Abstract
Long-lived iteroparous species often show aging-related changes in reproduction that may be explained by 2 non-mutually exclusive hypotheses. The terminal investment hypothesis predicts increased female reproductive effort toward the end of the life span, as individuals have little to gain by reserving effort for the future. The senescence hypothesis predicts decreased female reproductive output toward the end of the life span due to an age-related decline in body condition. Nonhuman primates are ideal organisms for testing these hypotheses, as they are long lived and produce altricial offspring heavily dependent on maternal investment. In this study, we integrated 50 years of continuous demographic records for the Cayo Santiago rhesus macaque (Macaca mulatta) population with new morphometric and behavioral data to test the senescence and terminal investment hypotheses. We examined relationships between maternal age and activity, mother and infant body condition, interbirth intervals, measures of behavioral investment in offspring, and offspring survival and fitness to test for age-associated declines in reproduction that would indicate senescence, and for age-associated increases in maternal effort that would indicate terminal investment. Compared with younger mothers, older mothers had lower body mass indices and were less active, had longer interbirth intervals, and spent more time in contact with infants, but had infants of lower masses and survival rates. Taken together, our results provide strong evidence for the occurrence of reproductive senescence in free-ranging female rhesus macaques but are also consistent with some of the predictions of the terminal investment hypothesis.
Keywords: aging, life history, maternal investment, offspring fitness, reproductive senescence, rhesus macaques
According to the terminal investment hypothesis (Williams 1966), a female’s reproductive strategies should change according to her future reproductive and survival prospects. When a female is in her reproductive prime, she should minimize investment in existing offspring so that she has adequate resources for future reproduction. When a female has surpassed her reproductive prime and her future reproductive potential is low, she should expend more reproductive effort in existing offspring.
Numerous studies have provided strong evidence in favor of the terminal investment hypothesis. Correlational studies have shown, for example, greater birth weights and improved survival rates of red deer (Cervus elaphus) infants born to older mothers (Clutton-Brock 1984), that more older than younger female red squirrels (Sciurus vulgaris) attempt to breed twice within a single season (Descamps et al. 2008), and that older Nile lechwe (Kobus megaceros) females produce a disproportionate number of sons, the costlier, heavier sex that is more frequently linked to maternal death (Bercovitch et al. 2009). In addition to correlational evidence, experimental studies conducted on several animal species have also demonstrated that investment in reproduction can increase in both sexes when condition is compromised. For example, in response to experimental immune challenges, female house sparrows (Passer domesticus) produce larger clutches (Bonneaud et al. 2004), female eiders (Somateria mollissima) increase brood tending (Hanssen 2006), male blue-footed boobies (Sula nebouxii) have increased reproductive success (Velando et al. 2006), and male mealworms (Tenebrio molitor) increase investment in sexual signaling (Sadd et al. 2006).
Though authors from some studies have reported both correlational and experimental evidence for the terminal investment hypothesis, other authors have concluded that their data do not support it. For example, older pipefish males (Syngnathus typhle) exposed to increased predation risk do not differ in the rate at which they attempt to reproduce when compared with younger males (Billing et al. 2007), and older reindeer (Rangifer tarandus; Weladji et al. 2002) and tsetse flies (Glossina morsitans morsitans; Langley and Clutton-Brock 1998) do not give birth to heavier or larger offspring, respectively. In the absence of any evidence for terminal investment, authors have frequently cited senescence, which is age-associated physiological deterioration, as the most likely explanation for results showing age-specific changes in reproductive output (e.g., Weladji et al. 2002). Evidence for senescence is becoming more widespread in animal populations (e.g., Parus major, Bouwhuis et al. 2009; Arctocephalus tropicalis, Beauplet et al. 2006; Ovis canadensis, Berube et al. 1999). Observations of declining reproductive output with age support the senescence hypothesis, whereas observations of increasing investment with age support the terminal investment hypothesis (Weladji et al. 2010). These 2 hypotheses are not mutually exclusive explanations for age-related changes in reproductive output. Older mothers, for example, may have reduced resources due to senescence, and although they may invest a greater proportion of these resources in their offspring, offspring mass and/or survival may decline nonetheless, providing evidence for both processes (Weladji et al. 2010).
Primates have relatively long life spans and produce highly altricial offspring whose survival depends on months, if not years, of maternal investment, making them ideal organisms for testing the terminal investment and senescence hypotheses. Reproductive senescence in primates has been well documented (Walker and Herndon 2008), whereas several studies have reported evidence for social senescence (e.g., withdrawal from social interactions and declines in social network size; Veenema et al. 1997; Corr 2000). Evidence for terminal investment in primate species, however, is mixed. For example, although some studies have shown that infants of older mothers have greater birth weights and survival rates than those of younger mothers (Presbytis entellus, Dolhinow et al. 1979; Macaca mulatta, Silk et al. 1993; M. sylvanus, Paul et al. 1993), other studies have not found this relationship (Semnopithecus entellus, Borries and Koenig 2008; Propithecus edwardsi, Wright et al. 2008; Pan troglodytes, Fessler et al. 2005). Furthermore, although it has been reported that older mothers exhibit longer interbirth intervals than younger mothers (e.g., M. mulatta, Gagliardi et al. 2007; Gorilla beringei beringei, Robbins et al. 2006; M. sylvanus, Paul et al. 1993), this finding is consistent with both hypotheses (Paul et al. 1993; Robbins et al. 2006). To detect age-associated changes in interbirth interval and offspring survival patterns, long-term data are needed, whereas assessment of body condition and behavior relies on data that are typically restricted to short-term data sets. Previous studies of nonhuman primates have not utilized both long- and short-term data sets to analyze multiple measures of females and to disentangle potential senescence and terminal investment effects.
