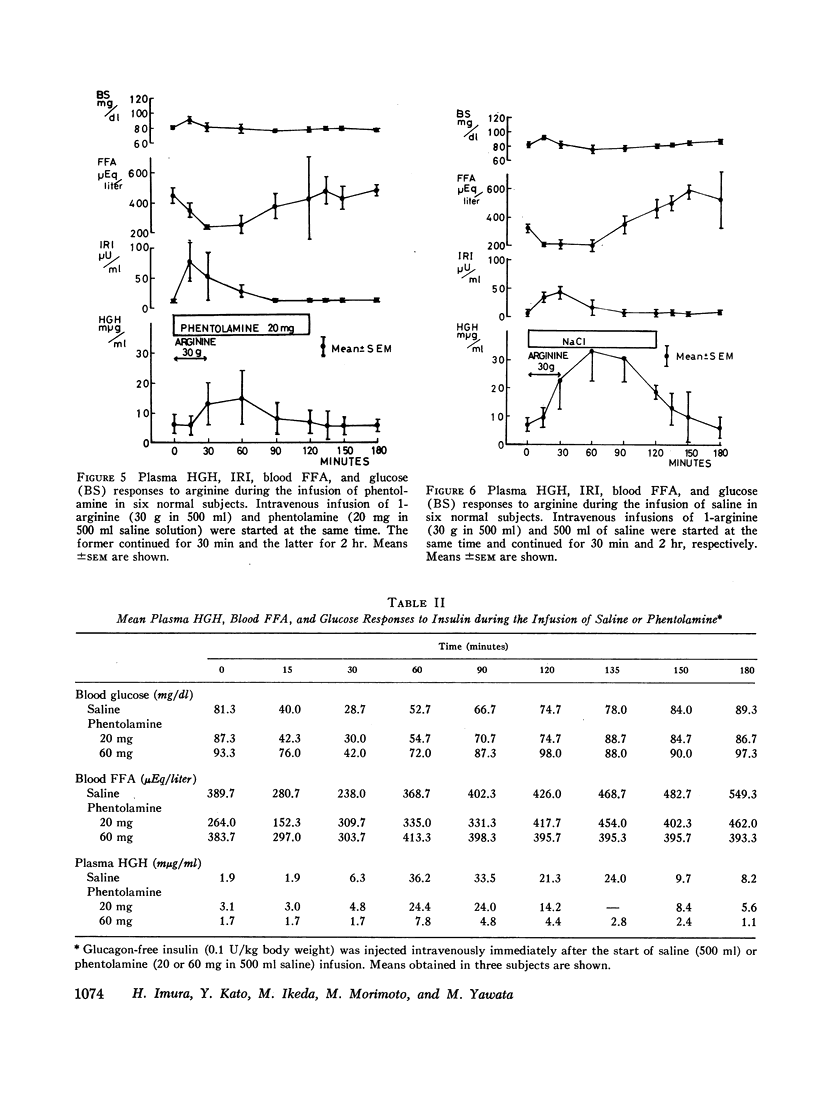
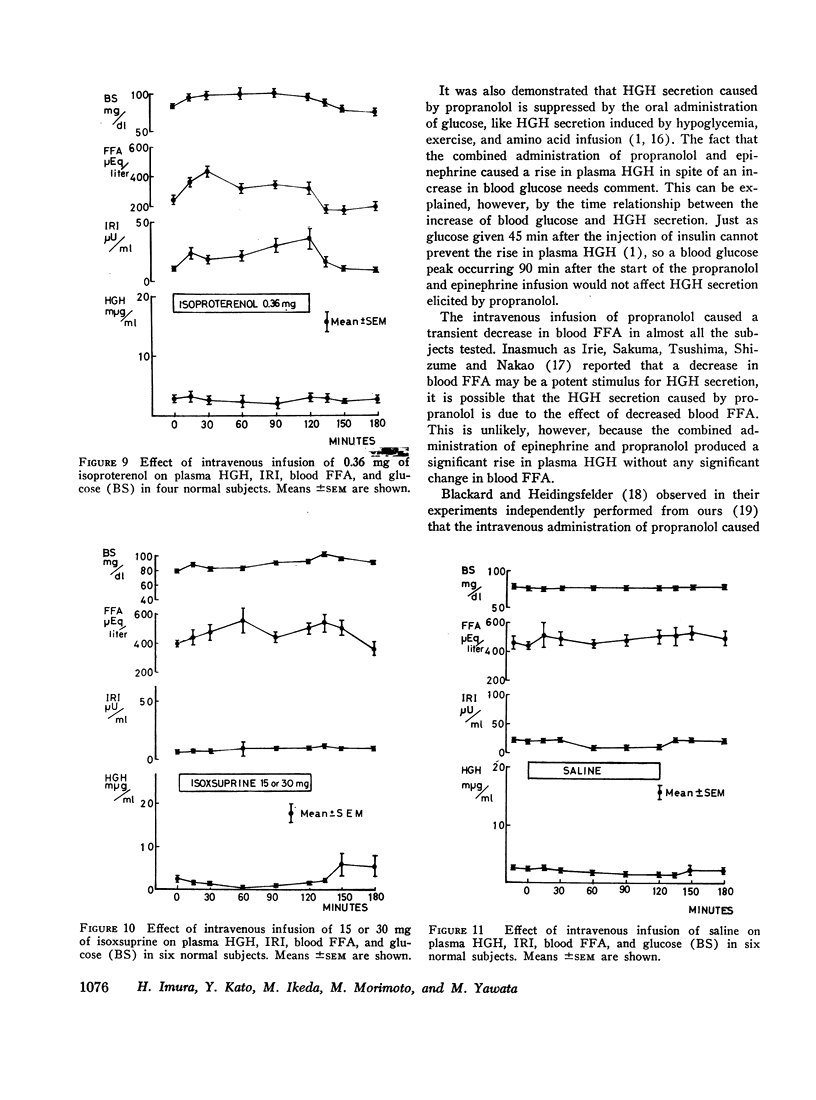
The intravenous infusion of propranolol, a beta adrenergic-blocking agent, caused a rise in plasma HGH, a transient decrease in blood FFA, and no significant change in plasma insulin. This increase in plasma HGH was inhibited either by the combined administration of isoproterenol, a beta adrenergic-stimulating agent, along with propranolol or by oral glucose loading immediately before the start of propranolol infusion. The concomitant administration of epinephrine and propranolol brought about a rise in plasma HGH comparable with that produced by propranolol alone, without any significant change in blood FFA. Alpha adrenergic blockade by the intravenous infusion of phenotolamine significantly suppressed plasma HGH responses to insulin-induced hypoglycemia and to arginine infusion, and enhanced plasma insulin response to arginine infusion. It also stimulated lipid mobilization significantly.
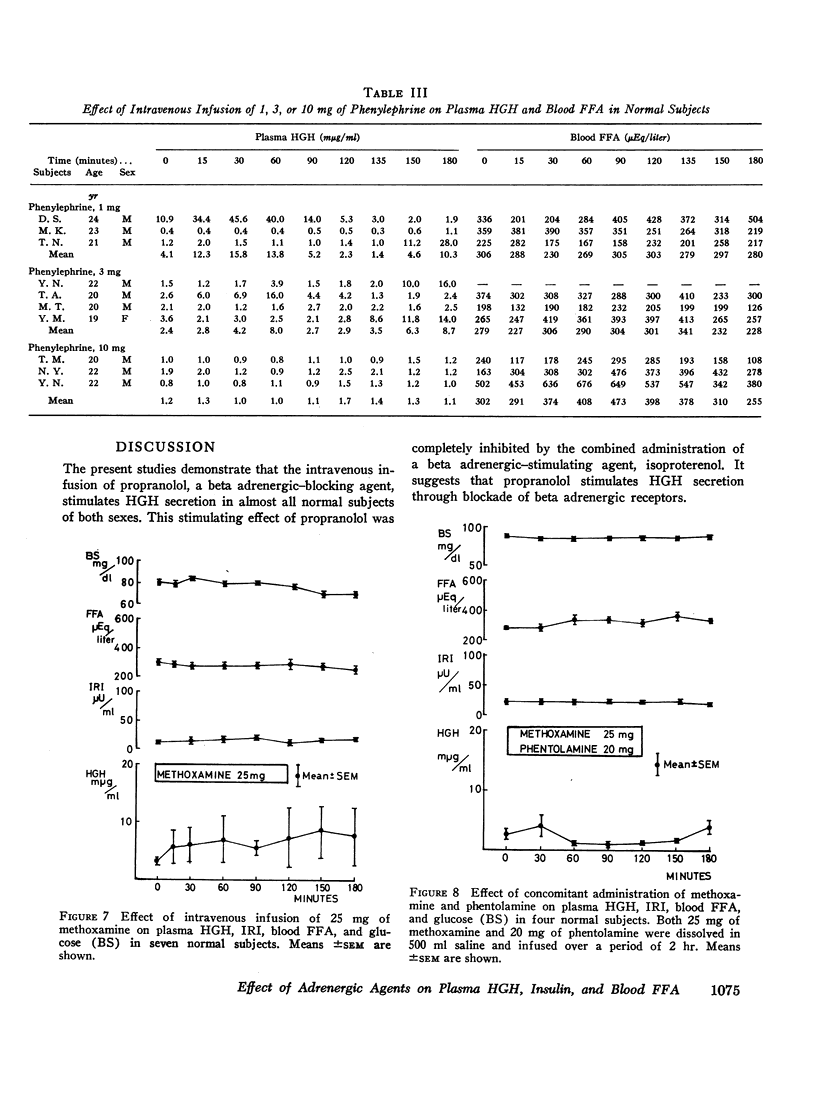
The intravenous infusion of alpha adrenergic-stimulating agents, phenylephrine and methoxamine, caused an increase in plasma HGH, a slight decrease in blood FFA, and no significant change in plasma insulin. This increase in plasma HGH was significantly inhibited by the simultaneous administration of phentolamine along with methoxamine. On the contrary, a beta adrenergic stimulant, isoproterenol, raised plasma insulin and blood FFA, and abolished the plasma HGH response to propranolol. Another beta stimulator, isoxsuprine, raised blood FFA but not plasma insulin.
