Abstract
Vascular disease, such as atherosclerosis, is accompanied by changes in the mechanical properties of the vessel wall. Although altered mechanics is thought to contribute to disease progression, the molecular mechanisms whereby vessel wall stiffening could promote vascular occlusive disease remain unclear. It is well known that platelet-derived growth factor (PDGF) is a major stimulus for the abnormal migration and proliferation of vascular smooth muscle cells (VSMCs) and contributes critically to vascular disease. Here we used engineered substrates with tunable mechanical properties to explore the effect of tissue stiffness on PDGF signaling in VSMCs as a potential mechanism whereby vessel wall stiffening could promote vascular disease. We found that substrate stiffness significantly enhanced PDGFR activity and VSMC proliferation. After ligand binding, PDGFR followed distinct routes of activation in cells cultured on stiff versus soft substrates, as demonstrated by differences in its intensity and duration of activation, sensitivity to cholesterol extracting agent, and plasma membrane localization. Our results suggest that stiffening of the vessel wall could actively promote pathogenesis of vascular disease by enhancing PDGFR signaling to drive VSMC growth and survival.
Keywords: PDGF, substrate stiffness, vascular smooth muscle cell, atherosclerosis
Introduction
Uncontrolled proliferation and migration of vascular smooth muscle cells (VSMCs) coupled with increased deposition of extracellular matrix (ECM) are major causes of intimal thickening during the development of vascular occlusive disease (Imanaka-Yoshida et al., 2001; Nikkari et al., 1994; Thyberg, 1998; Thyberg et al., 1997). Moreover, cellular and molecular compositional changes in the vessel wall modulate the mechanical properties of the vessel (Glagov, 1990; Jacot et al., 2004; Lee et al., 1991; Matsumoto et al., 2002). For example, the elastic modulus of a normal vessel is around 30 kPa, but for a diseased vessel with increased VSMC number and collagen content, the modulus is in the range of 80 kPa (Matsumoto et al., 2002).
Substrate stiffness influences adhesion, proliferation and differentiation in a variety of cell types (Engler et al., 2004b; Friedland et al., 2009; Paszek and Weaver, 2004; Paszek et al., 2005; Pelham and Wang, 1997; Wang et al., 2000). Specifically, we and others have found that VSMC migration and proliferation increase with substrate stiffness (Brown et al., 2005; Engler et al., 2004a; Isenberg, 2009; McDaniel et al., 2007; Peyton and Putnam, 2005; Wong et al., 2003). Such findings suggest that VSMC behavior could be modified due to increased vessel stiffness during the development of vascular disease.
Cells sense the mechanical properties of their substrate through integrins (Ingber, 2003b; Katsumi et al., 2004; Paszek et al., 2005; Wang et al., 2001). Changes in substrate stiffness can potentially influence cellular responses to growth factors through cross-talk between integrin and growth factor receptor (GFR) signaling pathways. As reviewed in an article by Lee and Juliano, common downstream signaling molecules such as Ras, Rho, FAK and PI3K integrate simultaneous inputs from integrins and GFRs to generate a single mitogenic output (Lee and Juliano, 2004; Miranti and Brugge, 2002).
Integrin and GFR cross-talk often occurs in lipid rafts. Lipid rafts are cholesterol-rich, organized lipid membrane microdomains. They play a central role in the signal transduction of cell surface receptors because many essential downstream signaling molecules are targeted to the rafts from the cytosol through post-translational modification (Resh, 2004; Simons and Ehehalt, 2002; Simons and Toomre, 2000). By bringing the cell surface receptors and their downstream effectors into close proximity to each other, these organized lipid membrane microdomains increase the efficiency of these signaling processes. It has been demonstrated by many groups that disruption of lipid rafts by sequestering cholesterol significantly decreases the activities of the cell surface receptors (Arcaro et al., 2007; Decker and ffrench-Constant, 2004; Makoto et al., 2004; Stehr et al., 2003).
PDGF plays a major role in VSMC migration and proliferation during the development of vascular occlusive disease (reviewed in (Raines, 2004)). Although PDGF mRNA has been detected in both healthy and diseased vessels (Barrett and Benditt, 1988), VSMC migration and proliferation has only been found to occur in diseased vessels. Despite the fact that increased vessel stiffness is characteristic of vascular disease, little is known about the influence of substrate stiffness on the stimulatory effect of PDGF. We hypothesize that substrate stiffness modulates VSMC response to PDGF. In this study, we used a defined model system that mimicked the stiffness of healthy and diseased vessels to investigate the effects of substrate stiffness on VSMC response to PDGF and its underlying mechanisms.
Materials and Methods
Substrate preparation
Our engineered model system was comprised of polyacrylamide (PAAm) gels with RGD peptides incorporated into the bulk of the gel. Acryloyl-PEG-GRGDSP was synthesized according to the method first described by Hubbell’s group (Jackman et al., 1999; Stegemann et al., 2007). Briefly, equal molar of acryloyl-PEG-N-hydroxysuccinimide ester (Nektar Therapeutics, San Carlos, CA) and GRGDSP peptide (American Peptide, Sunnyvale, CA) were incubated in sodium bicarbonate buffer (pH 8.5) at room temperature for 2 hr. Un-reacted peptide and sodium bicarbonate were removed by dialysis in dI water. PAAm substrates were prepared as described previously (Leach et al., 2007). Briefly, prior to making the gel solution, 25 mm round cover slips were activated. Cover slips were passed through a Bunsen burner flame, treated sequentially with 0.1 M NaOH and 3-aminopropyltrimethyoxy silane (Sigma, St. Louis, MO), washed with distilled water, and then incubated with 0.5% glutaraldehyde (Polysciences, Warrington, PA) for 30 min at room temperature. A typical acrylamide/bis-acrylamide mixture contained 10% acrylamide (Bio-Rad, Hercules, CA), 0.03–0.3% bis-acrylamide (Bio-Rad), 0.05 M HEPES, 0.15% TEMED (Sigma), 0.05% ammonium persulfate (Sigma) and 10 µM acryloyl-PEG-GRGDSP. The stiffness of the PAAm substrate was modulated by changing the bis-acrylamide concentration. A 30 µl drop of the gel solution was cast between an activated and non-activated cover slip and allowed to polymerize at room temperature. The non-activated cover slip was then removed, and the substrates were incubated in 2M Glycine overnight to quench the residual unreacted acryloyl-PEG-N-hydroxysuccinimide ester in the gel. All gels were washed thoroughly with PBS before each experiment.
Substrate characterization
The stiffness of the substrates was determined by a micro-indentation method (Jacot et al., 2006). Briefly, a 100 µm diameter glass bead attached to a glass fiber was used as indenter tip. Images were captured as the tip was lowered into the substrate. Indenter tip displacement and deflection were measured from the images, and the elastic modulus of the substrate was calculated from the unloading portion of the indenter tip force versus displacement curve.
The rate of acryloyl-PEG-GRGDSP incorporation into the substrate was determined using I-125 labeled peptide. Briefly, 10 µCi I-125-labeled YRGDS (5 µCi/µg, Phoenix Pharmaceuticals, Belmont, CA) was mixed with 1 mg GRGDSP and acryloyl-PEG- N-hydroxysuccinimide ester to generate I-125-labeled ligand. The resulting product was then used to cast gels with different stiffness values, and the radioactivity of the substrates was measured with a gamma-counter. The amount of ligand per square micron of each substrate throughout the thickness of the gel was calculated assuming that I-125 labeled YRGDS and unlabeled GRGDSP had the same incorporation rate.
The surface ligand density was determined by ELISA using biotin-labeled peptide (GRGDSPY-biotin, American Peptide). Briefly, biotin-labeled GRGDSPY was mixed with GRGDSP at a 1:100 molar ratio to generate a mixture of biotinylated and non-biotinylated ligand, which were then used to cast substrates. After blocking with 2% BSA and 0.1% Tween-20, the substrates were incubated first with monoclonal rabbit anti-Biotin antibody (Abcam, Cambridge, MA) at 1:5,000 dilution and then with HRP-labeled anti-rabbit antibody at 1:10,000 dilution (Jackson ImmunoResearch Labs, West Grove, PA). Soluble TMB substrate (Thermo Fischer, Rockford, IL) was used for colorimetric measurements (OD450) to determine the surface ligand density.
Cell culture and manipulations
Primary bovine vascular smooth muscle cells (VSMCs, AG08504, Coriell Cell Repositories, Camden, NJ) were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% bovine calf serum, 200 mM L-glutamine, and penicillin-streptomycin in a 5% CO2 humidified incubator. All cell culture reagents were from Invitrogen Inc., San Diego, CA. Cells with passage number between 10 and 15 were used for the experiments.
For projected cell area analysis, VSMCs were serum starved for 2 days before being seeded onto the substrates in serum-free media at a density of 104cells/cm2. After 4 hr of attachment, non-adherent cells were removed and phase contrast images were captured at ten random positions of each substrate. Projected cell area was analyzed using ImageJ software (National Institute of Health). The areas of at least 100 cells were determined for each substrate.
For cell growth studies, VSMCs cultured on soft and stiff substrates were serum starved for 24 hr before stimulation with 0.5% serum with or without 10 ng/ml PDGF-BB (R&D Systems, Minneapolis, MN). The number of cells at the day of stimulation (day 0) and 3 days after stimulation (day 3) was analyzed using the acid phosphatase assay. The rate of cell growth was calculated by dividing the number of cells on day 3 by the number of cells on day 0.
