Abstract
p63 is distinct from its homologue p53 in that its role as a tumour suppressor is controversial, an issue complicated by the existence of two classes of p63 isoforms1. Here we show that TAp63 isoforms are robust mediators of senescence that inhibit tumorigenesis in vivo. Whereas gain of TAp63 induces senescence, loss of p63 enhances sarcoma development in mice lacking p53. Using a new TAp63-specific conditional mouse model, we demonstrate that TAp63 isoforms are essential for Ras-induced senescence, and that TAp63 deficiency increases proliferation and enhances Ras-mediated oncogenesis in the context of p53 deficiency in vivo. TAp63 induces senescence independently of p53, p19Arf and p16Ink4a, but requires p21Waf/Cip1 and Rb. TAp63-mediated senescence overrides Ras-driven transformation of p53-deficient cells, preventing tumour initiation, and doxycycline-regulated expression of TAp63 activates p21Waf/Cip1, induces senescence and inhibits progression of established tumours in vivo. Our findings demonstrate that TAp63 isoforms function as tumour suppressors by regulating senescence through p53-independent pathways. The ability of TAp63 to trigger senescence and halt tumorigenesis irrespective of p53 status identifies TAp63 as a potential target of anti-cancer therapy for human malignancies with compromised p53.
Determining the role of p63 in cancer has been challenging, largely because p63 encodes six different proteins. Isoforms containing or lacking an amino-terminal p53-like transactivation domain are referred to as TA and ΔN isoforms, respectively, and they have distinct, even opposing, functions1,2. ΔNp63 isoforms enhance proliferation3,4 and inhibit apoptosis5, whereas TAp63 isoforms induce apoptosis6,7 and inhibit cell-cycle progression8. Although these findings are in line with ΔNp63 and TAp63 proteins promoting and suppressing tumorigenesis, respectively, this has not been directly demonstrated.
Cellular senescence is a tumour-suppressive mechanism that prevents progression of pre-malignant lesions in vivo9 and its activation by p53 induction leads to tumour regression and clearance10,11. We previously reported that p63 deficiency induces cellular senescence in epidermal keratinocytes12. This former study ablated all p63 isoforms and therefore did not address which p63 isoform(s) regulates senescence. Here, we asked whether TAp63 isoforms, which are most similar to p53 in structure and transactivation capabilities1, could induce senescence in mouse embryonic fibroblasts (MEFs). We chose these cells as TAp63 is expressed within the dermis, the tissue from which MEFs are derived (Supplementary Information, Fig. S1a). Expression of TAp63α, TAp63β and TAp63γ in wild-type MEFs induced morphological characteristics of cellular senescence, increased senescence-associated β-galactosidase (SA-β-Gal) activity, enhanced expression of Rb and decreased proliferation (Fig. 1a; Supplementary Information, Fig. S1b, c). TAp63β and TAp63γ were more potent than TAp63α in each of these assays. As TAp63 had been associated with apoptosis in transformed cells1,6 as well as in oocytes7, we investigated whether apoptosis was occurring by analysing the sub-G1 population. Consistent with the absence of microscopically visible apoptotic cells, TAp63 expression did not alter the percentage of apoptotic cells (0.7%, 1.1%, 0.8% for vector control, TAp63β and TAp63γ, respectively). We asked whether TAp63 isoforms also induced senescence in human cells, and indeed found that this was the case (Fig. 1b). TAp63 isoforms also induced senescence in p63−/− MEFs, however ΔNp63α and ΔNp63γ could not induce senescence in either p63−/− or wild-type MEFs (Supplementary Information, Fig. S1d–g). Furthermore, ΔNp63α, but not ΔNp63γ, blocked TAp63γ-induced senescence (Supplementary Information, Fig. S1e, g). These findings indicate that expression of TAp63 is sufficient to induce cellular senescence in both mouse and human cells.
Figure 1.