In this study, we utilized long-term and short-term data sets to test the senescence and terminal investment hypotheses in free-ranging rhesus macaques (M. mulatta) on Cayo Santiago, examining maternal body condition, interbirth intervals, offspring sex ratio, and maternal behavior in relation to age. Additionally, we examined offspring’s body mass, survival to 30 days and 1 year, and future reproduction to determine if maternal investment increases with advancing age and whether any extra investment does result in improved infant fitness. In the Cayo Santiago rhesus macaque population, females begin reproducing at 3–5 years and continue producing offspring every year or every other year until the end of their life span, which can extend up to 30 years (Rawlins et al. 1984; Bercovitch and Berard 1993). Long-term data, which can be used to assess variation in reproductive output and life history by age, can be combined with short-term studies assessing the interaction between age and behavioral and morphometric measures of maternal investment. This is important, as with only long-term life-history data, it is difficult to distinguish between senescence and terminal investment effects. Finally, in the Cayo Santiago rhesus macaque population, there is a larger sample of old females than is found in the wild as a result of food provisioning and the absence of predators. Collectively, these factors allow for a comprehensive test of the senescence and terminal investment hypotheses.
We examined the effects of maternal age on interbirth intervals and offspring fitness using 50 years of continuous demographic records for the entire Cayo Santiago rhesus macaque population. In addition, from a sample of females that ranged from 6 to 26 years, we collected data on maternal behavior in the first postpartum month and maternal and offspring body mass. Because male rhesus macaques weigh significantly more at birth than females and because females in the Cayo Santiago population reproduce more slowly after the birth of sons (Bercovitch et al. 2000), we also tested whether offspring sex influenced maternal behavioral investment. We predicted that 1) older females (>15 years of age) should exhibit physical and behavioral signs of aging (e.g., lower body mass index [BMI], less time engaging with conspecifics, and more time resting when compared with younger females), as well as changes in reproductive output and offspring fitness, in accordance with the senescence hypothesis; 2) behaviors associated with maternal investment should increase with age in accordance with the terminal investment hypothesis; 3) older females should produce more sons and offspring with greater fitness as a consequence of increased maternal investment; and 4) interbirth intervals should increase as a function of age in accordance with both the senescence and terminal investment hypotheses.
MATERIALS AND METHODS
Field site and subjects
Cayo Santiago is a 15.2-ha island located 1 km off the southeastern coast of Puerto Rico. The rhesus macaque colony on this island was established in 1938, with free-ranging individuals captured in India (Rawlins and Kessler 1986). Since then, no new individuals have been introduced into the population, except through births. To maintain a stable population size, a fraction of the yearlings and 2-year-olds are transferred off the island each year. Monkeys living on Cayo Santiago forage on vegetation and are provisioned with rainwater and commercial monkey chow. Rhesus macaques are seasonal breeders, and in this population, the 6-month mating season begins in March and is followed by a 6-month birth season that begins in September (Hoffman et al. 2008). The data for the present study came from 2 different data sets, which will be referred to as “short-term data” and “long-term data,” respectively.
Short-term data
This data set comprised of body measurements and behavioral data collected between April 2007 and February 2008. At that time, approximately 850 animals distributed among 6 naturally formed social groups resided on Cayo Santiago. Between January and February 2008, we collected morphometric data from 40 adult females from 4 social groups who were between 7 and 26 years of age (mean age = 15.8 years). Trained staff members captured these females and their infants in a feeding corral, approximately 100 m2, which was provisioned daily with monkey chow. Infants remained with their mothers during the entire trapping and data collection process. Trapping generally occurred between 0830 and 1200 h. The staff members netted or captured the monkeys by hand in 1 of 3 feeding corrals, transferred them to a holding cage (0.62 × 0.42 × 0.62 m), and moved them to a small field laboratory. The monkeys remained in a standard squeeze cage with their infants for overnight housing. The following morning, veterinary technicians anesthetized the adult females and their infants with ketamine (ca. 10 mg/kg via intra-muscular injection), and we weighed the anesthetized females and infants separately in a standard hanging scale. We used a large anthropometer (Lafayette Instruments, Lafayette, IN) to measure crown–rump length of each anesthetized adult female while she was placed in standardized position with her back fully straight. We calculated BMI for each adult female by dividing mass (kilograms) by the square of crown–rump length (square meter) (Campbell and Gerald 2004).
Subjects that were the focus of behavioral data collection ranged between 6 and 22 years of age (mean age = 15.0 years). We collected behavioral data during prebirth observations (those occurring from 1 April 2007 until a female gave birth) and postbirth observations (occurring from the date a focal female gave birth until mid-December 2007). We conducted instantaneous sampling (Altmann 1974) at 1-min intervals during 30-min focal follows to determine the proportion of time focal animals spent feeding, resting, traveling, and engaging in grooming interactions. The grooming measure combined grooming given to and received from social partners 3 years and above. During the postbirth period, we recorded the amount of time mothers and infants were in ventro-ventral contact during focal follows. We used time spent in ventro-ventral contact as a proxy for the amount of time infants had the opportunity to suckle. In the first month of infant life, time spent in ventro-ventral contact is mostly controlled by the mother and can therefore be interpreted as an indicator of the mother’s willingness to invest in the offspring (Maestripieri 2002).