It is concluded that either beta adrenergic blockade or alpha stimulation enhances HGH secretion and inhibits insulin secretion and fat mobilization, whereas either alpha blockade or beta stimulation stimulates insulin secretion and fat mobilization and inhibits HGH secretion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARIENS E. J., SIMONIS A. M. Autonomic drugs and their receptors. Arch Int Pharmacodyn Ther. 1960 Sep 1;127:479–496. [PubMed] [Google Scholar]
- Blackard W. G., Heidingsfelder S. A. Adrenergic receptor control mechanism for growth hormone secretion. J Clin Invest. 1968 Jun;47(6):1407–1414. doi: 10.1172/JCI105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks M. H., Guha A., Danforth E., Jr, Weinstein J. J., Barry K. G. Pheochromocytoma: observations on mechanism of carbohydrate intolerance and abnormalities associated with development of Goldblatt kidney following removal of tumor. Metabolism. 1969 Jun;18(6):445–459. doi: 10.1016/0026-0495(69)90137-1. [DOI] [PubMed] [Google Scholar]
- DEUBEN R. R., MEITES J. STIMULATION OF PITUITARY GROWTH HORMONE RELEASE BY A HYPOTHALAMIC EXTRACT IN VITRO. Endocrinology. 1964 Mar;74:408–414. doi: 10.1210/endo-74-3-408. [DOI] [PubMed] [Google Scholar]
- Edgar P., Rabinowitz D., Merimee T. J. Effects of amino acids on insulin release from excised rabbit pancreas. Endocrinology. 1969 Apr;84(4):835–843. doi: 10.1210/endo-84-4-835. [DOI] [PubMed] [Google Scholar]
- Fain J. N. Adrenergic blockade of hormone-induced lipolysis in isolated fat cells. Ann N Y Acad Sci. 1967 Feb 10;139(3):879–890. doi: 10.1111/j.1749-6632.1967.tb41257.x. [DOI] [PubMed] [Google Scholar]