For analysis of PDGFR activation, VSMCs were serum starved for 24 hr. Just before PDGF-BB stimulation, VSMCs were treated with or without 10 mM methyl-β-cyclodextrin (MBCD, Sigma) for 1 hr. After PDGF-BB stimulation, cells were lysed in a buffer containing 20 mM Tris pH 7.4, 150 mM NaCl, 1% Triton 100, 5 mM EDTA, 5 mM EGTA, 1 mM PMSF, 1 µg/ml leupeptin, 1 µg/ml aprotinin and 0.2 mM Na3VO4.
Western blot
Cell lysates with equal amounts of total protein were separated on denaturing SDS-polyacrylamide gels and transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA). Phosphorylated PDGFR was detected with PY20 (BD Transduction Laboratories, San Diego, CA), and total PDGFR was detected with PDGFR-β anti-serum (a generous gift from Dr. Kazlauskas at the Schepens Eye Research Institute). The blots were analyzed with ImageJ software, and the level of PDGFR phosphorylation was normalized to total PDGFR. Fold PDGFR activation in PDGF-BB treated samples was calculated against untreated samples.
Immunocytochemistry and fluorescent microscopy
Adherent VSMCs were fixed with 3% phosphate buffered formalin for 20 min and permeabilized with 0.5% TritonX-100. F-actin was stained with Rhodamine-labeled Phalloidin (Invitrogen, Carlsbad, CA), and cell nuclei were stained with Hoechst 33342 (Invitrogen, Carlsbad, CA). To visualize PDGFR activation and lipid microdomains, samples were subjected to MBCD and/or PDGF treatment as described above. After fixing and permeabilization, nonspecific binding was first blocked with 2.5% goat serum (Vector Laboratories, Burlingame, CA) for 1 hr. The first primary antibody incubation was with rabbit polyclonal phospho-PDGFR β (Abcam Inc. Cambridge, MA) at a dilution of 1:50 for 1 hr, followed by a second incubation with FITC-conjugated goat anti-rabbit antibody (Vector Laboratories) for 1 hr. Samples were then washed 3 × 15 min with PBS and blocked with 2.5% horse serum (Vector Laboratories) for 1 hr. The second primary antibody incubation was with mouse monoclonal Flotillin-1 (BD Transduction Laboratories, Franklin Lakes, NJ) at a dilution of 1:250 for 1 hr, followed by an incubation with Texas Red-conjugated horse anti-mouse antibody (Vector Laboratories) for 1 hr, followed by a 3 × 15 min PBS wash. Both secondary antibodies were used at the manufacturer’s suggested concentrations. All incubations and washes were at room temperature. Fluorescent images were captured with confocal microscopy (Olympus FV1000).
Statistical analysis
Statistics were performed using Student’s t-test assuming equal variances for paired comparisons and ANOVA for more than two groups of data.
Results
Characterization of polyacrylamide substrates
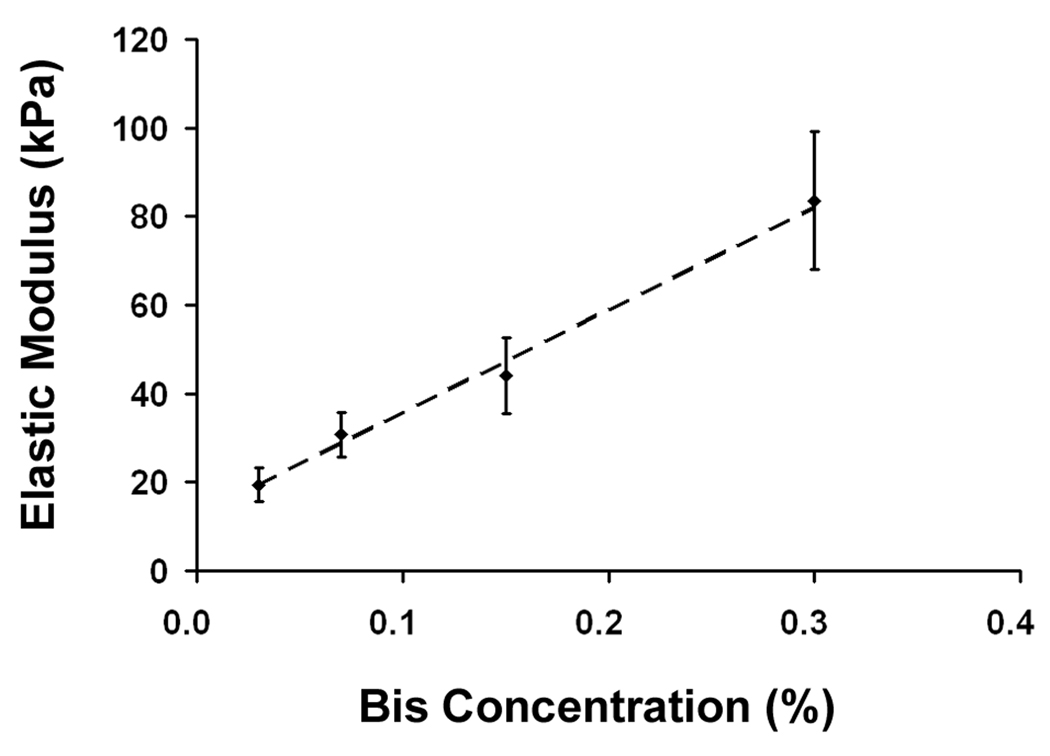
We prepared a series of PAAm substrates with 10% acrylamide with varying bis-acrylamide concentrations (0.03 – 0.3%). As measured by micro-indentation, when the bis-acrylamide concentration was increased from 0.03% to 0.3%, the elastic modulus increased linearly from 19 to 84 kPa (Fig. 1A). These values are in the range of stiffness that has been reported for normal and atherosclerotic vessels (Matsumoto et al., 2002); thus, we were able to tune the mechanical properties of our model substrates to mimic the stiffness of healthy and diseased vessels simply by varying the bis-acrylamide concentration.
Fig. 1.
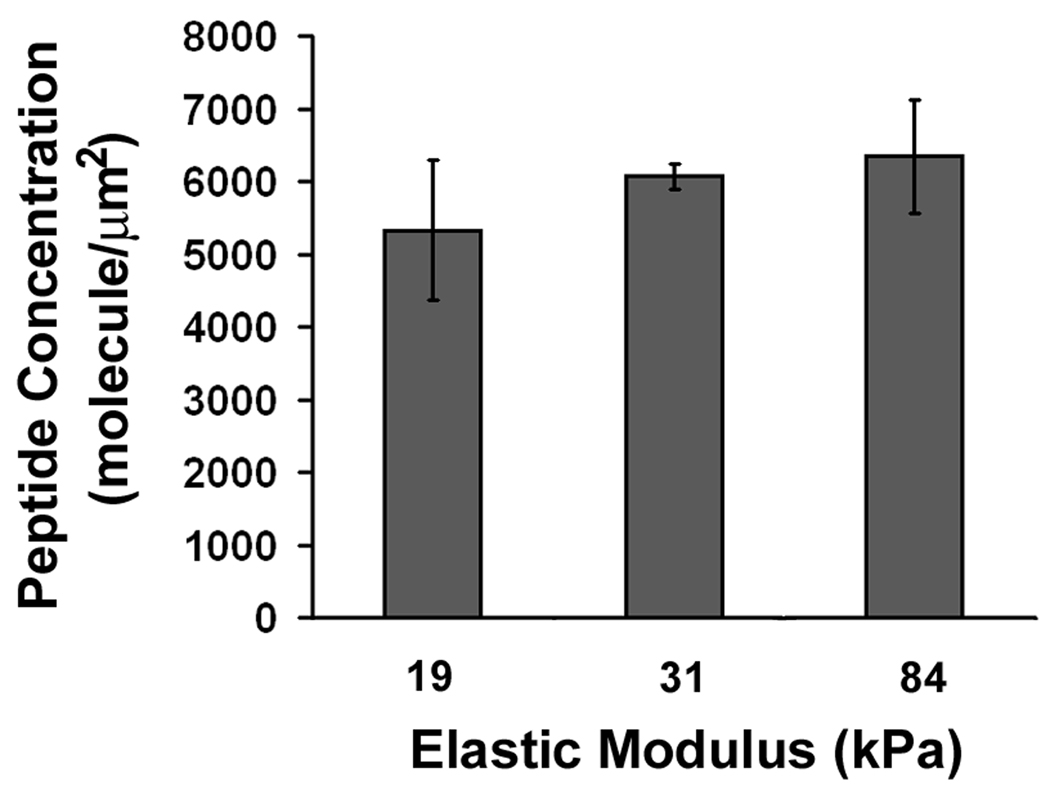
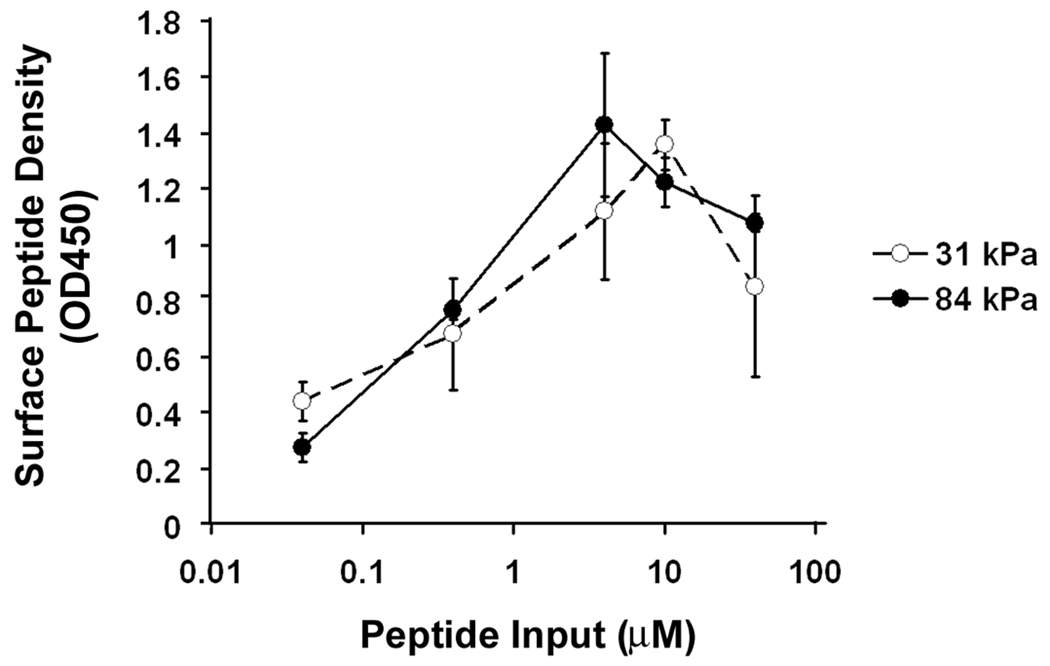
Substrate characterization. A: Substrate stiffness increases with bis-acrylamide concentration (n=10). B: Ligand concentration in the bulk of the substrate remains the same as substrate stiffness increases (n=3). C: Surface ligand densities are dependent on input peptide concentration but not substrate stiffness (n=3). All error bars represent standard deviation.