TAp63 isoforms mediate senescence in mouse and human cells. (a, b) TAp63 isoforms induce cellular senescence and inhibit proliferation. (a) Wild-type MEFs infected with retroviral vectors expressing cDNA encoding TAp63α, TAp63β, TAp63γ were assayed for SA-β-Gal activity 4 days after infection (upper panels), or pulsed with BrdU 6 days after infection (lower panels). The percentage of SA-β-Gal or BrdU positive cells is shown (right). Values represent mean ± s.d. (n = 3 fields). (b) Human BJ cells transduced with TAp63α, TAp63β or TAp63γ were assayed for SA-β-Gal activity and BrdU incorporation as in a. Values represent mean ± s.d. (n = 3 fields). (c) TAp63-induced senescence requires p21 and Rb. Wild-type MEFs were co-infected with MSCV vectors expressing a TAp63 isoform with a vector control or with an shRNA specific for p21, Rb or p16. Infected cells were subjected to BrdU incorporation and flow cytometric analysis. The x-axis shows DNA content and the y-axis shows BrdU incorporation. The percentage of BrdU-positive cells is indicated. (d–f) The knockdown efficiencies of the shRNA constructs are shown. Values represent mean ± s.d. from a triplicate experiment. Scale bars in a and b, 100 μm. Vector, empty control vector.
To determine whether TAp63 functions through p21Waf1/Cip1 (p21), p16Ink4a (p16) or Rb, we used short hairpin RNAs (shRNAs) to knock down expression of these senescence mediators, and assessed whether TAp63 isoforms could still induce senescence. Focusing on TAp63β and TAp63γ, because they were the most robust senescence inducers, we found that whereas MEFs expressing TAp63β or TAp63γ became senescent, the morphological changes of senescence normally induced by TAp63 did not occur when p21 was simultaneously knocked down (Supplementary Information, Fig. S2a). Whereas 33% of empty vector-expressing control cells were proliferating, expression of TAp63β and TAp63γ decreased the BrdU (5-bromo-2-deoxyuridine)-positive populations to 18.3% and 13.7%, respectively (Fig. 1c). However, knockdown of p21 restored proliferation to levels comparable to those of empty vector-expressing control cells (Fig. 1c, d), indicating that p21 is required for TAp63-induced cell-cycle arrest. Consistent with it being essential for TAp63-induced senescence, p21 was induced upon TAp63γ expression (Supplementary Information, Fig. S2b). We also found that knockdown of Rb, but not p16, inhibited the ability of TAp63 to induce senescence (Fig. 1c, e, f; Supplementary Information, Fig. S2a), although the effect was more dramatic when p21 was depleted. As the level of p16 knockdown was not extensive, we tested whether p16 (as well as p19Arf, p19) was required for TAp63-induced senescence by expressing TAp63 isoforms in NIH 3T3 cells, which lack the Ink4a/Arf locus that encodes both p16 and p19 (Supplementary Information, Fig. S2c)13. TAp63 induced senescence in NIH 3T3 cells (Supplementary Information, Fig. S2d), indicating that p16 and p19 are dispensable for this process. These findings indicate that TAp63-induced cellular senescence is p21- and Rb-dependent, consistent with endogenous p21 and Rb being induced by TAp63.
To determine whether p63 is required for oncogene-induced senescence (OIS), a tumour-suppressive program that has been reported to require an intact p53 pathway14, we generated MEFs from p63-deficient mice15 and assessed their ability to undergo OIS. Whereas H-RasV12 induced senescence in wild-type MEFs, OIS was dramatically compromised in p63-deficient MEFs, and these cells had enhanced proliferation (Fig. 2a, b). Ras-mediated p21 expression was compromised in p63-deficient MEFs (Fig. 2c), a finding consistent with our observation that TAp63 induces p21 during senescence. Even though p63−/− cells failed to senescence in response to Ras, p63−/− Ras-expressing MEFs were distinct from p53−/− Ras-expressing MEFs in that they did not form colonies in soft agar or tumours in vivo (Supplementary Information, Fig. S3; X.G. and A.A.M., personal observation). These findings indicate that although p63 deficiency compromises Ras-induced senescence, it is distinct from p53 deficiency. This is probably due to the fact that other barriers to oncogenesis can compensate in this context.