- GLICK S. M., ROTH J., YALOW R. S., BERSON S. A. THE REGULATION OF GROWTH HORMONE SECRETION. Recent Prog Horm Res. 1965;21:241–283. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood F. C., Landon J. Growth hormone secretion in response to stress in man. Nature. 1966 Apr 30;210(5035):540–541. doi: 10.1038/210540a0. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITAYA K., UI M. COLORIMETRIC DETERMINATION OF FREE FATTY ACIDS IN BIOLOGICAL FLUIDS. J Lipid Res. 1965 Jan;6:16–20. [PubMed] [Google Scholar]
- Imura H., Kato Y., Ikeda M., Morimoto M., Yawata M., Fukase M. Increased plasma levels of growth hormone during infusion of propranolol. J Clin Endocrinol Metab. 1968 Jul;28(7):1079–1081. doi: 10.1210/jcem-28-7-1079. [DOI] [PubMed] [Google Scholar]
- KNOPF R. F., CONN J. W., FAJANS S. S., FLOYD J. C., GUNTSCHE E. M., RULL J. A. PLASMA GROWTH HORMONE RESPONSE TO INTRAVENOUS ADMINISTRATION OF AMINO ACIDS. J Clin Endocrinol Metab. 1965 Aug;25:1140–1144. doi: 10.1210/jcem-25-8-1140. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Mayhew D. A possible role for the adenylcyclase system in insulin secretion. J Clin Invest. 1967 Nov;46(11):1724–1734. doi: 10.1172/JCI105663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller E. E., Sawano S., Arimura A., Schally A. V. Blockade of release of growth hormone by brain norepinephrine depletors. Endocrinology. 1967 Mar;80(3):471–476. doi: 10.1210/endo-80-3-471. [DOI] [PubMed] [Google Scholar]
- Pecile A., Müller E., Falconi G., Martini L. Growth hormone-releasing activity of hypothalamic extracts at different ages. Endocrinology. 1965 Aug;77(2):241–246. doi: 10.1210/endo-77-2-241. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr A receptor mechanism for the inhibition of insulin release by epinephrine in man. J Clin Invest. 1967 Jan;46(1):86–94. doi: 10.1172/JCI105514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte D., Jr Beta adrenergic stimulation of insulin release in man. Diabetes. 