Behavioral data collection included 28 females initially. Each subject was multiparous and belonged to 1 of 3 social groups (F = 11, R = 9, S = 7). All 28 females gave birth during the birth season. Five of those females (F = 2, R = 2, S = 1), however, did not give birth until the end of the birth season, and consequently, no postbirth data were collected from these females. We conducted focal follows on 3 additional females (F = 1, S = 2), beginning with the week they gave birth. Thus, we collected behavioral data on 28 females during the prebirth period and 26 females during the postbirth period.
Dominance ranks were assigned on the basis of behavioral data on aggressive (i.e., threats, chases) and submissive (i.e., withdrawals, screams, grimaces) interactions collected by trained observers. The data were used to create a linear dominance hierarchy for each group’s females. Each female was assigned a number based on where she fell within the group’s female hierarchy, with the highest ranking female receiving 1. Using those assigned numbers, a proportional rank was calculated by dividing each female’s rank number by the total number of females in the group (e.g., Nelson et al. 2010). Thus, of all the females in a group, the highest ranking female received the proportional rank closest to 0 and the lowest ranking female’s proportional rank was 1. In addition to utilizing these proportional ranks in analyses, we also undertook all analyses using absolute rank categories (high, middle, and low); results from analyses of these 2 different rank types did not differ qualitatively in any analysis, and we present the results of proportional rank analyses only.
Long-term data
This data set comprised of records maintained in the Cayo Santiago database, which includes information on each animal’s genealogy and dates of birth and death, as well as a history of each individual’s group membership and reproduction. Throughout the period of interest to this study (1957–2007), Cayo Santiago staff updated colony records with daily censuses of all animals. Using this long-term data set, we calculated median life span, interbirth intervals, and infant survival rates for all reproductively active females between the ages of 4 and 24 years who were born after 1 January 1957, lived their entire lives on the island, and died prior to 1 May 2007 (n = 637). For every age at which a female gave birth to a live infant, we assessed the number of days between that birth and her previous birth. Additionally, we used the data to determine whether the female had given birth in the previous birth season. After rounding female ages at birth to the nearest year, we calculated for each age the average interbirth interval and the proportion of females skipping a birth season.
We measured the effects of maternal age and offspring sex on average offspring life span and on rates of infant mortality during the first 30 days and 1 year of life (Gagliardi et al. 2007). We examined female offspring reproductive success by calculating birth rate for female offspring who lived their entire lives on Cayo Santiago. To calculate birth rate, we divided the number of offspring born during a female’s life span by the number of birth seasons during which that female was reproductively mature, beginning with the first birth season during which a female gave birth. We also used long-term data to determine the age at which each female offspring first gave birth. Because the Cayo Santiago database does not contain accurate information about each animal’s dominance rank, this variable was not analyzed.
Data analysis
We undertook linear mixed modeling (LMM) to investigate our short-term data, as this allowed us to control for social group by including this variable as a random factor in all models reported below.
Physical and behavioral signs of senescence
Using the short-term data set, we employed LMM to test the relationships between female age, proportional rank, and BMI. We also used LMM to determine relationships between age, proportional rank, and the proportion of time females spent resting, grooming, feeding, and traveling prebirth (i.e., when cycling or pregnant), and during the first month postbirth. We did not have a sufficient sample size of females with infants to analyze behavioral data occurring beyond this.
Age and time spent in ventro-ventral contact with infants
Using the short-term data set, we employed LMM to test relationships between the average number of minutes mother–infant pairs spent in ventro-ventral contact per hour during each infant’s first month and maternal age, proportional rank, and infant sex. We also used LMM to assess relationships between infant birth date and maternal age, proportional rank, and infant sex. We performed linear regression to determine whether infant birth date was related to time in ventro-ventral contact.
Age and offspring body mass, sex, survival, and reproductive success
For analyses involving infant mass from the short-term data set, we excluded infants younger than 60 days at the time of measurement because rhesus growth is linear only between 2 and 11 months (Smith and Small 1982). We used LMM to determine the relationship between infant mass and maternal age, infant age, and infant sex. To determine whether offspring sex ratio changes with age, we calculated from the long-term data set the proportion of offspring that were male born to females at each year of life (years 4–24) and then ran linear and quadratic regressions to determine whether sex ratio changed with maternal age. We performed linear and quadratic regressions to determine relationships between maternal age and the proportion of offspring surviving to 30 days and 1 year in the long-term database (Gagliardi et al. 2007). We also conducted chi-square analyses to determine if offspring sex was related to offspring survival to these time points and performed linear regression to determine whether maternal age at offspring birth was related to female offspring’s age of first reproduction or annual birth rate.
Age and interbirth interval
We performed linear and quadratic regression analyses on data from the long-term data set to determine relationships between female age and both interbirth intervals and the probability of skipping a birth season.
We conducted all statistical data analyses in SPSS 17.0. All tests were two tailed, and we considered P < 0.05 as significant.