Figure 2.
p63 deficiency compromises Ras-induced senescence and enhances sarcoma development in vivo. (a) p63 is required for Ras-induced senescence. Wild-type (left) or p63−/− (middle) MEFs expressing oncogenic H-RasV12 were assayed for SA-β-Gal activity, which was quantified (right). Values represent mean ± s.d. (n = 6 fields). (b) p63 deficiency confers a growth advantage in Ras-expressing MEFs. Colony formation assays were performed by plating cells at the indicated numbers and staining with crystal violet. (c) Immunoblot analysis for p53 and p21 in wild-type, p53−/− and p63−/− cells expressing Ras, relative to wild-type control cells. The band depicted by an asterisk is detectable in Ras-expressing MEFs. (d) p63+/−; p53−/− mice have increased sarcoma development. Tumour data is expressed as the percentage of tumours that are sarcomas (upper panels) as well as the percentage of the cohort that developed sarcomas (n = 78 and 28 for the p53−/− and p63+/−; p53−/− cohorts, respectively). (e) p63 is not expressed in mouse or human sarcomas. Histology (left) and immunohistochemical analysis for p63 expression (right) are presented, showing nuclear immunostaining for p63 in non-neoplastic squamous epithelium overlying the subcutaneous sarcomas. H&E, hematoxylin and eosin; WT, wild-type; T, tumour; N, non-neoplastic. Scale bars in a and e, 100 μm. A full scan of the blot in c is shown in Supplementary Information, Fig. S8.
We previously conducted a spontaneous tumour study in p63-compromised mice16. Analysis of tumorigenesis in the p53-deficient cohort indicated that heterozygosity of p63 significantly enhanced sarcoma development (Fig. 2d). Importantly, p63 was not detectable in sarcomas that developed in p63+/−; p53−/− mice (Fig. 2e, upper panels). We also assessed whether human sarcomas were similarly devoid of p63 expression. Whereas p63 expression was robust in non-neoplastic epithelial tissue in these sections, it was not detectable in tumours (Fig. 2e, lower panels). This absence of p63 expression in both mouse and human sarcomas is consistent with results from a previous study where 375 out of 385 human sarcomas analysed were p63-negative17, supporting the idea that p63 loss facilitates sarcomagenesis.
As OIS was compromised in the absence of p63 and TAp63 isoforms mediate senescence, we investigated whether TAp63 isoforms were essential for OIS by examining Ras-mediated senescence in cells specifically lacking TAp63. Using chromosomal engineering18, we generated a TAp63-conditional mouse model in which TAp63-specific exons were flanked by loxP sites (Supplementary Information, Fig. S4). In this model, TAp63 isoforms (but not ΔNp63 isoforms) were ablated by Cre-mediated recombination in both cultured cells and in vivo (Supplementary Information, Fig. S4d–f). Whereas MEFs prepared from both wild-type and TAp63F/F siblings were susceptible to OIS, and tamoxifen-induced Cre expression did not prevent OIS in wild-type MEFs, Cre activation in TAp63F/F MEFs compromised OIS (Fig. 3a–c). When cultured for an extended period, Ras-expressing TAp63-deficient MEFs grew to high confluency, whereas Ras-expressing wild-type MEFs remained senescent (Fig. 3a, lower panels). We converted the TAp63 floxed conditional allele to a TAp63 null allele by crossing TAp63F/+ mice with CMV-Cre mice and generated MEFs from TAp63−/− embryos (which did not have an overt phenotype) and sibling controls, allowing us to demonstrate that TAp63−/− MEFs had enhanced proliferation and compromised OIS (Fig. 3d, e). Furthermore, endogenous TAp63 was induced by Ras at the transcript level, and TAp63γ was the predominant p63 protein detectable (Fig. 3f). To directly assess the tumour-suppressive activity of TAp63 in vivo, we investigated whether TAp63 deficiency exacerbated tumori-genesis of p53-compromised, Ras-driven cells. Indeed, TAp63−/− MEFs in which p53 was knocked down in the context of oncogenic Ras had increased tumour size and decreased latency relative to control cells (Fig. 3g). These findings indicate that TAp63 inhibits proliferation, facilitates OIS and prevents tumorigenesis in vivo.
Figure 3.