1967 Mar;16(3):150–155. doi: 10.2337/diab.16.3.150. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr, Graber A. L., Kuzuya T., Williams R. H. The effect of epinephrine on immunoreactive insulin levels in man. J Clin Invest. 1966 Feb;45(2):228–236. doi: 10.1172/JCI105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz D., Merimee T. J., Burgess J. A., Riggs L. Growth hormone and insulin release after arginine: indifference to hyperglycemia and epinephrine. J Clin Endocrinol Metab. 1966 Oct;26(10):1170–1172. doi: 10.1210/jcem-26-10-1170. [DOI] [PubMed] [Google Scholar]
- SCHALCH D. S., PARKER M. L. A SENSITIVE DOUBLE ANTIBODY IMMUNOASSAY FOR HUMAN GROWTH HORMONE IN PLASMA. Nature. 1964 Sep 12;203:1141–1142. doi: 10.1038/2031141a0. [DOI] [PubMed] [Google Scholar]
- Schalch D. S. The influence of physical stress and exercise on growth hormone and insulin secretion in man. J Lab Clin Med. 1967 Feb;69(2):256–269. [PubMed] [Google Scholar]
- Schally A. V., Arimura A., Bowers C. Y., Kastin A. J., Sawano S., Reeding T. W. Hypothalamic neurohormones regulating anterior pituitary function. Recent Prog Horm Res. 1968;24:497–588. doi: 10.1016/b978-1-4831-9827-9.50016-2. [DOI] [PubMed] [Google Scholar]
- Schneider H. P., McCann S. M. Possible role of dopamine as transmitter to promote discharge of LH-releasing factor. Endocrinology. 1969 Jul;85(1):121–132. doi: 10.1210/endo-85-1-121. [DOI] [PubMed] [Google Scholar]
- Werrbach J. H., Gale C. C., Goodner C. J., Conway M. J. Effects of autonomic blocking agents on growth hormone, insulin, free fatty acids and glucose in baboons. Endocrinology. 1970 Jan;86(1):77–82. doi: 10.1210/endo-86-1-77. [DOI] [PubMed] [Google Scholar]