RESULTS
Physical and behavioral signs of senescence
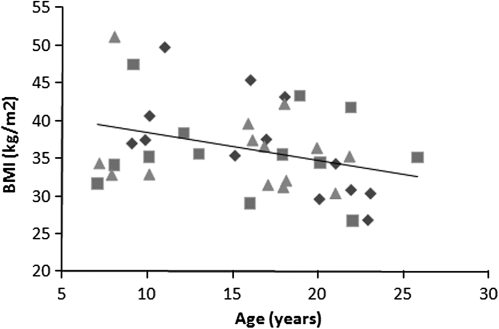
There was no relationship between female age and proportional rank (F1,26.00 = 0.11, P = 0.74). Age was a significant predictor of BMI (F1,37.73 = 6.41, P = 0.02), with BMI decreasing with increasing age (Figure 1). Rank was unrelated to BMI (P > 0.10; Figure 1).
Figure 1.
Relationships between female age and body mass index. Diamonds denote high-ranking females, squares denote middle-ranking females, and triangles denote low-ranking females.
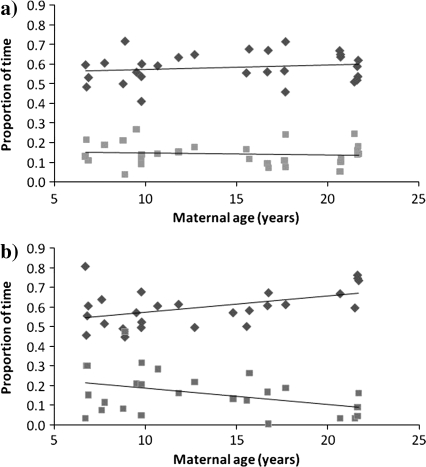
Female age and rank were unrelated to the proportion of time females spent resting, grooming, or traveling prebirth (all P > 0.10; for resting and grooming results, see Figure 2a). Prior to giving birth, female age and rank were significant predictors of proportion of time females spent feeding (age: F1,25.00 = 5.37, P = 0.03; rank: F1,25.00 = 7.63, P = 0.01), with older females spending less time feeding than younger females and low-ranking females spending less time feeding than high-ranking females. The relationship between age and proportion of time spent traveling prebirth was approaching significance (F1,24.58 = 3.62, P = 0.07), but there was no relationship between rank and proportion of time spent traveling (P > 0.10).
Figure 2.
Relationships between maternal age and proportion of time spent resting (diamonds) and grooming (squares): (a) prebirth and (b) postbirth.
During the first month postbirth, female age predicted proportion of time spent resting and grooming, with older females spending more time resting and less time grooming (resting: F1,24.00 = 6.24, P = 0.02; grooming: F1,23.05 = 4.34, P = 0.049; Figure 2b). Neither age nor rank predicted proportion of time spent feeding in the first postpartum month (P > 0.10), and there was no relationship between age and proportion of time spent traveling (P > 0.10). The relationship between rank and proportion of time spent traveling postbirth was approaching significance (F1,22.22 = 3.34, P = 0.08).
Age and time spent in ventro-ventral contact with infants
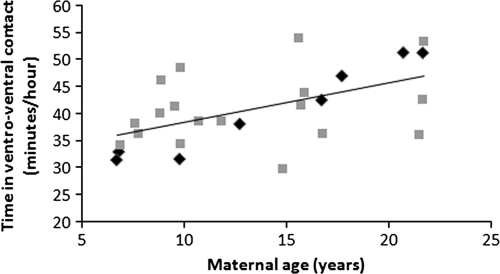
The behavioral data indicate that females, on average, spent 40.1 ± 6.8 min/h in ventro-ventral contact with their infants during their infant’s first month. Maternal time in ventro-ventral contact with infants during infant’s first month was not affected by rank or infant sex (for both P > 0.10) but increased with female age (F1,22.26 = 10.55, P = 0.004; Figure 3). Because older females tended to give birth later in the birth season than younger females (F1,24.00 = 6.75, P = 0.02), we examined whether there was a relationship between infant birth date and time in ventro-ventral contact but found no relationship (F1,24 = 1.10, P = 0.31).
Figure 3.
Relationship between maternal age and time spent in ventro-ventral contact with female (diamonds) and male (squares) infants during the first 30 days postbirth.
Age and offspring body mass, sex, survival, and reproductive success
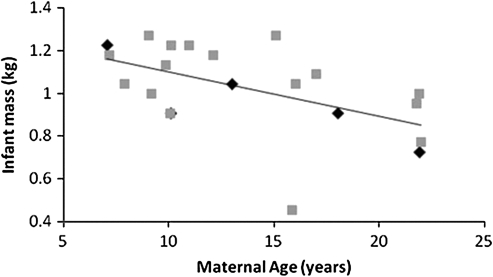
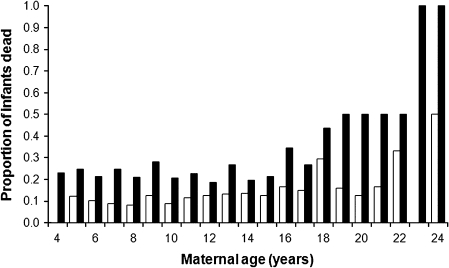
There was no relationship between infant mass and infant age or sex (for both P > 0.10), but there was a relationship between infant mass and maternal age (F1,17.82 = 6.94, P = 0.02; Figure 4). Infant mass was lower for those infants born to older mothers. In the long-term data, we found no relationship between maternal age and sex ratio (linear: F1,19 = 0.71, P = 0.41; quadratic: F2,18 = 1.74, P = 0.20). Early offspring survival decreased as a function of increasing maternal age, with a greater proportion of infants born to older mothers than to younger mothers dying by 30 days and 1 year, even when mothers dying prior to these time points were excluded from analyses (30 days: F2,18 = 4.65, P = 0.02; 1 year: F2,18 = 57.61, P < 0.001; Figure 5). Infant sex was unrelated to the likelihood of infant survival to 30 days or 1 year (all chi-square analyses, P > 0.10). Relationships between maternal age and infant survival to these time points were still significant when 23- and 24-year-old mothers were excluded from the analyses (all regression analyses: P ≤ 0.001). None of the infants born to 23- and 24-year-olds survived beyond 5 months, even though 4 of the 6 mothers giving birth during this age range survived beyond their infant’s first 5 months; in fact, 2 of the 3 mothers that gave birth at 24 years survived an additional 4–6 years. Therefore, low rates of survival among offspring of old mothers were not due to maternal death.