Changes in ligand density influence cell adhesion, migration and proliferation (Engler et al., 2004a; Gaudet et al., 2003; Rajagopalan et al., 2004; Rowley and Mooney, 2002). To ensure that substrate stiffness was the only variable in our model system, we used I-125 labeled YRGDS to calculate the rate of peptide incorporation. When the elastic modulus increased from 19 to 84 kPa, we found no statistical differences in the peptide concentration in the bulk of the gel (5846±452 molecule/µm2, p=0.37, Fig. 1B). We also compared the amount of peptide on the gel surface available for cell binding using biotinylated GRGDSPY peptide and anti-biotin antibody. When the input peptide concentration in the initial gel mix was increased from 0.04 µM to 40 µM, the peptide surface concentration increased linearly at first, which then reached a maximum at 4 µM input peptide concentration for the 84 kPa gels and 10 µM for the 31 kPa gels (Fig. 1C). We did not detect any statistical differences in the surface peptide density between 31 kPa gels and 84 kPa gels at peptide input concentrations above 0.04 µM. In order to ensure maximum and equal surface ligand concentration on our substrates, we used a peptide input of 10 µM throughout our study.
Increase in substrate stiffness leads to increased cytoskeletal organization and VSMC proliferation
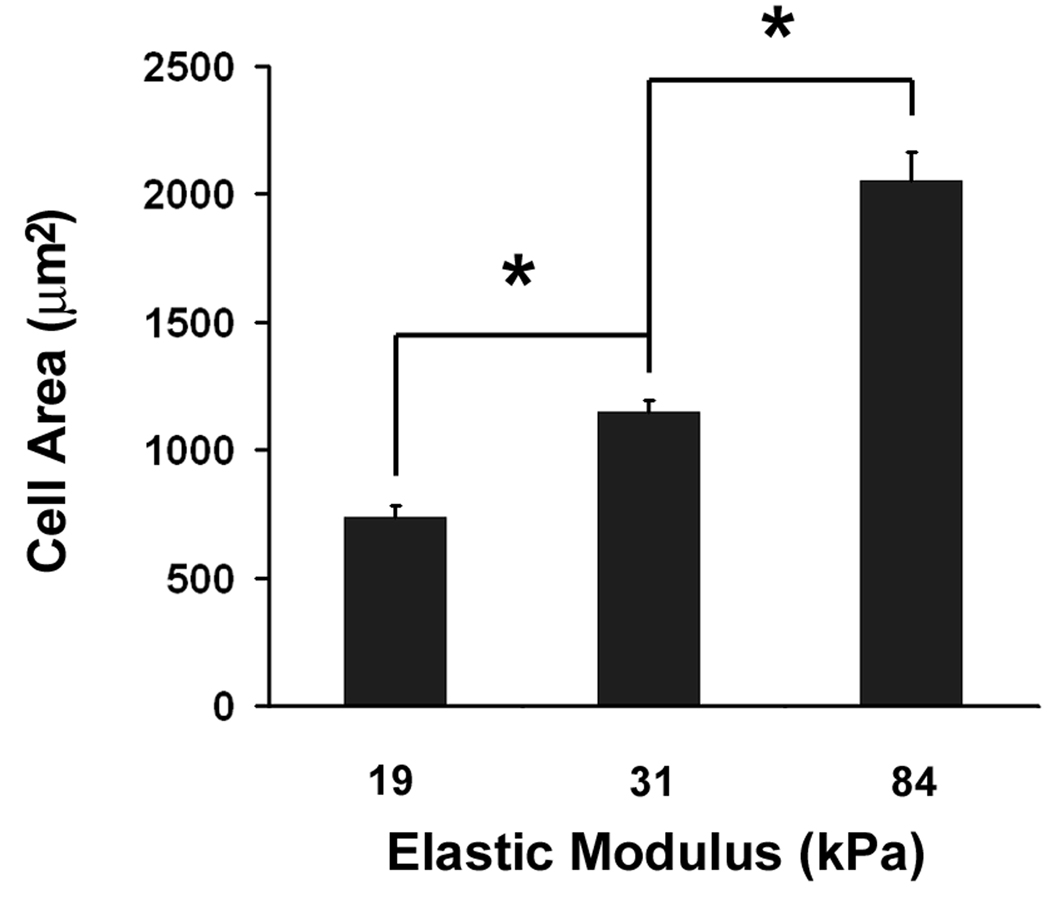
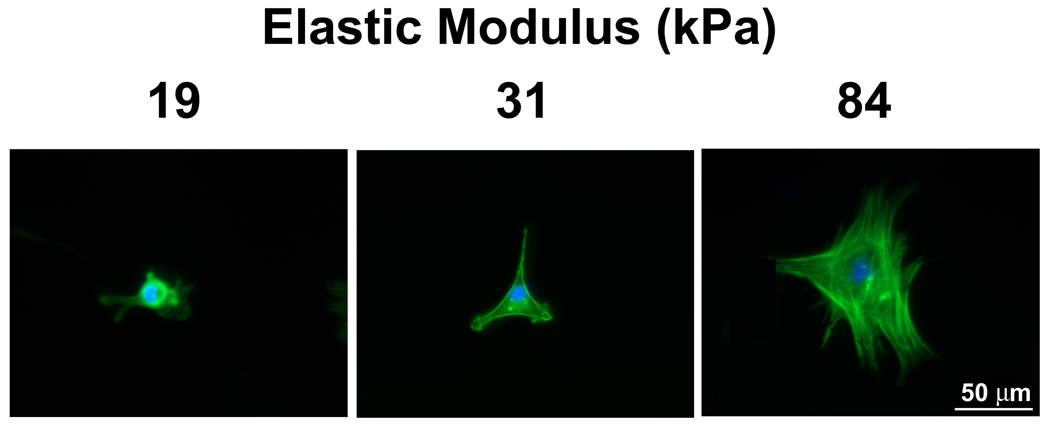
Using our model system, we first investigated whether changes in mechanical properties of substrates mimicking vessel stiffness would affect VSMC survival and proliferation. For anchorage-dependent cells, cell adhesion regulates cell survival and proliferation. Successful cell adhesion is indicated by an increase in projected cell area and the formation of stress fibers. From short term (4 hours) adhesion studies, we found that projected cell area and the formation of stress fibers increased significantly with increased substrate stiffness (Fig. 2A and Fig. 2B). The projected cell area on the 84 kPa substrates was 1.8-fold larger than on the 31 kPa substrates (p < 0.01), which in turn was 1.6-fold larger than on the 19 kPa substrates (p <0.01) (Fig. 2A). On 19 kPa substrates, F-actin was diffuse and concentrated around the nucleus. In contrast, on 84 kPa substrates, stress fibers were fully formed in the cell body (Fig. 2B).
Fig. 2.
Substrate stiffness regulates cytoskeletal organization and VSMC proliferation. A: VSMC projected cell area increases with substrate stiffness (n>100). Error bars represent S.E.M. (* p<0.01). B: Fluorescent images of VSMC show more defined stress fibers as substrate stiffness increases. F-actin (green) was stained with Rhodamine-labeled Phalloidin and cell nuclei (blue) were stained with Hoechst 33342. C: The rate of VSMC proliferation is higher on stiff substrates (n=3). Error bars represent standard deviation (* p<0.01).