TAp63 deficiency compromises Ras-induced senescence, enhances proliferation and increases tumorigenesis. (a–c) TAp63 is essential for Ras-induced senescence. (a) MEFs from wild-type (left) or TAp63 conditional (TAp63F/F, right) embryos were co-infected with CreER and H-RasV12 and assayed for senescence (a, b) and proliferation (c) in the absence or presence of the inducer 4-OHT. Values in b and c represent mean ± s.d. (n = 3 fields). (d) TAp63−/− cells have enhanced proliferation. Values represent mean ± s.d. (n = 3 wells). (e) TAp63−/− cells bypass Ras-induced senescence. MEFs expressing Ras were subjected to a foci formation assay. (f) Endogenous TAp63 is induced by oncogenic Ras at both the transcript and the protein level. Wild-type MEFs expressing either vector control or H-RasV12 were subjected to qRT-PCR analysis for TAp63 expression (left panel). Values represent mean ± s.d. (n = 3 independent experiments). Wild-type, p53−/− or p63−/− MEFs expressing Ras, or MEFs expressing exogenous TAp63α, TAp63β or TAp63γ were subjected to western blotting analysis (right panel). (g) TAp63 deficiency enhances tumorigenesis. MEFs from TAp63−/− or wild-type sibling control mice that had been simultaneously infected with H-RasV12 and shRNA against p53 were subcutaneously injected in nude mice and tumour growth was monitored. P value determined using two-way ANOVA test is shown (n = 10 sites injected). Data represent mean ± s.e.m. Scale bar in a, 100 μm. A full scan of the blot in f is shown in Supplementary Information, Fig. S8. WT, wild-type; Vector, empty control vector.
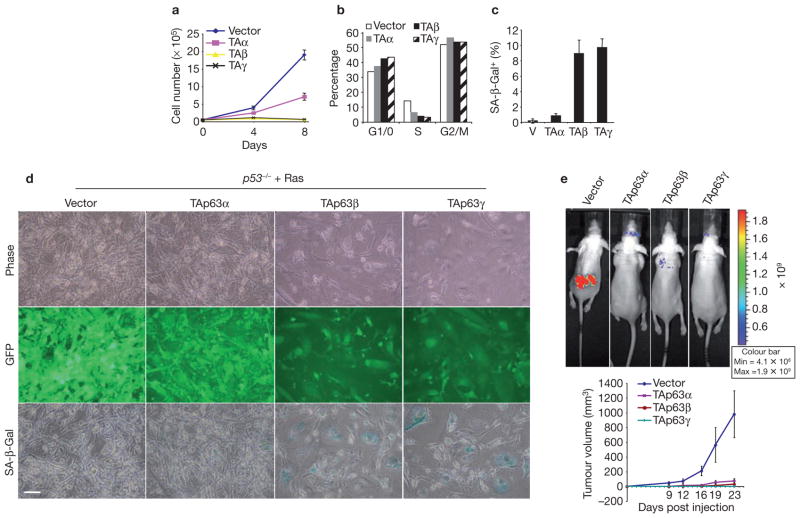
The finding that TAp63 inhibits tumorigenesis in Ras-expressing cells in which p53 has been knocked down suggests that p53 is dispensable for the tumour-suppressive function of TAp63. To demonstrate this more rigorously, we showed that TAp63α, TAp63β and TAp63γ inhibit proliferation of p53−/− MEFs (Fig. 4a). As we found previously, TAp63α was less efficient at inhibiting proliferation than the other TAp63 isoforms, which was most likely due to this isoform having a carboxy-terminal inhibitory domain19. The G1/0 population was increased, whereas the S-phase population was decreased, in TAp63 isoform-expressing p53−/− MEFs, indicating that TAp63 isoforms promote cell-cycle arrest at the G1/S transition (Fig. 4b; Supplementary Information, Fig. S5a). As in wild-type MEFs, TAp63 isoforms induced senescence in p53−/− MEFs (Fig. 4c; Supplementary Information, Fig. S5b). These observations demonstrate that TAp63 induces senescence independently of p53.
Figure 4.