Figure 4.
Relationship between maternal age and mass of infant daughters (diamonds) and sons (squares).
Figure 5.
Relationship between maternal age and infant death by 30 days (white bars) and 1 year (black bars). Only infants whose mothers were still alive at each point in time were included in the analyses.
For female offspring who survived to reproductive age, their mother’s age at the time of their birth was unrelated to their age of first reproduction (4.3 ± 0.1 years; F1,15 = 1.89, P = 0.19) or their annual birth rate (0.83 ± 0.02 offspring per year; F1,13 = 0.99, P = 0.34). Fewer than 28% of daughters born to females over 15 years survived to 4 years, however.
Age and interbirth intervals
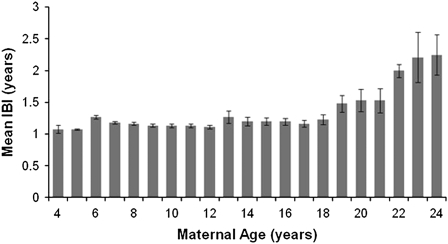
For females in the long-term data set who survived to at least 4 years of age (n = 631), median life span was 15.0 years and maximum life span was 31.4 years; the oldest female to give birth did so at 24.2 years. Interbirth intervals for these females ranged from 300 to 2591 days (X = 431.6 days, standard deviation [SD] = 147.4, n = 1800 births) and increased significantly with increasing maternal age (F2,18 = 66.93, p < 0.01; Figure 6). Average interbirth intervals of females between 4 and 18 years of age ranged between 392 and 463 days (X = 425.6 days, SD = 140.1, n = 1741 births), but the average interbirth interval rose to 541 days at 19 years and continued climbing thereafter (X = 607.2 days, SD = 228.1, n = 59 births). The proportion of females skipping a birth season began increasing when females were in their late teens. Between 4 and 18 years, 15% of females skipped at least 1 year between births, whereas 25% did so at 19 years of age, and more than 50% did so at 22 years of age. As such, there was an overall significant positive relationship between female age and the probability of skipping a breeding season (F2,18 = 54.17, P < 0.001).
Figure 6.
Relationship between maternal age and interbirth interval.
DISCUSSION
In our study of the Cayo Santiago rhesus macaque population, we integrated long-term demographic data on survival and reproduction with new morphometric and behavioral data collected from a sample of mothers and infants. The association between old age and declines in physical condition and activity levels, decreases in reproductive output, and a reduction in early offspring survival, constitute evidence in favor of the senescence hypothesis. Although we had predicted that offspring sex ratio would be skewed toward the costlier sex in accordance with the terminal investment hypothesis, this prediction was not met. However, increased time spent in ventro-ventral contact may suggest that older mothers invest more in their offspring and therefore represents potential evidence in favor of the terminal investment hypothesis. Longer interbirth intervals among older mothers could be interpreted as evidence for either process. As such, our study provides strong evidence for senescence and some weaker evidence for terminal investment.
As previously observed in a captive rhesus macaque population (Gagliardi et al. 2007), infant survival on Cayo Santiago decreases sharply as females enter their late teens. Our data highlight at least 2 factors that may lead to this. First, although there were no age-related differences in grooming or resting prebirth, older mothers were involved in less grooming interactions after the birth of their infant than younger mothers were. Unfortunately, previous studies that have characterized rhesus behavior in wild habitats have not included female age, rank, or infant presence in their analyses (e.g., Teas et al. 1980), preventing comparison of these results with wild populations. Given that cercopithecine non-mothers and mothers with young infants tend to be attracted to females with young infants (e.g., Papio anubis, Frank and Silk 2009; Papio cynocephalus ursinus, Silk et al. 2003; M. mulatta, Whitham et al. 2007), this reduced grooming time may mean that the offspring of older rhesus females are less attractive to other females. This observed decrease in sociality may affect the likelihood of infant survival, given the potential links between social support and survivorship in primates (Silk et al. 2006). In baboons, strong social bonds may increase infant survival and benefit infants by providing mothers with support in agonistic interactions, lowering mother’s basal cortisol levels, increasing infant protection from harassment, or making more resources accessible to mother–infant dyads (Silk et al. 2003).
One might expect that by engaging in fewer social interactions and resting more, older females are reserving energetic resources for lactation. However, morphometric data show that older females’ infants weigh less than younger females’ infants. This may be related to the fact that older females had lower BMIs than younger females, which in turn may be attributable to reduced feeding time before birth among older females compared with younger females. Low infant mass is predictive of infant mortality in common marmosets (Callithrix jacchus, Tardif et al. 2002) and may be a second factor leading to high mortality rates for rhesus infants born to older mothers.