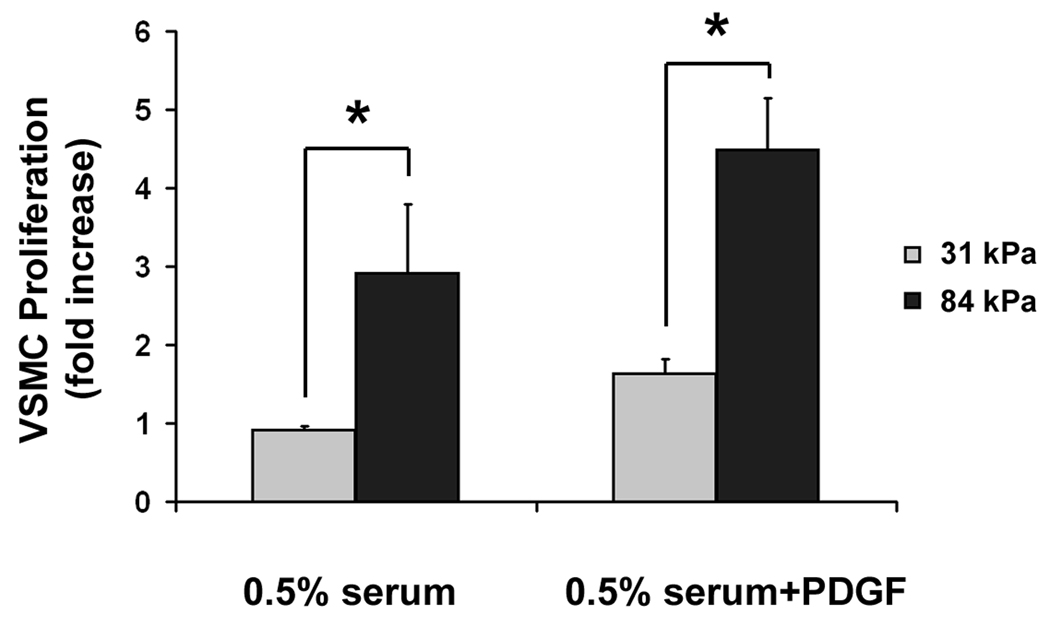
VSMCs did not adhere well on the 19 kPa substrate and did not survive overnight culture. We therefore concentrated our study on the 31 kPa substrate, which mimicked the stiffness of a healthy vessel, and the 84 kPa substrate, which mimicked the stiffness of a diseased vessel. After 3 days of serum stimulation, there was no statistically significant change in the number of VSMCs on 31 kPa substrates; the number of VSMCs on 84 kPa substrates, however, was almost 3-fold the original number before the start of stimulation (Fig. 2C). After 3 days of combined serum and PDGF-BB stimulation, the number of VSMCs on 31 kPa substrates was only 1.6 fold of the original number, while the number of VSMCs on 84 kPa substrates reached 4.5 fold the original number (Fig. 2C). These results indicate that substrate stiffness promotes VSMC survival and proliferation under both serum and PDGF stimulation.
Substrate stiffness enhances the stimulating effect of PDGF-BB
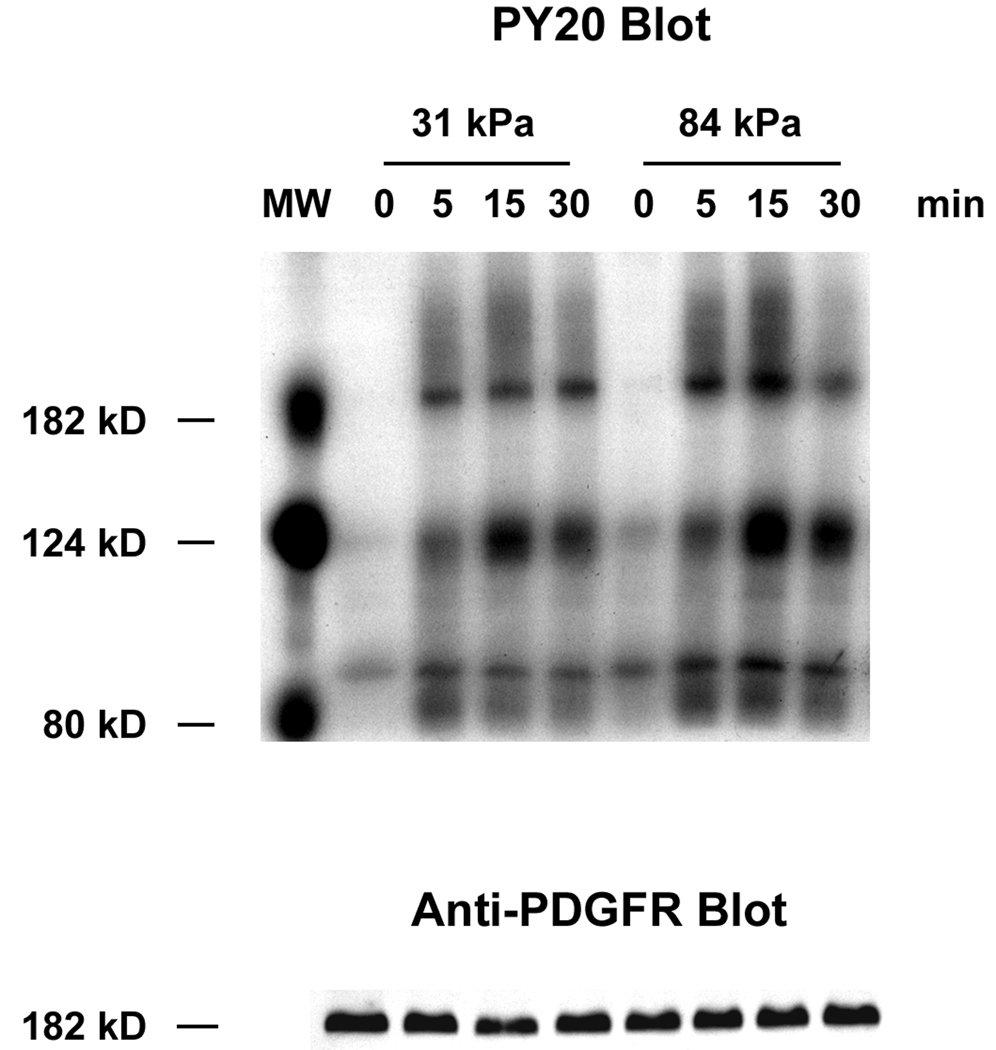
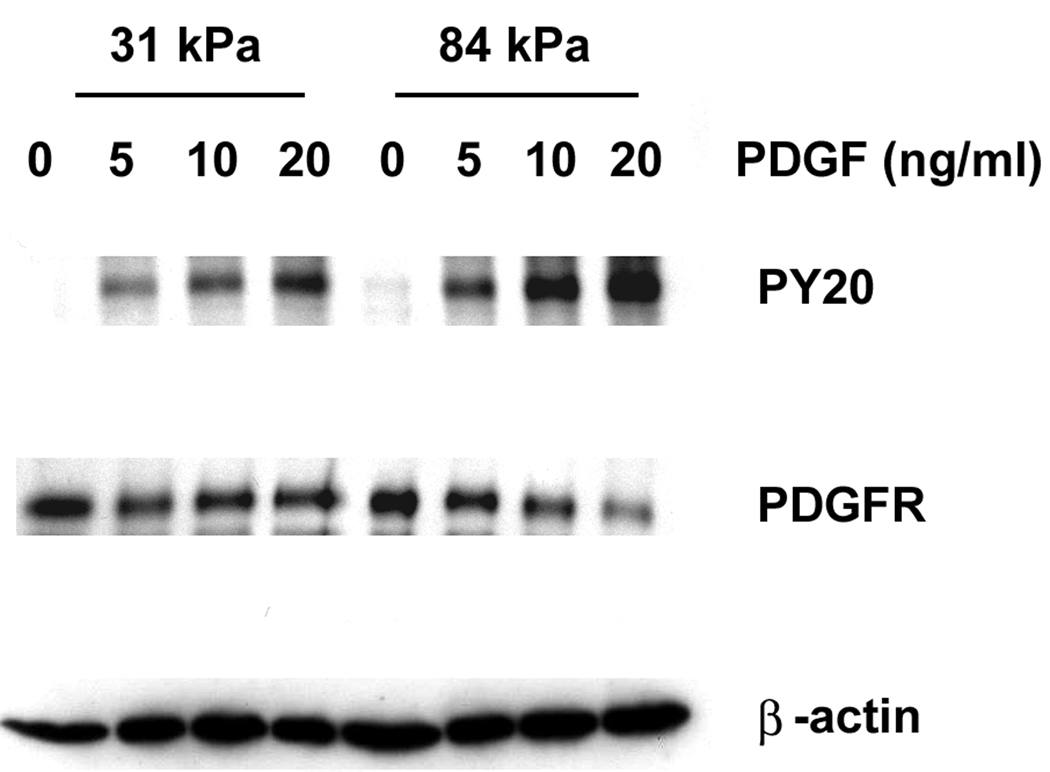
In light of our observed changes in vessel stiffness during the progression of vascular disease, an important question to address is the role of mechanical stiffness in VSMC response to PDGF. We already observed an increase in the rate of VSMC proliferation with increased substrate stiffness when cells were cultured in the presence of both serum and PDGF-BB. To eliminate the possible activation of PDGFR by serum, we exposed serum-starved VSMCs to PDGF-BB and investigated the level of PDGFR activation on 31 kPa and 84 kPa substrates. Using PY20, a general antibody that recognizes phosphorylated Tyrosine, we identified three phosphorylated proteins in VSMC lysates at 190 kD, 125 kD and 90 kD (Fig. 3A). Because 190 and 125 kD are the molecular weights (MW) for PDGFR and FAK, respectively (Heldin et al., 1983), it is likely that the 190 kD protein corresponds to phosphorylated PDGFR, and the 125 kD protein corresponds to phosphorylated FAK. In addition, we detected proteins at the same MW positions using PDGFR-β antiserum and monoclonal anti-FAK antibody (Fig. 3A and data not shown).