TAp63 blocks Ras-driven transformation and tumour formation of p53−/− cells in vivo. (a–c) TAp63 isoforms induce senescence independently of p53. (a) TAp63 isoforms inhibit the proliferation of p53−/− MEFs. 5 × 104 cells were plated in duplicate in 6-well plates and infected with retroviral vectors expressing TAp63 isoforms, and cell numbers were counted at the indicated time points. Values represent mean ± s.d. (n = 2 wells). (b) TAp63 induces cell-cycle arrest. DNA content analysis was performed in p53−/− MEFs expressing TAp63 isoforms, and the percentage of cells in each phase of the cell cycle was plotted. (c) In p53−/− MEFs, TAp63 increases SA-β-Gal activity. p53−/− MEFs infected with MSCV vectors expressing TAp63 isoforms were assayed and SA-β-Gal activity was quantified. Values represent mean ± s.d. (n = 3 fields). (d) TAp63 isoforms induce cellular senescence and block Ras-driven transformation. p53−/− MEFs co-infected with retroviral vectors expressing H-RasV12 and either empty vector or TAp63–IRES–GFP were viewed using phase contrast (upper panels) and fluorescence (middle panels) before being assayed for SA-β-Gal activity (bottom panels). (e) TAp63 isoforms inhibit tumour formation in vivo. p53−/− MEFs expressing Ras plus empty MSCV-IRES-GFP (MIG) vector or Ras plus MIG expressing TAp63 isoforms were injected subcutaneously in nude mice and tumour growth was monitored. Representative mice were imaged at day 23. Tumour volume (bottom panel) was calculated as length × width2 × π/6. Values represent mean ± s.d. (n = 6 sites). Scale bar in d, 100 μm. Vector, empty control vector.
Aberrant mitogenic signals cooperate with tumour-suppressor loss to cause cellular transformation and tumour formation9; however, senescence can override this process. To evaluate the ability of TAp63 to induce senescence in the context of both p53 loss and oncogenic Ras expression, we co-expressed H-RasV12 and TAp63 isoforms in p53−/− MEFs. Whereas H-RasV12 transforms p53−/− MEFs, causing these cells to form rapidly growing tumours when injected subcutaneously in nude mice14,20, we found that co-expression of TAp63 isoforms and RasV12 in p53−/− MEFs led to senescence (Fig. 4d; Supplementary Information, S5). These findings are in agreement with our observations that TAp63 is essential for regulating proliferation, senescence and tumorigenesis in vivo.
To examine whether TAp63 isoforms prevent tumour development in vivo, we injected p53−/− cells expressing RasV12 plus GFP (control) or RasV12 plus TAp63 isoforms subcutaneously into nude mice and monitored for tumorigenesis. The constructs containing cDNAs encoding TAp63 proteins also expressed GFP, providing a marker for TAp63 expression that also facilitated in vivo imaging. Whereas p53−/− cells expressing RasV12 plus GFP formed rapidly growing tumours, the formation of tumours was dramatically inhibited in p53−/− cells expressing RasV12 in combination with TAp63α, TAp63β or TAp63γ; again, TAp63β and TAp63γ were the most effective (Fig. 4e). As we did not drug select for infected cells, TAp63-non-expressing cells were probably present in the injected pool. Indeed, the small tumours that eventually developed in mice injected with cells transduced with RasV12 plus TAp63α (5 out of 6) or TAp63β (6 out of 6) were GFP-negative, indicating that they were derived from RasV12-expressing p53−/− cells in which TAp63 was not expressed. These observations indicate that exogenous expression of TAp63 isoforms prevents oncogene-mediated tumour formation of p53-deficient cells.