Most infants born to older females do not survive to reproductive age. However, for those daughters who do survive to reproductive age, mother’s age at daughter’s birth does not affect age at first parturition or annual birth rate. It may be that the mothers of those few daughters who survive to reproductive maturity were in exceptionally good body condition for their age or were high ranking, but unfortunately, long-term records do not contain morphometric or hierarchical information. From the short-term data set, however, we did observe that high-ranking females spent more time feeding during pregnancy than low-ranking females, and this may confer fitness-related advantages to the offspring of high-ranking females.
Although, according to the terminal investment hypothesis, increasing interbirth intervals and suckling time may increase infant survival for older females, this is not the case for females in our study. Consistent with a vast body of literature on primate life history (e.g., Malik et al. 1992; Okamoto et al. 2000; Higham et al. 2009), we might expect interbirth intervals to be shorter for females whose infants die, but we see no evidence of this; whereas infant survival was lower for the older females in our study, their interbirth intervals were longer. These patterns are consistent with general reproductive senescence in these females. Additional data are needed to determine if lengthening interbirth intervals are associated with hormonal or behavioral changes. Hormonal data would indicate whether the increasing interbirth intervals are associated with increasingly irregular ovulatory patterns, as they are in captive rhesus populations (Gilardi et al. 1997). Furthermore, behavioral data collected from mothers and infants between 2 and 8 months post-parturition would reveal whether age-related differences in suckling intensity affect time between parturition and the resumption of mating (see Johnson et al. 1998). If extended interbirth intervals among older females are clearly related to increased lactational effort, this would be consistent with a terminal investment interpretation for longer interbirth intervals. Conversely, if extended interbirth intervals occur among older females even though overall lactational effort is similar across the adult lifespan, this would be consistent with a senescence interpretation of the effect.
Our findings show that although rhesus macaque females on Cayo Santiago have the physical and physiological ability to conceive and give birth virtually until the end of their life span, their ability to sustain lactation and guarantee infant survival decreases steadily but markedly in their last 5–10 years of life. Because the probability of offspring survival for females older than 23 years is virtually zero, extremely old females would fare better if they ceased reproducing and, instead, invested their resources in their existing offspring. In wild populations of macaques, predators, disease, and unpredictable food sources make it unlikely that adult females will survive into their third decade (Johnson et al. 1991; Jones-Engel et al. 2006) such that selective pressures for early termination of reproduction are probably negligible or nonexistent in the wild. As such, the absence of strong evidence for terminal investment in our population, even when females would apparently benefit from it, may be related to the provisioned nature of our population and consequent increased female longevity. Although it is possible that longer interbirth intervals of females in the mid- to late-teen years may alleviate the negative effects of their declining physical condition on the probability of offspring survival, there are no apparent fitness benefits associated with increased maternal investment for rhesus females who continue reproducing into very old age (i.e., above 20 years).
In conclusion, we detected a number of age-related changes in behavior, body condition, reproduction, and infant mortality among Cayo Santiago mothers and their infants. Although female rhesus macaques do show some signs of increased maternal investment, they do not increase investment by producing larger offspring as red deer do (Clutton-Brock 1984), increasing reproductive rate as red squirrels do (Descamps et al. 2008), or producing a larger number of sons as Nile lechwe do (Bercovitch et al. 2009), perhaps because they suffer such strong senescence effects. Taken together, our results provide strong evidence for senescence and only weak evidence for terminal investment. Our approach, which utilized multiple measures of individuals and both long-term and short-term data sets, is useful for assessing which of the 2 hypotheses best explain age-related changes in reproduction within a given species. Although we analyzed a great deal of data, we lacked information about mother–infant interactions beyond the first month postpartum. Future tests of these hypotheses should combine life-history and morphometric data with more extensive behavioral observations on maternal investment throughout infant and juvenile development.
FUNDING
National Institutes of Health (NIH; grant number R21-AG029862 to D.M). This publication was made possible by grant number CM-5-P40RR003640 from the NIH National Center for Research Resources to the Caribbean Primate Research Center of the University of Puerto Rico. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of National Center for Research Resources or NIH.
Supplementary Material
Acknowledgments
We thank Richelle Fulks, Geoff Gallice, Bianca Giura, and Jake Reeder for assistance with data collection; the staff scientists and census takers of the Caribbean Primate Research Center (CPRC) for creating and maintaining the database through the years; and the staff of the CPRC for logistical support and assistance with animal capturing and handling. This study was conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. The protocol for this study was approved by the Institutional Animal Care and Use Committee, Medical Sciences Department, University of Puerto Rico.