Fig. 3.
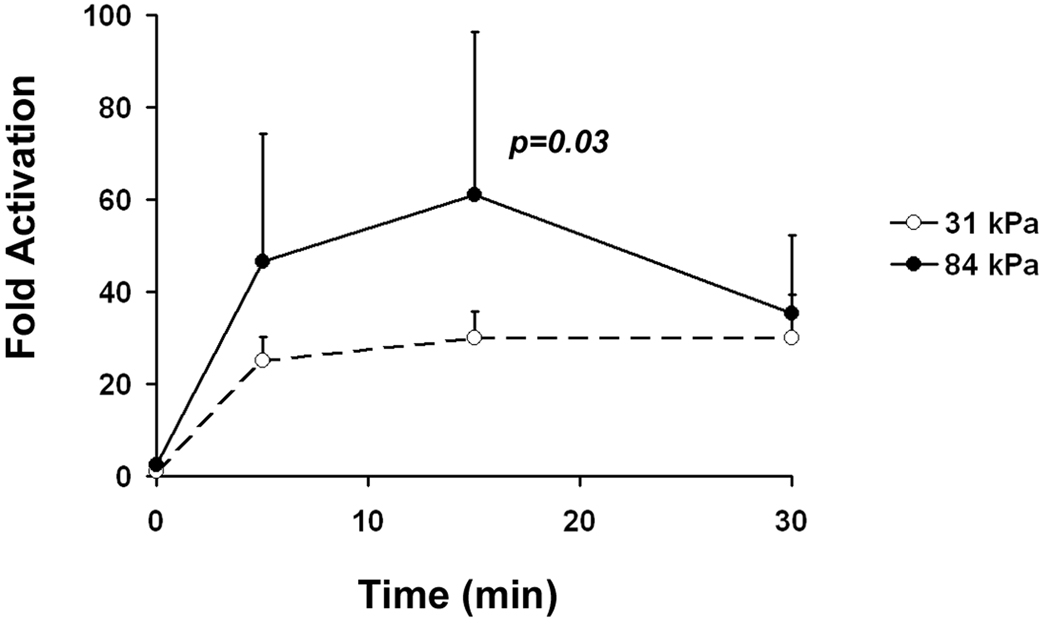
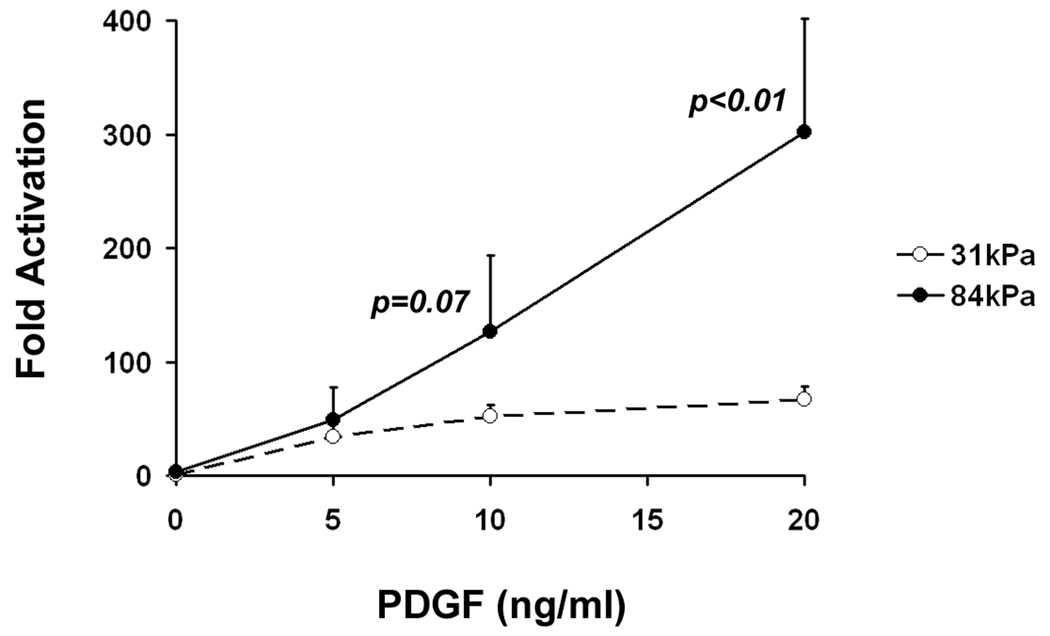
Substrate stiffness enhances the stimulating effect of PDGF in VSMCs. A: Representative Western blots of VSMCs stimulated with 10 ng/ml PDGF over a 30 minute time course. B: VSMCs on stiff substrates show higher levels of PDGFR phosphorylation during the first 30 minutes of PDGF stimulation (n=3). C: Representative Western blots of VSMCs stimulated with increasing concentrations of PDGF for 10 minutes. D: VSMCs on stiff substrates show higher levels of PDGFR phosphorylation over a range of PDGF concentrations (n=3). Error bars represent standard deviation.
After normalizing to total PDGFR protein level, we calculated changes in the level of phosphorylated PDGFR in cells exposed to PDGF-BB compared to cells that were not exposed to PDGF-BB (fold activation). We found after the 10 ng/ml PDGF-BB stimulation, fold activation of PDGFR in cells on 31 kPa substrates remained low throughout a 30 min time course (Fig. 3A and Fig. 3B). On 84 kPa substrates however, fold activation of PDGFR was twice as much as on 31 kPa substrates during the first 15 min and decreased rapidly to the same level as on 31 kPa substrates at 30 min. This indicates that substrate stiffness affects the intensity and dynamics of PDGFR activation in VSMCs.
We further investigated the effect of substrate stiffness on PDGFR activation in VSMCs by exposing VSMCs to increasing amounts of PDGF-BB (5, 10 and 20 ng/ml) for 10 minutes. Fold activation of PDGFR increased in a dose-dependent manner in VSMCs on both 31 and 84 kPa substrates (Fig. 3C and Fig. 3D). However, levels of PDGFR activation were significantly higher in VSMCs on 84 kPa substrates than on 31 kPa substrates, and VSMCs responded more significantly to increasing amounts of PDGF-BB on 84 kPa substrates. When PDGF concentration increased four-fold from 5 ng/ml to 20 ng/ml, the level of PDGFR activation increased six-fold on 84 kPa substrates versus two-fold on 31 kPa substrates. The time course experiment and dose response experiment together demonstrate that substrate stiffness enhances the stimulating effect of PDGF in VSMCs. In addition, we also observed a decrease in total PDGFR protein level in cells on 84 kPa substrates exposed to high concentrations of PDGF (20 ng/ml), but not in cells on 31 kPa substrates, indicating a difference in the regulation of PDGFR in cells on soft vs. stiff substrates.