Given our findings that TAp63 induces senescence and blocks tumour initiation, we investigated whether TAp63γ was potent enough to induce senescence in cells that were already transformed and tumorigenic. We constructed a tetracycline-inducible system where TAp63γ was expressed under the control of the TREtight promoter, and coupled it to an IRES-GFP cassette, which allows the monitoring of TAp63γ expression in cultured cells as well as in vivo (Supplementary Information, Fig. S6a). p53−/− MEFs were co-transduced with a bicistronic retrovirus expressing N-RasG12D along with the tetracycline reverse transactivator protein (rtTA), as well as a retrovirus expressing either TRE–GFP or TRE–TAp63γ/GFP. In the absence of the inducer, doxycycline (Dox), TAp63γ/GFP expression was not detectable and p53−/− cells harbouring either TRE–GFP control or TRE–TAp63γ/GFP showed a spindle-shaped morphology characteristic of transformed cells. However, in the presence of Dox, TAp63γ, GFP and p21 were induced (Supplementary Information, Fig. S6b, c). In response to Dox, cells infected with TRE–GFP continued to proliferate, whereas cells infected with TRE–TAp63γ/GFP became senescent in a dose-dependent manner (Supplementary Information, Fig. S6b, d–f). Regulated expression of TAp63γ also induced senescence in human BJ (Supplementary Information, Fig. S7a), IMR90 and LFS041 cells (X.G. and A.A.M., personal observation). These findings indicate that TAp63γ induces cellular senescence and inhibits proliferation of transformed cells.
To test whether TAp63 could cease the progression of tumours in vivo, we injected p53−/− cells expressing rtTA/N-Ras plus TRE–TAp63γ/GFP into nude mice. Whereas these cells formed large tumours in untreated mice, tumours did not progress in Dox-treated mice (Fig. 5a). Dox administration caused robust GFP expression 24 h after treatment, with the GFP intensity declining rapidly during the following days (Fig. 5a, middle panels). Whereas tumours in Dox-treated mice stopped growing and did not progress during the 4 days following Dox treatment, tumours eventually formed, which was most likely due to the outgrowth of cells with low or absent TAp63 expression. Indeed, as we described above, these escapers were GFP-negative (Fig. 5a, middle panels). Thus, regulated expression of TAp63γ effectively halts the progression of established tumours in vivo.
Figure 5.
Regulated expression of TAp63γ induces senescence and halts tumour progression in vivo. (a) TAp63 expression halts tumour progression. p53−/− MEFs expressing N-RasG12D/rtTA and TRE-TAp63γ/GFP were injected subcutaneously into nude mice, and tumour growth was monitored in untreated mice (upper panels) or mice treated with Dox (middle panels) by measuring and in vivo imaging. Representative tumour growth in untreated (red) and Dox-treated (black) mice is shown (lower panel). (b) Hematoxylin and eosin (H&E) staining (upper panels) and SA-β-Gal assay (lower panels) of tumour sections from untreated mice or mice treated with Dox for 2 days. (c) Immunofluorescence for p63 and p21 expression in tumours. (d) Immunoblotting analysis for p63 and β-actin. Scale bars in b and c, 50 μm. A full scan of the blot in d is shown in Supplementary Information, Fig. S8.
To determine whether this abrupt shutdown of tumour progression was due to cellular senescence, we performed SA-β-Gal assays on tumour sections. We found that senescent cells were much more prevalent in tumours from Dox-treated mice than in tumours from untreated mice (Fig. 5b). Immunofluorescent analysis revealed that at day 2 both TAp63γ and its target, p21, were significantly induced in tumours from Dox-treated mice (Fig. 5c). This enhanced p21 expression observed at the protein level is consistent with our finding that p21 transcript is induced and that p63 is bound to the p21 promoter in TAp63γ-expressing p53−/− MEFs (Supplementary Information, Fig. S7b). By day 4, TAp63γ-expressing cells were virtually undetectable in the tumour (Fig. 5c, d), suggesting that TAp63γ-expressing tumour cells are rapidly lost. This clearance of cells that had become senescent in response to TAp63γ induction could be through the innate immune response10. These findings indicate that regulated expression of TAp63γ induces p21 and cellular senescence, which halts tumour progression in vivo.
Our observations demonstrate that TAp63 isoforms are potent inducers of senescence that prevent cancer. The findings that p53 is dispensable for TAp63-mediated senescence and that TAp63 deficiency increases the tumorigenicity of p53-deficient Ras-expressing cells highlight the potent tumour-suppressive function of TAp63. Unlike p53, which induces senescence in response to oncogenic stimuli21, TAp63 can induce senescence on its own. TAp63 isoforms induce senescence in wild-type cells, as well as in cells that have lost nodal tumour-suppressive pathways, such as p16, p19 and p53. Notably, TAp63 triggers cellular senescence in p53−/− cells both before and after they have been transformed by oncogenic Ras, halting tumour progression. Thus, expression of TAp63 is tumour protective at both the initiation and progression stages.