References
- Altmann J. Observational study of behavior—sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Beauplet G, Barbraud C, Dabin W, Kussener C, Guinet C. Age-specific survival and reproductive performances in fur seals: evidence of senescence and individual quality. Oikos. 2006;112:430–441. [Google Scholar]
- Bercovitch FB, Berard JD. Life history costs and consequences of rapid reproductive maturation in female rhesus macaques. Behav Ecol Sociobiol. 1993;32:103–109. [Google Scholar]
- Bercovitch FB, Loomis CP, Rieches RG. Age-specific changes in reproductive effort and terminal investment in female Nile lechwe. J Mammal. 2009;90:40–46. [Google Scholar]
- Bercovitch FB, Widdig A, Nurnberg P. Maternal investment in rhesus macaques (Macaca mulatta): reproductive costs and consequences of raising sons. Behav Ecol Sociobiol. 2000;48:1–11. [Google Scholar]
- Berube CH, Festa-Bianchet M, Jorgenson JT. Individual differences, longevity, and reproductive senescence in bighorn ewes. Ecology. 1999;80:2555–2565. [Google Scholar]
- Billing AM, Rosenqvist G, Berglund A. No terminal investment in pipefish males: only young males exhibit risk-prone courtship behavior. Behav Ecol. 2007;18:535–540. [Google Scholar]
- Bonneaud C, Mazuc J, Chastel O, Westerdahl H, Sorci G. Terminal investment induced by immune challenge and fitness traits associated with major histocompatibility complex in the house sparrow. Evolution. 2004;58:2823–2830. doi: 10.1111/j.0014-3820.2004.tb01633.x. [DOI] [PubMed] [Google Scholar]
- Borries C, Koenig A. Reproductive and behavioral characteristics of aging in female Asian colobines. In: Atsalis S, Margulis SW, Hof PR, editors. Primate reproductive aging: cross-taxon perspectives. Basel (Switzerland): Karger; 2008. pp. 80–102. [DOI] [PubMed] [Google Scholar]
- Bouwhuis S, Sheldon BC, Verhulst S, Charmantier A. Great tits growing old: selective disappearance and the partitioning of senescence to stages within the breeding cycle. Proc Biol Sci. 2009;276:2769–2777. doi: 10.1098/rspb.2009.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BC, Gerald MS. Body composition, age and fertility among free-ranging female rhesus macaques (Macaca mulatta) J Med Primatol. 2004;33:70–77. doi: 10.1111/j.1600-0684.2004.00055.x. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH. Reproductive effort and terminal investment in iteroparous animals. Am Nat. 1984;123:212–229. [Google Scholar]
- Corr JA. The effects of aging on social behavior in male and female rhesus macaques of Cayo Santiago [dissertation] 2000 Ohio State University. [Google Scholar]
- Descamps S, Boutin S, Berteaux D, Gaillard J. Age-specific variation in survival, reproductive success and offspring quality in red squirrels: evidence of senescence. Oikos. 2008;117:1406–1416. [Google Scholar]
- Dolhinow P, McKenna JJ, Laws JVH. Rank and reproduction among aging female langur monkeys: aging and improvement (they're not just getting older, they're getting better) Aggress Behav. 1979;5:19–30. [Google Scholar]
- Fessler DMT, Navarrete CD, Hopkins W, Izard MK. Examining the terminal investment hypothesis in humans and chimpanzees: associations among maternal age, parity, and birth weight. Am J Phys Anthropol. 2005;127:95–104. doi: 10.1002/ajpa.20039. [DOI] [PubMed] [Google Scholar]
- Frank RE, Silk JB. Grooming exchange between mothers and non-mothers: the price of natal attraction in wild baboons (Papio anubis) Behaviour. 2009;146:889–906. [Google Scholar]
- Gagliardi C, Liukkonen JR, Phillippi-Falkenstein KM, Harrison RM, Kubisch HM. Age as a determinant of reproductive success among captive female rhesus macaques (Macaca mulatta) Reproduction. 2007;133:819–826. doi: 10.1530/REP-06-0323. [DOI] [PubMed] [Google Scholar]
- Gilardi KVK, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod. 1997;57:335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- Hanssen SA. Costs of an immune challenge and terminal investment in a long-lived bird. Ecology. 2006;87:2440–2446. doi: 10.1890/0012-9658(2006)87[2440:coaica]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Higham JP, Warren Y, Adanu J, Umaru BN, MacLarnon AM, Sommer V, Ross C. Living on the edge: life-history of olive baboons at Gashaka-Gumti National Park, Nigeria. Am J Primatol. 2009;71:293–304. doi: 10.1002/ajp.20651. [DOI] [PubMed] [Google Scholar]
- Hoffman CL, Ruiz-Lambides AV, Davila E, Maldonado E, Gerald MS, Maestripieri D. Sex differences in survival costs of reproduction in a promiscuous primate. Behav Ecol Sociobiol. 2008;62:1711–1718. doi: 10.1007/s00265-008-0599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RL, Malik I, Berman CM. Age- and dominance-related variation in feeding time among free-ranging female rhesus monkeys. Int J Primatol. 1991;12:337–356. [Google Scholar]
- Johnson RL, Malik I, Berman CM. On the quantification of suckling intensity in primates. Am J Phys Anthropol. 1998;105:33–42. doi: 10.1002/(SICI)1096-8644(199801)105:1<33::AID-AJPA4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Jones-Engel L, Engel GA, Schillaci MA, Lee B, Heidrich J, Chalise M, Kyes RC. Considering human-primate transmission of measles virus through the prism of risk analysis. Am J Primatol. 2006;68:868–879. doi: 10.1002/ajp.20294. [DOI] [PubMed] [Google Scholar]