Substrate stiffness modulates VSMC response to PDGF through organized membrane domains
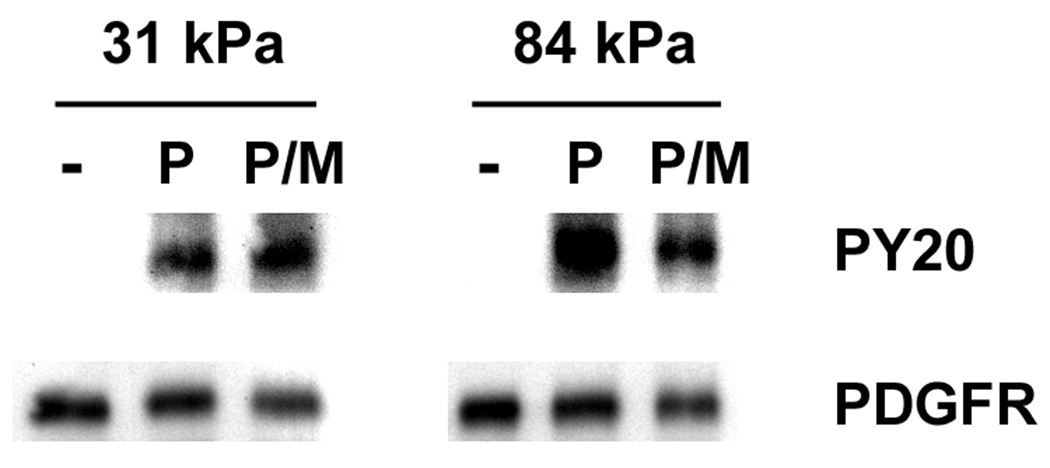
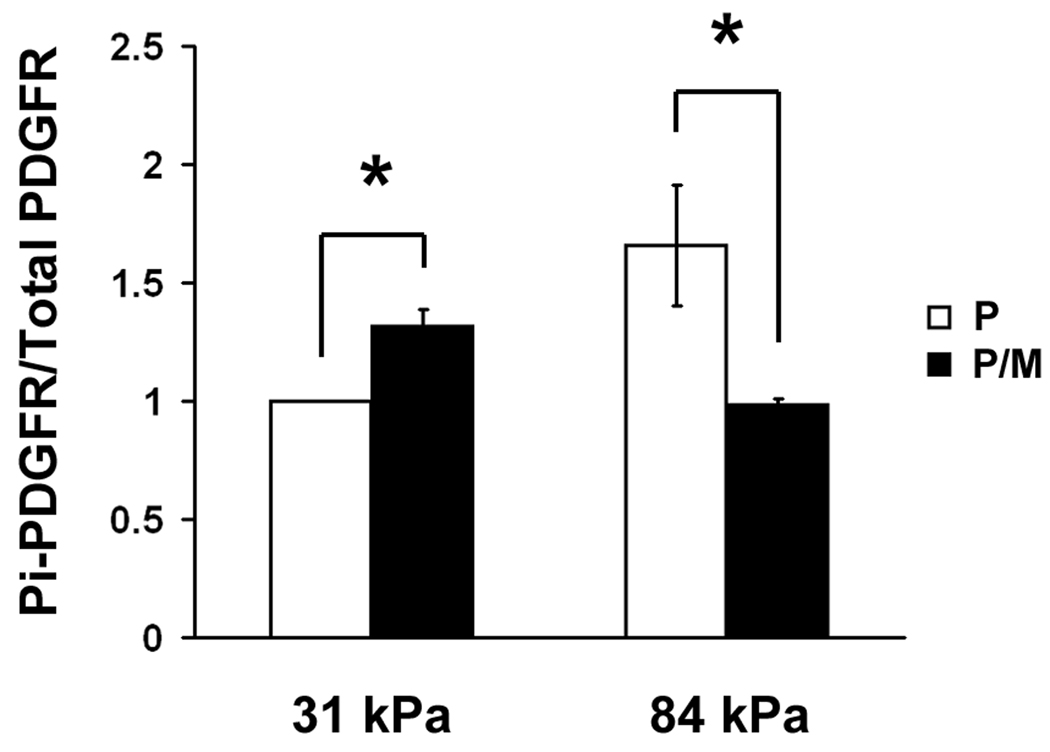
We wanted to dissect out the possibility that substrate stiffness modulates PDGFR signaling in organized membrane microdomain lipid rafts. In order to address this, we treated VSMCs with MBCD – an agent that extracts cholesterol from the cells and disrupts lipid rafts. In MBCD treated cells, the level of PDGFR activation was significantly reduced on 84 kPa substrates, suggesting lipid rafts are involved in PDGFR activation (Fig. 4A and Fig. 4B). On 31 kPa substrates, however, the level of PDGFR activation in PDGF stimulated cells increased slightly after MBCD treatment, suggesting a lipid raft independent mechanism of PDGFR activation.
Fig. 4.
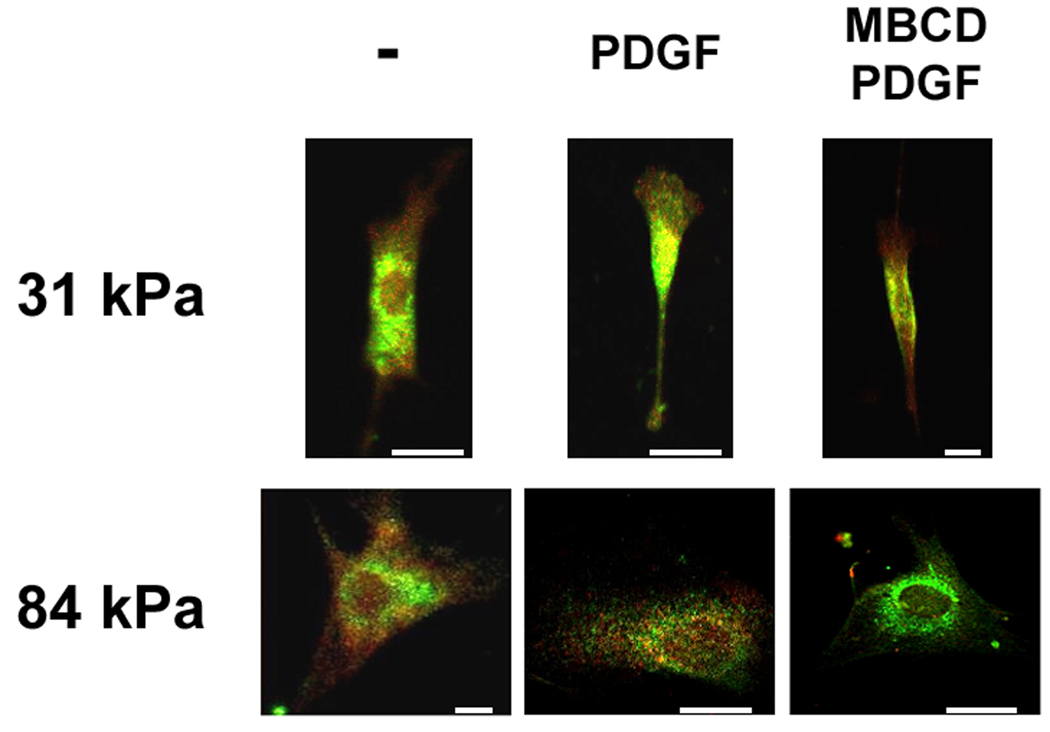
Lipid rafts are involved in PDGFR activation in VSMCs on stiff substrates. A: Representative Western blots of MBCD treated and PDGF stimulated VSMCs. B: PDGFR phosphorylation is diminished by MBCD treatment on stiff substrates only (n=3). Error bars represent standard deviation (* p<0.01). C: Double-label fluorescence immunocytochemistry for flotillin-1 (green) and phospho-PDGFRβ (red) as a function of substrate stiffness in the presence or absence of MBCD and/or PDGF. Scale bars represent 10 microns.
We also examined the phosphorylation of PDGFR in VSMCs using confocal microscopy. We observed positive staining for phosphorylated PDGFRβ (Fig. 4C, red) and lipid raft marker flotillin-1 (Fig. 4C, green) in cells on both 31 kPa and 84 kPa substrates. The staining pattern for phospho-PDGFRβ and flotillin-1 appeared diffuse in cells before PDGF stimulation on both substrates (Fig. 4C, left panels). After PDGF stimulation, phospho-PDGFRβ and flotillin-1 were rearranged to a punctate state on the 84 kPa substrate (Fig. 4C, center bottom panel), whereas on the 31 kPa substrate, the staining remained diffuse (Fig. 4C, center top panel). In PDGF stimulated cells, MBCD eliminated phosphorylation of PDGFRβ in VSMCs on the 84 kPa substrate (Fig. 4C, bottom right panel). In contrast, MBCD had no effect on the phosphorylation of PDGFRβ in VSMCs on the 31 kPa substrate (Fig. 4C top right panel). These data further suggest that ligand-dependent PDGFR activation on the 84 kPa substrate is lipid raft dependent, whereas PDGFR signaling on the 31 kPa substrate is independent of these membrane domains.
Discussion
Using a model system that represents the stiffness of a healthy vessel (31 kPa) and a diseased vessel (84 kPa), here we showed that substrate stiffness enhances the stimulating effect of PDGF on VSMCs. VSMCs on stiff (84 kPa) substrates had significantly higher growth in response to PDGF compared to VSMCs on soft (31 kPa) substrates. On the stiff substrate, PDGFR activation by PDGF was strong and appeared to be dependent on cholesterol rich lipid rafts. However, on the soft substrate, PDGFR phosphorylation was weak and appeared to be independent of lipid rafts.
In addition to the different dependencies on lipid rafts during PDGFR activation, we also observed other differences in the regulation of PDGFR signaling in VSMCs when substrate stiffness is changed. After PDGF stimulation, the high level of PDGFR activation on stiff substrates (84 kPa) was transient (Fig. 3A and Fig. 3B), whereas the low level of PDGFR activation on soft substrates remained steady in the time frame we examined. When treated with increasing concentrations of PDGF, total PDGFR protein decreased in VSMCs on stiff substrates (84 kPa, Fig. 3C), indicating PDGFR internalization and degradation through ligand-bound PDGFR, which could also explain the transient nature of PDGFR activation on stiff substrates. In contrast, on soft substrates (31 kPa) total PDGFR protein did not decrease in the concentration range used in this study. Despite decreased total PDGFR protein levels, the level of phosphorylated PDGFR increased with PDGF concentration on stiff substrates. As a matter of fact, after normalization to PDGFR protein level, the level of PDGFR phosphorylation increased significantly more on stiff substrates compared to soft substrates with increasing PDGF concentrations (Fig. 3D). This suggests an increased sensitivity to PDGF when VSMCs are on stiffer substrates.