We reported previously that p63 deficiency causes cellular senescence in proliferating keratinocytes of the skin, leading to accelerated ageing in vivo 12. Although the role of different p63 isoforms was not addressed, we found recently that ΔNp63α — the predominant isoform expressed in keratinocytes1,22, which has a pro-proliferative function3,4,23 — regulates senescence in this setting (W.M.K. and A.A.M., unpublished data). Here, we identify TAp63γ as the isoform induced in MEFs in response to oncogenic Ras and define TAp63 isoforms as essential mediators of senescence. In yet another setting — skin-derived progenitor cells of the hair follicle, which are distinct from both keratinocytes and MEFs — specific loss of TAp63 isoforms has been reported to induce hyperproliferation that culminates in senescence24. Whereas loss of TAp63 in both of these settings enhances proliferation, it remains to be determined whether TAp63 is an essential mediator for OIS in skin-derived progenitor cells, as we found in MEFs. Thus, the finding that TAp63 induces senescence in fibroblasts complements and augments our previous discovery in keratinocytes, adding to the growing list of tissue-specific settings in which senescence occurs, and indicating isoform-specific roles of p63 proteins in senescence regulation.
Since senescence is a barrier against tumour progression in vivo, activation of this program in tumour cells10,11, especially in those that are resistant to chemotherapy-induced cell death due to genetic lesions such as p53 loss, provides a rationale for anti-cancer therapy. Our study suggests that robust induction of TAp63 is one such therapeutic approach. This strategy is especially promising in light of the p53- and p16-independence of senescence induction by TAp63 and given the fact that most human cancers have defective p53 (ref. 25) or frequent loss of INK4a (ref. 26), but retain the p63 locus27–29. Additional strategies that stabilize TAp63 (refs 8, 30) or to inhibit pathways that negatively regulate TAp63 activity, such as the common negative regulators of p53 family members, iASPP (refs 31–33), could provide effective approaches for cancer therapy.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturecellbiology/.
Supplementary Material
Acknowledgments
We thank D. Burgess, A. Bric, R. Dickins, P. Moody and L. Rodgers for suggestions, and L. Bianco and staff for assistance. A.A.M. and X.G. were supported by an American Cancer Society Research Scholar Award.
Footnotes
Note: Supplementary Information is available on the Nature Cell Biology website.
AUTHOR CONTRIBUTIONS
X.G. and A.A.M. designed and performed experiments, analysed data and prepared the manuscript. W.M.K. performed BrdU immunohistochemistry and immunofluorescent staining; C.P. and W.L. performed western blotting analyses; H.V. performed histopathology; J.Z. and S.W.L. designed and constructed the Tet-on system, which formed the basis of the TAp63-specific inducible expression system.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Yang A, et al. p63, a p53 homolog at 3q27–29, encodes multiple products with trans-activating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 2.Wu G, et al. ΔNp63α and TAp63α regulate transcription of genes with distinct biological functions in cancer and development. Cancer Res. 2003;63:2351–2357. [PubMed] [Google Scholar]
- 3.Hibi K, et al. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci USA. 2000;97:5462–5467. doi: 10.1073/pnas.97.10.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patturajan M, et al. ΔNp63 induces β-catenin nuclear accumulation and signaling. Cancer Cell. 2002;1:369–379. doi: 10.1016/s1535-6108(02)00057-0. [DOI] [PubMed] [Google Scholar]
- 5.Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Gressner O, et al. TAp63α induces apoptosis by activating signaling via death receptors and mitochondria. EMBO J. 2005;24:2458–2471. doi: 10.1038/sj.emboj.7600708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh EK, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–628. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- 8.Gallegos JR, et al. SCF TrCP1 activates and ubiquitylates TAp63γ. J Biol Chem. 2008;283:66–75. doi: 10.1074/jbc.M704686200. [DOI] [PubMed] [Google Scholar]