- Langley PA, Clutton-Brock TH. Does reproductive investment change with age in tsetse flies, Glossina morsitans morsitans (Diptera: Glossinidae)? Funct Ecol. 1998;12:866–870. [Google Scholar]
- Maestripieri D. Parent-offspring conflict in primates. Int J Primatol. 2002;23:923–951. [Google Scholar]
- Malik I, Johnson RL, Berman CM. Control of postpartum mating behavior in free-ranging rhesus monkeys. Am J Primatol. 1992;26:89–95. doi: 10.1002/ajp.1350260203. [DOI] [PubMed] [Google Scholar]
- Nelson E, Hoffman CL, Gerald MS, Shultz S. Digit ratio (2D:4D) and dominance rank in female rhesus macaques (Macaca mulatta) Behav Ecol Sociobiol. 2010;64:1001–1009. [Google Scholar]
- Okamoto K, Matsumura S, Watanabe K. Life history and demography of wild moor macaques (Macaca maurus): summary of ten years of observations. Am J Primatol. 2000;52:1–11. doi: 10.1002/1098-2345(200009)52:1<1::AID-AJP1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Paul A, Kuester J, Podzuweit D. Reproductive senescence and terminal investment in female Barbary macaques (Macaca sylvanus) at Salem. Int J Primatol. 1993;14:105–124. [Google Scholar]
- Rawlins RG, Kessler MJ. The Cayo Santiago macaques: history, behavior, and biology. Albany (NY): SUNY Press; 1986. [Google Scholar]
- Rawlins RG, Kessler MJ, Turnquist JE. Reproductive performance, population dynamics and anthropometrics of the free-ranging Cayo Santiago rhesus macaques. J Med Primatol. 1984;13:247–259. [PubMed] [Google Scholar]
- Robbins AM, Robbins MM, Gerald-Steklis N, Steklis HD. Age-related patterns of reproductive success among female mountain gorillas. Am J Phys Anthropol. 2006;131:511–521. doi: 10.1002/ajpa.20474. [DOI] [PubMed] [Google Scholar]
- Sadd B, Holman L, Armitage H, Lock F, Marland R, Siva-Jothy MT. Modulation of sexual signalling by immune challenged male mealworm beetles (Tenebrio molitor, L.): evidence for terminal investment and dishonesty. J Evol Biol. 2006;19:321–325. doi: 10.1111/j.1420-9101.2005.01062.x. [DOI] [PubMed] [Google Scholar]
- Silk J, Short J, Roberts J, Kusnitz J. Gestation length in rhesus macaques (Macaca mulatta) Int J Primatol. 1993;14:95–104. [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302:1231–1234. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- Silk JB, Altmann J, Alberts SC. Social relationships among adult female baboons (Papio cynocephalus) I. variation in the strength of social bonds. Behav Ecol Sociobiol. 2006;61:183–195. [Google Scholar]
- Smith DG, Small MF. Selection and the transferrin polymorphism in rhesus monkeys (Macaca mulatta) Folia Primatol. 1982;37:127–136. doi: 10.1159/000156025. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Layne DG, Cancino L, Smucny DA. Neonatal behavioral scoring of common marmosets (Callithrix jacchus): relation to physical condition and survival. J Med Primatol. 2002;31:147–151. doi: 10.1034/j.1600-0684.2002.02005.x. [DOI] [PubMed] [Google Scholar]
- Teas J, Richie T, Taylor H, Southwick C. Population patterns and behavioral ecology of rhesus monkeys (Macaca mulatta) in Nepal. In: Lindburg DG, editor. The macaques: studies in ecology, behavior and evolution. New York: Van Nostrand Reinhold Company; 1980. pp. 247–262. [Google Scholar]
- Veenema HC, Spruijt BM, Gispen WH, van Hooff JARAM. Aging, dominance history, and social behavior in Java-monkeys (Macaca fascicularis) Neurobiol Aging. 1997;18:509–515. doi: 10.1016/s0197-4580(97)00107-3. [DOI] [PubMed] [Google Scholar]
- Velando A, Drummond H, Torres R. Senescent birds redouble reproductive effort when ill: confirmation of the terminal investment hypothesis. Proc Biol Sci. 2006;273:1443–1448. doi: 10.1098/rspb.2006.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ML, Herndon JG. Menopause in nonhuman primates? Biol Reprod. 2008;79:398–406. doi: 10.1095/biolreprod.108.068536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weladji RB, Holand O, Gaillard J, Yoccoz NG, Mysterud A, Nieminen M, Stenseth NC. Age-specific changes in different components of reproductive output in female reindeer: terminal allocation or senescence? Oecologia. 2010;162:261–271. doi: 10.1007/s00442-009-1443-5. [DOI] [PubMed] [Google Scholar]
- Weladji RB, Mysterud A, Holand O, Lenvik D. Age-related reproductive effort in reindeer (Rangifer tarandus): evidence of senescence. Oecologia. 2002;131:79–82. doi: 10.1007/s00442-001-0864-6. [DOI] [PubMed] [Google Scholar]
- Whitham JC, Gerald MS, Maestripieri D. Intended receivers and functional significance of grunt and girney vocalizations in free-ranging female rhesus macaques. Ethology. 2007;113:862–874. [Google Scholar]
- Williams GC. Adaptation and natural selection: a critique of some current evolutionary thought. Princeton (NJ): Princeton University Press; 1966. [Google Scholar]
- Wright P, King SJ, Baden A, Jernvall J. Aging in wild female lemurs: sustained fertility with increased infant mortality. In: Atsalis S, Margulis SW, Hof PR, editors. Primate reproductive aging: cross-taxon perspectives. Basel (Switzerland): Karger; 2008. pp. 17–28. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.