Cells sense matrix stiffness through integrins (Friedland et al., 2009; Ingber, 2003b; Katsumi et al., 2004; Paszek et al., 2005; Wang et al., 2001). Paszek et al. showed increased stroma stiffness during breast cancer development leads to integrin clustering, enhanced ERK and ROCK activation (Paszek et al., 2005). A recent paper by Friedland et al. showed that an increase in substrate stiffness switches the conformation of α5β1 integrins from a relaxed state to a tensioned state, which strengthens the association between integrins and fibronectin and leads to higher level of FAK phosphorylation at Y397 position (Friedland et al., 2009). We observed increased projected cell area and stress fiber formation in VSMCs on stiff substrates, indicating enhanced integrin signaling (Fig. 2A and Fig. 2B). Our preliminary data also showed increased FAK and ERK phosphorylation in VSMCs on stiff substrates (data not shown). Further investigation is required to elucidate the specific mechanism of integrin activation in our model system, i.e. determining whether activation is achieved through integrin clustering and/or via changes in integrin conformation. We note that these two proposed mechanisms are not mutually exclusive.
Increase in substrate stiffness enhances integrin activation, whereas integrin engagement and activation is essential for the formation and stabilization of organized lipid membrane microdomains (Echarri et al., 2007; Gaus et al., 2006; Pankov et al., 2005). In addition, the expression levels of lipid raft associated molecules were found to be up-regulated when VSMCs were cultured on stiff tissue culture plastic when compared with a soft spherical culture (Monastyrskaya et al., 2003). Mistuda and Stehr observed that, although phosphorylation of PDGFR upon PDGF binding might be independent of the localization of the receptor, PDGFR signals more efficiently when localized in lipid rafts (Mitsuda et al., 2002; Stehr et al., 2003). We observed that on stiff (84 kPa) substrates, phosphorylated PDGFR molecules aggregate into punctate domains in PDGF stimulated VSMCs (Fig. 4C bottom panels), and the level of PDGFR phosphorylation on stiff substrates is high but significantly diminished after disruption of lipid rafts by using a cholesterol extracting agent (Fig. 4A and Fig. 4B). On soft (31 kPa) substrates however, the localization of phosphorylated PDGFR is dispersed (Fig. 4C top panels), and the level of PDGFR phosphorylation is low but not further reduced by the cholesterol extracting agent (Fig. 4A and Fig. 4B). In fact, we observed a slight increase in the phosphorylation of PDGFR (Fig. 4B), which is possibly due to ligand independent activation of PDGFR by cholesterol extracting agents (Liu et al., 2007). Our data highlight the fact that PDGFR activation is regulated differently when substrate stiffness is changed. On the stiff substrate, PDGFR activation appears to be lipid raft dependent, as depleting cholesterol decreases the level of PDGFR phosphorylation. In contrast, on the soft substrate, PDGFR activation seems to be lipid raft independent, as depleting cholesterol increases the level of PDGFR phosphorylation in a ligand independent manner.
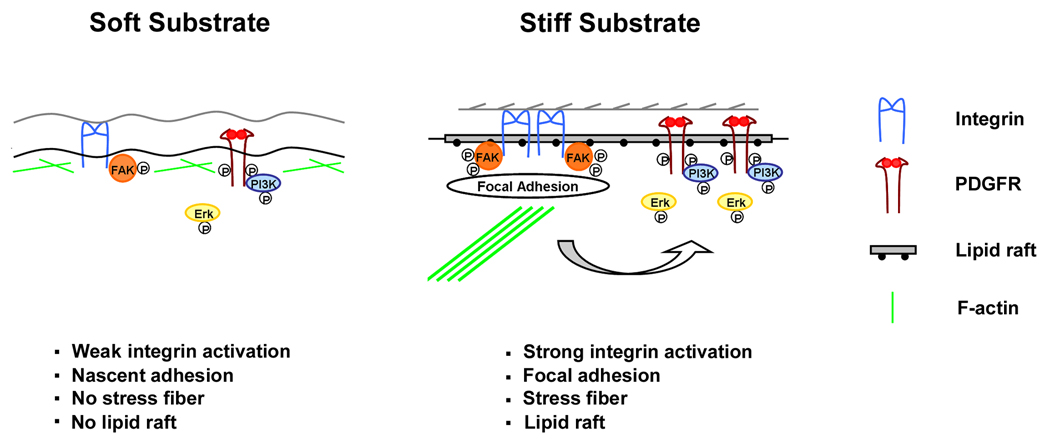
Based on previous reports and our own observations, we propose the following mechanism (Fig. 5): when cells are attached to a stiff substrate, there is enough force to induce high levels of integrin activation (clustering or conformational changes) and the formation of organized membrane domain lipid rafts. PDGFR and its downstream effectors are recruited to these membrane microdomains, and PDGF-induced signaling follows the similar pathway that we observe on tissue culture plastic surfaces. When cells are attached to a soft substrate, there is not enough integrin activation to stabilize lipid rafts. As a consequence, PDGF signaling follows a lipid raft-independent pathway.
Fig. 5.
A proposed model for the regulation of PDGFR signaling by substrate stiffness. When cells are attached to a soft substrate, there is not enough force to induce high level of integrin activation, and there is little organized membrane domain lipid rafts. As a consequence, PDGFR activation is independent of lipid rafts and weak. When cells are attached to a stiff substrate, there is enough force to induce high level of integrin activation and formation of lipid rafts. PDGFR and its downstream effectors are recruited to these membrane domains, and PDGFR activation is strong.
Changes in ECM stiffness occurs during many physiological and pathological conditions (Beloussov et al., 1997; Czirok et al., 2004; Ingber, 2003a; Paszek and Weaver, 2004). Using a model system that mimics normal and diseased vessel stiffness, we found that VSMCs respond differently to a growth factor (i.e. PDGF) and a pharmacological agent (i.e. MBCD) when substrate stiffness is changed (Fig. 3 and Fig. 4). The fact that VSMCs on substrates with the stiffness of a normal vessel (31 kPa) respond to PDGF stimulation with lipid raft-independent and low-level PDGFR activation suggests that the softer, healthy vessel keeps VSMCs in a quiescent state even in the presence of a growth factor. However, that VSMCs on substrates with the stiffness of a diseased vessel (84 kPa) respond to PDGF stimulation with lipid raft-dependent and high-level PDGFR activation indicates that the stiffer, diseased vessel promotes the stimulation of VSMCs by the growth factor. Increased sensitivity of VSMC to PDGF with increased vessel stiffness may result in a positive feedback loop that leads to further VSMC migration and proliferation, and thus, further progression of vascular disease. Although we do not have direct evidence to show whether survival and proliferation signals are relatively insensitive to perturbations in PDGF level in the healthy vessel wall in vivo, other in vitro findings suggest that substrate biochemical composition in a healthy vessel renders VSMC relatively insensitive to PDGF stimulation. For example when VSMCs are cultured on laminin, FAK is not phosphorylated and PDGF does not stimulate VSMC proliferation; in contrast, on fibronectin, FAK is phosphorylated and PDGF does stimulate VSMC proliferation (Morla and Mogford, 2000). As we know, in the healthy vessel, the basement membrane is intact and laminin is the major component of the ECM underlining VSMCs; however in a diseased vessel, the basement membrane is damaged and fibronectin is the major component of the ECM underlining VSMCs (Newby and Zaltsman, 2000; Thyberg et al., 1997). Morla and Mogford’s finding together with our data presented here indicate that changes in the properties of the ECM (mechanical or biochemical) during the development of vascular occlusive disease can potentially increase the sensitivity of VSMCs to PDGF stimulation.
Regardless of the initial insult that causes the onset of vascular occlusive disease, vessel stiffness increases during the development of the disease. Hence, stiffness changes can play a major role in supporting the progressive nature of the disease by enhancing PDGF signaling. Here, we offer a possible mechanism for the progression of vascular occlusive disease that should be taken under consideration when developing therapeutic interventions.
Acknowledgments
We thank Dr. Matthew Nugent at Boston University School of Medicine for helpful discussions. We also thank Dr. Andrius Kazlauskas at the Schepens Eye Research Institute for the generous gift of PDGFR-β anti-serum. The authors also acknowledge contribution to the discussion of an anonymous reviewer. This work is supported by NIH R01 HL072900 grant awarded to J.Y. Wong and DOD W81XWH-05-1-0330 grant awarded to V.M. Weaver.