- 9.Narita M, Lowe SW. Senescence comes of age. Nature Med. 2005;11:920–922. doi: 10.1038/nm0905-920. [DOI] [PubMed] [Google Scholar]
- 10.Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ventura A, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 12.Keyes WM, et al. p63 deficiency activates a program of cellular senescence and leads to accelerated aging. Genes Dev. 2005;19:1986–1999. doi: 10.1101/gad.342305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quelle DE, Zindy F, Ashmun RA, Sherr CJ. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995;83:993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 14.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 15.Mills AA, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 16.Keyes WM, et al. p63 heterozygous mutant mice are not prone to spontaneous or chemically induced tumors. Proc Natl Acad Sci USA. 2006;103:8435–8440. doi: 10.1073/pnas.0602477103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CH, et al. Gene expression profiling identifies p63 as a diagnostic marker for giant cell tumor of the bone. Mod Pathol. 2008;21:531–539. doi: 10.1038/modpathol.3801023. [DOI] [PubMed] [Google Scholar]
- 18.Zheng B, Mills AA, Bradley A. A system for rapid generation of coat color-tagged knockouts and defined chromosomal rearrangements in mice. Nucleic Acids Res. 1999;27:2354–2360. doi: 10.1093/nar/27.11.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serber Z, et al. A C-terminal inhibitory domain controls the activity of p63 by an intramolecular mechanism. Mol Cell Biol. 2002;22:8601–8611. doi: 10.1128/MCB.22.24.8601-8611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka N, et al. Cellular commitment to oncogene-induced transformation or apoptosis is dependent on the transcription factor IRF-1. Cell. 1994;77:829–839. doi: 10.1016/0092-8674(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 21.Ferbeyre G, et al. Oncogenic ras and p53 cooperate to induce cellular senescence. Mol Cell Biol. 2002;22:3497–3508. doi: 10.1128/MCB.22.10.3497-3508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liefer KM, et al. Down-regulation of p63 is required for epidermal UV-B-induced apoptosis. Cancer Res. 2000;60:4016–4020. [PubMed] [Google Scholar]
- 23.Westfall MD, Mays DJ, Sniezek JC, Pietenpol JA. The ΔNp63 α phospho-protein binds the p21 and 14-3-3 σ promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Mol Cell Biol. 2003;23:2264–2276. doi: 10.1128/MCB.23.7.2264-2276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su X, et al. TAp63 prevents premature aging by promoting adult stem cell maintenance. Cell Stem Cell. 2009;5:64–75. doi: 10.1016/j.stem.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 26.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 27.Osada M, et al. Cloning and functional analysis of human p51, which structurally and functionally resembles p53. Nature Med. 1998;4:839–843. doi: 10.1038/nm0798-839. [DOI] [PubMed] [Google Scholar]
- 28.Sunahara M, et al. Mutational analysis of p51A/TAp63γ, a p53 homolog, in non-small cell lung cancer and breast cancer. Oncogene. 1999;18:3761–3765. doi: 10.1038/sj.onc.1202972. [DOI] [PubMed] [Google Scholar]
- 29.Moll UM, Slade N. p63 and p73: roles in development and tumor formation. Mol Cancer Res. 2004;2:371–386. [PubMed] [Google Scholar]
- 30.MacPartlin M, Zeng SX, Lu H. Phosphorylation and stabilization of TAp63γ by IkappaB kinase-β. J Biol Chem. 2008;283:15754–15761. doi: 10.1074/jbc.M801394200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergamaschi D, et al. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nature Genet. 2003;33:162–167. doi: 10.1038/ng1070. [DOI] [PubMed] [Google Scholar]
- 32.Robinson RA, Lu X, Jones EY, Siebold C. Biochemical and structural studies of ASPP proteins reveal differential binding to p53, p63, and p73. Structure. 2008;16:259–268. doi: 10.1016/j.str.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Bell HS, et al. A p53-derived apoptotic peptide derepresses p73 to cause tumor regression in vivo. J Clin Invest. 2007;117:1008–1018. doi: 10.1172/JCI28920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.