Contract grant sponsor: NIH
Contract grant number: R01 HL072900
Contract grant sponsor: DOD
Contract grant number: W81XWH-05-1-0330
Literature cited
- Arcaro A, Aubert M, Espinosa del Hierro ME, Khanzada UK, Angelidou S, Tetley TD, Bittermann AG, Frame MC, Seckl MJ. Critical role for lipid raft-associated Src kinases in activation of PI3K-Akt signalling. Cellular Signalling. 2007;19(5):1081. doi: 10.1016/j.cellsig.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Barrett TB, Benditt EP. Platelet-derived growth factor gene expression in human atherosclerotic plaques and normal artery wall. Proc Natl Acad Sci U S A. 1988;85(8):2810–2814. doi: 10.1073/pnas.85.8.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloussov LV, Kazakova NI, Luchinskaia NN, Novoselov VV. Studies in developmental cytomechanic. Int J Dev Biol. 1997;41(6):793–799. [PubMed] [Google Scholar]
- Brown XQ, Ookawa K, Wong JY. Evaluation of polydimethylsiloxane scaffolds with physiologically-relevant elastic moduli: interplay of substrate mechanics and surface chemistry effects on vascular smooth muscle cell response. Biomaterials. 2005;26(16):3123–3129. doi: 10.1016/j.biomaterials.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Czirok A, Rongish BJ, Little CD. Extracellular matrix dynamics during vertebrate axis formation. Dev Biol. 2004;268(1):111–122. doi: 10.1016/j.ydbio.2003.09.040. [DOI] [PubMed] [Google Scholar]
- Decker L, ffrench-Constant C. Lipid Rafts and Integrin Activation Regulate Oligodendrocyte Survival. J Neurosci. 2004;24(15):3816–3825. doi: 10.1523/JNEUROSCI.5725-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echarri A, Muriel O, Del Pozo MA. Intracellular trafficking of raft/caveolae domains: insights from integrin signaling. Semin Cell Dev Biol. 2007;18(5):627–637. doi: 10.1016/j.semcdb.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004a;86(1 Pt 1):617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004b;166(6):877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323(5914):642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- Gaudet C, Marganski WA, Kim S, Brown CT, Gunderia V, Dembo M, Wong JY. Influence of type I collagen surface density on fibroblast spreading, motility, and contractility. Biophys J. 2003;85(5):3329–3335. doi: 10.1016/S0006-3495(03)74752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaus K, Le Lay S, Balasubramanian N, Schwartz MA. Integrin-mediated adhesion regulates membrane order. J Cell Biol. 2006;174(5):725–734. doi: 10.1083/jcb.200603034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glagov S, Newman WP, III, Schaffer SA. Pathology of the human atherosclerotic plaque. Berlin, Heidelberg, New York: Springer; 1990. [Google Scholar]
- Heldin CH, Ek B, Ronnstrand L. Characterization of the receptor for platelet-derived growth factor on human fibroblasts. Demonstration of an intimate relationship with a 185,000-Dalton substrate for the platelet-derived growth factor- stimulated kinase. J Biol Chem. 1983;258(16):10054–10061. [PubMed] [Google Scholar]
- Imanaka-Yoshida K, Matsuura R, Isaka N, Nakano T, Sakakura T, Yoshida T. Serial extracellular matrix changes in neointimal lesions of human coronary artery after percutaneous transluminal coronary angioplasty: clinical significance of early tenascin-C expression. Virchows Arch. 2001;439(2):185–190. doi: 10.1007/s004280000390. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003a;35(8):564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanosensation through integrins: Cells act locally but think globally. PNAS. 2003b;100(4):1472–1474. doi: 10.1073/pnas.0530201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg BC, DiMilla PA, Walker M, Kim S, Wong JY. Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength. Biophys J. 2009;97:1313–1322. doi: 10.1016/j.bpj.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman RJ, Duffy DC, Cherniavskaya O, Whitesides GM. Using Elastomeric Membranes as Dry Resists and for Dry Lift-Off. Langmuir. 1999;15:2973–2984. [Google Scholar]
- Jacot JG, Abdullah I, Belkin M, Gerhard-Herman M, Gaccione P, Polak JF, Donaldson MC, Whittemore AD, Conte MS. Early adaptation of human lower extremity vein grafts: Wall stiffness changes accompany geometric remodeling. J Vasc Res. 2004;39:547–555. doi: 10.1016/j.jvs.2003.09.045. [DOI] [PubMed] [Google Scholar]
- Jacot JG, Dianis S, Schnall J, Wong JY. A simple microindentation technique for mapping the microscale compliance of soft hydrated materials and tissues. J Biomed Mater Res A. 2006;79(3):485–494. doi: 10.1002/jbm.a.30812. [DOI] [PubMed] [Google Scholar]
- Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279(13):12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- Leach JB, Brown XQ, Jacot JG, Dimilla PA, Wong JY. Neurite outgrowth and branching of PC12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity. J Neural Eng. 2007;4(2):26–34. doi: 10.1088/1741-2560/4/2/003. [DOI] [PubMed] [Google Scholar]
- Lee JW, Juliano R. Mitogenic signal transduction by integrin- and growth factor receptor-mediated pathways. Mol Cells. 2004;17(2):188–202. [PubMed] [Google Scholar]
- Lee RT, Grodzinsky AJ, Frank EH, Kamm RD, Schoen FJ. Structure-dependent dynamic mechanical behavior of fibrous caps from human atherosclerotic plaques. Circulation. 1991;83(5):1764–1770. doi: 10.1161/01.cir.83.5.1764. [DOI] [PubMed] [Google Scholar]
- Liu YT, Song L, Templeton DM. Heparin suppresses lipid raft-mediated signaling and ligand-independent EGF receptor activation. J Cell Physiol. 2007;211(1):205–212. doi: 10.1002/jcp.20924. [DOI] [PubMed] [Google Scholar]
- Makoto Y, Kazuo N, Tetsuya T. Roles of lipid rafts in integrin-dependent adhesion and gp130 signalling pathway in mouse embryonic neural precursor cells. Genes to Cells. 2004;9(9):801–809. doi: 10.1111/j.1365-2443.2004.00764.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Abe H, Ohashi T, Kato Y, Sato M. Local elastic modulus of atherosclerotic lesions of rabbit thoracic aortas measured by pipette aspiration method. Physiol Meas. 2002;23(4):635–648. doi: 10.1088/0967-3334/23/4/304. [DOI] [PubMed] [Google Scholar]
- McDaniel DP, Shaw GA, Elliott JT, Bhadriraju K, Meuse C, Chung KH, Plant AL. The stiffness of collagen fibrils influences vascular smooth muscle cell phenotype. Biophys J. 2007;92(5):1759–1769. doi: 10.1529/biophysj.106.089003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4(4):E83. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- Mitsuda T, Furukawa K, Fukumoto S, Miyazaki H, Urano T, Furukawa K. Overexpression of Ganglioside GM1 Results in the Dispersion of Platelet-derived Growth Factor Receptor from Glycolipid-enriched Microdomains and in the Suppression of Cell Growth Signals. J Biol Chem. 2002;277(13):11239–11246. doi: 10.1074/jbc.M107756200. [DOI] [PubMed] [Google Scholar]
- Monastyrskaya K, Babiychuk EB, Schittny JC, Rescher U, Gerke V, Mannherz HG, Draeger A. The expression levels of three raft-associated molecules in cultivated vascular cells are dependent on culture conditions. Cell Mol Life Sci. 2003;60(12):2702–2709. doi: 10.1007/s00018-003-3307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morla AO, Mogford JE. Control of smooth muscle cell proliferation and phenotype by integrin signaling through focal adhesion kinase. Biochem Biophys Res Commun. 2000;272(1):298–302. doi: 10.1006/bbrc.2000.2769. [DOI] [PubMed] [Google Scholar]
- Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190(3):300–309. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Nikkari ST, Jarvelainen HT, Wight TN, Ferguson M, Clowes AW. Smooth muscle cell expression of extracellular matrix genes after arterial injury. Am J Pathol. 1994;144(6):1348–1356. [PMC free article] [PubMed] [Google Scholar]
- Pankov R, Markovska T, Hazarosova R, Antonov P, Ivanova L, Momchilova A. Cholesterol distribution in plasma membranes of beta1 integrin-expressing and beta1 integrin-deficient fibroblasts. Arch Biochem Biophys. 2005;442(2):160–168. doi: 10.1016/j.abb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9(4):325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J Cell Physiol. 2005;204(1):198–209. doi: 10.1002/jcp.20274. [DOI] [PubMed] [Google Scholar]
- Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15(4):237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Rajagopalan P, Marganski WA, Brown XQ, Wong JY. Direct comparison of the spread area, contractility, and migration of balb/c 3T3 fibroblasts adhered to fibronectin- and RGD-modified substrata. Biophys J. 2004;87(4):2818–2827. doi: 10.1529/biophysj.103.037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD. Membrane targeting of lipid modified signal transduction proteins. Subcell Biochem. 2004;37:217–232. doi: 10.1007/978-1-4757-5806-1_6. [DOI] [PubMed] [Google Scholar]
- Rowley JA, Mooney DJ. Alginate type and RGD density control myoblast phenotype. J Biomed Mater Res. 2002;60(2):217–223. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002;110(5):597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1(1):31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Stegemann JP, Kaszuba SN, Row SL. Advances in Vascular Tissue Engineering Using Protein-Based Biomaterials. Tissue Eng. 2007;13(11):2601–2613. doi: 10.1089/ten.2007.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehr M, Adam RM, Khoury J, Zhuang L, Solomon KR, Peters CA, Freeman MR. Platelet derived growth factor-BB is a potent mitogen for rat ureteral and human bladder smooth muscle cells: dependence on lipid rafts for cell signaling. J Urol. 2003;169(3):1165–1170. doi: 10.1097/01.ju.0000041501.01323.b9. [DOI] [PubMed] [Google Scholar]
- Thyberg J. Phenotypic modulation of smooth muscle cells during formation of neointimal thickenings following vascular injury. Histol Histopathol. 1998;13(3):871–891. doi: 10.14670/HH-13.871. [DOI] [PubMed] [Google Scholar]
- Thyberg J, Blomgren K, Roy J, Tran PK, Hedin U. Phenotypic modulation of smooth muscle cells after arterial injury is associated with changes in the distribution of laminin and fibronectin. J Histochem Cytochem. 1997;45(6):837–846. doi: 10.1177/002215549704500608. [DOI] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Hanks SK, Wang Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci U S A. 2001;98(20):11295–11300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279(5):C1345–C1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- Wong JY, Velasco A, Rajagopalan P, Pham Q. Directed Movement of Vascular Smooth Muscle Cells on Gradient-Compliant Hydrogels. Langmuir. 2003;19(5):1908–1913. [Google Scholar]